Abstract
Backgroud
Heart responds to physiologic and pathologic conditions and sympathetic drive plays an important role. It has been documented that LV base is more dominantly affected by sympathetic drive compared to the other regions. LV base is more dominantly exposed to wall stress in the initial period of remodeling due to pressure-overload, since LV cavity is the largest at base. Basal septal hypertrophy (BSH) in cross-sectional data is associated with the early phase of hypertensive heart disease. BSH was confirmed by 3rd generation microscopic ultrasound in small animals. BSH as the closest location to increased afterload could be detected in variety of stress stimuli and result in a huge septal hypertrophy in advance cases possibly related to earlier exposure of hemodynamic stress to septal wall.
Conclusion
Effective geometric and functional evaluation of initial remodeling due to hemodynamic stress is important according to both human and animal data. These findings possibly contribute to early recognition of adaptive phase of hypertensive remodeling and more effective management in a timely fashion.
Keywords: Hemodynamic stress, Adaptive phase, Remodeling, Basal septal hypertrophy, Hypertension
Highlights
-
•
Microimaging provides the documentation of regional evolution of left ventricular remodeling due to hemodynamic stress prospectively beyond cross-sectional clinical findings.
-
•
Both human and animal data show that novel quantitative imaging methods provide documentation of geometric and functional details of adaptive phase during hemodynamic stress-mediated remodeling.
-
•
Basal septal hypertrophy and hyperdynamic tissue response to hemodynamic stress could improve the clinical assessment of individuals with adaptive phase.
1. Hemodynamic stress and heart
Physiologic and pathologic stimuli leads to cardiac remodeling and sympathetic drive is an important part of this longstanding course [1]. Dynamic blood pressure fluctuations is one of the most important stimuli leading to cardiac tissue remodeling [2]. Although types of stress induction could be different in secondary cardiomyopathy, myocardial response to acute or chronic conditions may represent some specific aspects [[2], [3], [4]]. Hypertension is the most important factor for heart failure [1] and associated with multiple end-organ involvement that includes an important subclinical cardiac damage, left ventricular hypertrophy (LVH) which is approximately 12 % among patients with borderline hypertension and 20 % among patients with relatively mild hypertension [5]. LVH is a detectable target organ damage in population with previously undiagnosed hypertension [6]. LVH may not always be an adaptive process and its prevalence has ranged from 36 % to 41 % in the pooled population [7]. Therefore, it is crucial to use specific imaging findings representing early adaptive phase to hemodynamic stress with increased afterload prior to LV dysfunction and maladaptation.
2. Regional geometry and sympathetic drive
Original heart geometry is associated with the largest region at base, while the distance decreases towards apex [8]. Because of larger volume at the LV base than midapical segment, LV basal wall stress is the greatest compared to other segments. Secondary LVH to hypertension is associated with a diminished basal volume detected by the real-time 3 dimensional echocardiography (3 DE), [9]. Sympathetic innervation was found relatively predominant in LV base compared to the other regions by histopathologic data long ago [10]. Regional stress-induced predominant morphology and related basal cavity narrowing in 3DE possibly related to sympathetic overdrive which was clearly documented in the precisely designed studies [11]. Additionally, distribution of circulating norepinephrine levels and sympathetic nerves are well correlated in the body parts, especially in the heart [12]. I-meta-iodobenzylguanidine (I-mIBG) is a norepinephrine analog and acts as same as norepinephrine in uptake, storage and releasing stages. Further studies reported the increased sympathetic activity in basal septum via I-meta-iodobenzylguanidine (I-mIBG) scintigraphy [13]. Shimizu et al.‘s study supports our paper in which they addressed that hypertension can lead to an asymmetric septal hypertrophy and basal hypertrophy, and increased I-mIBG activity (norepinephrine analog) in patients with hypertension [14].
3. Microimaging and regional remodeling
Cross-sectional data have shown a regional remodeling of LV base (Fig. 1a, b, c) named basal septal hypertrophy (BSH) could be detected in hypertensive patients [15,16]. We and the others previously speculated that this finding could be related to increased cardiac dynamics since BSH is developed in the location which is the closest myocardial part to increased afterload and is associated with a high rate-pressure product in hypertension [17,18]. However, none of these observations had prospective value, since they all were cross-sectional human studies. Quantitative echocardiographic follow-up could be beneficial for monitoring cardiac remodeling due to pressure-overload and preventing the progressive process using effective medical management [19]. Recently, we have planned a study to document evolution of the LV segmental remodeling prospectively using 3rd generation microscopic ultrasonography. We have confirmed this early segmental tissue torsion at LV base in this small animal study and noted that BSH is the early imaging biomarker in pressure-overload model for the first time, while hypertrophy progresses to midapical portion later on (Fig. 2), [20]. Microimaging in this animal experiment has provided an opportunity for regional geometric and functional analysis during LV remodeling. Furthermore, this cardiac imaging biomarker during the early period of hypertensive heart disease may be used in clinical evaluation to avoid more untreated patients with target organ damage, since LV remodeling is not rare in this group [5,6].
