Abstract
Postmarketing surveillance of Japanese patients with unresectable, previously treated, advanced or recurrent non‐small‐cell lung cancer treated with nivolumab was undertaken during the conditional approval period. The study aim was to evaluate the occurrence of treatment‐related adverse events of nivolumab in the real world. Patients were registered between December 2015 and March 2016 at 536 sites. Nivolumab was given intravenously (3 mg/kg every 2 weeks); the observation period was 12 months after the first dose of nivolumab. Patients were evaluated for safety (n = 3601; 18.2% ≥75 years, 22.4% ECOG performance status ≥2) and effectiveness (n = 3570). The frequencies of any grade and grade 3 or higher treatment‐related adverse events were 47.1% and 15.9%, respectively. The most frequent treatment‐related adverse events (any grade) were interstitial lung disease (6.4%), hypothyroidism (5.7%), and diarrhea (4.4%). Treatment‐related adverse events of special interest (priority items) occurring at a frequency of 5% or more were adverse events related to interstitial lung disease, thyroid dysfunction, liver dysfunction, colitis/severe diarrhea, infusion reaction, and infusion reaction within 24 hours. Significant risk factors for these priority items were identified by competing risk analysis: interstitial lung disease (previous/comorbid interstitial lung disease, abnormal findings on chest imaging, and smoking history); liver dysfunction (previous/comorbid liver disease, smoking history, and metastasis); thyroid dysfunction (previous/comorbid thyroid disease and performance status); and colitis/severe diarrhea (treatment line 2 vs ≥3). The 12‐month survival rate was 40.7%. In conclusion, the safety profile of nivolumab in this postmarketing surveillance was similar to that in clinical trials, and no new safety signals were identified. The study was registered with the Japan Pharmaceutical Information Center (clinicaltrials.jp: Japic‐163271).
Keywords: Japan, nivolumab, non‐small‐cell lung cancer, postmarketing surveillance, real‐world data
Postmarketing surveillance of Japanese patients with unresectable, previously treated, advanced or recurrent non‐small‐cell lung cancer treated with nivolumab was undertaken during the conditional approval period. The safety profile of nivolumab in this postmarketing surveillance was similar to that in clinical trials, and no new safety signals were identified.

Abbreviations
- AE
adverse event
- CT
computed tomography
- ICI
immune checkpoint inhibitor
- ILD
interstitial lung disease
- NSCLC
non‐small‐cell lung cancer
- OS
overall survival
- PMS
postmarketing surveillance
- PS
performance status
- SAF
safety analysis set
- TRAE
treatment‐related adverse event
1. INTRODUCTION
Worldwide, lung cancer is a leading cause of cancer‐related deaths. 1 In Japan, lung cancer has the highest incidence (13.5%) and mortality rate (19.6%) compared with other major cancer types. 2 The predominant type of lung cancer is NSCLC, which can be divided into squamous and nonsquamous histological subtypes. 3 Traditionally, treatment for stage IV NSCLC consisted of platinum‐containing chemotherapy in the first instance, and among patients with disease progression, treatment then consisted of docetaxel with limited alternative regimens. 4 However, new drugs with novel mechanisms of action—including antiangiogenesis agents, ICIs, and tyrosine kinase inhibitors—have provided new treatment options. 4 , 5 Currently, first‐line standard treatment is centered on ICIs, and an ICI is recommended in second‐line treatment for patients who did not receive an ICI in the first line. 4 , 5
Nivolumab is a programmed cell death‐1 ICI. This fully humanized mAb is approved for the treatment of a number of cancer types, including NSCLC. 6 Two international phase III studies (CheckMate 017 and CheckMate 057) were instrumental in the approval of nivolumab for the treatment of NSCLC. 7 , 8 Superior OS and response rates were reported with nivolumab treatment compared with docetaxel in patients with previously treated NSCLC. 7 , 8 The efficacy and safety of nivolumab were examined in two phase II studies in Japanese patients with advanced or recurrent squamous 9 (ONO‐4538‐05; N = 35) or nonsquamous 10 (ONO‐4538‐06; N = 76) NSCLC. The primary end‐point was overall response rate assessed by independent radiology review, which was 25.7% and 22.4% in squamous 9 and nonsquamous NSCLC, 10 respectively, and the safety profile was acceptable. Although the results of these phase II studies supported the approval of nivolumab in the treatment of Japanese patients with squamous or nonsquamous NSCLC, the number of patients examined was small. Therefore, as a condition for the approval of the use of nivolumab for unresectable advanced/recurrent NSCLC in Japan, all‐case PMS was required in order to acquire real‐world safety data.
The purpose of this PMS was to understand the occurrence of TRAEs of nivolumab in the real‐world setting after approval, especially those described as important identified risks (TRAEs of special interest) in the drug risk management plan, and to examine the patient characteristics that could affect safety. The 6‐ and 12‐month survival rates were evaluated as effectiveness outcomes.
2. MATERIALS AND METHODS
2.1. Study design
This prospective all‐case PMS was undertaken at 536 medical facilities in Japan. Patient registration occurred between 17 December 2015 and 30 June 2019. This report presents results from patients registered between 17 December 2015 and 31 March 2016. For PMS, signed contracts were required between the facilities and the study sponsor. The study was registered with the Japan Pharmaceutical Information Center (clinicaltrials.jp: Japic‐163271) and was carried out in accordance with the Good Postmarketing Study Practice Ministerial Ordinance in Japan. Data were collected by case report forms.
