Abstract
Stroke is a leading cause of morbidity and mortality worldwide. It inflicts immeasurable suffering on patients and their loved ones and carries an immense social cost. Efforts to mitigate the impact of stroke have focused on identifying therapeutic targets for the prevention and treatment. The gut microbiome represents one such potential target given its multifaceted effects on conditions known to cause and worsen the severity of stroke. Vitamin B12 (VB12) serves as a cofactor for two enzymes, methylmalonyl-CoA synthase and methionine synthase, vital for methionine and nucleotide biosynthesis. VB12 deficiency results in a buildup of metabolic substrates, such as homocysteine, that alter immune homeostasis and contribute to atherosclerotic disorders, including ischemic stroke. In addition to its support of cellular function, VB12 serves as a metabolic cofactor for gut microbes. By shaping microbial communities, VB12 further impacts local and peripheral immunity. Growing evidence suggests that gut dysbiosis-related immune dysfunction induced by VB12 deficiency may potentially contributes to stroke pathogenesis, its severity, and patient outcomes. In this review, we discuss the complex interactions of VB12, gut microbes and the associated metabolites, and immune homeostasis throughout the natural history of ischemic stroke.
Keywords: Stroke, Ischemic stroke, Cerberovascular Disease, Atherosclerosis, gut microbiome, microbiome, gut-brain axis, metabolism, metabolites, Neuroinflammation, Vitamin B12, Cobalamin, Homocysteine, innate immunity, Microglia, Inflammation
Search Strategy and Criteria.
Data for this Review were identified by searches of MEDLINE, PubMed, Google Scholar and references from relevant articles using the search terms “vitamin B12”, “gut microbiome”, “ischemic stroke”, “homocysteine”. and “cobalamin”. Only articles published in English between 1973 and 2021 were included.
Alt-text: Unlabelled box
1. Introduction
Despite major diagnostic and therapeutic advancements for the management of stroke, it remains a leading global cause of death and disability. Thus, the identification of reversible risk factors, reliable biomarkers, therapeutic targets in acute management and interventions to promote neurologic recovery are paramount to combat this disease. Growing evidence supports the notion that the gut microbial dysbiosis may affect development of stroke risk, toxic neuroinflammation in the immediate aftermath of stroke, and stroke recovery. VB12, a vital cofactor for amino acid and nucleotide biosynthesis and metabolic substrate for gut microbes, confers protection against the induction and severity of stroke in appropriately selected patients [1]. In this review, we discuss the link between the gut microbiome, stroke risk factor pathogenesis and stroke-mediated neuroinflammation, as well as report data that illuminates the potential impact of VB12 and its associated substrates on gut microbial composition and gut-brain immune homeostasis throughout the natural history of ischemic stroke.
2. Vitamin B12 absorption, biosynthesis, and activity
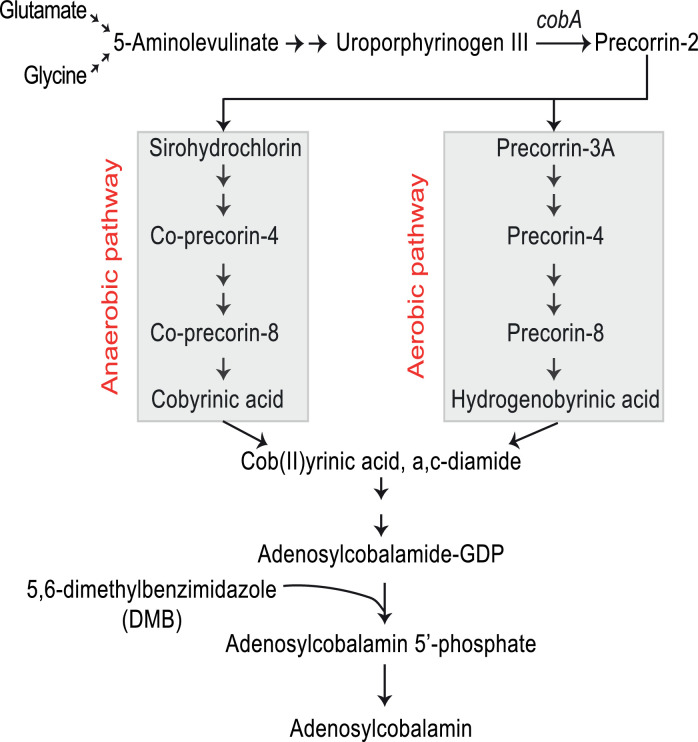
VB12, also known as cobalamin, is synthesized in nature by specific bacteria and archaea [2]. VB12 consists of a corrin ring in which cobalt is positioned centrally and coordinated with upper and lower ligands [3]. Cobalamins and naturally-occurring chemical compounds, cobamides, contain cobalt in corrinoid family macrocyclic complexes, which can be distinguished by a lower ligand made up of 5, 6-dimethylbenzimidazole (DMB). Mechanistically, the lower ligand DMB is pivotal for binding VB12 to the intrinsic factor (IF) to form an IF-VB12 complex recognized by cubilin and megalin which facilitate receptor mediated-endocytosis by intestinal epithelial cells (iECs) [4]. IF is then degraded in lysosomes and VB12 is released in the microenvironment by iECs to influence ileal cellular homeostasis [5]. Importantly, VB12 is involved in nucleotide synthesis, regulation of branched-chain amino acids (BCAAs), long-chain fatty acid metabolism and cellular development [6]. VB12 is also a vital cofactor in cytoplasmic methionine synthase and in mitochondrial methylmalonyl-CoA mutase, directing the methylation of homocysteine to methionine and the conversion of methylmalonyl-CoA to succinyl-CoA, respectively [7]. Moreover, VB12 is a critical cofactor used by other gut commensals to regulate the breakdown of VB12-dependent short-chain fatty acids (SCFAs), including, butyrate, propionate and acetate [8]. VB12 can be produced through two routes, aerobic and anaerobic, whereupon nearly 30 different enzymes are required for its biosynthesis (Fig. 1). Several genes, both within and external to the cob operon express enzymes critical for de novo biosynthesis [9]. Very few specific bacteria, including the Propionibacterium P. UF1 strain, harbor the genetic machinery necessary for VB12 synthesis. The capacity of VB12 to alter immune homeostasis in the gut, promote microbial metabolite utilization and support cellular metabolism make it a vital factor in facilitating resilience to ischemic stroke pathogenesis and severity.
