Abstract
Objective
COVID-19 pandemic disease has profound consequences for physical and mental health. In this regard, health care for chronic diseases, especially epilepsy is neglected The purpose of this systematic review study was to investigate the epidemic effect of COVID-19 on increasing the prevalence of mental disorders such as depression, anxiety, and sleep disorders in people with epilepsy (PWE).
Methods
We systematically searched MEDLINE, Cochrane, Embase, Web of science, Scopus, and Psych info databases for studies that estimate the prevalence of mental disorders in PWE during the COVID-19 until December 2020. Inclusion criteria included samples of population, with a confirmed diagnosis of epilepsy.
Results
Irrespective of PWE or people without epilepsy (PWOE), all experienced stress and anxiety during COVID-19 pandemic. Most of the studies showed that PWE and even PWOE during the pandemic, suffer from depression. The highest rate of depression was attributed to female PWE with financial problems (66.7%) and the lowest rate of depression in PWE was reported in 8.6%. 7.1–71.2% and 28.2% of patients reported sleep disorders and insomnia, respectively. Less than 2% experienced a sleep improvement.
Limitations
Due to a large amount of heterogeneities across the results, we could not evaluate the exact rate of prevalence in spite of using effective measures.
Conclusions
People with epilepsy were considered as a susceptible group to the impact of the pandemic. Therefore, great attention should be paid to PWE and adequate psychological supports provided in this period to relieve or inhibit risks to mental health in PWE.
Keywords: Epilepsy, COVID-19, Depression, Anxiety, Psychological distress
1. Introduction
The advent of SARS-CoV-2 (COVID-19) pandemic in December 2019 has led to unprecedented changes in our lives, with profound consequences for physical and mental well-being [1]. The spread of COVID-19 quickly reached pandemic proportions and severely affected the health systems of several countries [2]. According to the WHO Situation Report published on March 3, 2021, there are >114,315,846 confirmed COVID-19 cases and >2,539,427 death tolls globally [3]. The pandemic has forced governments to crack down on people, including forced house arrests, in order to curb the spread of the infection. The coronavirus crisis, quarantine, isolation leading to feelings of loneliness, boredom, anger, and anxiety threaten public mental health [4]. In this regard, extensive research on mental health has shown that emotional distress is present in affected populations everywhere – a finding that should be replicated in COVID-19 pandemic-affected populations [5].
Epilepsy is a chronic neurological disease that has affected more than 50 million people worldwide. In addition to the physical problems caused by epilepsy, this disease has also other significant neurological, cognitive, and psychosocial consequences [6]. In today’s society, depressive disorders and anxiety are among the most mental disorders [7]. Meanwhile, insomnia, a common sleep complaint, may trigger epileptic seizures. Insomnia also affects the control of epileptic seizures; alongside, assessing insomnia is an important element in delivering comprehensive epilepsy care [8]. According to the reports, it is believed that 9.4% of people in the target population suffer from anxiety disorder; 8.2% of them show the symptoms of depressive disorder at every 12-month interval. In addition, population-based research on people with epilepsy (PWE) shows that concurrent depressive disorders and anxiety are more prevalent in PWE than in the general population [7]. Stress plays a major role in the recurrence of epileptic seizures. Also, it is a commonly reported factor for seizure recurrence [9]. Today, there is no doubt that stress associated with health concerns, limited access to health care, and lifestyle changes due to confinement at home and remote work can potentially contribute to seizures and the overall well-being of PWE [2]. Meanwhile, the prevalence of anxiety in PWE is 20.2% higher than that of patients with drug-resistant epilepsy [10].
Regarding PWE, COVID-19 pandemic has led to delays in outpatient visits, cancellation of epilepsy tests such as electroencephalogram (EEG) test and magnetic resonance imaging (MRI), and even problems in emergency attendance [4]. With respect to the mental health of PWE during COVID-19, there has been scant evidence so far that COVID-19 can directly affect seizures; however, there is evidence that the COVID-19 pandemic has led to increased psychological distress among PWE [11].
Research shows that PWE are at a higher risk of depression and anxiety than healthy controls and patients with other illnesses. Also, depression and anxiety have a significantly negative impact on the quality of life of PWE [12]. To this end and given the potential negative effect of COVID-19 on PWE, we set out the present systematic review to investigate the effect of COVID-19 pandemic on the prevalence of mental disorders, namely depression, anxiety, and sleep disorders among PWE.
2. Methods
2.1. Data sources and search strategy
The present systematic review was conducted according to the PRISMA guidelines, and registered with PROSPERO (International Prospective Register of Systematic Reviews) under No: CRD42021244383 [13]. We initially performed a systematic search in Cochrane, PubMed, Embase, Web of Science, Scopus, and Psych info. In the next step, we included all relevant studies published between December 29, 2019 and December 31, 2020. Also, we searched the reference lists of the selected studies. A detailed search strategy including keywords is presented in the Supporting Information section (Supporting Appendix 1). Also, we combined the search term ‘COVID-19’ with a term related to psychological indicators and epilepsy.
2.2. Eligibility criteria
In this study, we included only papers reported in English. Also, we included samples of children and adolescents with a confirmed diagnosis of epilepsy. In addition, both cross-sessional and observational studies aiming to assess the effects of COVID-19 on psychological indicators among PWE were considered eligible. With respect to exclusion criteria, we excluded letters to editors, review articles, conference abstracts, case reports, and animal studies. Meanwhile, studies that did not investigate the diagnosis of anxiety or depressive disorders or stress in PWE during the COVID19 pandemic were excluded. Also, we excluded studies whose population was not based on epilepsy, or their results have not been obtained during the pandemic. One of the authors (MT) of this study screened titles and abstracts of identified references; full texts of eligible studies were then independently retrieved and reviewed by two authors (MT and ZB). The viewpoints of a third reviewer were also used in cases of disagreement.
2.3. Data extraction
Data extraction was conducted and documented independently by two reviewers (MT and ZB). We used a data extraction form to collect data on the setting, population, sample size, data source, study design, gender, depression assessment, anxiety assessment, psychological distress assessment, epilepsy diagnosis, number of disorders, and findings.
2.4. Quality assessment
The Joanna Briggs Institute (JBI) Prevalence Critical Appraisal tool was used to assess the methodological quality of the included studies [14]. Two authors of the present study (ZB and MT) independently conducted the assessment of the risk of bias. Disagreements were solved first by discussion, and then by consulting a third reviewer, if disagreements persisted. Finally, the entire body of studies was summarized descriptively. Also, we performed a qualitative synthesis of results.