2.2. Study population and treatment protocol
Patients with unresectable, previously treated, advanced or recurrent NSCLC received nivolumab intravenously at a dosing regimen of 3 mg/kg (body weight) every 2 weeks. The observation period was 12 months after the first dose of nivolumab. For patients who discontinued nivolumab within 12 months, outcomes were followed up as much as possible until the end of the observation period.
2.3. Patient characteristics
Demographic data included age and sex. Baseline clinical data included the following: ECOG PS, smoking history, previous or comorbid liver disease, kidney disease, lung disease, thyroid disease, or autoimmune disease, abnormal findings except for lung cancer on chest imaging, NSCLC histological type, metastases, and treatment line.
2.4. Outcome measure
Details of AEs were collected by physicians. Type, grade, and frequency of AEs were assessed and evaluated using the Japanese translation of the NCI’s Common Terminology Criteria for Adverse Events version 4.0. Adverse events were classified using the Medical Dictionary for Regulatory Activities/Japanese version 21.1 and indicated by system organ class and preferred term. Priority items were TRAEs related to ILD, myasthenia gravis or myositis, colitis or severe diarrhea, type 1 diabetes, liver dysfunction, thyroid dysfunction, nerve disorder, renal disorder, adrenal disorder, encephalitis, severe skin disorder, venous thromboembolism, infusion reaction at any time, infusion reaction within 24 hours, and cardiac disorders (eg, atrial fibrillation, bradycardia, and premature ventricular contraction) and were treated as TRAEs of special interest. Each TRAE of special interest consisted of an aggregation of TRAEs with related preferred terms. Survival rate at 6 and 12 months after the initiation of nivolumab was calculated as the proportion of patients who were confirmed to be alive at 6 and 12 months, respectively.
2.5. Statistical analysis
The SAF consisted of patients who received one or more doses of nivolumab (Figure 1). The effectiveness analysis set consisted of patients in the SAF excluding those with an indication other than NSCLC, patients on first‐line treatment, and patients with a deviation from the nivolumab dosing regimen (Figure 1).
FIGURE 1.

Patient flow diagram showing the recruitment of Japanese patients with non‐small‐cell lung cancer treated with nivolumab
Subgroup analysis based on patient characteristics (Table S1) was carried out on the incidence of TRAEs. Fisher's exact test, Wilcoxon rank sum test, or χ2 test was used to compare between the subgroups. To identify patient characteristics that could affect the development of TRAEs of special interest occurring at a frequency of 5% or more, competing risk analyses were undertaken using the Fine and Gray proportional subdistribution hazards model. 11 The details of model selection are provided in Document S1 and Tables S2‐S5. All statistical analyses were carried out using SAS statistical software (SAS Institute Japan) version 9.4 TS1M4.
3. RESULTS
3.1. Patient characteristics
A total of 3789 patients who registered between 17 December 2015 and 31 March 2016 were targeted for inclusion in this study; of these, 3612 were selected for collection of case report forms. A total of 3601 patients were included in the safety analysis, and 3570 were included in the effectiveness analysis (Figure 1). The reasons for exclusion from the safety analysis were no nivolumab dose received (n = 6) and consent for inclusion in this publication was not provided (n = 5). A total of 31 patients were excluded from the effectiveness analysis because they had not received prior chemotherapy treatment (n = 29) and off‐label use of nivolumab (primary malignant melanoma of the lung, n = 1; kidney cancer, n = 1). The majority of patients in the safety analysis were male (71.9%), aged less than 75 years (81.8%), with a smoking history (73.8%) (Table 1). Patients with an ECOG PS 2‐4 (22.4%) were included in this study. The main NSCLC histological type was adenocarcinoma (66.0%), and metastasis was present in 79.6% of patients. Previous or comorbid liver disease was reported by 6.3% of patients, and previous or comorbid ILD was reported by 6.1% of patients. The majority of patients (78.2%) were on their third line of treatment.
TABLE 1.
Characteristics of Japanese patients with non‐small‐cell lung cancer treated with nivolumab
| Characteristic | N = 3601 |
|---|---|
| Sex | |
| Male | 2590 (71.9) |
| Female | 1011 (28.1) |
| Age | |
| <75 years | 2944 (81.8) |
| ≥75 years | 657 (18.2) |
| ECOG PS | |
| 0‐1 | 2793 (77.6) |
| 2‐4 | 807 (22.4) |
| Unknown | 1 (<0.1) |
| Smoking history | |
| Nonsmoker | 871 (24.2) |
| Current or past smoker | 2656 (73.8) |
| Unknown | 74 (2.1) |
| Histology | |
| Adenocarcinoma | 2378 (66.0) |
| Squamous cell carcinoma | 1020 (28.3) |
| Other | 237 (6.6) |
| Previous or comorbid disease | |
| Liver | 228 (6.3) |
| Kidney | 172 (4.8) |
| ILD | 221 (6.1) |
| Thyroid | 168 (4.7) |
| Autoimmune disease | 127 (3.5) |
| Abnormal findings on chest imaging (CT) | |
| Yes | 777 (22.6) |
| No | 2648 (77.2) |
| Unknown | 7 (0.2) |
| Metastasis | 2867 (79.6) |
| Treatment line | |
| Second | 748 (20.8) |
| Third | 2816 (78.2) |
| Number of nivolumab doses received, median (range) | 4 (1‐46) |
Data are n (%), except where indicated.