Fig. 1.
Vitamin B12 Biosynthetic Pathways.
De Novo biosynthesis occurs with divergent enzymatic pathways based on the availability of oxygen. Gut microbes with necessary biochemical machinery convert glutamate and glycine to 5-aminolevulinate, which is then converted to uroporphyrinogen III (UroIII). The cobA gene product converts UroIII to precorrin-2, the last common intermediate between the anaerobic and aerobic pathways which converge with the production of Cob(II)yrinic acid, a,c-diamide, which, through an additional series of reactions, is then converted to adenosylcobalamin (VB12).
3. Neurologic complications of vitamin B12 deficiency
Severe VB12 deficiency causes myelopathy (subacute combined degeneration), ataxia, optic neuropathy, peripheral neuropathy and dementia with psychosis, all of which may be reversible through VB12 supplementation. Early histopathological analyses of VB12-deficient patients demonstrated glial dysfunction and characteristic “spongy” lesions of the brain and spinal cord. While the precise mechanisms by which VB12 deficiency induces neuropsychiatric conditions remains unclear, several vital functions of VB12 are likely to be relevant. VB12 protects against oxidative injury and inflammatory stress by directly scavenging free radical species and suppressing oxidation-induced injury, supporting glutathione activity, reducing endoplasmic reticulum stress and resisting neuronal apoptosis in several models of neurological disease [10].
VB12 deficiency also contributes to ischemic stroke risk and severity in the absence of other neurologic stigmata of VB12 deficiency. It operates as a crucial cofactor for methylation in fatty acid biosynthesis, while substrates that accumulate as a result of VB12 deficiency, such as methylmalonyl-CoA, can suppress or disturb normal lipid synthesis [11]. Thus, VB12 promotes, and its deficiency potentially hinders, the recovery of lost myelinated tracts following ischemic stroke. Mice deficient in TClR2/CD320, the extracellular receptor for VB12, demonstrate enlarged nodes of Ranvier and reduced myelin content compared to controls, as well as increased microglial activation and proinflammatory cytokine expression [12]. VB12 is also integral for production of S-Adenosylmethionine (SAM), a methyl donor critical for epigenetic modulation. One genome-wide association study of several large prospective cohorts demonstrated several categorical differentially-methylated regions in groups of individuals with VB12-replete diets compared to those with less intake of foods rich in VB12 [13]. In one study of patients with ischemic stroke, investigators unveiled differential DNA methylation patterns of the methyltetrahydrofolate reductase (MTHFR) gene, implicated in ischemic stroke risk [14]. Data also supports a role for VB12 in the differentiation of helper T (Th) cells toward an immunoregulatory Treg phenotype, which play a critical role in mitigating post-stroke neuroinflammation. Patients with VB12 deficiency show lower levels of Tregs, and VB12 supplementation decreases the inflammatory circulating cytokine profiles [15]. Further, experimental animals with hereditary hyperhomocysteinemia supplemented with VB12 exhibit significantly more circulating Tregs and less proinflammatory Th17 cells compared to controls and those fed methionine-rich diets [16].
In addition to exerting its own characteristic neurological syndrome, many plausible mechanisms exist by which VB12 deficiency impacts the natural history of ischemic stroke, including combatting apoptotic signaling, promoting myelination, modulating transcription of thrombotic gene products, altering immune homeostasis and influencing the makeup of the gut microbiome, as discussed further below.
4. Vitamin B12 for ischemic stroke prevention
Numerous clinical analyses of VB12 and its associated metabolism point to a link between VB12 deficiency and ischemic stroke. Both retro- and prospective cohorts showed that VB12 and folate deficiency, as manifested by reduced VB12 or folate levels, or elevated methylmalonic acid, positively correlated with stroke risk [17]. Studies have additionally demonstrated secondary homocysteine elevations in patients with coronary artery disease, venous thromboembolism, stroke and transient ischemic attack (TIA) [18,19]. Several clinical trials have evaluated the safety and efficacy of B vitamins for stroke prevention with mixed results [20]. In the Supplementation with Folate, VB6 and VB12 and/or Omega-3 fatty acids (Su.Fol.OM3) trial, treatment reduced risk of incident stroke by 43% [21]. In the China Stroke Primary Prevention Trial Supplementation (CSPPT), folate supplementation reduced risk of incident stroke [22]. The more recent HOPE-2 trial, in which patients received folate, VB6 and VB12 supplementation demonstrated a 25% absolute risk reduction of future stroke as well [23]. However, data from two large randomized clinical trials, Vitamin Intervention for Stroke Prevention (VISP) and VITAmins TO Prevent Stroke (VITATOPS), failed to demonstrate significant stroke reduction with B vitamin therapy with the exception of certain subgroups [24]. In the Diabetic Intervention with Vitamins to Improve Nephropathy (DIVINe) trial, VB6, folate, and VB12 appeared to accelerate renal functional decline and double the incidence of cardiovascular events in patients with diabetic nephropathy [25]. Similarly, the NORVIT trial showed harm with high doses of VB12 supplementation without reducing risk of stroke [26]. A meta-analysis of patient-level data in the latter two trials demonstrated cyanocobalamin dosing and level of renal dysfunction drove poor outcomes in these patients in correlation with elevated levels of toxic thiocyanate [27]. Importantly, the harmful effects of VB12 in patients with renal dysfunction masked the benefit of vitamin B supplementation in other groups, as one subgroup analysis exhibited vitamin B therapy clearly reduced risk of stroke, myocardial infarction, and vascular death by 34% in patients without renal dysfunction [1]. Collectively, these important findings indicate that, with appropriate patient selection, B vitamin supplementation effectively mitigates the risk of ischemic stroke.
Collectively, in the wake of the of the findings from VITATOPS and VISP, it was postulated that vitamin B therapy for stroke prevention was inefficacious despite compelling findings from the subgroup and meta-analyses discussed above. However, these subsequent studies suggest that with the appropriate patient cohort, these vitamins indeed reduce ischemic stroke risk. Unfortunately, clinical data acquired in these trials do not provide much insight into the mechanism of stroke risk reduction by VB12 and other B vitamins. However, compelling evidence points to the influence of VB12 on the gut microbiome as one such protective strategy.