3. Results
3.1. Included studies
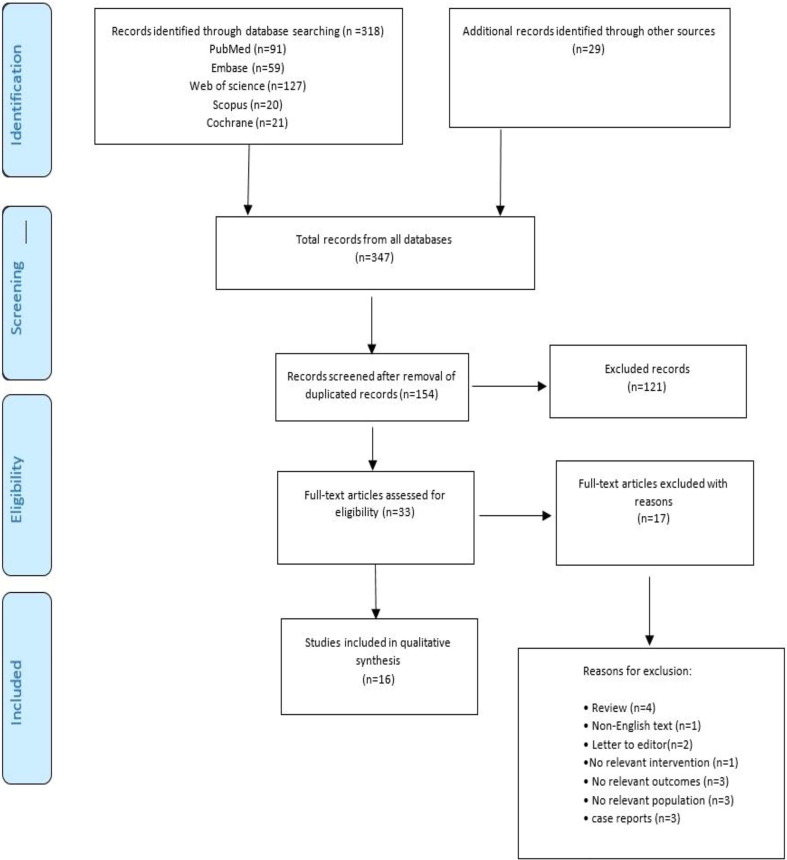
After a meticulous search across databases, we were able to initially find 347 studies. In the next step, we removed duplicated articles, and then came up with 154 studies. Based on the exclusion/inclusion criteria, we evaluated the titles and abstracts of these 154 studies, and were able to select 33 papers. In the next step, we reviewed these 33 articles and excluded 17 studies; details with regard to why these 17 studies have been excluded are given in PRISMA flow diagram (see Fig. 1 ). Finally, we came to the conclusion that only 16 studies met the inclusion/exclusion criteria, and thus were selected as the final included studies in this systematic review. The outcomes of search processes and the selection of the various sources are also presented in Fig. 1.
Fig. 1.
PRISMA flow diagram for study selection. Population: patients with epilepsy; Intervention: COVID-19 pandemic; Comparison: Period before COVID-19; Outcomes: Stress, depression, anxiety, and insomnia.
3.2. Data extraction
16 studies examined the effect of the COVID-19 on anxiety, depression, stress, and sleep disorder based on questionnaires developed for PWE and/or their caregivers. The data extracted from these studies are summarized in Table 1 . These 16 studies were conducted in nine countries/regions, namely Italy: 5, China: 2, United Kingdom: 1, Belgium:1, Iran:1, spain:2, Saudi Arabia:1, united states:2, and Lithuania:1. In all of the 16 studies, both genders participated; however, the female participants were more than those of male [6], [15]; therefore, the results of this study cannot be generalized to the whole community.
Table 1.
Features of the online questionnaires.
| First author (publish year)/country | Sample size/number of disorder/gender | Data source | Study design | Assessment of depression/anxiety/psychological distress/sleep disorder | Epilepsy diagnosis/prevalence | Significant predictors in multivariate analysis | Findings |
|---|---|---|---|---|---|---|---|
| Amal Alkhotani | 156/156/Female | An electronic self-administered questionnaire was distributed to PWE via their treating neurologist. | Cross-sectional | Self-report/an electronic self-administered questionnaire | Self-report/NR | 59.4% of PWE self-reported a significant increase in stress, 71.2% of PWE experienced a significant change in their sleep. | Patients with uncontrolled epilepsy, sleep disorders, and self-reported stress who were also unable to take medication were more likely to have seizures during the epidemic. |
| (2020)/Saudi Arabia | n = 97(62%) | (self-reported stress/self-reported stress/disturbed sleep | Anxiety (15.4%) increased, depression (7.1%) increased significantly. | ||||
| Male n = 59 (38%) | |||||||
| Jacopo Lanzone (2020)/Italy | 879/427/Female | The survey was spread by the efforts of LICE [Lega Italiana Contro l’Epilessia, the Italian | Case control | Polarization score/Survey | Self-report/NR | Distribution between group (significant) | During the lockdown, PWOE increased reactive stress responses to the restrictions. PWE, instead, appeared to internalize more the damage of lockdown. Interestingly, PWE also reported positive feelings about the restrictions more frequently than PWOE. |
| n = 327 | chapter of the International League Against Epilepsy (ILAE)] | NLP methods/NR/ | increased frequency in the occurrence of words with positive “Affin” score in the PWE group compared to | ||||
| 77% | Foundation and included clinical data and psychometric scales. | the PWOE (X2 = 5.3953, df = 1, p = 0.045, PWE = 10.2%, 29/282; PWOE = 5.2%, 17/323). | |||||
| Male | Mean polarization value of − 1.28 in PWE and − 1.39 in PWOE (not significant) | ||||||
| n = 100 | |||||||
| 23% | |||||||
| Giovanni Assenza | 928/456/Female | An online survey was created using the free open-access Google TM Forms (https://www.google.com/forms/about/) | Case control | BDI-II/GAD-7/NR/PSQI for sleep | Survey/NR | Anxiety: 39.5% had normal, 28.9% had mild, 21.3% had moderate, and 10.3% had severe | During the pandemic, many patients had increased number of seizures and had difficulty following-up, especially patients with chronic ASM and poor sleep quality. |
| (2020)/Italy | n = 344 | application. | (not significant). | ||||
| (78%) | PSQI scale scores did not differ between PWE and PWOE depressive: (65.1%) had normal, 15.1% had mild, 11.6% had moderate, 8.1% had severe depressive (not significant). | ||||||
| Male | |||||||
| n = 112 | |||||||
| (22%) | |||||||
| Stijn Van Hees | 399/399/Female | Online questionnaire surveys were developed in Belgium, English, Dutch, French, and Portuguese. | Cross-sectional | PHQ-9 and HADS/PHQ-9 and HADS/NR/NR | Follow-up consultation with the treating physician | 50.4% of cases showed positive anxiety according | Patients were faced with problems accessing ASM during the pandemic. 50% of the patients had been measured positive for depression. They showed less positive symptoms of anxiety. Also, almost 50% of the respondents cancelled their follow-up consultation with the physicians. |
| (2020)/Belgium | n = 320 | or | to the HADS; also, 39.8% and 46.9% PWE showed positive symptoms for depression via the HADS and PHQ-9 scale, respectively. Female and thus PWE with financial problems experience depression and anxiety. | ||||
| 80.2% | visit via telephone or online consult./YES | ||||||
| Male | |||||||
| n = 79 | |||||||
| 19.8% | |||||||
| Corrado Cabona | 189/189/Female N = 103 | Three Italian Epilepsy Centers set up telephone consultations using a semi-structured interview, allowing collection of data on seizure course during the pandemic. | Cohort | NR/numeric rating scale/NR | Follow-up visit via telephone/NR | Median score for COVID-19 concern was 6 (IQR: 3–8) | Telephone counseling was set up using a semi-structured interview with the aim of managing seizures and treating AED. |
| (2020)/Italy | (55%) | (not significant). | |||||
| Male | |||||||
| N = 86 | |||||||
| (45%) | |||||||
