Abstract
Pancreatic cancer frequently involves cancer‐associated thromboembolism, which is strongly associated with poor prognosis. Tissue factor, a blood coagulation factor largely produced in cancer patients as a component of extracellular vesicles, plays a key role in the incidence of cancer‐associated thromboembolism in patients with pancreatic cancer. However, no prospective studies have been published on the relationship between tissue factor and cancer‐associated thromboembolism or patient clinical characteristics, including recent chemotherapy regimens. Thus, we aimed to address this in a Japanese cohort of 197 patients and 41 healthy volunteers. Plasma tissue factor levels were measured by ELISAs preevaluated by tissue factor specificity. Multivariable analysis was used to identify independent predictors of cancer‐associated thromboembolism. We found that the cancer‐associated thromboembolism rate in the patient cohort was 6.6% (4.6%, venous thromboembolism; 2.0%, arterial thromboembolism). Tissue factor levels of 100 pg/mL or higher at patient registration were predictive of cancer‐associated thromboembolism, with positive and negative predictive values of 23.1% and 94.6%, respectively. Multivariable analysis showed that plasma tissue factor levels were an independent predictive factor for cancer‐associated thromboembolism, with a risk ratio of 5.54 (95% confidence interval, 1.02‐30.09). Unlike in healthy volunteers and patients without cancer‐associated thromboembolism, tissue factor levels were highly correlated with extracellular vesicles’ procoagulant activity in patients developing cancer‐associated thromboembolism. Taken together, our data show that the tissue factor levels at patient registration were a predictive factor for cancer‐associated thromboembolism in this cohort of patients with pancreatic cancer.
Keywords: extracellular vesicle, pancreatic neoplasm, risk factor, thromboembolism, tissue factor
Tissue factor levels were highly correlated with extracellular vesicles’ procoagulant activity in patients with pancreatic cancer who developed cancer‐associated thromboembolism. Tissue factor levels before the start of systemic chemotherapy were a predictive factor of cancer‐associated thromboembolism in a Japanese cohort of patients with pancreatic cancer.
Abbreviations
- ATE
arterial thromboembolism
- BMI
body mass index
- CA19‐9
carbohydrate antigen 19‐9
- CAT
cancer‐associated thromboembolism
- CI
confidence interval
- CT
computed tomography
- EV
extracellular vesicle
- fVIIa
activated factor VII
- FOLFIRINOX
oxaliplatin, irinotecan, fluorouracil, and leucovorin
- FXa
activated factor X
- HV
healthy volunteer
- PCA
procoagulant activity
- PS
performance status
- S‐1
oral fluoropyrimidine
- TF
tissue factor
- TF‐EV
TF‐positive EV
- VTE
venous thromboembolism
1. INTRODUCTION
Pancreatic adenocarcinoma is the fourth leading cause of cancer‐related death in the United States and Japan, accounting for approximately 48 000 and 36 000 deaths each year, respectively. 1 , 2 Surgical resection is the only curative treatment; however, recurrence is not rare, even after administration of adjuvant chemotherapy. 3
Cancer‐associated thromboembolism is a phenomenon of venous and arterial thromboembolism occurring in patients with cancer. Venous thromboembolism and ATE have some common risk factors, 4 and the former is more common in CAT. 4 Cancer‐associated thromboembolism has been reported to be the second most frequent cause of death, after cancer progression, in patients with cancer. 5
The classical risk model of CAT reported by Khorana et al 6 includes cancer site, increased platelet count, anemia, use of erythropoiesis‐stimulating agents, increased leukocyte count, and obesity. Pancreatic cancer was associated with the highest risk of developing CAT in the Khorana et al 6 model. Indeed, the reported incidence of CAT in pancreatic cancer was 12%‐45% in the United States and Europe, 7 , 8 which is considerably higher than that in breast (0.4%‐8.1%) and prostate (0.5%‐1.3%) cancers 7 ; however, few prospective studies 8 , 9 , 10 have been published on the relationship between CAT and pancreatic cancer.
In addition, Khorana’s model has some limitations as it uses obesity as a factor to calculate the risk score. This factor is affected significantly by ethnicity. 11 Furthermore, anticancer treatment can affect the risk of CAT partly due to molecules derived from dead tumors cells 12 and because the standard treatment for unresectable pancreatic cancer has significantly changed in recent years in comparison with that in the era of the Khorana risk score development. 13 , 14
Tissue factor is the receptor for blood coagulation factor VII, which is responsible for initiating the extrinsic blood coagulation cascade. Several studies 15 , 16 have reported that TF can be secreted from cancer and immune cells as a component of cell‐derived EVs. Tissue factor is often highly expressed in resected tumor specimens, and TF‐EVs are shed more abundantly in the plasma of patients with pancreatic cancer than in that of healthy individuals. 9 , 17 Indeed, patients who developed VTE showed high plasma TF levels at the start of chemotherapy. 9 Thus, it is likely that the secretion of TF‐EVs from cancer cells associated with fVIIa, followed by fibrin deposition, is a primary determinant of VTE, especially in patients with pancreatic cancer. 8 , 16 , 18 Moreover, TF‐EVs can induce platelet aggregation, thereby leading to VTE. 19 Tissue factor secreted by macrophages and monocytes is a cause of ATE in cardiovascular diseases. However, the role of TF derived from both cancer and noncancer cells in cancer‐associated ATE has not been fully elucidated. 10 , 20 Other molecular mechanisms involving neutrophil extracellular traps, increased platelet counts, and cytokines might also participate in CAT. 10
Tissue factor in blood is measured using commercial ELISA kits. 21 , 22 , 23 However, recent studies 24 , 25 have reported that the results can vary depending on the commercial ELISA kit used, presumably due to the type of anti‐TF Abs included. Thus, it is uncertain whether these ELISA kits can accurately quantify TF in blood samples. Tissue factor has also been evaluated by measuring the PCA of EVs, given that PCA directly causes CAT. 21 , 22 , 26 , 27 Procoagulant activity can be regulated by conformational change mechanisms, such as the encryption‐decryption process. 28 , 29 It is not yet understood how TF levels and PCA are associated with the incidence of CAT. Therefore, we aimed to prospectively evaluate the incidence of CAT and its risk factors in Japanese patients with pancreatic cancer, focusing on the relationship between plasma TF levels and PCA. Clinical trial registration ID (UMIN000010910).
[Correction added on 7 October 2021, after first online publication: Clinical trial registration ID has been added at the end of the Introduction section.]
2. MATERIALS AND METHODS
2.1. Patients and healthy controls
This study was approved by the Institutional Review Board of Kanagawa Cancer Center, Kanagawa, Japan, and conforms to the Declaration of Helsinki’s provisions. All participants provided written informed consent. We prospectively enrolled patients with pancreatic adenocarcinoma treated at our hospital from June 2013 to November 2016 who met the following inclusion criteria: (a) pathologically proven pancreatic cancer; (b) no history of treatment for pancreatic cancer, except for surgical resection in recurrent cases; and (c) provided written informed consent. The exclusion criteria were as follows: (a) prescribed use of anticoagulation drugs, such as warfarin or direct oral anticoagulants; (b) history of thromboembolism within 5 years or apparent concomitant thromboembolism at the time of registration; (c) allergy to contrast media in CT; or (d) jaundice or pancreatitis estimated not to have improved within a few days. We also analyzed 41 healthy controls (26 male and 15 female individuals; median age, 60 years [range, 40‐69 years]).