5. Vitamin B12 shapes gut microbial communities
Humans acquire VB12 almost exclusively through the diet, particularly from animal protein and in fermented foods. Approximately half of known resident gut microbes rely on VB12, cobamides, and structurally similar corrinoids for nucleotide and methionine biosynthesis [28]. VB12 makes up approximately 2% of the total corrinoid content in the human intestine [29]. Given the limited capacity of gut microbes to synthesize cobalamin de novo, the microbiota may not significantly contribute to host endogenous VB12 levels [30]. Indeed, individuals with high bacterial loads in the small intestine have lower levels of VB12 [31]. Thus, gut microbes compete with host intrinsic factor for dietary VB12. Microbial competition for VB12 and other corrinoids shapes the microbial community composition. Degnan and colleagues postulated that provision of dietary corrinoids thus represents a potential means to manipulate gut microbial ecology, nourishing beneficial bacterial taxa, while suppressing growth of pathogenic strains [30]. Several studies support this hypothesis. VB12 supplementation exerts a protective effect on the intestinal epithelium in several models of gastrointestinal disease [32]. Numerous studies demonstrate that VB12-deficient patients supplemented with VB12 have significant increases in alpha diversity, increased abundance of Firmicutes and a decrease in Bacteroidetes [33]. Further data also illustrated the effects of corrinoids on microbial metabolite biosynthesis in patients with VB12 deficiency, highlighting that methylcobalamin supplementation increased the relative abundance of Acinetobacter and reduced Bacteroides, Enterobacteriaceae and Ruminococceae spp. Methylcobalamin supplementation also stimulated production of SCFAs, including propionate and butyrate, by gut microbes [34]. An analysis of intestinal biopsies and gut microbiota revealed a positive correlation between a VB12 and folate-rich diet and the abundance of SCFA-producing Akkermansia and Faecalibacterium [35]. VB12, by nurturing growth of SCFA-producing microorganisms, may thus alter the immunological milieu and thereby attenuate toxic neuroinflammation after ischemic stroke.
6. The gut microbiome and homocysteine
VB12 and folate are critical cofactors for methionine synthase, which converts homocysteine to methionine. Deficiency of VB12 causes accumulation of homocysteine, which promotes atherogenesis and risk of ischemic stroke. Gut microbes influence homocysteine levels in part by competing for available dietary VB12 and folate. Aberrant gut microbial metabolism of homocysteine may also play a role in atherogenesis. Gut microbes from patients with atherosclerotic cardiovascular disease showed a diminished capacity to produce tetrahydrofolate and metabolize homocysteine [36]. Elevated homocysteine induces atherogenesis largely in part by promoting local inflammation at the endothelial layer. It stimulates endothelial expression of proinflammatory vascular cell adhesion molecule-1 (VCAM-1), intracellular adhesion molecule-1 (ICAM-1), E- and P-selectin, β1-integrin, IL-8, and monocyte chemoattractant protein (MCP-1), which facilitates inflammatory cell migration into vascular intima. Homocysteine also activates nuclear factor kappa B (NF-kappa B) nuclear translocation and mitogen-activated protein kinase (MAPK), further contributing to inflammation and induced MCP-1 and IL-8 secretion in human monocytes [37]. Additional data demonstrated that folate reduced endotoxin-induced chemokine responsivity in monocytes of patients with hereditary homocystinuria by reducing homocysteine concentrations [38]. Folate also diminishes inflammatory cell adhesion in animals fed methionine-rich diets [39]. Homocysteine can induce differentiation of naïve helper T cells to Th1 cells, the proinflammatory and predominant T cell subtype within atheromatous plaque engaged in signaling amplification loops with local macrophages to enhance inflammation and atherogenesis via matrix metalloproteinases (MMPs) and proinflammatory cytokine storm [40]. Studies have also demonstrated B cell abundance, activated complement and elevated immunoglobulins in atheromatous plaque [41]. Homocysteine can both directly induce B cell activation and immunoglobulin production, as well as potentiate endotoxin-induced activation [42]. The influence of microbiota composition on circulating homocysteine and its contribution to atherosclerotic disease, however, still requires further mechanistic elucidations to determine the impact of competitive microbial digestion of VB12 on homocysteine-induced atherogenesis.
7. Gut dysbiosis and stroke risk factors
VB12 promotes symbiosis and nourishes SCFA-producing commensal bacteria in the gut. VB12 may thus promote resilience against the development of well-established stroke risk factors associated with gut dysbiosis, including hypertension, diabetes, atrial fibrillation, and dyslipidemia.
Epidemiologic studies evaluating the impact of diet on blood pressure provided some of the early insights into the potential implication of the gut microbiome in augmenting stroke risk factors. Several meta-analyses concluded that high-fiber diets reduce blood pressure [43]. We first demonstrated a link between hypertension and dysbiosis, establishing in rats and in patients that hypertensive phenotypes are highly associated with reduced microbial diversity and decreased SCFA-producing bacteria [44]. Others have subsequently established critical associations between dietary interventions known to mitigate hypertension, including high-fiber diets and protective SCFA-producing microbial communities [45]. SCFAs synthesized by gut microbes also drive immune homeostasis, which may regulate hypertension pathogenesis via their agonism of G protein-coupled receptors 109A (GPR109A), 43 (GPR43) and by inhibition of histone deacetylases (HDACs) on circulating leukocytes [46]. Studies in animal models have consistently demonstrated reduced microbial diversity in association with hypertension development. Additionally, butyrate inhibits (pro)renin receptor (PRR) activation and activates olfactory receptor 78 (Olfr-78) in the renal afferent arteriole, enacting endogenous antihypertensive effects [47].
A study observing responses of germfree (GF) mice – mice bred without a gut microbiome – to fecal transplantation from obese and non-obese monozygotic twins demonstrated that dietary differences resulting in different gut microbial compositions initiated the development of obesity, a frequent comorbidity with ischemic stroke [48]. Longitudinal studies employing obese patients also showed that while microbial species vary considerably, the ratio of Firmicutes to Bacteroides positively correlated with weight gain [49]. Correspondingly, SCFAs induce hormonal changes that alter systemic metabolism and act centrally to induce satiety via stimulation of peptide YY and ghrelin production [50]. Many investigators have implicated gut dysbiosis in the destabilization of glucose homeostasis and promotion of insulin insensitivity, known risk factors for ischemic stroke. Abundance of certain bacteria that synthesize, or cannot utilize (and thereby increase host bioavailability of) branched-chain amino acids (BCAAs), including Prevotella copri and Bacteroides vulgatus, correlated with insulin insensitivity in a large Dutch cohort of non-diabetics. Further evidence suggested changes in γ-proteobacteria and Verrucomicrobia may also play a key role in the pathogenesis of diabetes [51].