| Mehri Salari | 900/141/Female | The online questionnaire with two referrals at Shahid Beheshti | Cross-sectional | NR/(BAI-II)/NR/NR | Participants before entering the survey by telephone call/NR | The mean BAI-II total score between patient (11.82 ± 9.71) and control (10.28 ± 8.98) (no significant). | The total level of anxiety in PWE did not increase compared with the general population. Anxiety in men of younger ages was significantly higher in the control group. There was no correlation between anxiety, age, and gender in the patient group. |
| (2020)/Iran | n = 78 | and Isfahan Universities of Medical Sciences. | and case–control survey | 13.5% of patients and 6.9% of controls have experienced severe level of anxiety (significant). | |||
| (55.40%) | |||||||
| Male | |||||||
| n = 63 | |||||||
| (44.6%) | |||||||
| Elena Fonseca | 255/255/Female | The Vall d’ Hebron Research Institute Clinical Research Ethics Committee | Cross-sectional | Routine clinical telephone visits with a neurologist | Medical records/NR | Sixty-eight (26.7%) patients reported confinement-related anxiety, | A significant number of patients experienced an increase in the frequency of seizures, and many of them reported anxiety, depressive symptoms, and insomnia. It was found that insomnia and decreased economic status are risk factors for increasing the frequency of tumor-related seizures and drug-resistant epilepsy. |
| (2020)/Span | N = 121 (47.5%) | and | and a 19-item questionnaire were systematically completed/routine clinical telephone visits with a neurologist | 22 (8.6%) depression, | |||
| Male | a neurologist administered | and 19-item questionnaire were systematically completed/No/ | 31 (12.2%) both, and 72 (28.2%) insomnia. | ||||
| N = 134 (52.5%) | a 19-item questionnaire to all participants. | questionnaire | (significant). | ||||
| Alfonso Giordano | 40/40/Female | Outpatient epilepsy clinic of the | Cohort | Hospital Anxiety and Depression | Outpatient clinic during the pre-lockdown period | Anxiety sub-score (8.7%) increased. | A stressful event such as the outbreak of COVID-19 could adversely affect the course of epilepsy. |
| (2020)/Italy | N = 32 | First Division of Neurology at the University of Campania “Luigi Vanvitelli” (Naples, Italy). | Scale | medical records/NR | Depression sub-score (4.7%) increased. | ||
| (80%) | (HADS)/Hospital Anxiety and Depression | Insomnia Severity Index (7.0%) increased | |||||
| Male | Scale | (significant). | |||||
| N = 8 | (HADS)/Scale-Revised (IES-R)/sleep disturbances were measured by Insomnia Severity Scale (ISI) | ||||||
| (80%) | |||||||
| Xiaoting Hao | 504/252/Female | Electronic clinical records in Epilepsy Center in West China Hospital. | Cross-sectional and case-control | NR/NR/K-6 scale/NR | Diagnosed with epilepsy at least 1 year before, and they had to be followed up monthly by an epilepsy specialist | Severe psychological distress (defined as K-6 score > 12; both P ≤ 0.001). | During an outbreak, public health, physicians, and caregivers should focus not only on seizure control but also on the mental health of patients with epilepsy, especially drug-resistant patients with epilepsy. |
| (2020)/China | N = 132 | and | 1.6% of the control group and 13.1% of PWE reported severe psychological distress (p < 0.001) | ||||
| (52.3%) | they self-reported seizures within 48 hours before completing the questionnaires/NR | (significant). | |||||
| Male | |||||||
| N = 120 | |||||||
| (47.6%) | |||||||
| Colin Reilly | 201/Female PWE: 61 (86%) caregiver: 64 | An anonymous online survey hosted on Survey Monkey. | Cross-sectional | NR/online survey/online survey/online survey | Self-reports/NR | The majority of young people had poor sleep (72 %) and bad mood (64 %). | The pandemic and associated restrictions have had a negative effect on young PWE. Increases in seizures and reluctance to go to hospital is likely to affect epilepsy handling. |
| (2021)/UK | Male PWE: 10 (14%) caregiver: 66 | ||||||
| Barbara Mostacci | 222/222 Female N = 128 | Epilepsy Center clinicians and telephone survey. | Cross-sectional | NR/NR/NR/questionnaire for sleep disorder | Self-reported/YES | 23.9% of PWE reported sleep changes. 8.5% reported various degrees of disturbed sleep, 11.3% a change of sleep pattern, and 1.8% of them an improvement in sleep. | Most PWE did not report a significant alteration in their clinical conditions during the COVID-19. However, the severity of epilepsy, attendant disability, comorbid psychiatric conditions, sleep disorders, and limited availability to healthcare may impact their health condition. |
| (57.64%) | |||||||
| (2020)/Italy | Male: N = 94 (42.23%) | ||||||
| Shanshan Huang/(2020)/China | 362/362/ | Via online questionnaires in Wuhan, and its surrounding cities. | Cross-sectional | PHQ-9 scale/GAD-7 scale/Patient health questionnaire to identify insomnia and sleep disorders: Insomnia Severity Index (ISI) | Questionnaire/NR | Depression among PWE and PWOE increased; number of seizures was 22.58% versus 12.08%, respectively (significant). | A small proportion of PWE experienced seizure exacerbations during the COVID-19 outbreaks. Stress is an independent factor in causing seizures. |
| Female | Anxiety among patients with or without an increased number of seizures was 19.35% versus 8.46% (not significant) | ||||||
| N = 166 | Among patients with or without an increased number of seizures 25.81% versus 19.03% (not significant). | ||||||
| (45.86%) | |||||||
| Male | |||||||
| N = 196 | |||||||
| (54.14%) | |||||||
| Wendy R. Miller/(2020)/USA | 94/74/Female | A questionnaire including 65 questions, shared via social media like Facebook pages & Twitter, and also | Cross-sectional and descriptive study | NR/PROMIS anxiety scale/NR | Medical records/NR | Through using the PROMIS anxiety scale, it was asked to rate how they were coping with the current pandemic on a scale of 0–10, with 0 being not at all and 10 being very well, the mean score was 7.08 (SD: 1.89; range: 0–10) | During the pandemic, patients’ medical needs were not met; in some cases, it also increased. |