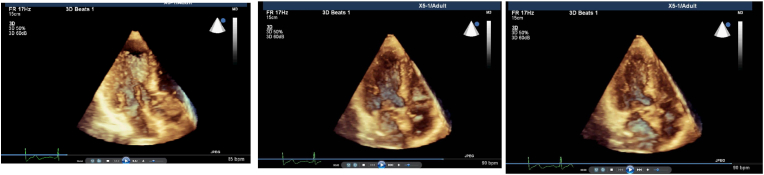
Fig. 1.
a: Real-time 3 dimensional imaging from apical 4 chamber view shows the predominant regional LV septal base during end-diastole in a patient with systemic hypertension. b: Increased prominency of LV septal base during early-systole from apical 4 chamber view of real-time 3 dimensional imaging in the same patient. c: LV basal cavity obliteration by septal base during end-systole from the same echocardiographic window in the same patient.
Fig. 2.
Cardiac images of a mice using 3rd generation microscopic ultrasound show normal cardiac geometry, relatively prominent LV septal base at 4 week after stress induction due to pressure-overload (TAC: transverse aortic construction) and global remodeling at 8 week, respectively.
Cardiac morphology and function in mice can be assessed non-invasively using a high-frequency, high-resolution in vivo microimaging echocardiography system designed especially for small animal imaging research. High-resolution images can be obtained in the parasternal and apical orientations. Standard B-mode 2 dimensional images of the heart, pulsed Doppler images of the mitral valve in flow and pulse wave tissue Doppler imaging of the myocardial tissue velocities can be determined quantitatively. Animal model and microscopic ultrasonography is a perfect opportunity for scientists to validate their findings in cross-sectional human studies using segmental tissue details in disease course prospectively.
4. Regional dynamics and pressure-overload
Recently, we have validated functional evolution of pressure-overload by determination of intracavitary gradients using 3rd generation microscopic ultrasonography and noted that the early imaging biomarker, BSH is associated with compensatory hyperfunction prior to maladaptation to increased stress [21]. In this study, incremental LV intracavitary blood velocity in adaptive phase of remodeling has become blunted sharply after global remodeling. Cross-sectional human studies were consistent with the validated quantitative animal data showing that stress-mediated LV hyperfunction as a component of the clinical spectrum in early stage hypertension [[22], [23], [24]]. Regular microscopic evolution of LV remodeling in mice [20,21] supports that variety of stress stimuli could be combined in human data, because of quite heterogenous morphology [3,4,[17], [18], [19],25,26].
Enhanced ventricular contractility in mild hypertension was documented by quantitative functional parameters [27]. LV hyperfunction was also determined in borderline blood pressure elevations and higher blood pressures in holter monitoring that was associated with a greater myofibriller shortening [28]. In patients with chest pain and normal coronary arteries, hyperdynamic LV was determined by wall motion analysis [29]. In this study, hyperdynamic LV was more common in hypertensive patients than the others. In the early adaptive phase, BSH is associated with hyperfunction under stress that we previously detected using combined stress echocardiography and tissue Doppler imaging quantitatively [30]. Basal septal involvement during hypertensive process could be huge in advance cases that presumably related to early stress exposure of septal base and alcohol septal ablation could become a solution for advance hypertensive cases [4,19,25]. Quantitative imaging techniques are able to depict regional motion including tissue velocity or displacement and sensitive quantitative markers of myocardial tissue function in both animal models and humans [31,32]. In addition to LV hyperfunction at stress on tissue level that could be determined by quantitative tissue imaging, increased cardiac index and ejection fraction determination at stress test could also be determined by nuclear studies.
5. Myocardial dynamics in hypertension and radyonuclid studies
Hyperdynamic tissue response to stress are not rare in patients with high blood pressure and high rate-pressure product, respectively [29,30]. In fact, radionuclide myocardial perfusion imaging showed hyperfunctional myocardial response to stress induction associated with diastolic dysfunction and speculated that dyspnea may be related to diastolic dysfunction [33]. Moreover, we found this conclusion sceptical and emphasized the importance of possible exercise hypertension for mechanism of hyperdynamic LV function [34]. First-pass radionuclide angiography is associated with LV hyperfunction and exercise hypertension, while those individuals were normotensive at rest [35]. Nevertheless, if diagnosis of adaptive phase of hypertensive disease is missed [6], LVH leads to LV dysfunction which is detectable by variety of modalities as echocardiography, magnetic resonance and nuclear imaging [[36], [37], [38], [39]].