2.2. Plasma sample collection and patient background
Blood samples were collected in glass tubes containing a mixture of citrate, theophylline, adenosine, and dipyridamole (BD Vacutainer) at the time of registration and after 3, 6, and 12 months, or until CAT development. Samples were centrifuged within 2 hours after collection at 1500 g for 15 minutes, followed by 13 000 g for 2 minutes. The supernatants, corresponding to platelet‐free plasma, 24 were stored as aliquots at −80°C until required for TF evaluation. Immediately after sample collection, the following laboratory parameters were measured: blood count, hematocrit, total proteins, albumin, creatinine, potassium, glycohemoglobin, total cholesterol, triglycerides, high‐density lipoprotein cholesterol, carcinoembryonic antigen, CA19‐9, prothrombin time, activated partial prothrombin time, fibrinogen, and D‐dimer. Clinical factors, including ECOG‐PS, height, body weight, and clinical‐stage diagnosed by CT imaging according to the UICC staging system (7th edition), were also evaluated. Body mass index was calculated as follows: body weight (kg) / (height [m])2.
The Khorana score was calculated according to the original article. 6 The primary cancer site was the pancreas in all cases in this study, and the related score was 2 points for all patients; platelet count ≥350 × 109/L, hemoglobin <10 g/L, white blood cell count >11 × 109/L, and BMI ≥35 kg/m2 were allocated one point each. Erythropoiesis‐stimulating agents were not used because they are not approved for cancer chemotherapy in Japan; the corresponding score was 0 points for all patients.
2.3. Cell culture
The OVSAYO, OVISE, OVSAHO, MCAS, and MDA‐MB‐231 cell lines were as previously described. 30 The AsPC‐1, PANC‐1, and TIME (CRL‐4025) cell lines were purchased from ATCC. The YMB‐1, KP2, KP3, BxPC‐3, and KATOIII cell lines were purchased from the Japanese Collection of Research Bioresources Cell Bank. Cell lines, except for TIME, were maintained in RPMI‐1640 medium with 10% FCS under normoxia and hypoxia, as previously described. 31 TIME cells were cultured according to the manufacturer’s instructions.
2.4. Preparation of whole‐cell lysates and western blot analysis
Preparation of whole‐cell lysates was carried out using Cell Lysis Buffer 1 (R&D Systems) according to the manufacturer’s protocol. Briefly, 2 × 105 cells were seeded and cultured for 2 days in 35‐mm dishes under normoxia and hypoxia, as previously described. 31
2.5. Protein quantification
Protein levels were quantified using the Micro BCA protein assay kit (Thermo Fisher Scientific) according to the manufacturer’s instructions.
2.6. Western blot analysis
The procedures used for western blotting were the same as those described previously. 31 Briefly, whole‐cell lysates were analyzed using the NuPAGE system (Thermo Fisher Scientific). The primary anti‐TF Abs used (5G9, 9C3, 6B4, and 10H10) were as previously described. 31 The VD8 Ab was purchased from SEKISUI Diagnostics. The anti‐vinculin Ab was purchased from Sigma (#V9131). Detection was carried out using the Amersham ECL Select reagent (RPN2235; Cytiva). Quantification of band intensity was undertaken using ImageJ software (https://imagej.nih.gov/ij/). [Correction added on 7 October 2021, after first online publication: In the 2nd sentence of section 2.6, the texts 'corresponding to 20 μg protein,' have been deleted.]
2.7. Immunoassays
We carried out ELISA according to the manufacturers’ protocols. The kits used in this study included the Imubind Tissue Factor ELISA (Sekisui Diagnostics), Zymutest (Hyphen BioMed), CD142 ELISA (Abcam), and Quantikine ELISA (R&D Systems).
2.8. Real‐time RT‐PCR analysis
Real‐time RT‐PCR analysis of TF expression in various cell lines was carried out, as previously described. 31
2.9. Extracellular vesicle preparation and PCA assay
Extracellular vesicles were prepared from plasma samples, as described previously. 9 , 24 Briefly, plasma samples (100 µL) were diluted to 200 µL with PBS because sample viscosity can potentially affect the efficient recovery of EVs. 32 Diluted samples were then centrifuged at 20 000 g for 15 minutes. The supernatant was carefully removed, and the resulting pellets were washed with PBS. Procoagulant activity was then evaluated as the FXa generation activity as follows: EVs were washed once with reaction buffer (10 mmol/L HEPES [pH 7.5], 150 mmol/L NaCl, 4 mmol/L KCl, 11 mmol/L glucose, 5 mmol/L Ca2+, and 1 mg/mL BSA). Extracellular vesicle pellets were then resuspended in reaction buffer containing 175 nmol/L bovine factor X (BCX‐1050; Haematologic Technologies) and 10 nmol/L human fVIIa (HCVIIA‐0031; Haematologic Technologies) at 37℃ for 2 hours. A 20‐μL aliquot was removed and mixed with EDTA‐containing stopping solution. A total of 100 µL of the above reaction mixture was mixed with the same volume of 0.5 mmol/L Spectrozyme FXa (BioMedica Diagnostics), and the development of optical absorbance at 405 nm was measured. The FXa concentration in the samples was determined by a standard curve using bovine FXa as a standard (BCXA‐1060; Haematologic Technologies). [Correction added on 7 October 2021, after first online publication: In the 3rd sentence of section 2.9, the unit value '12 000 g' was changed to '20 000 g'.]
2.10. Diagnosis of CAT
Computed tomography was regularly undertaken to evaluate the efficacy of the anticancer treatment every 6‐8 weeks. Thus, CAT diagnosis was based on patient CAT symptoms or asymptomatic thrombus CT detection. Routine screening of thrombi was not carried out in this study.
2.11. Cancer and thromboembolism treatment
Patients were treated with systemic chemotherapy in unresectable cases and best supportive care in cases with some contraindication for chemotherapy, such as an ECOG‐PS of 3‐4 and advanced age. All systemic chemotherapy regimens were given at the physicians’ discretion: gemcitabine‐based regimens (gemcitabine monotherapy, gemcitabine plus erlotinib, gemcitabine plus nab‐paclitaxel), 13 , 33 , 34 or FOLFIRINOX. 13 The treatment regimen of resectable cases consisted of surgical resection followed by adjuvant chemotherapy using S‐1 alone, with or without neoadjuvant chemotherapy using gemcitabine plus S‐1. Prophylactic anticoagulation therapy was not given. Continuous infusion of unfractionated heparin and direct oral anticoagulants were adopted as the best treatment options to resolve thromboembolism as much as possible, although low molecular weight heparin could not be used because it is not approved for CAT in Japan.
2.12. Calculation of parameters and definition of cut‐off values
Hemoglobin levels, white blood cell counts, platelet counts, BMI, and D‐dimer levels were divided into two categories using the respective cut‐off values of the Khorana and Vienna scores. 35
We set the cut‐off value of the plasma TF level based on its distribution (see Results) and divided patients into two groups according to high and low plasma TF levels. For the remaining factors, we used the median values to divide the patients into two groups. The positive and negative predictive values were calculated to evaluate the influence of each factor on the incidence of CAT.
2.13. Statistical analyses
Missing data were assumed to be missing at random and imputed using the multiple imputation method with 10 repeated imputations. The complete dataset was also used in the following analysis to compensate for the influence of missing data. Categorical variables were compared using Fisher’s exact test, and continuous variables were compared using the Mann‐Whitney U test, or the Kruskal‐Wallis test, as appropriate. The relationships between two continuous values were evaluated using simple linear regression analysis. Statistical significance was presented together with the coefficient of determination. To evaluate the risk factors for CAT, we undertook logistic regression analysis using the forward stepwise selection method, with variables that had a P value of less than .05 in the univariable analysis. All statistical analyses were carried out using SPSS statistical software (version 26) (IBM SPSS).