In addition to the influence of gut microbes on the development of hypertension and diabetes mellitus, gut dysbiosis may also directly contribute to structural cardiac diseases. Atrial fibrillation (AF) confers significantly elevated risk for atrial appendage thrombus formation and subsequent embolization to the intracranial vasculature resulting in ischemic stroke. Studies also demonstrated remarkable differences in the gut microbiome of individuals with AF compared to age-matched controls. In AF patients, data illuminated the abundance of Ruminococcus, Streptococcus, and Enterococcus, and reduced levels of Faecalibacterium, Alistipes, Oscillobacter, and Bilophila. Additionally, metabolomic analysis highlighted several critical pathways that are differentially generated by microbes in patients with AF [52].
Elevated low-density lipoprotein (LDL) and cholesterol levels also predispose hosts to stroke, and there are several studies pointing to the impact of the gut microbiome on circulating lipids and dysregulated lipid metabolism. Interestingly, a large cohort study revealed numerous gut bacterial taxa whose abundance positively correlated with body mass index and blood lipids. In cross-validation analysis, inclusion of gut microbial data explained about 26% of the variance in protective high-density lipoprotein (HDL) levels [53]. Certain gut microbiota-associated metabolites, including trimethylamine N-oxide (TMAO) may also confer increased risk of atherosclerosis and stroke. Intestinal microbes metabolize L-carnitine, found primarily in red meat, to TMAO, which accelerates atherosclerosis and increases risk of stroke and cardiovascular disease. In patient populations, TMAO predicted risk of stroke in association with undesired metabolic activity in circulating monocytes [54]. Our epidemiologic data also shed light on several etiologic categories of gastrointestinal disorders associated with subsequent ischemic stroke, suggesting that primary injury to the gut may independently predict future stroke [55].
While robust data illustrate the prevalence of gut dysbiosis in established risk factors for ischemic stroke, causality between gut dysbiosis and these conditions is not yet fully established. Utilization of interventions to modify the gut microbiome may effectually reduce the incidence of these stroke risk factors and ischemic stroke by extension. VB12 may thus represent a promising candidate for ischemic stroke prevention due to its ability to nourish a healthy, diverse, SCFA-rich gut microbiome [35,56].
8. Neuroinflammation following acute ischemic stroke
In the immediate aftermath of ischemic stroke, a complex, dynamic inflammatory response guides recovery from tissue damage but also inflicts collateral injury on viable tissue. The attenuation of the cellular toxic inflammatory response shortly after ischemic stroke represents another therapeutic target for which VB12 may exert protective effects by promoting gut symbiosis and immune attenuation.
Microglia, the resident macrophages of the brain, constitute up to 20% of all glia. In steady state conditions, microglia extend and retract their processes surveilling for local distress signals, foreign bodies and tissue debris to phagocytose. Within hours following ischemic stroke, microglia become morphologically altered to form “amoeboid bodies” with ramified processes and enlarged cell bodies and become phenotypically polarized toward proinflammatory phagocytic cells. These cells express MHCII, CD40, and highly secrete proinflammatory IFNγ, TNFα, IL-1β, IL-6, CXCL10, NO, matrix metalloproteinase (MMP)-3 and -9. Activation of this inflammatory cascade induces local tissue injury, breakdown of the blood-brain barrier (BBB), and subsequent infiltration of circulating peripheral inflammatory cells, resulting in secondary tissue injury [57] (Fig. 2).
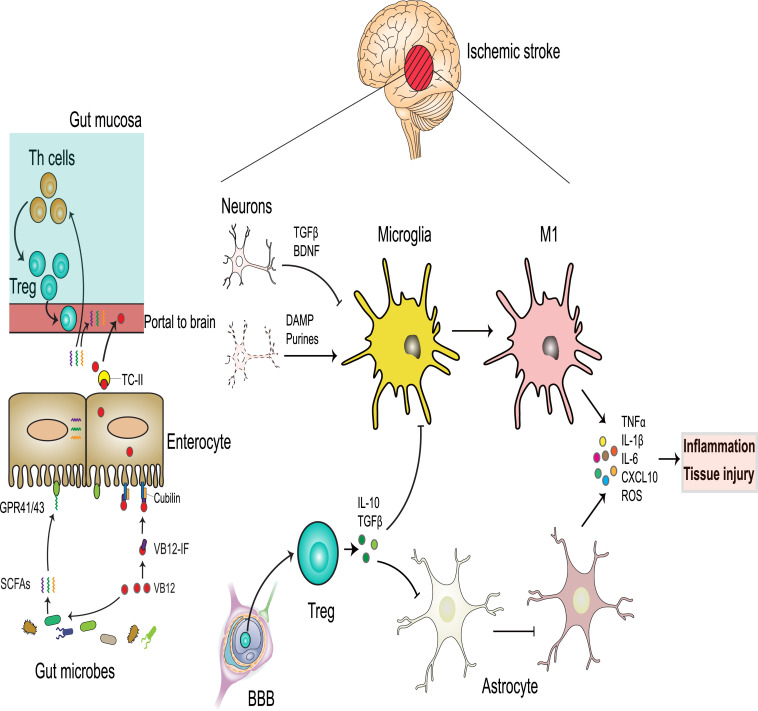
Fig. 2.
VB12 absorption and impact on neuroinflammation after ischemic stroke.
Gut microbes compete with IF for dietary VB12. VB12 promotes growth of SCFA-producing bacteria. SCFAs bind GPR41/43 in the intestinal epithelium and promote differentiation of naïve Th cells to functional FoxP3+ Tregs in the intestinal submucosa. Peripheral circulating immune cells are recruited to the brain parenchyma by crossing the disrupted BBB. Centrally-recruited Helios+ Tregs suppress highly activated astrocytes and microglia, which produce tissue injury and exacerbation of the inflammatory cascade by secreting inflammatory cytokines and injurious ROS. By contrast, dysfunctional Tregs lack the regulation of overactivated microglia with proinflammatory M1 phenotype and astrocytes, all of which further exacerbate toxic inflammation, potentially resulting in scar formation and tissue injury.