| N = 47 (50%) | via the link embedded in the online advertisement. (hashtags: #COVID-19, #epilepsy, #pandemic, and #research). | (significant). | |||||
| Male | |||||||
| N = 47 (50%) | |||||||
| Kristijonas Puteikis/(2020)/Lithuania | 143/143/ | A questionnaire in Lithuanian was designed to gather information | Cross-sectional | NR/GAD-7/survey/survey | Questionnaire/NR | The majority of young people and caregivers expressed that the young person’s mood (PWE: 64%; caregiver: 61%) and sleep (PWE: 72%; caregiver: 56%) had worsened since COVID-19 lockdown | Quarantine during the pandemic may lead to worsening seizure control and health status in some PWE. |
| Female | about the clinical characteristics of PWE, their health changes and altered the use of healthcare services during a national lockdown. | (significant). | |||||
| N = 84 | |||||||
| (58.7%) | |||||||
| Male | |||||||
| N = 59 | |||||||
| (41.3%) | |||||||
| Jillian L. Rosengard/(2020)/USA | 177/177 | The questionnaire was created by neurologists in the | Cross-sectional and cohort | NR/survey | Medical records/YES | Subjects with poorer seizure control were more likely to express increased or worsened stress (80.6% versus 50.0%, P = 0.002) (significant). | The poor access to care and difficulty in receiving anticonvulsant medication are important factors in inducing stress. Many patients reported stress as the main factor in recurrent seizures. |
| Female | Montefiore Health System. | Worse sleep increased in those PWE who reported no change or improved seizure control and those who reported worsened seizure control were 41.8% and 48.4% respectively | |||||
| N = 120 | (no significant). | ||||||
| (67.8%) | |||||||
| Male | |||||||
| N = 57 | |||||||
| (32.2%) | |||||||
| Alvaro Sanchez-Larsen/(2020)/Spain | 100/100/Female | In an epilepsy outpatient clinic of a tertiary center in Albacete University General Hospital in the province of Albacete (Castilla-La Mancha, Spain). | Observational and retrospective study based on prospective data collection | Survey/survey/survey/survey | Patients who attended an epilepsy outpatient clinic either face-to-face or via phone during the months of the COVID-19 outbreak and national state of emergency/NR | Exacerbation of stress/anxiety (21 [77.8%] vs 21 [28.8%], OR: 8.67, 95% CI: 3.07–24.50; p < 0.001), | Anxiety and stress due to the confinement were reported as the most important factors in changing the frequency of seizures. Also, seizure control of the patients worsened during the pandemic. |
| N = 52 | sadness/depression (15 [55.6%] vs 20 [27.4%], OR: 3.31, 95% CI: 1.32–8.29; p = 0.01), | ||||||
| (52%) | sleep deprivation (16 [59.3%] vs 15 [20.5%], OR: 5.62, 95% CI:2.16–14.61; p < 0.001) | ||||||
| Male | (significant). | ||||||
| N = 48 | |||||||
| (48%) | |||||||
Note: PHQ-9: 9-item of Patient Health Questionnaire (PHQ-9); NLP methods: Natural Language Processing methods; HADS: Hospital Anxiety and Depression Scale; BAI-II: Beck Anxiety Inventory II-Persian; GAD7: General Anxiety Disorder-7; IES-R: Impact of Event Scale-Revised; ISI: Insomnia Severity Index; BDI- II: Beck Depression Inventory scale II; PSQI: Pittsburg Sleep Quality Index; K-6 scale: 6-item Kessler Psychological Distress scale; PWE: People With Epilepsy; PWOE: People Without Epilepsy; NR: Not Reported.
3.3. Risk of bias within studies
Due to the fact that all qualified studies used surveys, we decided to use the Joanna Briggs Institute Critical Appraisal tool for studies that reported the prevalence and incidence of data [16], [17]. In 43% of the studies, the sample was representative of the target population. Considering the fact that most of the participants were women, this result cannot be generalized to the general population. Also, 75% of the studies reported a suitable sample size. In addition, only 37.5% of the study subjects and the settings were described in detail. 68.75% of the data analysis were also conducted with sufficient coverage of the identified sample. Meanwhile, 68.75% of studies had appropriate statistical analysis, and 25% of them were objective, for which standard criteria were used for the measurement of the condition (Table 2 ).
Table 2.
Risk of bias assessment (Joanna Briggs Institute Critical Appraisal tool) for prevalence studies.
| First author/year | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Amal Alkhotani/2020 | Y | Y | Y | N | Y | Y | NA | Y | N | Y |
| Giovanni Assenza/2020 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Corrado Cabona/2020 | Y | Y | Y | N | N | Y | NA | N | NA | Y |
| Elena Fonseca/2020 | Y | Y | Y | Y | Y | Y | Y | Y | N | Y |
| Alfonso Giordano/2020 | N | Y | N | N | N | Y | Y | N | N | Y |
| Xiaoting Hao/2020 | Y | Y | Y | Y | N | Y | Y | N | NA | Y |
| Shanshan Huang/2020 | N | Y | Y | N | Y | Y | Y | Y | Y | Y |
| Wendy R. Miller/2020 | N | Y | Y | N | N | Y | Y | N | UC | Y |
| Kristijonas Puteikis/2020 | N | Y | Y | N | Y | Y | Y | Y | UC | Y |
| Jillian L. Rosengard/2020 | N | Y | N | N | Y | Y | Y | UC | UC | Y |
| Alvaro Larsen/2020 | N | Y | N | N | Y | Y | Y | Y | UC | Y |
| Shanshan Huang/2020 | N | Y | Y | N | Y | Y | N | Y | N | Y |
| Barbara Mostacci/2020 | Y | Y | Y | Y | Y | Y | Y | Y | N | Y |
| Mehri Salari/2020 | N | Y | N | Y | N | Y | Y | Y | Y | Y |
| Stijn Van Hees/2020 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Jacopo Lanzone/2020 | N | Y | Y | N | Y | Y | N | Y | N | Y |
| The percentage of “yes” scores | 43.75% | 100% | 75% | 37.5% | 68.75% | 100% | 75 % | 68.75% | 25% | 100% |
1: Was the sample representative of the target population?
2: Were study participants recruited in an appropriate way?
3: Was the sample size adequate?
4: Were the study subjects and the setting described in detail?
5: Was the data analysis conducted with sufficient coverage of the identified sample?
6: Were objective and standard criteria used for the measurement of the condition?
7: Was the condition measured reliably?
8: Was there appropriate statistical analysis?
9: Are all important confounding factors/sub-groups/differences identified and accounted for?
10: Were sub-populations identified using objective criteria?