6. Conclusion
Basal septal hypertrophy and hyperdynamic tissue response to hemodynamic stress could improve the clinical assessment of individuals with adaptive phase of remodeling according to both human and animal data.
Acknowledgement
There is no potential conflict of interest to declare.
Footnotes
FY and HY are supported by the US Government Fulbright Program, Washington DC, USA.
References
- 1.Hill J.A., Olson E.N. Cardiac plasticity. N. Engl. J. Med. 2008;358:1370–1380. doi: 10.1056/NEJMra072139. [DOI] [PubMed] [Google Scholar]
- 2.Yalçin F., Yalçin H., Abraham T.P. Exercise hypertension should be recalled in basal septal hypertrophy as the early imaging biomarker in patients with stressed heart morphology. Blood Pres. Monit. 2020;25:118–119. doi: 10.1097/MBP.0000000000000429. [DOI] [PubMed] [Google Scholar]
- 3.Yalçin F., Yalçin H., Abraham T.P. Stress-induced regional features of left ventricle is related to pathogenesis of clinical conditions with both acute and chronic stress. Int. J. Cardiol. 2010;145:367–368. doi: 10.1016/j.ijcard.2010.02.041. [DOI] [PubMed] [Google Scholar]
- 4.Yalçin F., Topaloglu C., Kucukler N., Ofgeli M., Abraham T.P. Could early septal involvement in the remodeling process be related to the advance hypertensive heart disease? Int. J. Cardiol. Heart Vasc. 2015;7:241–245. doi: 10.1016/j.ijcha.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hammond I.W., Devereux R.B., Alderman M.H., et al. The prevalance and correlates of echocardiographic left ventricular hypertrophy among employed patients with uncomplicated hypertension. J. Am. Coll. Cardiol. 1986;7:639–650. doi: 10.1016/s0735-1097(86)80476-4. [DOI] [PubMed] [Google Scholar]
- 6.Korhonen P.E., Kautiainen H., Järvenpää S., Kantola I. Target organ damage and cardiovascular risk factors among subjects with previously undiagnosed hypertension. Eur. J. Prev. Cardiol. 2013;21:980–988. doi: 10.1177/2047487312474530. [DOI] [PubMed] [Google Scholar]
- 7.Cuspidi C., Sala C., Negri F., Mancia G., Morganti A. Prevalence of left-ventricular hypertrophy in hypertension: an updated review of echocardiographic studies. J. Hum. Hypertens. 2012;26:343–349. doi: 10.1038/jhh.2011.104. [DOI] [PubMed] [Google Scholar]
- 8.Frielingsdorf J., Franke A., Kühl H.P., Hess O.M., Flachskampf F.A. Evaluation of septal hypertrophy and systolic function in diseases that cause left ventricular hypertrophy: a 3-dimensional echocardiography study. J. Am. Soc. Echocardiogr. 2001;14:370–377. doi: 10.1067/mje.2001.112674. [DOI] [PubMed] [Google Scholar]
- 9.Yalçin F., Shiota T., Odabashian J., et al. Comparison by real-time three-dimensional echocardiography of left ventricular geometry in hypertrophic cardiomyopathy versus secondary left ventricular hypertrophy. Am. J. Cardiol. 2000;85:1035–1038. doi: 10.1016/s0002-9149(99)00929-7. [DOI] [PubMed] [Google Scholar]
- 10.Angelakos E.T., Davis R.W. Regional distribution of catecholamines in dog heart. Circ. Res. 1965;16:39–44. doi: 10.1161/01.res.16.1.39. [DOI] [PubMed] [Google Scholar]
- 11.Schlaich M.P., Kaye D.M., Lambert E., Sommerville M., Socratous F., Esler M.D. Relation between cardiac sympathetic activity and hypertensive left ventricular hypertrophy. Circulation. 2003;108:560–565. doi: 10.1161/01.CIR.0000081775.72651.B6. [DOI] [PubMed] [Google Scholar]
- 12.Kimata S. Chemical evaluation of the distribution of catecholamines in the heart. Jpn. Circ. J. 1965;29:7–10. doi: 10.1253/jcj.29.7. [DOI] [PubMed] [Google Scholar]