3. RESULTS
3.1. Reactivity of TF Abs to TF expressed in various cell types
Recent studies 21 , 24 , 25 have suggested that plasma TF levels measured by commercial ELISA kits do not necessarily reflect actual TF protein amounts. One of the causes of this problem was deduced as the differential glycosylation patterns of TF, which might affect the reactivity of anti‐TF Abs. 36 , 37 Thus, we first sought to test whether and how TF Abs reacted with TF, expressed in multiple cancer cell lines, by using several mouse mAbs (Figure 1). In addition to OVSAYO and OVISE, cells cultured under normoxia (OVSAYO‐N and OVISE‐N) and the same cells cultured under hypoxia (1% O2; OVSAYO‐H and OVISE‐H) were used to test TF expression because hypoxic stress is known to upregulate TF in these cells. Western blot analysis of whole‐cell lysates was carried out using mouse mAbs raised against full‐length TF purified from the human brain 38 (clones 10H10, 9C3, 6B4, and 5G9) and apoprotein (amino acids 1‐25) of human TF (clone VD8).
FIGURE 1.
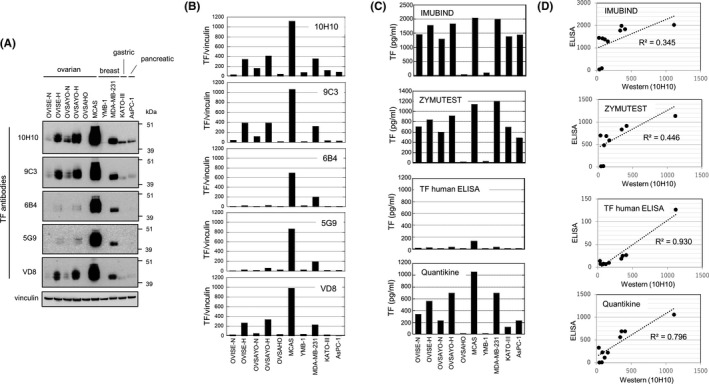
Relationship between tissue factor (TF) levels detected by western blotting and those evaluated by ELISA. A, Western blot analysis of TF expression in various cancer cell lines with the same concentration (5 μg/mL) of primary Abs. Vinculin was used as the protein loading control. B, Densitometrical quantification of TF levels expressed in cancer cells by ImageJ software (https://imagej.nih.gov/ij/). Data were normalized to vinculin expression. C, ELISA TF expression in cancer cells. Data were calculated based on the TF standard included in each kit. Data reported are mean values of duplicate wells of a 96‐well plate. D, Linear regression analysis of the relationship between western blot and ELISA TF levels [Correction added on 7 October 2021, after first online publication: In Figure 1A caption, the value '(2 mg/mL)' was changed to '(5 μg/mL)'.]
We found considerably different patterns depending on the Abs used (Figure 1A,B). For example, TF expressed in OVSAYO‐H and OVISE‐H lysates was only weakly detected by 6B4 and 5G9 Abs. Moreover, TF expressed in KATOIII and AsPC‐1 cells was clearly detected only by the 10H10 Ab. Tissue factor was not detected by any Ab in OVSAHO cells. These data suggest that the reactivity of TF Abs differs among cell types. Furthermore, real‐time RT‐PCR analysis with a negative control human endothelial cell line (TIME) revealed that the pattern of TF mRNA expression in these cancer cell lines (Figure S1A) was consistent with the TF protein expression pattern (Figures 1B and S1B). From these experimental findings, we surmised that the 10H10 Ab reflects the TF levels in cancer cells and is currently the best for TF protein detection.
3.2. Selection of ELISA kit for evaluating plasma TF levels
Current ELISA kits mostly use the sandwich‐ELISA methodology to detect TF with two different mAbs; therefore, the results are significantly affected by the Abs used. Furthermore, unfortunately, the identity of the Abs used in commercial ELISA kits is usually not stated. Therefore, we next evaluated the commercial ELISA assays with the 10H10 Ab to select the most suitable kit for the present study. The ELISA experiments, using the same lysates prepared for the earlier western blot experiments, indicated that TF levels significantly differed depending on the kit used (Figure 1C). As reported previously, 24 , 25 the Imubind ELISA kit detected high TF levels. In contrast, the TF human ELISA kit detected very low TF levels in cell lysates (Figure 1C). We next tested whether the TF levels evaluated by ELISA corresponded with those evaluated by western blotting using the 10H10 Ab. Linear regression analysis showed that there was better agreement with the Quantikine kit (r 2 = .796) than with the Zymutest (r 2 = .446) and Imubind (r 2 = .345) kits (Figure 1D). We observed the best linearity with the TF human ELISA kit (Figure 1D). However, most TF levels estimated by this kit were close to the detection limit of the concentration range of the TF calibrator curve (6.75‐400 pg/mL) (Figure S2), suggesting the low sensitivity of this kit to detect cancer cell‐derived TF (Figure 1C). Indeed, unlike western blotting, TF levels in KATOIII and AsPC‐1 lysates evaluated by this kit were almost identical to those in OVSAHO and YMB‐1 lysates (Figure S2). Furthermore, TF levels in multiple pancreatic cancer cell lines evaluated by western blotting using the 10H10 Ab corresponded well with those quantified using the Quantikine kit (Figure S3). The TF level in TIME cell lysate was indeed very low and lower than that in OVSAHO and YMB‐1 cells (Figure S4). Therefore, we decided to use the Quantikine kit to evaluate plasma TF levels in this study.
3.3. Patients and incidence of CAT
Patient characteristics are shown in Table 1. A total of 197 patients were included in this study, with a median age of 68 (range, 41‐85) years; most patients had an ECOG‐PS of 0‐1. The number of patients with resectable, locally advanced, and metastatic disease was 8 (4%), 54 (27%), and 135 (69%), respectively. In unresectable cases, the first‐line treatment for pancreatic cancer was gemcitabine plus nab‐paclitaxel (n = 88), gemcitabine monotherapy (n = 77), FOLFIRINOX (n = 20), S‐1 (n = 2), or best supportive care only (n = 2).
TABLE 1.