Abbreviations: CT, computed tomography; ECOG, Eastern Cooperative Oncology Group; ILD, interstitial lung disease; PS, performance status.
3.2. Nivolumab treatment
The median (range) number of nivolumab doses received by patients in the SAF was four (1‐46) (Table S6). For patients aged 75 years or older, the median (range) was four (1‐41), and for those patients with an ECOG PS 2‐4, the median (range) was two (1‐31).
3.3. Safety
The overall incidence of TRAEs (any grade) was 47.1% (1697/3601 patients); TRAEs occurring at an incidence of 2% or higher in either the PMS or phase II clinical studies 9 , 10 are presented in Table 2. The most frequent TRAEs by preferred term occurring in more than 4% of patients in the SAF were ILD (6.4%), hypothyroidism (5.7%), and diarrhea (4.4%). The incidence of TRAEs of grade 3 or higher was 15.9%. Overall, 87.0% of patients had discontinued treatment with nivolumab; reasons for discontinuation were progression of the underlying disease (including death) (48.9%), lack of effectiveness (31.3%), an AE (17.9%), changing treating hospital (1.7%), other (1.5%), and effectiveness confirmed (1.2%). For 96 deaths, a relationship to nivolumab could not be ruled out. The most common TRAEs resulting in death were ILD (n = 27), disseminated intravascular coagulation (n = 6), pneumonia (n = 6), and lung disorder (n = 6).
TABLE 2.
Treatment‐related adverse events (any grade) occurring at an incidence of 2% or higher either before approval or during this postmarketing surveillance (PMS) of nivolumab in Japanese patients with non‐small‐cell lung cancer
|
System organ class Preferred term, n (%) |
Japanese phase II studies 9 , 10 (N = 111) | PMS study (N = 3601) |
|---|---|---|
| Blood and lymphatic system disorders | ||
| Anemia | 3 (2.7) | 18 (0.5) |
| Endocrine disorders | ||
| Hyperthyroidism | 2 (1.8) | 80 (2.2) |
| Hypothyroidism | 7 (6.3) | 205 (5.7) |
| Metabolism and nutritional disorders | ||
| Hyponatremia | 4 (3.6) | 14 (0.4) |
| Decreased appetite | 16 (14.4) | 80 (2.2) |
| Nervous system disorders | ||
| Dizziness | 4 (3.6) | 5 (0.1) |
| Dysgeusia | 3 (2.7) | 11 (0.3) |
| Respiratory, thoracic, and mediastinal disorders | ||
| Interstitial lung disease | 5 (4.5) | 232 (6.4) |
| Oropharyngeal pain | 3 (2.7) |
0 (0) |
| Gastrointestinal disorders | ||
| Constipation | 6 (5.4) | 17 (0.5) |
| Diarrhea | 6 (5.4) | 160 (4.4) |
| Nausea | 11 (9.9) | 48 (1.3) |
| Stomatitis | 4 (3.6) | 20 (0.6) |
| Vomiting | 5 (4.5) | 18 (0.5) |
| Hepatobiliary disorders | ||
| Hepatic function abnormal | 1 (0.9) | 87 (2.4) |
| Skin and subcutaneous tissue disorders | ||
| Dermatitis acneiform | 5 (4.5) | 10 (0.3) |
| Erythema | 3 (2.7) | 6 (0.2) |
| Itching | 7 (6.3) | 62 (1.7) |
| Pruritus | 16 (14.4) | 134 (3.7) |
| Rash maculopapular | 6 (5.4) | 13 (0.4) |
| Musculoskeletal and connective tissue disorders | ||
| Arthralgia | 5 (4.5) | 19 (0.5) |
| Myalgia | 4 (3.6) | 18 (0.5) |
| General disorders and administration site conditions | ||
| Fatigue | 10 (9.0) | 23 (0.6) |
| Malaise | 16 (14.4) | 109 (3.0) |
| Peripheral edema | 3 (2.7) | 11 (0.3) |
| Pyrexia | 16 (14.4) | 128 (3.6) |
| Investigations | ||
| Alanine aminotransferase increased | 3 (2.7) | 92 (2.6) |
| Aspartate aminotransferase increased | 4 (3.6) | 117 (3.2) |
| Blood creatinine phosphokinase increased | 4 (3.6) | 14 (0.4) |
| Blood creatinine increased | 4 (3.6) | 20 (0.6) |
| Eosinophil count increased | 3 (2.7) | 7 (0.2) |
| Lymphocyte count decreased | 9 (8.1) | 1 (0.0) |
| Injury, poisoning, and procedural complications | ||
| Infusion‐related reaction | 3 (2.7) | 127 (3.5) |
Classified using the Medical Dictionary for Regulatory Activities/Japanese version 21.1.
In patients aged 75 years or older (18.2%), the incidence of TRAEs of grade 3 or higher was 16.4%. Treatment‐related AEs occurring at grade 3 or higher in five or more patients aged 75 years or older were ILD (3.3%), pneumonia (1.2%), diarrhea (0.9%), and hepatic function abnormal (0.8%) (Table S7). In patients with an ECOG PS 2 or higher (22.4%), the incidence of TRAEs grade 3 or higher was 17.8%. Treatment‐related AEs occurring at grade 3 or higher in five or more patients with an ECOG PS 2 or higher were: ILD (3.2%), pneumonia and increased alanine aminotransferase (0.7% each), and bacterial pneumonia, pneumonitis, abnormal hepatic function, malaise, and increased γ‐glutamyltransferase (0.6% each) (Table S8). In patients with an ECOG PS 3 or higher (5.6%), the main TRAEs of grade 3 or higher in three or more patients were ILD (3.0%) and pneumonia (1.5%) (Table S9).