- 13.Verschure D.O., van Eck-Smit B.L., Somsen G.A., Verberne H.J. Cardiac sympathetic activity in hypertrophic cardiomyopathy and Tako-tsubo cardiomyopathy. Clin. Transl. Imaging. 2015;3:379–385. doi: 10.1007/s40336-015-0133-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shimizu M., Ino H., Okeie K., et al. Cardiac sympathetic activity in the asymmetrically hypertrophied septum in patients with hypertension or hypertrophic cardiomyopathy. Clin. Cardiol. 2000;23:365–370. doi: 10.1002/clc.4960230512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Safar M., Benessiano J.R., Hornysk A.L. Editorial note Asymmetric septal hypertrophy and borderline hypertension. Int. J. Cardiol. 1982;2:103–108. doi: 10.1016/0167-5273(82)90015-8. [DOI] [PubMed] [Google Scholar]
- 16.Belenkie I., MacDonald R.P., Smith E.R. Localized septal hypertrophy: part of the spectrum of hypertrophic cardiomyopathy or an incidental echocardiographic finding? Am. Heart J. 1988;115:385–390. doi: 10.1016/0002-8703(88)90486-3. [DOI] [PubMed] [Google Scholar]
- 17.Krasnow N. Subaortic septal bulge simulates hypertrophic cardiomyopathy by angulation of the septum with age, independent of focal hypertrophy. An echocardiographic study. J. Am. Soc. Echocardiogr. 1997;10:545–555. doi: 10.1016/s0894-7317(97)70009-9. [DOI] [PubMed] [Google Scholar]
- 18.Yalçin F., Muderrisoglu H., Korkmaz M.E., Ozin B., Baltali M., Yigit F. The effect of dobutamine stress on left ventricular outflow tract gradients in hypertensive patients with basal septal hypertrophy. Angiology. 2004;55:295–301. doi: 10.1177/000331970405500309. [DOI] [PubMed] [Google Scholar]
- 19.Yalçin F., Yalçin H., Küçükler N., Abraham T.P. Quantitative left ventricular contractility analysis under stress: a new practical approach in follow-up of hypertensive patients. J. Hum. Hypertens. 2011;25:578–584. doi: 10.1038/jhh.2010.101. [DOI] [PubMed] [Google Scholar]
- 20.Yalçin F., Kucukler N., Cingolani O., Mbiyangandu B., Sorensen L., Abraham M.R., et al. Evolution of ventricular hypertrophy and myocardial mechanics in physiologic and pathologic hypertrophy. J. Appl. Physiol. 2019;126(1985):354–362. doi: 10.1152/japplphysiol.00199.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yalçin F., Kucukler N., Cingolani O., et al. Intracavitary gradient in mice with early regional remodeling at the compensatory hyperactive stage prior to left ventricular tissue dysfunction. J. Am. Coll. Cardiol. 2020;75(11) 10.1016/S0735- 1097(20)32212-9. [Google Scholar]
- 22.Come P.C., Bulkley B.H., Goodman Z.D., Hutchins G.M., Pitt B., Fortuin N.J. Hypercontractile cardiac states stimulating hypertrophic cardiomyopathy. Circulation. 1977;55:901–908. doi: 10.1161/01.cir.55.6.901. [DOI] [PubMed] [Google Scholar]
- 23.Verdecchia P., Porcellati C., Zampi I., et al. Asymmetric left ventricular remodeling due to isolated septal thickening in patients with systemic hypertension and normal left ventricular masses. Am. J. Cardiol. 1994;73:247–252. doi: 10.1016/0002-9149(94)90228-3. [DOI] [PubMed] [Google Scholar]
- 24.de Simone G., Di Lorenzo L., Costantino G., Moccia D., Buonissimo S., de Divitiis O. Supernormal contractility in primary hypertension without left ventricular hypertrophy. Hypertension. 1988;11:457–463. doi: 10.1161/01.hyp.11.5.457. [DOI] [PubMed] [Google Scholar]
- 25.Vieira M.L., Silva Filho R.M., Brito Filho F.S., et al. Selective contrast echocardiography in percutaneous transluminal septal myocardial ablation in an elderly patient with left ventricular concentric hypertrophy. Echocardiography. 2003;20:563–566. doi: 10.1046/j.1540-8175.2003.03096.x. [DOI] [PubMed] [Google Scholar]
- 26.Yalçin F., Muderrisoglu H. Tako-tsubo cardiomyopathy may be associated with cardiac geometric features as observed in hypertensive heart disease. Int. J. Cardiol. 2009;135:251–252. doi: 10.1016/j.ijcard.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 27.Lutas E.M., Devereux R.B., Reis G., et al. Increased cardiac performance in mild essential hypertension. Left ventricular mechanics. Hypertension. 1985;7:979–988. doi: 10.1161/01.hyp.7.6.979. [DOI] [PubMed] [Google Scholar]