Relationship between plasma tissue factor (TF) level and patient characteristics in a Japanese cohort with pancreatic cancer
| Factor | Low‐TF group, n = 184 | High‐TF group, n = 13 | P value | ||
|---|---|---|---|---|---|
| No. of patients (%) | Median (range) | No. of patients (%) | Median (range) | ||
| Age, y | 184 (100) | 69 (41‐85) | 13 (100) | 63 (44‐78) | .051 |
| Sex | |||||
| Male | 94 (51) | 7 (54) | |||
| Female | 90 (49) | 6 (46) | .540 | ||
| Disease status | 184 (100) | 13 (100) | |||
| Resectable | 6 (3) | 0 (0) | .047 | ||
| Locally advanced | 54 (29) | 0 (0) | |||
| Metastatic | 124 (67) | 13 (100) | |||
| ECOG‐PS | 184 (100) | 13 (100) | |||
| 0 | 76 (41) | 6 (46) | .860 | ||
| 1 | 100 (54) | 6 (46) | |||
| 2 | 7 (4) | 1 (8) | |||
| 3 | 1 (1) | 0 (0) | |||
| Hemoglobin, g/dL | 184 (100) | 12.3 (7.2‐17.1) | 13 (100) | 11.5 (8.1‐14.1) | .210 |
| Hematocrit, % | 184 (100) | 36.6 (21.3‐50.5) | 13 (100) | 34.2 (25.8‐41.3) | .220 |
| White blood cell count/μL | 184 (100) | 5700 (2500‐12 600) | 13 (100) | 7200 (4700‐13 400) | .019 |
| Platelet count, ×104/μL | 184 (100) | 21.2 (8.8‐46.3) | 13 (100) | 21.2 (11.1‐38.5) | .850 |
| Total protein, g/dL | 184 (100) | 6.7 (5.0‐8.0) | 13 (100) | 6.7 (5.8‐7.5) | .800 |
| Albumin, g/dL | 184 (100) | 3.8 (2.0‐4.7) | 13 (100) | 3.6 (2.3‐4.0) | .079 |
| Total bilirubin, mg/dL | 184 (100) | 0.7 (0.2‐6.4) | 13 (100) | 0.7 (0.4‐1.6) | .490 |
| Creatinine, mg/dL | 184 (100) | 0.65 (0.30‐1.43) | 13 (100) | 0.59 (0.45‐0.89) | .110 |
| HbA1c, % | 181 (98) | 6.5 (4.6‐13.5) | 12 (92) | 6.1 (4.7‐10.3) | .170 |
| T‐Cho, mg/dL | 179 (97) | 178 (95‐454) | 12 (92) | 188 (90‐228) | .400 |
| Triglyceride, mg/dL | 180 (98) | 99 (33‐540) | 12 (92) | 107 (61‐177) | .380 |
| HDL‐C, mg/dL | 180 (98) | 47 (12‐119) | 12 (92) | 43 (18‐73) | .350 |
| PT, % | 183 (99) | 89 (51‐122) | 13 (100) | 83 (47‐97) | .044 |
| APTT, s | 182 (99) | 29.5 (22.0‐45.0) | 13 (100) | 26.5 (22.0‐38.0) | .570 |
| Fibrinogen, mg/dL | 153 (83) | 375 (182‐627) | 11 (85) | 267 (146‐561) | .040 |
| D‐dimer, ng/mL | 149 (81) | 1.4 (0.5‐16.4) | 12 (92) | 10.2 (0.7‐31.5) | <.001 |
| CA19‐9, U/mL | 174 (95) | 1090 (2.3‐642 000) | 11 (85) | 56 061 (2.1‐8 910 300) | <.001 |
| BMI, kg/m2 | 184 (100) | 20.9 (14.9‐29.6) | 13 (100) | 20.3 (15.0‐29.8) | .610 |
| Khorana score | 184 (100) | 13 (100) | |||
| 2 | 15 (8) | 1 (8) | .900 | ||
| 3 | 160 (87) | 11 (85) | |||
| 4 | 7 (4) | 1 (8) | |||
| 5 | 2 (1) | 0 (0) | |||
| Anticancer therapy | 184 (100) | 13 (100) | |||
| GEM + nab‐PTX | 81 (44) | 7 (54) | .130 | ||
| GEM | 72 (39) | 5 (38) | |||
| FOLFIRINOX | 20 (11) | 0 (0) | |||
| Surgery | 8 (4) | 0 (0) | |||
| S‐1 | 2 (1) | 0 (0) | |||
| BSC | 1 (1) | 1 (8) | |||
Note: High‐TF group, initial plasma TF level ≥100 pg/mL; low‐TF group, initial plasma TF level <100 pg/mL.
Abbreviations: APTT, activated partial thromboplastin time; BMI, body mass index; BSC, best supportive care; CA19‐9, carbohydrate antigen 19‐9; FOLFIRINOX, fluorouracil, leucovorin, irinotecan, and oxaliplatin combination; GEM, gemcitabine; HbA1c, hemoglobin A1c; HDL‐C, high density lipoprotein cholesterol; nab‐PTX, nab‐paclitaxel; PS, performance status; PT, prothrombin time; S‐1, oral fluoropyrimidine drug; T‐Cho, total cholesterol.
The median observation period was 10.8 (range, 0.4‐42.6) months. The number of patients who developed CAT during the observation period was 13 (6.6%): asymptomatic deep venous thrombosis in seven, asymptomatic deep venous thrombosis and pulmonary embolism in two, and cerebral infarction in four patients. The median time to the occurrence of CAT was 342 (range, 1‐1265) days.
3.4. Plasma TF levels and PCA of HVs and patients with pancreatic cancer
The median plasma TF levels in HVs (n = 41), cancer patients who did not develop CAT (n = 184), and patients who did develop CAT (n = 13) were 39.1 (range, 16.8‐87.3), 47.2 (range, 0.0‐224.8), and 56.0 (range, 37.6‐318.7) pg/mL, respectively (Figure 2A). For patients with cancer, TF was measured at the time of registration (initial TF level). The initial TF level in patients with pancreatic cancer who did or did not develop CAT was higher than that in HVs, with P values of .002 and .009, respectively; however, the difference in the initial TF level between patients who developed CAT and those who did not was not statistically significant (P = .15).
FIGURE 2.
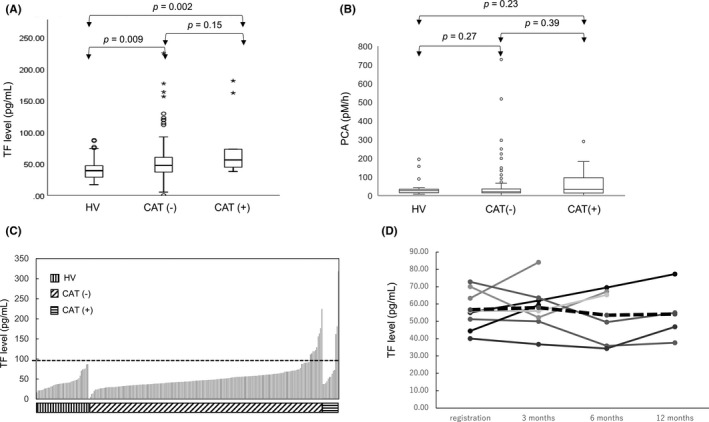
Plasma tissue factor (TF) levels and procoagulant activity (PCA) of healthy volunteers (HVs) and pancreatic cancer patients. A, Plasma TF levels of HVs, patients who did not develop thromboembolism (CAT−), and patients who developed thromboembolism (CAT+). Data shown are mean values of duplicate wells of a 96‐well plate. B, PCA in plasma samples. C, Sequential bar graph representation of TF levels. As shown by the dotted black line, we defined patients with an initial plasma TF level ≥100 pg/mL as the high‐TF group, and those with <100 pg/mL as the low‐TF group. D, Changes in plasma TF levels in eight CAT+ patients during the observation period. Solid lines represent the TF level in each case. Dotted line indicates the average value. Mean changes in plasma TF levels from registration were 1.28, −3.12, and −2.52 pg/mL, at 3, 6, and 12 mo, respectively
We next tested the PCA of EVs prepared from plasma samples of patients with cancer and HVs. The median PCA values in HVs, patients who did not develop CAT, and those who developed CAT were (30.3 (range7.9‐194.7), 21.0 (range: 0.3‐728.5) and 33.3 (range: 0.0‐289.8) pM/h), respectively (Figure 2B). There were no significant differences between HVs and patients who did not develop CAT (P = .27) or between HVs and patients who did develop CAT (P = .39). Thus, we decided to use protein levels to evaluate the predictive value of TF. [Correction made on 23 Sept 2021, after first online publication: In section 3.4, the PCA levels mentioned in the 2nd paragraph were corrected.]