Multiple patient characteristics were shown to have a statistically significant effect on the incidence of TRAEs (Table S10), including ECOG PS, smoking history, medical history, previous or comorbid disease (liver, kidney, heart, lungs, thyroid gland, ILD, emphysema/chronic obstructive pulmonary disease, and lung infection), and abnormal findings on chest imaging (X‐ray and CT). The incidence of TRAEs was low in patients with poor ECOG PS, and a statistically significant difference was observed (ECOG PS 1‐4, Wilcoxon rank sum test, P = .0409; ECOG PS 0‐1 vs 2‐4, Wilcoxon rank sum test, P = .0003) (Table S10).
3.4. Treatment‐related AEs of special interest
In this PMS, each TRAE of special interest consisted of an aggregation of TRAEs with related preferred terms. Any‐grade TRAEs of special interest occurring at a frequency of 5% or higher in the PMS were ILD (9.6%), infusion reaction (9.6%), thyroid dysfunction (9.1%), liver dysfunction (7.9%), infusion reaction within 24 hours (5.6%), and colitis/severe diarrhea (5.5%) (Table 3). Compared with the Japanese phase II clinical studies, ILD was the only TRAE of special interest (any grade) that occurred at more than one percentage point higher frequency in the PMS (Table 3). In comparison with the international phase III clinical studies, TRAEs of special interest (any grade) occurring at more than one percentage point higher frequency in the PMS were ILD, liver dysfunction, thyroid dysfunction, and adrenal disorder.
TABLE 3.
Treatment‐related adverse events (TRAEs) of special interest in postmarketing surveillance (PMS) compared with Japanese phase II and international phase III clinical studies of nivolumab for non‐small‐cell lung cancer
| TRAEs of special interest a | PMS (N = 3601) b | Japanese phase II studies 9 , 10 (N = 111) c | International phase III studies 7 , 8 (N = 418) c | |||
|---|---|---|---|---|---|---|
| Any grade | Grade ≥3 | Any grade | Grade ≥3 | Any grade | Grade ≥3 | |
| ILD d | 344 (9.6) | 141 (3.9) | 8 (7.2) | 2 (1.8) | 16 (3.8) | 4 (1.0) |
| Myasthenia gravis/myositis | 10 (0.3) | 5 (0.1) | 0 (0) | 0 (0) | 1 (0.2) | 1 (0.2) |
| Colitis/severe diarrhea | 199 (5.5) | 52 (1.4) | 7 (6.3) | 1 (0.9) | 33 (7.9) | 3 (0.7) |
| Type 1 diabetes | 13 (0.4) | 12 (0.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Liver dysfunction | 285 (7.9) | 72 (2.0) | 11 (9.9) | 2 (1.8) | 21 (5.0) | 4 (1.0) |
| Thyroid dysfunction | 326 (9.1) | 12 (0.3) | 15 (13.5) | 0 (0) | 32 (7.7) | 0 (0) |
| Nerve disorder | 27 (0.7) | 6 (0.2) | 2 (1.8) | 0 (0) | 5 (1.2) | 0 (0) |
| Renal disorder | 53 (1.5) | 10 (0.3) | 6 (5.4) | 0 (0) | 11 (2.6) | 1 (0.2) |
| Adrenal disorder | 48 (1.3) | 16 (0.4) | 2 (1.8) | 0 (0) | 0 (0) | 0 (0) |
| Encephalitis | 3 (0.1) | 2 (0.1) | 0 (0) | 0 (0) | 1 (0.2) | 1 (0.2) |
| Severe skin disorder | 16 (0.4) | 9 (0.2) | 0 (0) | 0 (0) | 2 (0.5) | 1 (0.2) |
| Venous thromboembolism | 22 (0.6) | 10 (0.3) | 1 (0.9) | 0 (0) | 1 (0.2) | 1 (0.2) |
| Infusion reaction (within 24 h) | 201 (5.6) | 12 (0.3) | NA | NA | NA | NA |
| Infusion reaction | 346 (9.6) | 38 (1.1) | 30 (27.0) | 1 (0.9) | 89 (21.3) | 4 (1.0) |
| Cardiac disorders | 27 (0.7) | 11 (0.3) | 2 (1.8) | 0 (0) | 7 (1.7) | 2 (0.5) |
Abbreviations: ILD, interstitial lung disease; NA, not available.
Includes all adverse events related to the TRAEs of special interest.
Safety analysis set.
Analysis period was from the start date of study drug to 28 days after the final study drug administration or the start date of posttreatment after the final study drug administration, whichever was earlier.
Includes the preferred terms: bronchiolitis, eosinophilic pneumonia, ILD, lung disorder, lung infiltration, organizing pneumonia, pneumonitis, pulmonary alveolar hemorrhage, pulmonary fibrosis, diffuse alveolar damage, and radiation pneumonitis.