In the resting state, neurons constitutively express and secrete signaling molecules such as brain-derived neurotrophic factor (BDNF) and TGFβ that control microglial activation. When neurons become injured through stroke-induced ischemia and inflammation, this constitutive suppressive effect is lost, and neurons release factors, including purines, glutamate, chemokines, and TREM2, which polarize microglia and initiate further cell death signaling [58]. Microglia can be alternatively stimulated to macrophages possessing the regulatory (M2) phenotype, whereby these cells secrete IL-10, TGFβ, IL-4, IL-13, and IGF1 (Fig. 2), leading to the resolution of inflammation and stimulation of tissue repair [59]. Thus, induction of regulatory M2 polarization as a protective mechanism represents a promising therapeutic target in stroke and other types of neuropathology [60]. In contrast, if overactivated microglia cells are not controlled by Tregs these can be differentiated into proinflammatory macrophages, the M1 cells (Fig. 2).
Astrocytes also play a crucial role in neuroinflammation and recovery following stroke. Under steady-state conditions astrocytes maintain the blood-brain barrier (BBB), attenuate excitotoxicity in neurons by clearing excess synaptic glutamate, and support myelination by providing metabolic support to oligodendrocytes. Upon induction of stroke, these cells express GFAP and STAT3 and can also induce pathogenic inflammation leading to neuronal injury. Activated microglia are highly implicated in stimulating astrocytes to promote astrogliosis, scar formation and tissue injury (Fig. 2) [61].
In addition to activation of brain-resident immune cells, cerebral ischemia induces central recruitment of immune cells from the periphery, which greatly influence the local neuroinflammatory response. Proinflammatory IL17+ γδ T cells recruited from lymphoid and intestinal tissue after stroke potentiate the progression of cerebral ischemic injury [62,63], whereupon centrally-recruited FoxP3+ Tregs critically orchestrate the feedback regulation of neuroinflammation after stroke [64]. Accordingly, Treg deficiency exacerbates reactive astrocytosis and stroke severity, while Treg supplementation significantly promotes disease recovery [65,66]. Importantly, at resting state, few Helios+ Tregs reside in the brain. However, in the subacute phase following stroke, these cells migrate centrally, likely primarily from the thymus, to promote stroke recovery [65,66], highlighting the crucial role of recruited Tregs [67].
The immune response to cerebral ischemia is both complex and dynamic. Effective therapies targeting damaging post-stroke inflammation may have numerous effects at different stages of the disease. While circumstantial evidence exists to support the hypothesis that VB12 can attenuate inflammation after stroke, targeted analyses of cellular effectors of immune-mediated injury can provide more definitive evidence of this potentially protective effect.
9. Gut Microbial Influence on Post-Stroke Inflammation and Injury
It is now known that the gut microbiome impacts the degree of cerebral ischemic injury. Antibiotic depletion of commensal gut microbes in hosts affected by ischemic stroke significantly impairs neurologic recovery and worsens mortality rate due to severe induced colitis, which can be mitigated through recolonization with healthy gut microbes [68]. Though, other preclinical data suggest that specific antibiotics may preferentially ablate pathogenic bacteria, reduce infarct size, and improve both short- and long-term outcomes [69]. In concert with these findings, gut dysbiosis in patients appears to predict the severity of injury and prognosis following ischemic stroke [70]. Data have demonstrated that functional gut microbes and the associated metabolites mediate stroke outcomes largely by modulating the phasic inflammatory response after stroke [71].
As noted above, microglia are major effectors of post-stroke immune-mediated tissue injury. The gut microbiome is known to play a paramount role in microglial activation and maturation [72]. GF animals exhibit marked dysfunction in microglial activation, suggesting that certain characteristics of the gut microbiome can alter activation of microglia. Emerging evidence indicates that SCFAs regulate microglial activation and promote neuronal plasticity following ischemic brain injury. Stroke-affected mice gavaged with SCFAs showed improved neurological recovery compared to controls, which was associated with enhanced synaptic plasticity and improved functional connectivity between both brain hemispheres. A comprehensive transcriptomic analysis showed that microglia are the main cellular target of SCFA-mediated post-stroke recovery. Two weeks after stroke, microglia from SCFA-treated animals displayed a more ramified morphology and reduced levels of CD68, suggesting less microglial activation compared to control animals. Given the critical role of microglia in synaptic plasticity, reduced microglial activation by SCFAs may explain the protective effects of these microbiota-derived metabolites on neurorepair mechanisms following ischemic stroke in hosts [73].
Stimulation of intestinal immune cell migration to the brain and lymphoid tissue represents one means by which dysbiosis exacerbates the neuroinflammatory response after stroke [63,74]. SCFAs act in gut mucosal tissue to induce differentiation of naïve T lymphocytes toward the functional Treg phenotype and away from the proinflammatory Th17 phenotype [75,76]. Thus, these data suggest that gut immune priming by microbial metabolites provides a larger number of Tregs than Th17 cells to tune the central immune response after stroke. Further, meningeal NK cells controlled by gut microbes promote immunoregulatory activity of astrocytes following ischemic stroke, presenting another indirect mechanism by which gut microbes and, by extension VB12, may modulate post-stroke neuroinflammation [77]. One comprehensive analysis further demonstrated that certain fungal and bacterial gut species are associated with distinct cytokine profiles from activated peripheral mononuclear cells. Thus, microbial priming of circulating immune cells may also impact post-stroke neuroinflammation [78]. Ischemic stroke also impairs gut motility and increases gut permeability allowing for microbial antigen presentation to gut immune cells, which may be a critical event in triggering systemic and neuroinflammation [79].
We now have a growing understanding of the various ways that gut microbes and the associated metabolites link gut-resident and central immune activation after stroke. Attenuating the post-stroke inflammatory response in the gut through microbial modulation may reduce tissue injury and improve short- and long-term outcomes after stroke. In addition to its potential as stroke prophylactic, VB12 also represents a promising therapeutic factor in the acute phase of stroke due to its capacity to promote symbiosis, induce immunoregulation and support the crucial molecular machinery of vulnerable cells. However, definitive evidence of the ability of VB12 to influence intestinal immune cell trafficking remains to be established.