The initial TF levels in HVs and patient groups are sequentially presented in Figure 2C. Varying the cut‐off value of TF resulted in only a small change in the negative predictive value (Figure S5). The positive predictive value increased as the cut‐off value increased. We selected a cut‐off value of 100 pg/mL as the test positive value should be higher than the incidence of CAT with the best positive prediction power (Figure S5). We defined patients with more than 100 pg/mL plasma TF as the high‐TF group and the remaining patients as the low‐TF group. The comparison of patient characteristics in the high‐ and low‐TF groups is shown in Table 1. All patients in the high‐TF group had metastatic disease. White blood cell counts, prothrombin time, fibrinogen levels, D‐dimer levels, and CA19‐9 levels were significantly different between the two groups, while the Khorana score was not.
3.5. Predictive value of plasma TF levels for the incidence of CAT
The relationships between the incidence of CAT and various laboratory parameters are shown in Table 2. High total protein, total cholesterol, TF, and low fibrinogen levels were significantly associated with CAT development, while Khorana scores of 3 or higher were not. The statistical significance did not differ between the actual and complete datasets (Table 2). Among total proteins, total cholesterol, fibrinogen, and TF, TF showed the best positive predictive value (10.4%, 10.6%, 10.3%, and 23.1%, respectively), with a comparable negative predictive value (97.8%, 97.8%, 97.0%, and 94.6%, respectively; Table 3). Additionally, the combined use of TF and D‐dimer levels improved the positive predictive value (33.3%) without reducing the negative predictive value (94.1%). Multivariable analysis showed that plasma TF levels were an independent predictive factor for CAT, with a risk ratio of 5.54 (95% CI, 1.02‐30.09) and a P value of .047 in the actual dataset and a risk ratio of 5.07 (95% CI, 1.09‐23.55) and a P value of .038 in the complete dataset (Table 4).
TABLE 2.
Relationship between cancer‐associated thromboembolism (CAT) incidence and background factors in pancreatic cancer patients
| Factor | Actual dataset | Completed dataset | ||||
|---|---|---|---|---|---|---|
| CAT+, n | CAT−, n | P value | CAT+, n | CAT−, n | P value | |
| Age, y | ||||||
| <65 | 4 | 65 | 4 | 65 | ||
| ≥65 | 9 | 119 | .50 | 9 | 119 | .50 |
| Sex | ||||||
| Male | 4 | 97 | 4 | 97 | ||
| Female | 9 | 87 | .11 | 9 | 87 | .11 |
| Disease status | ||||||
| Resectable | 1 | 5 | 1 | 5 | ||
| Locally advanced | 2 | 52 | 2 | 52 | ||
| Metastatic | 10 | 127 | .40 | 10 | 127 | .40 |
| ECOG‐PS | ||||||
| 0‐1 | 13 | 175 | 13 | 175 | ||
| 2‐3 | 0 | 9 | .53 | 0 | 9 | .53 |
| Hemoglobin, g/L | ||||||
| <100 | 2 | 18 | 2 | 18 | ||
| ≥100 | 11 | 166 | .39 | 11 | 166 | .39 |
| Hematocrit, % | ||||||
| <35 | 5 | 71 | 5 | 71 | ||
| ≥35 | 8 | 113 | .62 | 8 | 113 | .62 |
| White blood cell count, /μL | ||||||
| <11 000 | 13 | 178 | 13 | 178 | ||
| ≥11 000 | 0 | 6 | .66 | 0 | 6 | .66 |
| Platelet count, ×104/μL | ||||||
| <35 | 13 | 174 | 13 | 174 | ||
| ≥35 | 0 | 10 | .50 | 0 | 10 | .50 |
| Total protein, g/L | ||||||
| <67 | 2 | 89 | 2 | 89 | ||
| ≥67 | 11 | 95 | .019 | 11 | 95 | .019 |
| Albumin, g/L | ||||||
| <37 | 5 | 78 | 5 | 78 | ||
| ≥37 | 8 | 106 | .51 | 8 | 106 | .51 |
| Total bilirubin, mg/dL | ||||||
| <0.70 | 8 | 86 | 8 | 86 | ||
| ≥0.70 | 5 | 98 | .23 | 5 | 98 | .23 |
| Creatinine, mg/dL | ||||||
| <0.65 | 6 | 92 | 6 | 92 | ||
| ≥0.65 | 7 | 92 | .51 | 7 | 92 | .51 |
| HbA1c, % | ||||||
| <6.5 | 7 | 84 | 7.5 | 84.9 | ||
| ≥6.5 | 5 | 97 | .31 | 5.5 | 99.1 | .42 |
| T‐Cho, mg/dL | ||||||
| <178 | 2 | 91 | 2.5 | 93.4 | ||
| ≥178 | 10 | 88 | .021 | 10.5 | 90.6 | .028 |
| Triglyceride, mg/dL | ||||||
| <99 | 4 | 91 | 4 | 92.7 | ||
| ≥99 | 8 | 89 | .20 | 9 | 91.3 | .17 |
| HDL‐C, mg/dL | ||||||
| <46 | 4 | 92 | 4.3 | 93.8 | ||
| ≥46 | 8 | 88 | .19 | 8.7 | 90.2 | .21 |
| PT, % | ||||||
| <89 | 6 | 88 | 6.3 | 88 | ||
| ≥89 | 6 | 96 | .56 | 6.8 | 96 | .99 |
| APTT, s | ||||||
| <29 | 7 | 90 | 7.5 | 84.6 | ||
| ≥29 | 5 | 93 | .38 | 5.5 | 99.4 | .41 |
| Fibrinogen, mg/dL | ||||||
| <370 | 10 | 72 | 10.7 | 87.4 | ||
| ≥370 | 2 | 80 | .016 | 2.3 | 96.6 | .015 |
| D‐dimer, ng/mL | ||||||
| <1.44 | 5 | 74 | 5.4 | 78.8 | ||
| ≥1.44 | 7 | 75 | .41 | 7.6 | 105.2 | .93 |
| CA19‐9, U/mL | ||||||
| <1000 | 5 | 81 | 5 | 81 | ||
| ≥1000 | 8 | 91 | .38 | 8 | 103 | .38 |
| BMI, kg/m2 | ||||||
| <35 | 13 | 183 | 13 | 183 | ||
| ≥35 | 0 | 1 | .93 | 0 | 1 | .47 |
| Khorana score | ||||||
| 2 | 2 | 14 | 2 | 14 | ||
| 3‐5 | 11 | 170 | .29 | 11 | 170 | .29 |
| Anticancer therapy | ||||||
| GEM + nab‐PTX | 6 | 82 | .93 | 6 | 82 | .93 |
| GEM | 4 | 73 | 4 | 73 | ||
| FOLFIRINOX | 2 | 18 | 2 | 18 | ||
| Surgery | 1 | 7 | 1 | 7 | ||
| S‐1 | 0 | 2 | 0 | 2 | ||
| BSC | 0 | 2 | 0 | 2 | ||
| Tissue factor, pg/mL | ||||||
| <100 | 10 | 174 | 10 | 174 | ||
| ≥100 | 3 | 10 | .044 | 3 | 10 | .044 |
Abbreviations: APTT, activated partial thromboplastin time; BMI, body mass index; BSC, best supportive care; CA19‐9, carbohydrate antigen 19‐9; FOLFIRINOX, fluorouracil, leucovorin, irinotecan, and oxaliplatin combination; GEM, gemcitabine; HbA1c, hemoglobin A1c; HDL‐C, high density lipoprotein cholesterol; nab‐PTX, nab‐paclitaxel; PS, performance status; PT, prothrombin time; S‐1, oral fluoropyrimidine drug; T‐Cho, total cholesterol.
TABLE 3.