Time to onset for the TRAEs of special interest is shown in Figure 2. Median (range) time to onset for TRAEs of special interest occurring at a frequency of 5% or more in the PMS was 55 (1‐365) days for ILD, 57 (1‐344) days for thyroid dysfunction, 30 (1‐344) days for liver dysfunction, 16 (1‐338) days for infusion reaction, 2 (1‐338) days for infusion reaction within 24 hours, and 48 (1‐329) days for colitis/severe diarrhea.
FIGURE 2.
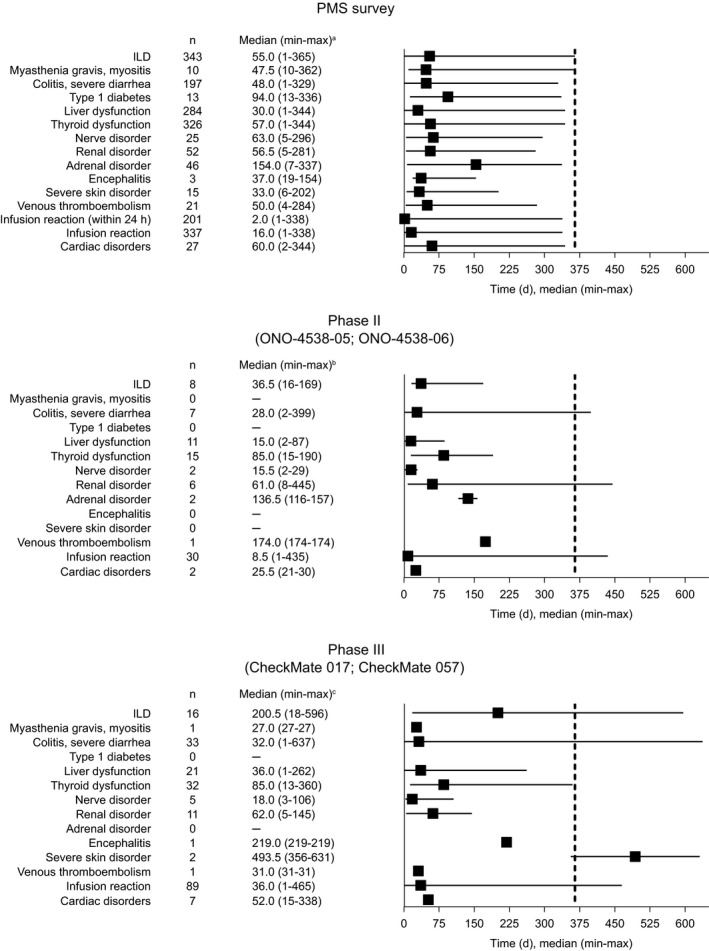
Summary statistics of time to onset of treatment‐related adverse events of special interest during postmarketing surveillance (PMS) of Japanese patients with non‐small‐cell lung cancer treated with nivolumab, the Japanese phase II clinical studies (ONO‐4538‐05 and ONO‐4538‐06), 9 , 10 and the international phase III studies (CheckMate 017 and CheckMate 057). 7 , 8 aIf an adverse event of special interest occurred more than once in the same patient, the earliest onset date was included in the analysis. bThe period from the start date of nivolumab treatment to 28 days after the final treatment with nivolumab or the start date of postnivolumab treatment, whichever is earlier. cThe analysis period was from first treatment with nivolumab to 30 days after the final treatment with nivolumab. Dashed line indicates the end of the observation period (12 months) for the PMS study. Abbreviations: ILD, interstitial lung disease; max, maximum; min, minimum
3.5. Risk factors for TRAEs of special interest occurring at a frequency of 5% or more
Patient characteristics that were identified by multivariate analyses as risk factors for the development of ILD, liver dysfunction, thyroid dysfunction, and colitis/severe diarrhea are shown in Table 4. Statistically significant risk factors were identified for the development of ILD (previous or comorbid ILD, abnormal findings on chest imaging [CT], and a smoking history), liver dysfunction (previous or comorbid liver disease, a smoking history, and presence of metastasis), thyroid dysfunction (previous or comorbid thyroid disease and ECOG PS), and colitis/severe diarrhea (treatment line 2 vs ≥3). The incidence of ILD was: 25.3% and 8.5% for those with and without previous or comorbid ILD, respectively; 16.0% and 7.8% for those with and without abnormal findings on chest imaging (CT), respectively; and 10.7% and 6.2% for those with a smoking history (current or past smoker) and without a smoking history (nonsmoker), respectively (Table S11).
TABLE 4.
