To the editor,
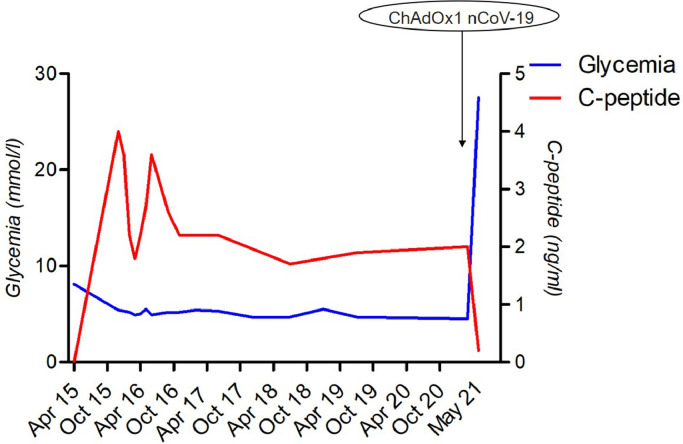
Solid organ transplant recipients are at high risk of developing severe forms of Covid-19 infection, requiring urgent access to vaccination [1]. mRNA vaccines are recommended for this population [2], but due to initial vaccine shortage in Europe, some patients were vaccinated using ChAdOx1 nCoV-19 (AstraZeneca). We report the case of a 51-year-old woman, recipient of a pancreas allograft 6 years ago because of type 1 diabetes. The patient was insulin-free on maintenance tacrolimus and mycophenolic acid. Pancreas function was optimal, as reflected by a glycemia at 4.5 mmol/l (normal range 4.1–5.9 mmol/l) and a C-peptide at 2 ng/mL (normal range 0.4–4 ng/ml) on March 6, 2021. There were no reported issues of therapeutic adherence, immunosuppressive drugs were well tolerated and CNI trough levels were stable around 8 ng/ml since the first months of transplantation. She received a first dose of ChAdOx1 nCoV-19 on March 12, 2021 and developed weakness and fever (38 °C) afterward, for 12 h. On May 4, 2021, she experienced polyuria and polydipsia requiring admission to hospital. Laboratory tests revealed hyperglycemia at 27 mmol/l, associated with ketoacidosis, elevation of lipasemia (181 IU/l, normal range 0 to 60 IU/l) and an abrupt decline of the C-peptide level to 0.1 ng/ml (Fig. 1 ). There was evidence of an eosinophilia at 2000/mm3, without features of parasitic infection nor recent drug introduction. SARS-Cov-2 serology was negative (electrochemiluminescence immunoassay, Roche Elecsys®). Auto-antibodies anti-ZnT8 were positive at 159 IU/ml (normal range < 15 IU/ml), anti-GAD 65 were positive at 6.3 IU/ml (normal range < 5 IU/ml), anti-islet cell antibodies were positive at 160 IU/ml (normal range < 5 IU/ml); anti IA2 and anti-insulin antibodies were negative. Computed tomography did not show any evidence of pancreas allograft thrombosis. Pancreas transplant biopsy revealed grade II acute rejection without any feature of antibody-mediated rejection (C4d negative) or significant islet alteration (Fig. 2 ). There were no circulating donor specific antibodies, as there was no evidence for histological recurrence of diabetes mellitus nor toxicity of tacrolimus. Immediate treatment including high-dose pulse steroids and anti-thymocyte globulins was initiated, with resolution of eosinophilia, but without significant impact on glycemic control; the patient remained insulin-dependent. Data were extracted from the French DIVAT cohort (www.divat.fr, approved by the national authorities, CNIL, no. 914,184) consisting of transplant recipients monitored in Nantes. The quality of the DIVAT data bank is validated by an annual cross-center audit, and all participants gave informed consent.
Fig. 1.
Representation of glycemia and C-peptide from the time of transplantation and occurrence of allograft dysfunction due to acute rejection following ChAdOx1 nCoV-19 injection.