Positive predictive value (PPV) and negative predictive value (NPV) for cancer‐associated thromboembolism (CAT) incidence in pancreatic cancer patients
| Actual dataset | Completed dataset | |||||
|---|---|---|---|---|---|---|
| CAT+, n | CAT−, n | P value | CAT+, n | CAT−, n | P value | |
| Total protein (g/L) | ||||||
| ≥67 | 11 | 95 | PPV: 10.4% | 11 | 95 | PPV: 10.4% |
| <67 | 2 | 89 | NPV: 97.8% | 2 | 89 | NPV: 97.8% |
| Total cholesterol (mg/dL) | ||||||
| ≥178 | 10 | 88 | PPV: 10.2% | 11 | 93 | PPV: 10.6% |
| <178 | 2 | 91 | NPV: 97.8% | 2 | 91 | NPV: 97.8% |
| Fibrinogen (mg/dL) | ||||||
| <370 | 10 | 72 | PPV: 12.2% | 10 | 87 | PPV: 10.3% |
| ≥370 | 2 | 80 | NPV: 97.6% | 3 | 97 | NPV: 97.0% |
| Tissue factor (pg/mL) | ||||||
| ≥100 | 3 | 10 | PPV: 23.1% | 3 | 10 | PPV: 23.1% |
| <100 | 10 | 174 | NPV: 94.6% | 10 | 174 | NPV: 94.6% |
TABLE 4.
Multivariable analysis of cancer‐associated thromboembolism incidence in pancreatic cancer patients
| Factor | Actual dataset | Completed dataset | ||||
|---|---|---|---|---|---|---|
| Risk ratio | 95% CI | P‐value | Risk ratio | 95% CI | P‐value | |
| Total protein ≥67 g/L | NA | NA | 4.47 | 0.94‐21.42 | .061 | |
| Total cholesterol ≥178 mg/dL | 6.84 | 1.36‐34.53 | .020 | 4.66 | 0.97‐22.26 | .054 |
| Fibrinogen <370 mg/dL | 5.38 | 1.08‐26.65 | .040 | NA | NA | |
| Tissue factor ≥100 pg/mL | 5.54 | 1.02‐30.09 | .047 | 5.07 | 1.090‐23.55 | .038 |
Abbreviations: CI, confidence interval; NA, not applicable.
In addition to TF levels at registration, we evaluated plasma TF levels at 3, 6, and 12 months in patients with CAT, except for five patients who developed CAT within 3 months after registration. Unlike a previous study 39 on the relationship between VTE and pancreatic cancer, we found that plasma TF levels were not significantly altered during the observation period; the mean changes compared with the values at registration were 1.28, −3.12, and −2.52 pg/mL at 3, 6, and 12 months, respectively (Figure 2D). Thus, the positive predictive value (23.1%) was not improved, even when the later TF levels were used for estimation.
3.6. Correlation between TF levels, PCA, and the incidence of CAT
We have shown that PCA was not significantly different between HVs and patients with and without CAT (Figure 2B). We further examined PCA at 3 months before the onset of CAT and found it to be significantly higher in patients with CAT than in HVs and patients without CAT (Figure 3A), although the TF levels in patients who developed CAT did not change during the observation period (Figure 2D). Thus, PCA potentially increased in patients with CAT. We next examined the correlation between TF levels and PCA. Linear regression analysis revealed that such correlation was weak in HVs and patients without CAT, with r 2 values of .29 and .21, respectively (Figure 3B,C). In contrast, we observed a strong linear correlation in patients who developed CAT (r 2 = .85) (Figure 3D).
FIGURE 3.
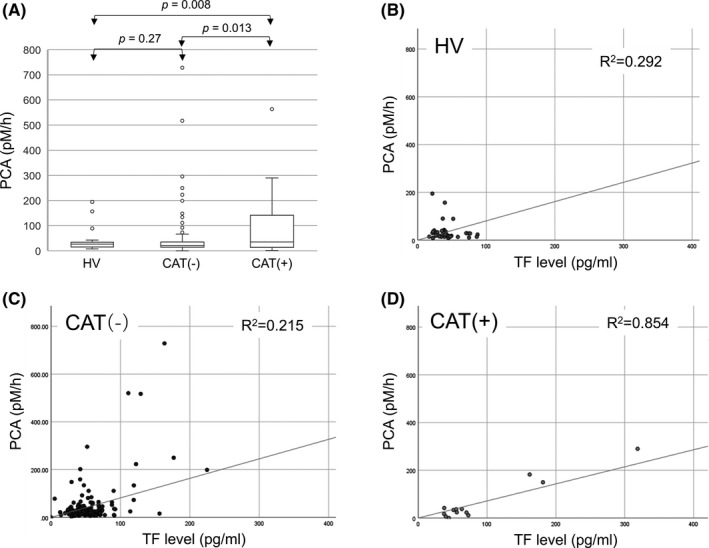
Relationship between procoagulant activity (PCA) and plasma tissue factor (TF) levels in healthy volunteers (HVs), patients who did not develop thromboembolism (CAT−), and patients who developed thromboembolism (CAT+). A, PCA before the onset of CAT (within 3 mo) was higher in CAT+ patients than in HVs and CAT− patients, while there was no difference between HVs and CAT− patients. B‐D, The linear relationship between PCA and TF level was strong in CAT+ patients, but weak in HVs and CAT− patients
4. DISCUSSION
This study determined that plasma TF levels at registration (>100 pg/mL), but not PCA, are associated with a higher risk of CAT, with a positive predictive value of 23.1% and a negative predictive value of 94.6%, which were better than those calculated based on D‐dimer levels and the Khorana risk scores. The initial TF levels were an independent predictive factor for CAT with a risk ratio of 5.54. We additionally found that PCA strongly correlated with plasma TF levels in patients who developed CAT. Thus, the use of initial TF level, followed by continuous monitoring of PCA, might improve the prediction of CAT.
First, it was revealed through this study that the commercially available ELISA kits detecting TF were different. Although all the kits evaluated were able to detect TF derived from various cancer cells, the reactivity differed considerably according to the kit. The human TF ELISA kit used in this study could accurately detect high TF levels but failed to detect low TF levels. It has also been reported 26 that, unlike the Imubind kit, the Zymutest kit failed to detect increased plasma TF levels in response to lipopolysaccharide treatment. Thus, the selection of ELISA kits composed of different anti‐TF Abs could be critical for evaluating TF levels, depending on the biological samples.
In our study cohort of Japanese patients, the incidence of VTE and ATE was 4.6% and 2.0%, respectively. Thus, the overall incidence of CAT was 6.6%, considerably lower than that observed in previous studies (12%‐45%) of patients with pancreatic cancer in Europe, the United States, 7 , 8 , 10 and Japan. 40 However, this study’s CAT rate is consistent with the VTE rate (6.7%) in the high‐risk group of the original Khorana score study. 6 Patients with more advanced tumors and poor general condition are generally excluded from prospective studies because it is difficult to obtain informed consent. In fact, our cohort included only two patients who received best supportive care. Moreover, we did not undertake periodic mandatory screenings, such as ultrasonography of lower limbs. These issues could explain why the CAT rate estimated in this study was relatively low.
Regarding the Khorana score, BMI is a predictive factor for the incidence of CAT. 6 In the present study, the median BMI was approximately 20 kg/m2, and no patient had a BMI higher than 35 kg/m2, which is evaluated in the Khorana score. Other factors potentially responsible for high Khorana scores in advanced cases with cachexia are high white blood cell counts, anemia, and high platelet counts. 41 However, such parameters were within the normal range in almost all patients in this study. These issues could partially explain why Khorana scoring failed to predict CAT in this Japanese cohort.