10. Translation to clinical practice
Recent scientific advances have sharpened our understanding of the pathophysiology of ischemic stroke with respect to gut-brain homeostasis. Investigations into VB12, gut-brain dynamics and ischemic stroke strongly suggest that VB12 plays a neuroprotective role in stroke prevention, secondary brain injury, and stroke recovery. Despite the mixed findings of prior clinical trials, studies that further define patients most likely to benefit may more clearly illuminate these beneficial effects. While no current clinical trials are exploring the therapeutic benefit of VB12 after stroke, ongoing mechanistic and translational studies are paving the way for the development of new clinical trials. Achieving gut symbiosis via VB12 supplementation, or enhancing VB12 availability with probiotic strains that possess the molecular machinery for de novo biosynthesis, represent promising and novel areas of growth for both primary and secondary stroke prevention. Prevention, or amelioration of the toxic immune response in the immediate aftermath of ischemic stroke through VB12 supplementation, fecal transplant, probiotic supplementation, or decontamination of the digestive system may curb the tide of secondary brain injury. In addition, promotion of feedback inhibition to the immune cascade following stroke by the aforementioned therapeutic interventions may potentiate recovery from ischemic stroke. VB12 in particular may promote remyelination of injured white matter tracts by promoting lipid synthesis, as well as suppress microglial activation and induce a robust Treg response in a microbe-dependent manner to promote long-term recovery. Further elucidation of the regulatory impact of VB12 on the gut epithelial microenvironment will undoubtedly contribute to identifying contexts in which VB12 deficiency may be particularly detrimental. Further, examining the metabolic and transcriptional impact of VB12 on the intestinal epithelial microenvironment may further illuminate mechanisms vital to cellular health, regulation of systemic immunity, and maintenance of epithelial barrier integrity. Building on our understanding of the protective role of VB12 in acute ischemic stroke with respect to intestinal homeostasis will likely provoke similar investigation into therapeutic strategies in other forms of acute brain injury, neurodegenerative disorders, and other inflammatory disorders of the nervous system.
10. Outstanding questions
The interrelationship between VB12 and the gut microbiome in ischemic stroke is complex. While existing data suggests significant promise for VB12 in the reduction and treatment of ischemic stroke, a great deal of work still remains to identify critical windows for the therapeutic effect of VB12 supplementation and identification of patients most likely to benefit. Further mechanistic work is needed to establish the dynamic and complex interaction of VB12 and its substrates in altering local gut and central immune homeostasis. Additional insights into the mechanisms of VB12, the gut microbiome, and the involved metabolites on immune circuit priming and provision of resilience to ischemic stroke promises to reveal more therapeutic targets and deepen our understanding of its pathophysiology.
11. Conclusions
Stroke takes an enormous toll on patients worldwide. While we have gained significant ground in the prevention, treatment and rehabilitation of stroke, it remains a leading contributor to the global disease burden. Recent investigations have illuminated the role of dysfunctional gut microbiota in influencing the development of stroke risk factors, the inflammatory response in the immediate aftermath of stroke and the capacity for stroke recovery. As a critical cofactor, VB12 not only mitigates these effects, in part through maintenance of amino acid metabolism and reprogramming the molecular machinery of host cells, but also fosters the maintenance of healthy gut microbiota and the associated metabolites, which in turn crucially support protective immune homeostasis in steady state and diseases. Further mechanistic studies will provide much-needed interventions for the management of patients afflicted with ischemic stroke.
Contributors
WR and MM contributed equally to the design, production, critical revision, and Fig. production of this review. The funders had no role in paper design, data collection, data analysis, interpretation, or writing of the manuscript. All authors read and approved the final version of the manuscript.
Declaration of Competing Interest
WR and MM report no relevant conflicts of interest.
Acknowledgments
Supported by R01 DK109560C and R01AI154630-01 (M. Mohamadzadeh).
Contributor Information
William Roth, Email: William.Roth@neurology.ufl.edu.
Mansour Mohamadzadeh, Email: Zadehm@uthscsa.edu.
References
- 1.Spence JD, Bang H, Chambless LE, Stampfer MJ. Vitamin intervention for stroke prevention trial: an efficacy analysis. Stroke. 2005;36(11):2404–2409. doi: 10.1161/01.STR.0000185929.38534.f3. [DOI] [PubMed] [Google Scholar]
- 2.Li J, Ge Y, Zadeh M, Curtiss R, 3rd Mohamadzadeh M. Regulating vitamin B12 biosynthesis via the cbiMCbl riboswitch in propionibacterium strain UF1. Proc Natl Acad Sci U S A. 2020;117(1):602–609. doi: 10.1073/pnas.1916576116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crofts TS, Seth EC, Hazra AB, Taga ME. Cobamide structure depends on both lower ligand availability and CobT substrate specificity. Chem Biol. 2013;20(10):1265–1274. doi: 10.1016/j.chembiol.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Andersen CB, Madsen M, Storm T, Moestrup SK, Andersen GR. Structural basis for receptor recognition of vitamin-B(12)-intrinsic factor complexes. Nature. 2010;464(7287):445–448. doi: 10.1038/nature08874. [DOI] [PubMed] [Google Scholar]