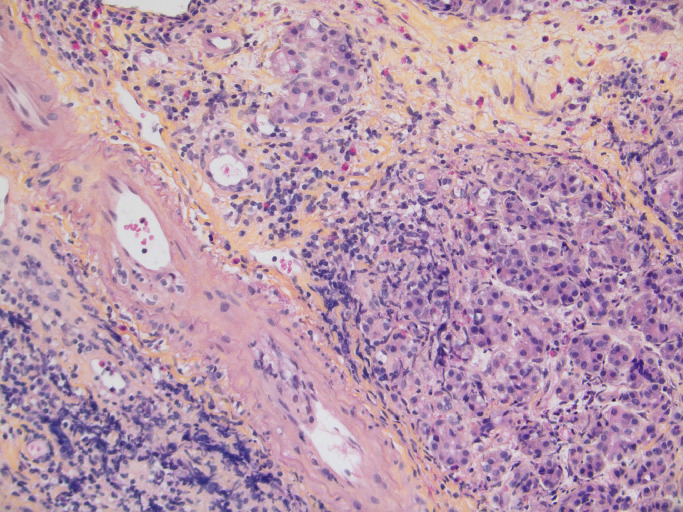
Fig. 2.
Moderate acute T-cell-mediated rejection characterized by septal and acinar inflammation composed of lymphocytes and eosinophils with mild acinar cell injury and mild intimal arteritis developed on stage II chronic allograft rejection lesion with chronic allograft arteriopathy. No feature of antibody-mediated rejection. HES, original magnification, x200.
To our knowledge, this is the first report of pancreas allograft rejection following Covid-19 vaccine. Presence of eosinophilia may suggest a delayed allergic response which could have triggered a larger immune response leading to allograft rejection, but can also directly reflect allograft rejection [3,4]. Currently, there are only two reports of allograft rejection following Covid-19 vaccine: kidney [5] and liver [6] rejection. Both were acute severe rejections consecutive to mRNA vaccines, with partial recuperation of allograft functions. Of note, presence of autoantibodies usually found in type 1 diabetes may suggest a part of auto-immune reactivity even if the biopsy did not show any typical characteristics [7]. Although the benefit of Covid-19 vaccination seems unquestionable for solid organ transplant recipients, these reports added to ours highlight the need for close follow-up to assess potential immune adverse events, such as acute rejection.
Declaration of Competing Interest
The authors declare no conflicts of interest.
Funding
No funds were provided for this report.
References
- 1.Caillard S., Anglicheau D., Matignon M., Dürrbach A., Greze C., Frimat L., et al. An initial report from the French SOT COVID Registry suggests high mortality due to Covid-19 in recipients of kidney transplants. Kidney Int. 2020;98:1549–1558. doi: 10.1016/j.kint.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kronbichler A., Anders H.J., Fernandez-Juarez G.M., Floege J., Goumenos D., et al. Recommendations for the use of COVID-19 vaccines in patients with immune-mediated kidney diseases. Nephrol Dial Transplant. 2021;36 doi: 10.1093/ndt/gfab064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drachenberg C.B., Odorico J., Arend L.J. Banff schema for grading pancreas allograft rejection: working proposal by a multi-disciplinary international consensus panel. Am J Transplant. 2008;8:1237–1249. doi: 10.1111/j.1600-6143.2008.02212.x. [DOI] [PubMed] [Google Scholar]
- 4.Weir M.R., Bartlett S.T., Drachenberg C.B. Eosinophilia as an early indicator of pancreatic allograft rejection: eosinophilia and pancreas graft rejection. Clin Transplant. 2012;26:238–241. doi: 10.1111/j.1399-0012.2011.01440.x. [DOI] [PubMed] [Google Scholar]
- 5.Del Bello A., Marion O., Delas A., Congy-Jolivet N., Colombat M., Kamar N. Acute rejection after anti–SARS-CoV-2 mRNA vaccination in a patient who underwent a kidney transplant. Kidney Int. 2021;100:238–239. doi: 10.1016/j.kint.2021.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vyhmeister R., Enestvedt C.K., VanSandt M., Schlansky B. Steroid-resistant acute cellular rejection of the liver after severe acute respiratory syndrome coronavirus 2 mrna vaccination. Liver Transpl lt. 2021;26097 doi: 10.1002/lt.26097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Occhipinti M., Lampasona V., Vistoli F., Bazzigaluppi E., Scavini M., Boggi U., et al. Zinc Transporter 8 autoantibodies increase the predictive value of islet autoantibodies for function loss of technically successful solitary pancreas transplant. Transplantation. 2011;92:674–677. doi: 10.1097/TP.0b013e31822ae65f. [DOI] [PubMed] [Google Scholar]