Y: yes, N: NO, UC: unclear, NA: Not applicable.
3.4. Psychiatric assessment
Anxiety, depression, stress, and sleep disorder were considered and measured using various methods.
3.5. The effect of COVID-19 pandemic on the depression of people with epilepsy
In seven studies, depression was measured via polarization score [18], such as Beck depression inventory scale II (BDI-Ⅱ) [19], 9-item Patient Health Questionnaire (PHQ-9) [6], [15], Hospital Anxiety and Depression Scale (HADS) [6], [20], a 19-item questionnaire [4], and a survey [21]. We used the PHQ-9 scale to measure the symptoms of depression. Also, we applied a threshold level of 10 points to diagnose depression severity. The adequate sensitivity and specificity PHQ-9 scale are 88.0% and 88.0%, respectively [22].
Results of one of the reviewed articles in this study showed that PWE (19%) and people without epilepsy (PWOE) (17%) have psychiatric disorders such as depression. Both groups (8%) received anti-depressant drugs at the moment of the survey. Also, results revealed that PWE showed more severe depressive symptoms (3.8%) (higher BDI-II scores) than PWOE [19].
Van Hees et al.’s study indicated that out of the 399 PWE, 159 (39.8%) and 187 (46.9%) PWE showed symptoms of depression measured by the HADS and PHQ-9 scales, respectively. They also claimed that women are more sensitive than men, so are more prone to psychiatric disorders. Meanwhile, their results showed that out of the 66 female PWE with financial problems, 44 cases (66.7%) suffered from depression [6]. In Huang et al.’s study (2020), depression was measured using PHQ-9. Results revealed that the worsening of depression among PWE and PWOE was associated with increasing the number of seizures (22.58% vs 12.08%, respectively) [15]. In addition, Sanchez-Larsenet al.’s study showed that depression in 8.6% of subjects appeared with lockdown-related symptoms [4]. In this regard, Sanchez-Larsen et al. claimed that depression was exacerbated in PWE [21].
3.6. The effect of COVID-19 pandemic on the stress of people with epilepsy
Seven studies evaluated stress through administering Impact of Event Scale-Revised (IES-R) [20], K6 scores [23], questionnaires [11], [15], [24], self-report [9], and Likert scale [25]. IES-R is one of the commonly used self-report tools within the trauma literature [26]. It has three subscales, namely (1) tapping intrusion like repeated thoughts about the occurrence, (2) avoidance like effortful attempts not to think about the occurrence, and (3) hyper arousal like anger, irritability, hypervigilance, and difficulty in concentrating measures related to the traumatic event [26]. According to the results of Giordano et al.’s study, higher scores on the IES-R intrusion subscale can be attributed to age, gender, and higher scores on the HADS. Meanwhile, higher scores on HADS could be related to higher scores on IES-R Hyperarousal subscales. Also, results revealed that higher scores on the IES-R Avoidance scale were associated with the higher age and higher scores on the HADS subscale. This result further suggests that there is a direct correlation between anxiety and stress levels [20].
Hao et al. measured psychological distress based on K-6 scores. They claimed that K-6 scores >12 indicated severe psychological distress. Results of their study revealed a significantly higher level of psychological distress among PWE compared to that of the control group. Also, results showed that 13.1% of PWE and 1.6% of the control group reported severe psychological distress (p < 0.001). Confinement-related anxiety and depression symptoms were reported by 68 (26.7%) and 31 patients (12.2%). The PWE, who submitted their answers online, experienced a higher level of stress and anxiety (p < 0.0001) accompanied by worse mental health condition during the COVID-19 lockdown (p = 0.015). Also, PWE who had changes in the use of AED one month before or during the lockdown expressed greater stress and GAD7scores [23].
Nearly 20% of subjects who reported worsened seizure control during the peak of COVID-19 showed more severe epilepsy and seizures accelerated by stress. The increased stress, associated with the pandemic, was directly correlated with the increased seizures in some PWE. 19.61% of patients reported moderate-to-critical concerns about the adverse effect of the pandemic on issues related to epilepsy. Also, 24.03% of patients expressed moderate-to-critical concerns about sudden seizures during the pandemic; alongside, 41.16% and 48.62% of patients worried about the loss of professional counselors and drug supply, respectively [15].
Rosengard et al. indicated that stress is the most important typical seizure trigger. They reported that patients with worsening seizure control showed more stress than patients with no change or improved seizure control (54.8% versus 26.0%, P = 002) [24]. Rosengard et al.’s study reported that stress level due to the COVID-19 pandemic was increased/worsened to 50% in those PWE who have no change or improved seizure control, and to 80.6% in those PWE who have worsened seizure control [24]. Therefore, based on the results of Rosengard et al., seizure control in patients during the COVID-19 pandemic can be effective in reducing the stress caused by COVID-19 [24]. Alongside, results of the Alkhotani et al.’s study indicated that a total of 59.4% of PWE reported a rise in self-reported stress during the COVID-19 lockdown [9].
3.7. The effect of COVID-19 pandemic on the anxiety of people with epilepsy
14 studies in this systematic review measured the level of anxiety in patients with epilepsy through collecting information from questionnaires [4], [9], [11], [19], [21], [24], behavioral scores such as promise anxiety scale [27], PHQ9 [6], GAD7 [15], [19], [25], HASD [6], [20], and BAI-Ⅱ [10]. The GAD-7 scale was applied to determine clinically important anxiety symptoms [22]. Results of the studies show that the level of anxiety increased in both PWE and PWOE; however, the rate of increase in anxiety is much more evident in PWE. In Assenza et al.’s study, 92 cases (28.9%) of PWE experienced mild anxiety symptoms, 162 (21.3%) reported moderate symptoms, and 47 (10.3%) had severe anxiety symptoms. With respect to PWOE, 128 cases (32.4%) reported mild anxiety symptoms, 142 (12.9%) and 34 (7.2%) reported moderate anxiety and severe anxiety symptoms, respectively [19]. Also, results revealed that PWE had higher anxiety symptoms than PWOE (higher GAD-7 scores; 8 ± 5.3 and 6.8 ± 4.9, respectively; p < 0.001). In addition, 44 PWE and 32 PWOE reported that they received new psychotropic drugs such as anxiolytics for depression and anxiety since the beginning of COVID-19 lockdown. However, no significant difference was observed with respect to GAD-7 values among responders from Italian region [19], GAD-7 scale was also used in Huang et al.’s study whose results showed that anxiety was observed among patients with or without an increased number of seizures, 19.35% and 8.46%, respectively. They observed no meaningful difference with respect to psychological reactions shown between the groups with and without seizure exacerbation [15].