- 5.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133(5):775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 6.Yajnik CS, Deshmukh US. Fetal programming: maternal nutrition and role of one-carbon metabolism. Rev Endocr Metab Disord. 2012;13(2):121–127. doi: 10.1007/s11154-012-9214-8. [DOI] [PubMed] [Google Scholar]
- 7.Nielsen MJ, Rasmussen MR, Andersen CB, Nexo E, Moestrup SK. Vitamin B12 transport from food to the body's cells–a sophisticated, multistep pathway. Nat Rev Gastroenterol Hepatol. 2012;9(6):345–354. doi: 10.1038/nrgastro.2012.76. [DOI] [PubMed] [Google Scholar]
- 8.Kasubuchi M, Hasegawa S, Hiramatsu T, Ichimura A, Kimura I. Dietary gut microbial metabolites, short-chain fatty acids, and host metabolic regulation. Nutrients. 2015;7(4):2839–2849. doi: 10.3390/nu7042839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Warren MJ, Raux E, Schubert HL, Escalante-Semerena JC. The biosynthesis of adenosylcobalamin (vitamin B12) Nat Prod Rep. 2002;19(4):390–412. doi: 10.1039/b108967f. [DOI] [PubMed] [Google Scholar]
- 10.de Queiroz KB, Cavalcante-Silva V, Lopes FL, Rocha GA, D'Almeida V, Coimbra RS. Vitamin B 12 is neuroprotective in experimental pneumococcal meningitis through modulation of hippocampal DNA methylation. J Neuroinflammat. 2020;17:1–12. doi: 10.1186/s12974-020-01763-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frenkel EP, Kitchens RL, Johnston JM. The effect of vitamin B12 deprivation on the enzymes of fatty acid synthesis. J Biol Chem. 1973;248(21):7540–7546. [PubMed] [Google Scholar]
- 12.Arora K, Sequeira JM, Alarcon JM, Wasek B, Arning E, Bottiglieri T, et al. Neuropathology of vitamin B12 deficiency in the Cd320−/− mouse. FASEB J. 2019;33(2):2563–2573. doi: 10.1096/fj.201800754RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mandaviya PR, Joehanes R, Brody J, Castillo-Fernandez JE, Dekkers KF, Do AN, et al. Association of dietary folate and vitamin B-12 intake with genome-wide DNA methylation in blood: a large-scale epigenome-wide association analysis in 5841 individuals. Am J Clin Nutr. 2019;110(2):437–450. doi: 10.1093/ajcn/nqz031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keat Wei L, Sutherland H, Au A, Camilleri E, Haupt LM, Gan SH, et al. A potential epigenetic marker mediating serum folate and vitamin B12 levels contributes to the risk of ischemic stroke. Biomed Res Int. 2015;2015 doi: 10.1155/2015/167976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boran P, Yildirim S, Karakoc-Aydiner E, Ogulur I, Ozen A, Haklar G, et al. Vitamin B12 deficiency among asymptomatic healthy infants: its impact on the immune system. Minerva Pediatr. 2016 doi: 10.23736/S2724-5276.16.04274-X. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Wang L, Li X, Geng J. Preliminary analysis of immunoregulatory mechanism of hyperhomocysteinemia‑induced brain injury in Wistar‑Kyoto rats. Experimental and Therapeutic Medicine. 2021;21(5):1–10. doi: 10.3892/etm.2021.9914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Guelpen B, Hultdin J, Johansson I, Stegmayr B, Hallmans Gr, Nilsson Tr K., et al. Folate, vitamin B12, and risk of ischemic and hemorrhagic stroke: a prospective, nested case-referent study of plasma concentrations and dietary intake. Stroke. 2005;36(7):1426–1431. doi: 10.1161/01.STR.0000169934.96354.3a. [DOI] [PubMed] [Google Scholar]
- 18.Boysen G, Brander T, Christensen H, Gideon R, Truelsen T. Homocysteine and risk of recurrent stroke. stroke. 2003;34(5):1258–1261. doi: 10.1161/01.STR.0000069017.78624.37. [DOI] [PubMed] [Google Scholar]
- 19.Nygård O, Nordrehaug JE, Refsum H, Ueland PM, Farstad M, Vollset SE. Plasma homocysteine levels and mortality in patients with coronary artery disease. N Engl J Med. 1997;337(4):230–237. doi: 10.1056/NEJM199707243370403. [DOI] [PubMed] [Google Scholar]
- 20.Spence JD. Homocysteine Lowering with B Vitamins for Stroke Prevention—A History. US Neurol. 2018;14:35–39. [Google Scholar]
- 21.Galan P, Kesse-Guyot E, Czernichow S, Briancon S, Blacher J, Hercberg S. Effects of B vitamins and omega 3 fatty acids on cardiovascular diseases: a randomised placebo controlled trial. BMJ. 2010:341. doi: 10.1136/bmj.c6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huo Y, Li J, Qin X, Huang Y, Wang X, Gottesman RF, et al. Efficacy of folic acid therapy in primary prevention of stroke among adults with hypertension in China: the CSPPT randomized clinical trial. JAMA. 2015;313(13):1325–1335. doi: 10.1001/jama.2015.2274. [DOI] [PubMed] [Google Scholar]
- 23.Lonn E. Heart Outcomes Prevention Evaluation (HOPE) 2 Investigators. Homocysteine lowering with folic acid and B vitamins in vascular disease. Nat Clin Pract Cardiovasc Med. 2006;3:414–415. [Google Scholar]
- 24.Group VTS. B vitamins in patients with recent transient ischaemic attack or stroke in the VITAmins TO Prevent Stroke (VITATOPS) trial: a randomised, double-blind, parallel, placebo-controlled trial. The Lancet Neurology. 2010;9(9):855–865. doi: 10.1016/S1474-4422(10)70187-3. [DOI] [PubMed] [Google Scholar]
- 25.House AA, Eliasziw M, Cattran DC, Churchill DN, Oliver MJ, Fine A, et al. Effect of B-vitamin therapy on progression of diabetic nephropathy: a randomized controlled trial. JAMA. 2010;303(16):1603–1609. doi: 10.1001/jama.2010.490. [DOI] [PubMed] [Google Scholar]
- 26.Bønaa KH, Njølstad I, Ueland PM, Schirmer H, Tverdal A, Steigen T, et al. Homocysteine lowering and cardiovascular events after acute myocardial infarction. N Engl J Med. 2006;354(15):1578–1588. doi: 10.1056/NEJMoa055227. [DOI] [PubMed] [Google Scholar]
- 27.Spence JD, Yi Q, Hankey GJ. B vitamins in stroke prevention: time to reconsider. The Lancet Neurology. 2017;16(9):750–760. doi: 10.1016/S1474-4422(17)30180-1. [DOI] [PubMed] [Google Scholar]