It is generally accepted that VTE correlates with poor prognosis in patients with cancer. 5 However, we believe that it is more important to clarify the mechanism of ATE in relation to these patients’ prognosis because we have routinely observed that ATE leads to greater impairment of patients’ daily living activities and is fatal more often than VTE. 42 A recent review 42 summarized the complex pathobiology of ATE in cancer, which includes many factors, such as: (a) traditional cardiovascular risk factors; (b) systemic chemotherapy and radiotherapy, potentially resulting in inflammation and endothelial apoptosis or injury, and release of TF‐EVs; (c) general risk factors, such as the use of blood products; and (d) nontumor cells potentially releasing TF‐EVs, cytokines, and neutrophil extracellular traps. Thus, future studies of ATE could lead to the prediction of patients with cancer requiring the prophylactic use of heparin or other anticoagulant agents.
To the best of our knowledge, this is the first report evaluating the incidence of CAT with recently developed regimens, such as FOLFIRINOX or gemcitabine plus nab‐paclitaxel, for advanced pancreatic cancer. In our study, we did not observe any differences in the incidence of CAT between regimens. It has been reported that different anticancer agents are associated with different levels of risk of CAT; gemcitabine was associated with a higher risk of CAT than other regimens, whereas another study showed that platinum‐based therapy was a risk factor and gemcitabine was not a risk factor for CAT. 43 , 44 In addition, PEGPH20, a pegylated recombinant human hyaluronidase that depletes the desmoplastic stroma, was associated with a high incidence of CAT in a phase II trial (HALO 202). 45 Nab‐paclitaxel is also known to deplete desmoplastic stroma, 46 and we speculated that gemcitabine plus nab‐paclitaxel would induce CAT more frequently than other regimens; however, it was not associated with an increase in the incidence of CAT in this study. Therefore, it would be valuable to elucidate the mechanisms by which PEGPH20 causes CAT. A larger sample size might be required to detect risk difference between the different regimens.
Tissue factor‐positive EV activity can be regulated by multiple molecular mechanisms, such as encryption‐decryption. 28 , 29 In encrypted conformation, TF could not associate with coagulation factor X, which halts coagulation activation. It is unclear how plasma TF levels and PCA are regulated in relation to the incidence of CAT. In this study, we found a significant linear relationship between plasma TF levels and PCA in patients who developed CAT. A possible explanation is that plasma TF adopts the less active configuration potentially through the encryption process in most patients without CAT and HVs, even if its level is high. However, some patients without CAT showed high PCA, which cannot be explained at present. Additionally, our data are inconsistent with those of a previously published study 9 in the United States, showing that patients’ plasma TF levels strongly correlated with PCA in all subjects, including patients without CAT. More detailed monitoring of PCA in patients without CAT is required to confirm that the increase in PCA is specific to patients with CAT.
Finally, there are some limitations of this study. First, the cut‐off value of plasma TF levels was set in a retrospective manner based on the incidence of CAT. It would be better to use a well‐validated cut‐off value; however, the evaluation method for plasma TF levels differed, including in terms of the use of platelet‐free or platelet‐poor plasma, among published studies. 24 Therefore, there was no reliable cut‐off level. A validation cohort will be needed to confirm the significance of this point. Second, the current study enrolled miscellaneous patients with different performance and disease statuses because patients received different chemotherapy regimens, potentially influencing the mechanisms of CAT. Prospective randomized trials including patients with different types and stages of cancer are generally characterized by small sample sizes. 47 Additionally, the sample size in the current study was relatively small. Thus, it would be worthwhile to undertake a large‐scale randomized controlled study of patients with advanced‐stage pancreatic cancer receiving the same chemotherapy regimen to increase the validity of TF as a marker of CAT in pancreatic cancer patients. Finally, from a molecular biology perspective, the current ELISA and PCA assays might not be able to evaluate the potential contribution of alternatively spliced soluble TF to CAT incidence. 48
In conclusion, we undertook a prospective cohort study in Japanese patients with pancreatic cancer to evaluate the influence of TF levels on the incidence of CAT. The results showed that the TF levels at patient registration were a predictive factor of CAT. Future detailed studies could lead to promising clinical applications, including the precise prediction of CAT.
DISCLOSURE
SK has received honoraria from Boston Scientific, Eisai Co., Ltd., Taiho Pharmaceutical Co., Ltd., and Yakult Honsha Co., Ltd. MU has received honoraria from MSD, Ono Pharmaceutical Co., Ltd., Taiho Pharmaceutical Co., Ltd., and Yakult Honsha Co., Ltd. The other authors have no conflicts of interest to disclose.
Supporting information
Fig S1‐5
ACKNOWLEDGMENTS
This research was supported by the Japan Society for the Promotion of Science (KAKENHI Grant No. 16H06277 [CoBiA]) from the Japanese Ministry of Education, Culture, Sports, Science and Technology. We would like to thank Editage for their writing support.
Kobayashi S, Koizume S, Takahashi T, et al. Tissue factor and its procoagulant activity on cancer‐associated thromboembolism in pancreatic cancer. Cancer Sci. 2021;112:4679–4691. 10.1111/cas.15106
Funding information
Japan Society for the Promotion of Science (JSPS) KAKENHI Grant (No. 16H06277 [CoBiA]) from the Japanese Ministry of Education, Culture, Sports, Science and Technology
REFERENCES
- 1. Torre L, Siegel R, Jemal A. Cancer facts & figures 2018. American Cancer Society. https://www.cancer.org/research/cancer‐facts‐statistics/all‐cancer‐facts‐figures/cancer‐facts‐figures‐2019.html. Accessed Nov 17, 2020.
- 2. Japan Ministry of Health, Labor and Welfare . Vital statistics Japan. Center for Cancer Control and Information Services, National Cancer Center, Japan; 2019. https://ganjoho.jp/reg_stat/statistics/dl/index.html#mortality. Accessed Nov 18, 2020.