Risk factors (multivariate analysis) for treatment‐related adverse events (TRAEs) of special interest occurring at a frequency of 5% or higher in Japanese patients with non‐small‐cell lung cancer (NSCLC) treated with nivolumab
|
TRAE of special interest Patient characteristic |
Comparison | HR | (95% CI) |
|---|---|---|---|
| ILD a | |||
| Sex | Male vs female | 1.03 | (0.74‐1.44) |
| Smoking history | Current or past smoker vs nonsmoker | 1.47 | (1.01‐2.15) |
| Previous or comorbid ILD | Yes vs no | 2.62 | (1.85‐3.70) |
| Previous or comorbid emphysema/COPD | Yes vs no | 1.33 | (0.98‐1.80) |
| Abnormal finding other than lung cancer on chest imaging (CT) | Yes vs no | 1.36 | (1.02‐1.81) |
| Past treatment for NSCLC (surgery) | Yes vs no | 1.12 | (0.88‐1.43) |
| Liver dysfunction | |||
| Smoking history | Current or past smoker vs nonsmoker | 1.44 | (1.06‐1.95) |
| Previous or comorbid liver disease | Yes vs no | 2.24 | (1.58‐3.19) |
| Presence of metastasis | Yes vs no | 1.66 | (1.16‐2.36) |
| Thyroid dysfunction | |||
| ECOG PS | 2‐4 vs 0‐1 | 0.50 | (0.36‐0.70) |
| Previous or comorbid thyroid disease | Yes vs no | 2.92 | (2.06‐4.14) |
| Colitis/severe diarrhea | |||
| Sex | Male vs female | 0.81 | (0.54‐1.20) |
| Age | ≥75 vs <75 years | 1.08 | (0.76‐1.54) |
| ECOG PS | 2‐4 vs 0‐1 | 0.97 | (0.69‐1.37) |
| Smoking history | Yes vs no | 1.21 | (0.78‐1.88) |
| NSCLC histology | Nonsquamous cell carcinoma vs squamous cell carcinoma | 0.89 | (0.64‐1.22) |
| Previous or comorbid autoimmune disease | Yes vs no | 0.88 | (0.39‐2.00) |
| Presence of metastasis | Yes vs no | 1.06 | (0.73‐1.52) |
| Treatment line | 2nd vs ≥3rd | 1.48 | (1.07‐2.03) |
Analysis results using Fine and Gray multivariate analysis.
Abbreviations: CI, confidence interval; COPD, chronic obstructive pulmonary disease; CT, computed tomography; HR, hazard ratio; ILD, interstitial lung disease.
Includes the preferred terms: bronchiolitis, eosinophilic pneumonia, ILD, lung disorder, lung infiltration, organizing pneumonia, pneumonitis, pulmonary alveolar hemorrhage, pulmonary fibrosis, diffuse alveolar damage, and radiation pneumonitis.
3.6. Effectiveness
The 6‐ and 12‐month survival rates were 58.0% and 40.7%, respectively. The survival rate after 12 months by ECOG PS was 48.0%, 14.9%, and 9.2% for PS 0‐1, PS 2, and PS 3‐4, respectively.
4. DISCUSSION
This is the first PMS in Japan to evaluate the safety and effectiveness of an ICI in more than 3000 patients with unresectable advanced or recurrent NSCLC in clinical practice. Results of this study have confirmed that the occurrence of TRAEs—especially TRAEs described as important identified risks—in clinical practice was comparable to that of clinical trials, and that no new safety concerns were identified. 9 , 10 , 12 These results support the approval of nivolumab for the treatment of Japanese patients with unresectable and advanced NSCLC.
Interstitial lung disease is an AE that has a high reporting rate in Japan compared with other countries 13 and is an immune‐related AE associated with nivolumab treatment of patients with NSCLC. 14 Therefore, the expression rate of ILD was expected to be high in this Japanese PMS. The incidence rate of ILD in this study was 9.6%, which is high in comparison with other real‐world studies of nivolumab treatment in non‐Japanese patients. In an Italian cohort of nivolumab‐treated patients with squamous NSCLC, the incidence of pneumonitis (any grade) was 1.0%, and in a cohort of Galician patients with NSCLC, the incidence was 2.7%. 15 , 16 The incidence of ILD was also lower in the two Japanese phase II studies combined (N = 111) than in the PMS (Table 3). The higher incidence of ILD in the PMS could be due to the inclusion of patients with a medical history of ILD or pulmonary fibrosis in the PMS, and—as we have shown in this report—a history of ILD or abnormal findings on chest imaging (CT) are risk factors for the development of ILD with nivolumab treatment. In the Japanese phase II studies, patients with a history of ILD or pulmonary fibrosis were excluded, and this could explain the lower incidence of ILD in the combined Japanese clinical studies.
Thyroid dysfunction caused by ICIs is a common immune‐related AE. 17 , 18 , 19 The incidence of thyroid dysfunction observed in the PMS was 9.1% compared with 13.5% in the combined Japanese phase II studies, and the median time to onset was 57 days compared with 85 days, respectively. Clinical trials are carried out according to a schedule and must adhere to defined testing procedures. In comparison, PMS facilities will differ in the method and frequency of testing for events such as thyroid dysfunction; therefore, the PMS might not be able to accurately follow the occurrence of such events, which is a limitation of this study. This difference in testing schedules could partly explain the difference in the median time to onset of thyroid dysfunction between the PMS and the clinical studies.
The CheckMate 153 phase IIIb/IV study assessed nivolumab treatment in patients with advanced NSCLC and included patients with poor ECOG PS status (ECOG PS 2) and patients with advanced age (70 years and older). 20 The incidence of TRAEs of grade 3 or higher in CheckMate 153 was 12% for patients with an ECOG PS 2 and 14% for patients aged 70 years or more. This PMS also included patients with poor ECOG PS (scores 2‐4) and elderly patients (aged 75 years or more). The incidence rate for TRAEs of grade 3 or higher was 17.8% in patients with an ECOG PS 2 or higher and 16.4% in elderly patients aged 75 years or older. These rates were similar to that observed for the whole PMS patient population (15.9%). Interestingly, in this study an ECOG PS 2‐4 was negatively associated with thyroid dysfunction (hazard ratio, 0.50 [95% confidence interval, 0.36‐0.70]). We hypothesize that the reduced risk of thyroid dysfunction for this patient subgroup might be a consequence of their shorter treatment period, as indicated by the lower median number of nivolumab doses received by patients with poor ECOG PS compared with the whole patient population (Table S6). Advanced age was not a risk factor for any TRAE of special interest, and the median number of nivolumab doses received by elderly patients in the PMS was the same as that for the whole population.