- 28.Hinderliter A.L., Light K.C., Willis P.W. Patients with borderline elevated blood pressure have enhanced left ventricular contractility. Am. J. Hypertens. 1995;8:1040–1045. doi: 10.1016/0895-7061(95)00256-1. [DOI] [PubMed] [Google Scholar]
- 29.Madaric J., Bartunek J., Verhamme K., Penicka M., Van Schuerbeeck E., Nellens P., et al. Hyperdynamic myocardial response to beta-adrenergic stimulation in patients with chest pain and normal coronary arteries. J. Am. Coll. Cardiol. 2005;46:1270–1275. doi: 10.1016/j.jacc.2005.06.052. [DOI] [PubMed] [Google Scholar]
- 30.Yalçin F., Yigit F., Erol T., Baltali M., Korkmaz M.E., Müderrisoglu H. Effect of dobutamine stress on basal septal tissue dynamics in hypertensive patients with basal septal hypertrophy. J. Hum. Hypertens. 2006;20:628–630. doi: 10.1038/sj.jhh.1002041. [DOI] [PubMed] [Google Scholar]
- 31.Derumeaux G., Mulder P., Richard V., et al. Tissue Doppler imaging differentiates physiological from pathological pressure-overload left ventricular hypertrophy in rats. Circulation. 2002;105:1602–1608. doi: 10.1161/01.cir.0000012943.91101.d7. [DOI] [PubMed] [Google Scholar]
- 32.Shan K., Bick R.J., Poindexter B.J., et al. Relation of tissue Doppler derived myocardial velocities to myocardial structure and beta-adrenergic receptor density in humans. J. Am. Coll. Cardiol. 2000;36:891–896. doi: 10.1016/s0735-1097(00)00786-5. [DOI] [PubMed] [Google Scholar]
- 33.Gorantla R.S., Ahmed S., Voruganti D., Menzies D.J. Hyperdynamic left ventricle on radionuclide myocardial perfusion imaging (RNMPI): a marker of diastolic dysfunction in patients presenting with dyspnea on exertion. Int. J. Cardiol. Heart Vasc. 2015;9:43–47. doi: 10.1016/j.ijcha.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yalçin F., Schindler T., Abraham T.P. Hypertension should be ruled out in hyperdynamic left ventricle in radionuclide myocardial perfusion imaging, diastolic dysfunction, dyspnea on exertion. Int. J. Cardiol. Heart Vasc. 2015;7:149–150. doi: 10.1016/j.ijcha.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iskandrian A.S., Heo J. Exaggerated systolic blood pressure response to exercise: a normal variant or a hyperdynamic phase of essential hypertension? Int. J. Cardiol. 1988;18:207–221. doi: 10.1016/0167-5273(88)90166-0. [DOI] [PubMed] [Google Scholar]
- 36.Wachtell K., Gerdts E., Palmieri V., et al. In-treatment midwall and endocardial fractional shortening predict cardiovascular outcome in hypertensive patients with preserved baseline systolic ventricular function: the Losartan Intervention for Endpoint reduction study. J. Hypertens. 2010;28:1541–1546. doi: 10.1097/HJH.0b013e328339f943. [DOI] [PubMed] [Google Scholar]
- 37.Elhendy A., Schinkel A.F., Van Domburg R.T., Bax J.J., Poldermans D. Prediction of cardiac death in hypertensive patients with suspected or known coronary artery disease by stress technetium-99m tetrofosmin myocardial perfusion imaging. J. Hypertens. 2003;21:1945–1951. doi: 10.1097/00004872-200310000-00023. [DOI] [PubMed] [Google Scholar]
- 38.Bigi R., Bestetti A., Strinchini A., et al. Combined assessment of left ventricular perfusion and function by gated single-photon emission computed tomography for the risk stratification of high-risk hypertensive patients. J. Hypertens. 2006;24 doi: 10.1097/01.hjh.0000217861.12617.33. [DOI] [PubMed] [Google Scholar]
- 39.Nakagawa Y., Kijima Y., Nishibe A. Myocardial fibrosis is associated with diastolic heart failure in hypertensive patients - noninvasive assessment by cardiac magnetic resonance. Eur. Heart J. 2010;31:S–457. [Google Scholar]