Meanwhile, Fonsece et al.’s study found that confinement-related anxiety was reported by 68 patients (26.7%); alongside, 31 patients (12.2%) showed symptoms of both depression and anxiety from the onset of quarantine [4]. Using the PROMIS anxiety scale in their study, Miller et al. reported that respondents showed a statistically significant higher level of post-pandemic anxiety compared to that of pre-pandemic [27]. Similar to the results of the Miller et al.’s study, Puteikis et al. found that PWE had a higher level of stress and anxiety (p < 0.0001), and reported worse mental health conditions (p = 0.015) during the quarantine [25]. In Reilly et al.’s study, caregivers reported that the level of stress (55%) and anxiety (52%) increased among young PWE during the COVID-19 lockdown [11]. According to the results of the HADS scale, out of the 399 PWE, 201 (50.4%) were screened positive with respect to anxiety [6].
Based on the total BAI-II score in Salari et al.’s study, a meaningful difference was observed between the control and patient groups on severe levels of anxiety (13.5% of patients versus 6.9% of controls). However, at a lower level of anxiety (45.4% versus 48.7%), no difference was observed. Results of Salari et al.’s study showed that men of younger ages experienced significantly higher levels of anxiety in the control group; however, no correlation was found among anxiety, age, and gender in the patient group. Also, no correlation was observed among anxiety level, epilepsy duration, and epilepsy type [10].
Results of Lanzone et al.’s study revealed that during the COVID-19 pandemic, the PWOE have been continually over reporting several terms showing anxiety (words such as prison, distance, loneliness, anguish, stress, and change) as a reactive response to the stress they experienced under lockdown. Also, Lanzone et al. argue that one of the main reasons for PWOE’ anxiety is being worried about practical issues and restrictions of lockdown. Meanwhile, words such as fear, boredom, sadness, and apathy have been reported frequently. Results of Lanzone et al.’s study showed that PWE feel restricted and anxious during the lockdown. Also, they were worried about their disease that would subsequently lead to developing depressive symptoms [18].
Sanchez-Larsen et al. conducted their study with 100 patients (52% women with an average age of 42.4). An increase of more than 50% in the frequency of seizures was observed in 27% of the patients. Increased stress/anxiety (odds ratios (OR): 5.78; p = 0.008) and a higher seizure frequency (OR: 12.4; p = 0.001) were associated with worsening of the seizures. Also, seizure control was improved in 9% of patients. Reducing stress/anxiety (OR: 0.05; p = 0.03) and using recent adjustment of antiepileptic drugs as protecting agents (OR: 0.07; p = 0.01) was effective. The risk factors (increases in depression, sleep disorder, less physical activity, and history of epilepsy operation) were statistically accompanying an important deterioration in seizure control: exacerbation of stress/anxiety (77.8% vs 28.8%, OR: 8.67, 95% CI: 3.07–24.50; p < 0.001), sad-ness/depression (55.6% vs 27.4%, OR: 3.31, 95% CI: 1.32–8.29; p = 0.01) [21].
3.8. The effect of COVID-19 pandemic on the sleep disorders of people with epilepsy
Ten studies that reviewed sleep disorders, [2], [4], [9], [11], [15], [19], [20], [24], [25], have shown that PWE experienced sleep disorders during the COVID-19. Out of these 10 studies, six studies were cross-sectional in which sleep disorders and insomnia were reported 7.1–71.2% [4], [9] and 28.2%, respectively [4]. Giordano et al. measured sleep disturbances through Insomnia Severity Scale (ISI). Their study reported a figure of 7% for sleep disorders [20]; however, Assenza et al.’s study showed that there was no meaningful difference in the sleep quality between PWE and healthy individuals (PWOE). Also, PWE’s and PWOE’s scores in PSQI scale were reported 6.8 ± 3.7 (46.9%) and 6.6 ± 3.8 (42.4%), respectively (p = 0.3117) [19]. Also, results of Reilly et al.’s study showed that insomnia measured through ISI among patients with or without an increased number of seizures was 25.81% versus 19.03%. Reilly et al. observed no significant difference with respect to sleep responses between the groups with and without a seizure [11]. Meanwhile, Rosengard et al.’s study found that sleep quality decreased due to the COVID-19 pandemic among those PWE who reported no change or improved seizure control (41.8%) as well as those PWE who reported worsened seizure control (48.4%) [24]. However, no significant difference was observed in the rates of worsened sleep across groups during this three-month period [24].
The majority of young people and caregivers expressed that their mood (PWE: 64%; caregiver: 61%) and sleep (PWE: 72%; caregiver: 56%) have worsened during the COVID-19 lockdown. Additionally, the majority of caregivers reported increased anxiety, stress, and sleep disorder as a result of the pandemic [25]. Also, results of Masaccio et al.’s study indicated that 1.8% of patients have experienced a kind of improvement in sleep; however, 23.9% of the cases reported fluctuations in sleep, 8.5% of them reported disturbed sleep, and 11.3% mentioned changes in their sleep patterns [2].
4. Discussion
In the present systematic review, we investigated the prevalence of psychological effects of COVID-19 pandemic, namely depression, stress, anxiety, and sleep disorders among PWE; we also assessed the relationship among these psychological symptoms during the pandemic. Findings of our systematic review revealed that PWE experienced a dramatic increase in the severity of mental problems. It is believed that COVID-19 pandemic has led to increased mental health problems as a result of death tolls, high level of infection, and rapid distribution among populations. Meanwhile, it has been reported that stress is more prevalent during the pandemic [28]. Alongside, recent studies have demonstrated that following the pandemic, anxiety and depression widely increased that is ultimately accompanied by sleep disorders in the general population [28]. In this respect, patients with epilepsy are at considerable risk to the psychosocial impact of the pandemic [29].
Diverse factors may involve in the high prevalence of psychological distress in PWE during the COVID-19 pandemic such as social separation and aloneness [30]. Putting restrictions in order to control the pandemic has a number of behavioral and social consequences that would in turn trigger epileptic seizures, and lower the threshold level of epileptic seizures in PWE [19]. Also, stress has been found to exacerbate epilepsy control. In fact, there is a direct correlation between stress and the frequency of epileptic seizures among PWE [31]. Results of studies on the effects of the COVID-19 pandemic on stress levels among PWE showed a higher stress and anxiety level in PWE; in addition, they also experienced a worse mental health condition during the COVID-19 restrictions [23]. Meanwhile, about 20% of subjects, who reported worsened seizure control during the pandemic, showed more severe epilepsy and seizures accelerated by stress. The increased stress, associated with the pandemic, has been shown to have a direct correlation with worsening seizures among some PWE [15].