The 1‐year OS rates observed in the international phase III studies of nivolumab in patients with advanced nonsquamous (CheckMate 057) and squamous (CheckMate 017) NSCLC were 51% and 42%, respectively, 7 , 8 and in the Japanese phase II studies in patients with advanced or recurrent nonsquamous and squamous NSCLC were 68% and 71.4%, respectively. 9 , 10 In CheckMate 153, which included elderly patients (≥70 years) and patients with a poor ECOG PS of 2, the 1‐year OS rate was 43%. 20 In a real‐world study in an Italian cohort of nivolumab‐treated patients with squamous NSCLC, the 1‐year OS rate was 39%, 16 and in a real‐world study of lung cancer patients in France, the 1‐year OS rate was higher at 48.6%. 21 In the PMS, the 12‐month survival rate was 40.7% for the whole population, which included heavily treated patients (three treatment lines), elderly patients, and patients with poor ECOG PS. Although a direct comparison of the survival rate in the PMS and those in the clinical studies should not be made because of different methodologies used to calculate the rates, these results indicate that the benefit of nivolumab treatment in the real‐world setting is comparable to the benefits observed in several other studies.
A strength of this study is that the results are derived from real‐world data based on a large study cohort of more than 3000 Japanese patients, which enabled the surveillance of the safety and effectiveness of nivolumab in patients with advanced and recurrent NSCLC. Moreover, the large cohort size enabled subgroup analyses. Limitations of this study were the absence of a control or comparator drug and the fact that the follow‐up/observation period was limited to 12 months.
In conclusion, the safety profile of nivolumab in Japanese patients with NSCLC in this PMS was similar to that in clinical studies, and no new safety concerns were identified.
DISCLOSURE
Ono Pharmaceutical Co., Ltd. was involved in the study design, data collection, data analysis, and preparation of the manuscript. Bristol‐Myers Squibb KK was involved in the study design and data collection. N. Yamamoto has received lecture fees, honoraria, or other fees from MSD KK, AstraZeneca KK, Chugai Pharmaceutical Co., Ltd, Eli Lilly Japan KK, Nippon Boehringer Ingelheim Co., Ltd, Pfizer Japan Inc, and Ono Pharmaceutical Co., Ltd, research funds from MSD KK, AstraZeneca KK, Chugai Pharmaceutical Co., Ltd, Eli Lilly Japan KK, Nippon Boehringer Ingelheim Co., Ltd, Pfizer Japan Inc., Ono Pharmaceutical Co., Ltd, Terumo Corporation, Toppan Printing Co., Ltd, Takeda Pharmaceutical Co., Ltd, Amgen Inc., Taiho Pharmaceutical Co., Ltd, and Janssen Pharmaceutical KK, and scholarship endowments or research grants from Ono Pharmaceutical Co., Ltd, Taiho Pharmaceutical Co., Ltd, Chugai Pharmaceutical Co., Ltd, and Daiichi‐Sankyo Co., Ltd. Y. Nakanishi has received research funds from Ono Pharmaceutical Co., Ltd. A. Gemma has received lecture fees, honoraria, or other fees from Nippon Kayaku Co., Ltd and Daiichi‐Sankyo Co., Ltd. K. Nakagawa has received lecture fees, honoraria, or other fees from AstraZeneca KK, MSD KK, Eli Lilly Japan KK, Ono Pharmaceutical Co., Ltd, Merck Biopharma Co., Ltd, Pfizer Japan Inc., and KYORIN Pharmaceutical Co., Ltd, research funds from MSD KK, AstraZeneca KK, Pfizer Japan Inc, ICON Japan KK, Astellas Pharma Inc, Eli Lilly Japan KK, Bristol‐Myers Squibb Company, CMIC Shift Zero KK, Taiho Pharmaceutical Co., Ltd, PAREXEL International Corp., Ono Pharmaceutical Co., Ltd, A2 Healthcare Corp., Kyowa Kirin Co., Ltd, Syneos Health, Chugai Pharmaceutical Co., Ltd, Daiichi‐Sankyo Co., Ltd, Nippon Boehringer Ingelheim Co., Ltd, IQVIA Services JAPAN KK, SymBio Pharmaceuticals Limited, and Sysmex Corporation, and scholarship endowments or research grants from Chugai Pharmaceutical Co., Ltd, Ono Pharmaceutical Co., Ltd, Bristol‐Myers Squibb Company, Daiichi‐Sankyo Co., Ltd, and Takeda Pharmaceutical Company Limited. T. Sakamoto and A. Akamatsu are employees of Ono Pharmaceutical Co., Ltd. Y. Ohe has received lecture fees, honoraria, or other fees from AstraZeneca KK, Chugai Pharmaceutical Co., Ltd, and KYORIN Pharmaceutical Co., Ltd, and research funds from Ono Pharmaceutical Co., Ltd, MSD KK, AstraZeneca KK, Chugai Pharmaceutical Co., Ltd, Bristol‐Myers Squibb Company, and Amgen Inc.