- 3. Conroy T, Hammel P, Hebbar M, et al. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med. 2018;379:2395‐2406. [DOI] [PubMed] [Google Scholar]
- 4. Poredoš P. Interrelationship between venous and arterial thrombosis. Int Angiol. 2017;36:295‐298. [DOI] [PubMed] [Google Scholar]
- 5. Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost. 2007;5:632‐634. [DOI] [PubMed] [Google Scholar]
- 6. Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy‐associated thrombosis. Blood. 2008;111:4902‐4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Geddings JE, Mackman N. Tumor‐derived tissue factor‐positive microparticles and venous thrombosis in cancer patients. Blood. 2013;122:1873‐1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bharthuar A, Khorana AA, Hutson A, et al. Circulating microparticle tissue factor, thromboembolism and survival in pancreaticobiliary cancers. Thromb Res. 2013;132:180‐184. [DOI] [PubMed] [Google Scholar]
- 9. Khorana AA, Francis CW, Menzies KE, et al. Plasma tissue factor may be predictive of venous thromboembolism in pancreatic cancer. J Thromb Haemost. 2008;6:1983‐1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Campello E, Ilich A, Simioni P, Key NS. The relationship between pancreatic cancer and hypercoagulability: a comprehensive review on epidemiological and biological issues. Br J Cancer. 2019;121:359‐371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zakai NA, Mcclure LA. Racial differences in venous thromboembolism. J Thromb Haemost. 2011;9:1877‐1882. [DOI] [PubMed] [Google Scholar]
- 12. Kasthuri RS, Hisada Y, Ilich A, Key NS, Mackman N. Effect of chemotherapy and longitudinal analysis of circulating extracellular vesicle tissue factor activity in patients with pancreatic and colorectal cancer. Res Pract Thromb Haemost. 2020;4:636‐643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817‐1825. [DOI] [PubMed] [Google Scholar]
- 14. Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab‐paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691‐1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Amin C, Mackman N, Key NS. Microparticles and cancer. Pathophysiol Haemost Thromb. 2008;36:177‐183. [DOI] [PubMed] [Google Scholar]
- 16. Grover SP, Mackman N. Tissue factor: an essential mediator of hemostasis and trigger of thrombosis. Arterioscler Thromb Vasc Biol. 2018;38:709‐725. [DOI] [PubMed] [Google Scholar]
- 17. Khorana AA, Ahrendt SA, Ryan CK, et al. Tissue factor expression, angiogenesis, and thrombosis in pancreatic cancer. Clin Cancer Res. 2007;13:2870‐2875. [DOI] [PubMed] [Google Scholar]
- 18. Hisada Y, Geddings JE, Ay C, Mackman N. Venous thrombosis and cancer: from mouse models to clinical trials. J Thromb Haemost. 2015;13:1372‐1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Geddings JE, Hisada Y, Boulaftali Y, et al. Tissue factor‐positive tumor microvesicles activate platelets and enhance thrombosis in mice. J Thromb Haemost. 2016;14:153‐166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tatsumi K, Mackman N. Tissue factor and atherothrombosis. J Atheroscler Thromb. 2015;22:543‐549. [DOI] [PubMed] [Google Scholar]
- 21. Claussen C, Rausch A‐V, Lezius S, et al. Microvesicle‐associated tissue factor procoagulant activity for the preoperative diagnosis of ovarian cancer. Thromb Res. 2016;141:39‐48. [DOI] [PubMed] [Google Scholar]
- 22. Cohen JG, Prendergast E, Geddings JE, et al. Evaluation of venous thrombosis and tissue factor in epithelial ovarian cancer. Gynecol Oncol. 2017;146:146‐152. [DOI] [PubMed] [Google Scholar]
- 23. Abu Saadeh F, Norris L, O’Toole S, et al. Tumour expression of tissue factor and tissue factor pathway inhibitor in ovarian cancer‐ relationship with venous thrombosis risk. Thromb Res. 2013;132:627‐634. [DOI] [PubMed] [Google Scholar]
- 24. Lee RD, Barcel DA, Williams JC, et al. Pre‐analytical and analytical variables affecting the measurement of plasma‐derived microparticle tissue factor activity. Thromb Res. 2012;129:80‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Parhami‐Seren B, Butenas S, Krudysz‐Amblo J, Mann KG. Immunologic quantitation of tissue factors. J Thromb Haemost. 2006;4:1747‐1755. [DOI] [PubMed] [Google Scholar]
- 26. Thaler J, Ay C, Mackman N, et al. Microparticle‐associated tissue factor activity, venous thromboembolism and mortality in pancreatic, gastric, colorectal and brain cancer patients. J Thromb Haemost. 2012;10:1363‐1370. [DOI] [PubMed] [Google Scholar]
- 27. Gezelius E, Flou Kristensen A, Bendahl PO, et al. Coagulation biomarkers and prediction of venous thromboembolism and survival in small cell lung cancer: a sub‐study of RASTEN – a randomized trial with low molecular weight heparin. PLoS One. 2018;13:e0207387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zelaya H, Rothmeier AS, Ruf W. Tissue factor at the crossroad of coagulation and cell signaling. J Thromb Haemost. 2018;16:1941‐1952. [DOI] [PubMed] [Google Scholar]
- 29. Ansari SA, Pendurthi UR, Rao LVM. Role of cell surface lipids and thiol‐disulphide exchange pathways in regulating the encryption and decryption of tissue factor. Thromb Haemost. 2019;119:860‐870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Koizume S, Ito S, Yoshioka Y, et al. High‐level secretion of tissue factor‐rich extracellular vesicles from ovarian cancer cells mediated by filamin‐A and protease‐activated receptors. Thromb Haemost. 2016;115:299‐310. [DOI] [PubMed] [Google Scholar]
- 31. Koizume S, Takahashi T, Yoshihara M, et al. Cholesterol starvation and hypoxia activate the FVII gene via the SREBP1‐GILZ pathway in ovarian cancer cells to produce procoagulant microvesicles. Thromb Haemost. 2019;119:1058‐1071. [DOI] [PubMed] [Google Scholar]
- 32. Witwer KW, Buzás EI, Bemis LT, et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles. 2013;2:20360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Burris HA, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first‐line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403‐2413. [DOI] [PubMed] [Google Scholar]
- 34. Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960‐1966. [DOI] [PubMed] [Google Scholar]
- 35. Thaler J, Ay C, Pabinger I. Venous thromboembolism in cancer patients – risk scores and recent randomised controlled trials. Thromb Haemost. 2012;108:1042‐1048. [DOI] [PubMed] [Google Scholar]
- 36. Krudysz‐Amblo J, Jennings ME 2nd, Mann KG, Butenas S. Carbohydrates and activity of natural and recombinant tissue factor. J Biol Chem. 2010;285:3371‐3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kothari H, Pendurthi UR, Rao LV. Tissue factor purified from different cellular sources and non‐glycosylated tissue factor show similar procoagulant activity. J Thromb Haemost. 2013;11:2066‐2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Morrissey JH, Fair DS, Edgington TS. Monoclonal antibody analysis of purified and cell‐associated tissue factor. Thromb Res. 1988;52:247‐261. [DOI] [PubMed] [Google Scholar]
- 39. Li H, Yu Y, Shi Q, et al. Prognostic significance of tissue factor in patients with pancreatic cancer: a systematic review protocol. BMJ Open. 2020;10:e037431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ishigaki K, Nakai Y, Isayama H, et al. Thromboembolisms in advanced pancreatic cancer: a retrospective analysis of 475 patients. Pancreas. 2017;46:1069‐1075. [DOI] [PubMed] [Google Scholar]
- 41. McMillan DC. Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care. 2009;12:223‐226. [DOI] [PubMed] [Google Scholar]
- 42. Aronson D, Brenner B. Arterial thrombosis and cancer. Thromb Res. 2018;164(Suppl 1):S23‐S28. [DOI] [PubMed] [Google Scholar]
- 43. Qi WX, Lin F, Sun YJ, Tang LN, Shen Z, Yao Y. Risk of venous and arterial thromboembolic events in cancer patients treated with gemcitabine: a systematic review and meta‐analysis. Br J Clin Pharmacol. 2013;76:338‐347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Moik F, van Es N, Posch F, et al. Gemcitabine and platinum‐based agents for the prediction of cancer‐associated venous thromboembolism: results from the Vienna Cancer and Thrombosis Study. Cancers. 2020;12:2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hingorani SR, Zheng L, Bullock AJ, et al. HALO 202: Randomized phase II study of PEGPH20 plus nab‐paclitaxel/gemcitabine versus nab‐paclitaxel/gemcitabine in patients with untreated, metastatic pancreatic ductal adenocarcinoma. J Clin Oncol. 2018;36:359‐366. [DOI] [PubMed] [Google Scholar]
- 46. Von Hoff DD, Ramanathan RK, Borad MJ, et al. Gemcitabine plus nab‐paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial. J Clin Oncol. 2011;29:4548‐4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zwicker JI, Liebman HA, Bauer KA, et al. Prediction and prevention of thromboembolic events with enoxaparin in cancer patients with elevated tissue factor‐bearing microparticles: a randomized‐controlled phase II trial (the Microtec study). Br J Haematol. 2013;160:530‐537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kocatürk B, Versteeg HH. Tissue factor isoforms in cancer and coagulation: May the best isoform win. Thromb Res. 2012;129:S69‐S75. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1‐5