Also, it has been estimated that one in every three patients with epilepsy experienced mental issues. In the meantime, psychological distress frequently occurred in their everyday life [32]; therefore, it is expected that in stressful situations such as COVID-19 restrictions, the incidence of depression and anxiety would likely increase. Among psychological distress, anxiety and depression are the most common epilepsy-related problems frequently observed among PWE during the COVID-19 period. Results of studies on COVID-19 in Italy showed that PWE (19%) and PWOE (17%) reported psychiatric disorders such as depression. However, PWE had more severe depressive symptoms than PWOE [19]. Additionally, Van Hees et al. believe that there are remarkably gender differences among PWE, as it was reported that females are at a higher risk of increased depression problems during the COVID-19 pandemic [6]. Excessive exposure to stressors not only exacerbates seizures in PWE but also triggers acute seizures even in people who have no history of epilepsy [33]. In this regard, Huang et al.’s study reported that depression among PWE and PWOE increased the number of seizures (22.58% vs 12.08%, respectively) [15]. Moreover, several studies conducted during the COVID-19 pandemic have indicated that there were gender and age differences in relation to the prevalence of stress and anxiety symptoms among PWE. In this respect, female and older individuals had higher scores on the HADS which showed an anxiety subscale [20]. Other studies also showed that both PWE and PWOE experienced anxiety symptoms during the pandemic; however, significantly higher prevalence of anxiety was observed in PWE [19].
On the other hand, it seems that imposed restrictions during COVID-19 pandemic could affect negatively the sleep quality in general population due to the increasing rate of psychological disorder prevalence, reducing exercise activity, staying home, and insufficient access to sunlight [34]. In this respect, results showed that 7.1–71.2% of PWE experienced sleep problems [4], [9], and the prevalence of insomnia was also 28.2% in patients with epilepsy [4]. However, one study reported that there was no significant difference in the sleep quality between PWE and healthy individuals [15]. Sleep disturbance in PWE is a significant activator that can lead to an increase in motor excitability which develops the frequency of epileptic seizures in these patients [35]. Generally, deficiency of the sleep-wake cycle during the COVID-19 restriction may influence both PWE and PWOE; however, sleep disturbance may lead to an increase in EEG epileptiform abnormalities resulting in exacerbation of seizure in PWE [36].
5. Conclusion
The present systematic review evaluated the prevalence of mental health disorders in PWE during the COVID-19 pandemic. Collectively, results of this study indicated that the prevalence of psychological disorders, namely stress, depression, anxiety, and sleep disruption in PWE was higher than that of PWOE. Based on the results of the present study, we argue that gender and age play a significant role in the level of psychological disorders, especially stress, in PWE. Some of the included papers in this study have shown that PWE above 60 years old, especially women, and young people with the age range of 12–25 have a higher risk of psychological stress compared to other groups in PWE. We think that the reason for the increase in prevalence of stress in these patients are as follows: (1) home confinement, social isolation, and other social restrictions imposed during the COVID-19 pandemic, (2) changes in sleep quality and lifestyle due to COVID-19 restrictions, (3) lack of adequate physical activity, and (4) following up the news and information about COVID-19 [6], [9], [10], [15], [20].
We thus believe that PWE, especially the above-mentioned groups within the PWE, are at considerable risk during the pandemic. Therefore, adequate psychological supports should be given to these patients in order to relieve the mental health risks among them. In doing so, we recommend that PWE alongside their families learn working with psychometric assessment tools in order to better grasp PWE’s emotional states. In addition, receiving meditation and relaxation training by experts can help cope with the worsening stress and anxiety. Meanwhile, we think that online follow-up sessions, known as telemedicine, alongside phone chats, and video calls offered by specialists in the field can be effective in reducing the risk of psychological disorders in PWE during the pandemic and post-pandemic era.
6. Limitations
Although the present study provided some significant lines of evidence demonstrating a high level of prevalence of psychological distress in PWE, this systematic review still has some limitations. First, due to a large amount of heterogeneities observed in the results, we could not evaluate the exact rate of prevalence in spite of using effective measures. Meanwhile, meta‐analysis and robust conclusions cannot be drawn because of the significant heterogeneity of the invalidated tools used. Second, most studies were conducted with a limited number of European population or in English-speaking countries; thus, we could not examine the prevalence of mental health problems in African and Asian populations. Third, due to lack of evidence from low-income countries that presumably have a higher level of stress during COVID-19 pandemic, we thus cannot generalize the results of this study to such countries.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledging funding
This study was not supported by any institution.
Consent for publication
All authors consented for publication.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.yebeh.2021.108410.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Varma P., Junge M., Meaklim H., Jackson M.L., et al. Younger people are more vulnerable to stress, anxiety and depression during COVID-19 pandemic: a global cross-sectional survey. Prog Neuropsychopharmacol Biol Psychiatry. 2021;109 doi: 10.1016/j.pnpbp.2020.110236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mostacci B., Licchetta L., Cacciavillani C., Di Vito L., Ferri L., Menghi V., et al. The impact of the COVID-19 pandemic on people with epilepsy. An Italian survey and a global perspective. Front Neurol. 2020;11:1725. doi: 10.3389/fneur.2020.613719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Organization, W.H., WHO Coronavirus (COVID-19) Dashboard. 2021: p. https://covid19.who.int/.
- 4.Fonseca E., Quintana M., Lallana S., Restrepo J.L., Abraira L., Santamarina E., et al. Epilepsy in time of COVID-19: A survey-based study. Acta Neurol Scand. 2020;142(6):545–554. doi: 10.1111/ane.13335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pfefferbaum B, North C, Mental health and the Covid-19 pandemic. N Engl J Med, 2020;383(6):510-512. [DOI] [PubMed]
- 6.Van Hees S., Siewe Fodjo J.N., Wijtvliet V., Van den Bergh R., de Moura Villela E.F., da Silva C.F., et al. Access to healthcare and prevalence of anxiety and depression in persons with epilepsy during the COVID-19 pandemic: a multicountry online survey. Epilepsy Behav. 2020;112 doi: 10.1016/j.yebeh.2020.107350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scott A.J., Sharpe L., Hunt C., Gandy M. Anxiety and depressive disorders in people with epilepsy: a meta-analysis. Epilepsia. 2017;58(6):973–982. doi: 10.1111/epi.13769. [DOI] [PubMed] [Google Scholar]