Supporting information
Table S1‐S11
Document S1
ACKNOWLEDGMENT
The authors would like to thank all study participants and their families. This study was sponsored by Ono Pharmaceutical Co., Ltd. and Bristol‐Myers Squibb KK.
Yamamoto N, Nakanishi Y, Gemma A, et al. Real‐world safety of nivolumab in patients with non‐small‐cell lung cancer in Japan: Postmarketing surveillance. Cancer Sci. 2021;112:4692–4701. 10.1111/cas.15117
Clinical Trial Register: https://www.clinicaltrials.jp/cti‐user/trial/List.jsp (Japic‐163271).
Funding information
This study was sponsored by Ono Pharmaceutical Co., Ltd. and Bristol‐Myers Squibb KK. Medical writing assistance was provided by Prudence Stanford, PhD, and Rebecca Lew, PhD, CMPP, of ProScribe – Envision Pharma Group, and was funded by Ono Pharmaceutical Co., Ltd. Bristol‐Myers Squibb KK. ProScribe's services complied with international guidelines for Good Publication Practice (GPP3).
REFERENCES
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394‐424. [DOI] [PubMed] [Google Scholar]
- 2. Ferlay J, Ervik M, Lam F, et al. Global Cancer Observatory: Cancer today. International Agency for Research on Cancer. Japan factsheet. 2018. https://gco.iarc.fr/today/data/factsheets/populations/392‐japan‐fact‐sheets.pdf. Accessed November 16, 2020 [Google Scholar]
- 3. Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. 2015;10:1243‐1260. [DOI] [PubMed] [Google Scholar]
- 4. Akamatsu H, Ninomiya K, Kenmotsu H, et al. The Japanese Lung Cancer Society Guideline for non‐small cell lung cancer, stage IV. Int J Clin Oncol. 2019;24:731‐770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ettinger DS, Wood DE, Aggarwal C, et al. NCCN guidelines insights: non‐small cell lung cancer, version 1.2020. J Natl Compr Canc Netw. 2019;17:1464‐1472. [DOI] [PubMed] [Google Scholar]
- 6. OPDIVO (nivolumab) [package insert]. Bristol‐Myers Squibb; 2021. [Google Scholar]
- 7. Borghaei H, Paz‐Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non‐small‐cell lung cancer. N Engl J Med. 2015;373:1627‐1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous‐cell non‐small‐cell lung cancer. N Engl J Med. 2015;373:123‐135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hida T, Nishio M, Nogami N, et al. Efficacy and safety of nivolumab in Japanese patients with advanced or recurrent squamous non‐small cell lung cancer. Cancer Sci. 2017;108:1000‐1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nishio M, Hida T, Atagi S, et al. Multicentre phase II study of nivolumab in Japanese patients with advanced or recurrent non‐squamous non‐small cell lung cancer. ESMO Open. 2017;1:e000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496‐509. [Google Scholar]
- 12. Morita R, Okishio K, Shimizu J, et al. Real‐world effectiveness and safety of nivolumab in patients with non‐small cell lung cancer: a multicenter retrospective observational study in Japan. Lung Cancer. 2020;140:8‐18. [DOI] [PubMed] [Google Scholar]
- 13. Wakao R, Taavola H, Sandberg L, et al. Data‐driven identification of adverse event reporting patterns for Japan in VigiBase, the WHO global database of individual case safety reports. Drug Saf. 2019;42:1487‐1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sata M, Sasaki S, Oikado K, et al. Treatment and relapse of interstitial lung disease in nivolumab‐treated patients with non‐small cell lung cancer. Cancer Sci. 2020;112:1506‐1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Areses Manrique MC, Mosquera Martínez J, García González J, et al. Real world data of nivolumab for previously treated non‐small cell lung cancer patients: a Galician lung cancer group clinical experience. Transl Lung Cancer Res. 2018;7:404‐415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Crinò L, Bidoli P, Delmonte A, et al. Italian cohort of nivolumab expanded access program in squamous non‐small cell lung cancer: results from a real‐world population. Oncologist. 2019;24:e1165‐e1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Barroso‐Sousa R, Barry WT, Garrido‐Castro AC, et al. Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens: a systematic review and meta‐analysis. JAMA Oncol. 2018;4:173‐182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Barroso‐Sousa R, Ott PA, Hodi FS, Kaiser UB, Tolaney SM, Min L. Endocrine dysfunction induced by immune checkpoint inhibitors: practical recommendations for diagnosis and clinical management. Cancer. 2018;124:1111‐1121. [DOI] [PubMed] [Google Scholar]
- 19. Torino F, Barnabei A, Paragliola R, Baldelli R, Appetecchia M, Corsello SM. Thyroid dysfunction as an unintended side effect of anticancer drugs. Thyroid. 2013;23:1345‐1366. [DOI] [PubMed] [Google Scholar]
- 20. Spigel DR, McCleod M, Jotte RM, et al. Safety, efficacy, and patient‐reported health‐related quality of life and symptom burden with nivolumab in patients with advanced non‐small cell lung cancer, including patients aged 70 years or older or with poor performance status (CheckMate 153). J Thorac Oncol. 2019;14:1628‐1639. [DOI] [PubMed] [Google Scholar]
- 21. Barlesi F, Dixmier A, Debieuvre D, et al. Effectiveness and safety of nivolumab in the treatment of lung cancer patients in France: preliminary results from the real‐world EVIDENS study. Oncoimmunology. 2020;9:1744898. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1‐S11
Document S1