- 8.Macêdo P.J.O.M., de Oliveira P.S., Foldvary-Schaefer N., da Mota Gomes M., et al. Insomnia in people with epilepsy: a review of insomnia prevalence, risk factors and associations with epilepsy-related factors. Epilepsy Res. 2017;135:158–167. doi: 10.1016/j.eplepsyres.2017.05.014. [DOI] [PubMed] [Google Scholar]
- 9.Alkhotani A., Siddiqui M.I., Almuntashri F., Baothman R. The effect of COVID-19 pandemic on seizure control and self-reported stress on patient with epilepsy. Epilepsy Behav. 2020;112 doi: 10.1016/j.yebeh.2020.107323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salari M., Etemadifar M., Gharagozli K., Etemad K., Ashrafi F., Ashourizadeh H. Incidence of anxiety in epilepsy during coronavirus disease (COVID-19) pandemic. Epilepsy Behav. 2020;112 doi: 10.1016/j.yebeh.2020.107442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reilly C., Muggeridge A., Cross J.H. The perceived impact of COVID-19 and associated restrictions on young people with epilepsy in the UK: young people and caregiver survey. Seizure. 2021;85:111–114. doi: 10.1016/j.seizure.2020.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gandy M., Sharpe L., Perry K.N. Psychosocial predictors of depression and anxiety in patients with epilepsy: a systematic review. J Affect Disord. 2012;140(3):222–232. doi: 10.1016/j.jad.2011.11.039. [DOI] [PubMed] [Google Scholar]
- 13.Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339 [PMC free article] [PubMed] [Google Scholar]
- 14.Munn Z., Moola S., Riitano D., Lisy K. The development of a critical appraisal tool for use in systematic reviews addressing questions of prevalence. Int J Health Policy Manage. 2014;3(3):123–128. doi: 10.15171/ijhpm.2014.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang S., Wu C., Jia Y., Li G., Zhu Z., Lu K., et al. COVID-19 outbreak: The impact of stress on seizures in patients with epilepsy. Epilepsia. 2020;61(9):1884–1893. doi: 10.1111/epi.16635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Briggs J. Joanna Briggs Institute; Australia: 2014. The systematic review of prevalence and incidence data. [Google Scholar]
- 17.Reynders R.M., Ronchi L., Ladu L., Di Girolamo N., de Lange J., Roberts N., et al. Barriers and facilitators to the implementation of orthodontic mini implants in clinical practice: a systematic review. Syst Rev. 2016;5(1):1–21. doi: 10.1186/s13643-016-0336-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lanzone J., Cenci C., Tombini M., Ricci L., Tufo T., Piccioli M., et al. Glimpsing the impact of COVID19 lock-down on people with epilepsy: a text mining approach. Front Neurol. 2020;11 doi: 10.3389/fneur.2020.00870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Assenza G., Lanzone J., Brigo F., Coppola A., Di Gennaro G., Di Lazzaro V., et al. Epilepsy care in the time of COVID-19 pandemic in Italy: risk factors for seizure worsening. Front Neurol. 2020;11:737. doi: 10.3389/fneur.2020.00737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giordano A., Siciliano M., De Micco R., Sant'Elia V., Russo A., Tedeschi G., et al. Correlates of psychological distress in epileptic patients during the COVID-19 outbreak. Epilepsy Behav. 2021;115 doi: 10.1016/j.yebeh.2020.107632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanchez-Larsen A., Gonzalez-Villar E., Díaz-Maroto I., Layos-Romero A., Martínez-Martín Á., Alcahut-Rodriguez C., et al. Influence of the COVID-19 outbreak in people with epilepsy: Analysis of a Spanish population (EPICOVID registry) Epilepsy Behav. 2020;112 doi: 10.1016/j.yebeh.2020.107396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kroenke K., Spitzer R.L., Williams J.B. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hao X., Zhou D., Li Z., Zeng G., Hao N., Li E., et al. Severe psychological distress among patients with epilepsy during the COVID-19 outbreak in southwest China. Epilepsia. 2020;61(6):1166–1173. doi: 10.1111/epi.16544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosengard J.L., Donato J., Ferastraoaru V., Zhao D., Molinero I., Boro A., et al. Seizure control, stress, and access to care during the COVID-19 pandemic in New York City: The patient perspective. Epilepsia. 2021;62(1):41–50. doi: 10.1111/epi.16779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Puteikis K., Jasionis A., Mameniškienė R. Recalling the COVID-19 lockdown: Insights from patients with epilepsy. Epilepsy Behav. 2021;115 doi: 10.1016/j.yebeh.2020.107573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beck J.G., Grant D.M., Read J.P., Clapp J.D., Coffey S.F., Miller L.M., et al. The impact of event scale-revised: psychometric properties in a sample of motor vehicle accident survivors. J Anxiety Disord. 2008;22(2):187–198. doi: 10.1016/j.janxdis.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller W.R., Von Gaudecker J., Tanner A., Buelow J.M. Epilepsy self-management during a pandemic: experiences of people with epilepsy. Epilepsy Behav. 2020;111 doi: 10.1016/j.yebeh.2020.107238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yuan K., Gong Y.-M., Liu L., Sun Y.-K., Tian S.-S., Wang Y.-J., et al. Prevalence of posttraumatic stress disorder after infectious disease pandemics in the twenty-first century, including COVID-19: a meta-analysis and systematic review. Mol Psychiatry. 2021:1–17. doi: 10.1038/s41380-021-01036-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.French J.A., Brodie M.J., Caraballo R., Devinsky O., Ding D., Jehi L., et al. Keeping people with epilepsy safe during the COVID-19 pandemic. Neurology. 2020;94(23):1032–1037. doi: 10.1212/WNL.0000000000009632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cénat J.M., Hosseinian-Far A., Jalali R., Vaisi-Raygani A., Rasoulpoor S., Mohammadi M., et al. Prevalence of symptoms of depression, anxiety, insomnia, posttraumatic stress disorder, and psychological distress among populations affected by the COVID-19 pandemic: A systematic review and meta-analysis. Glob Health. 2020 [Google Scholar]
- 31.McKee H.R., Privitera M.D.J.S. Stress as a seizure precipitant: identification, associated factors, and treatment options. Seizure. 2017;44:21–26. doi: 10.1016/j.seizure.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 32.Ribot R., Kanner A.M.J.E. Neurobiologic properties of mood disorders may have an impact on epilepsy: should this motivate neurologists to screen for this psychiatric comorbidity in these patients? Epilepsy Behav. 2019;98:298–301. doi: 10.1016/j.yebeh.2019.01.026. [DOI] [PubMed] [Google Scholar]
- 33.Christensen J., Li J., Vestergaard M., Olsen J. Stress and epilepsy: a population-based cohort study of epilepsy in parents who lost a child. Epilepsy Behav. 2007;11(3):324–328. doi: 10.1016/j.yebeh.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 34.Abokalawa F., Ahmad S.F., Al-Hashel J., Hassan A.M., Arabi M., et al. The effects of coronavirus disease 2019 (COVID-19) pandemic on people with epilepsy (PwE): an online survey-based study. Acta Neurol Belg. 2021:1–8. doi: 10.1007/s13760-021-01609-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manganotti P., Bongiovanni L.G., Fuggetta G., Zanette G., Fiaschi A. Effects of sleep deprivation on cortical excitability in patients affected by juvenile myoclonic epilepsy: a combined transcranial magnetic stimulation and EEG study. J Neurol Neurosurg Psychiatry. 2006;77(1):56–60. doi: 10.1136/jnnp.2004.041137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pratt K.L., Mattson R.H., Weikers N.J., Williams R. EEG activation of epileptics following sleep deprivation: a prospective study of 114 cases. Electroencephalogr Clin Neurophysiol. 1968;24(1):11–15. doi: 10.1016/0013-4694(68)90061-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.