- 28.Putnam EE, Goodman AL. B vitamin acquisition by gut commensal bacteria. PLoS Pathog. 2020;16(1) doi: 10.1371/journal.ppat.1008208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Allen RH, Stabler SP. Identification and quantitation of cobalamin and cobalamin analogues in human feces. Am J Clin Nutr. 2008;87(5):1324–1335. doi: 10.1093/ajcn/87.5.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Degnan PH, Taga ME, Goodman AL. Vitamin B12 as a modulator of gut microbial ecology. Cell Metab. 2014;20(5):769–778. doi: 10.1016/j.cmet.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Albert M, Mathan V, Baker S. Vitamin B12 synthesis by human small intestinal bacteria. Nature. 1980;283(5749):781–782. doi: 10.1038/283781a0. [DOI] [PubMed] [Google Scholar]
- 32.Lurz E, Horne RG, Määttänen P, Wu RY, Botts SR, Li B, et al. Vitamin B12 Deficiency Alters the Gut Microbiota in a Murine Model of Colitis. Frontiers in Nutrition. 2020;7:83. doi: 10.3389/fnut.2020.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boran P, Baris HE, Kepenekli E, Erzik C, Soysal A, Dinh DM. The impact of vitamin B12 deficiency on infant gut microbiota. Eur J Pediatr. 2020;179(3):385–393. doi: 10.1007/s00431-019-03517-2. [DOI] [PubMed] [Google Scholar]
- 34.Xu Y, Xiang S, Ye K, Zheng Y, Feng X, Zhu X, et al. Cobalamin (vitamin B12) induced a shift in microbial composition and metabolic activity in an in vitro colon simulation. Frontiers in microbiology. 2018;9:2780. doi: 10.3389/fmicb.2018.02780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gurwara S, Ajami NJ, Jang A, Hessel FC, Chen L, Plew S, et al. Dietary nutrients involved in one-carbon metabolism and colonic mucosa-associated gut microbiome in individuals with an endoscopically normal colon. Nutrients. 2019;11(3):613. doi: 10.3390/nu11030613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jie Z, Xia H, Zhong S-L, Feng Q, Li S, Liang S, et al. The gut microbiome in atherosclerotic cardiovascular disease. Nat Commun. 2017;8(1):1–12. doi: 10.1038/s41467-017-00900-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hofmann MA, Lalla E, Lu Y, Gleason MR, Wolf BM, Tanji N, et al. Hyperhomocysteinemia enhances vascular inflammation and accelerates atherosclerosis in a murine model. J Clin Invest. 2001;107(6):675–683. doi: 10.1172/JCI10588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang G, Dai J, Mao J, Zeng X, Yang X, Wang X. Folic acid reverses hyper-responsiveness of LPS-induced chemokine secretion from monocytes in patients with hyperhomocysteinemia. Atherosclerosis. 2005;179(2):395–402. doi: 10.1016/j.atherosclerosis.2004.10.033. [DOI] [PubMed] [Google Scholar]
- 39.Postea O, Krotz F, Henger A, Keller C, Weiss N. Stereospecific and redox-sensitive increase in monocyte adhesion to endothelial cells by homocysteine. Arterioscler Thromb Vasc Biol. 2006;26(3):508–513. doi: 10.1161/01.ATV.0000201039.21705.dc. [DOI] [PubMed] [Google Scholar]
- 40.Dawson H, Collins G, Pyle R, Deep-Dixit V, Taub DD. The immunoregulatory effects of homocysteine and its intermediates on T-lymphocyte function. Mech Ageing Dev. 2004;125(2):107–110. doi: 10.1016/j.mad.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 41.Parums D, Mitchinson M. Demonstration of immunoglobulin in the neighbourhood of advanced atherosclerotic plaques. Atherosclerosis. 1981;38(1-2):211–216. doi: 10.1016/0021-9150(81)90118-0. [DOI] [PubMed] [Google Scholar]
- 42.Chang L, Zhang Z, Li W, Dai J, Guan Y, Wang X. Liver-X-receptor activator prevents homocysteine-induced production of IgG antibodies from murine B lymphocytes via the ROS–NF-κB pathway. Biochem Biophys Res Commun. 2007;357(3):772–778. doi: 10.1016/j.bbrc.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 43.Streppel MT, Arends LR, van't Veer P, Grobbee DE, Geleijnse JM. Dietary fiber and blood pressure: a meta-analysis of randomized placebo-controlled trials. Arch Intern Med. 2005;165(2):150–156. doi: 10.1001/archinte.165.2.150. [DOI] [PubMed] [Google Scholar]
- 44.Yang T, Santisteban MM, Rodriguez V, Li E, Ahmari N, Carvajal JM, et al. Gut dysbiosis is linked to hypertension. Hypertension. 2015;65(6):1331–1340. doi: 10.1161/HYPERTENSIONAHA.115.05315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marques FZ, Mackay CR, Kaye DM. Beyond gut feelings: how the gut microbiota regulates blood pressure. Nat Rev Cardiol. 2018;15(1):20. doi: 10.1038/nrcardio.2017.120. [DOI] [PubMed] [Google Scholar]
- 46.Maslowski KM, Mackay CR. Diet, gut microbiota and immune responses. Nat Immunol. 2011;12(1):5–9. doi: 10.1038/ni0111-5. [DOI] [PubMed] [Google Scholar]
- 47.Wang L, Zhu Q, Lu A, Liu X, Zhang L, Xu C, et al. Sodium butyrate suppresses angiotensin II-induced hypertension by inhibition of renal (pro) renin receptor and intrarenal renin–angiotensin system. J Hypertens. 2017;35(9):1899–1908. doi: 10.1097/HJH.0000000000001378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013;341(6150) doi: 10.1126/science.1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Human gut microbes associated with obesity. Nature. 2006;444(7122):1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 50.Khan MJ, Gerasimidis K, Edwards CA, Shaikh MG. Role of gut microbiota in the aetiology of obesity: proposed mechanisms and review of the literature. Journal of obesity. 2016;2016 doi: 10.1155/2016/7353642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barlow GM, Yu A, Mathur R. Role of the gut microbiome in obesity and diabetes mellitus. Nutr Clin Pract. 2015;30(6):787–797. doi: 10.1177/0884533615609896. [DOI] [PubMed] [Google Scholar]
- 52.Zuo K, Li J, Li K, Hu C, Gao Y, Chen M, et al. Disordered gut microbiota and alterations in metabolic patterns are associated with atrial fibrillation. Gigascience. 2019;8(6):giz058. doi: 10.1093/gigascience/giz058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fu J, Bonder MJ, Cenit MC, Tigchelaar EF, Maatman A, Dekens JA, et al. The gut microbiome contributes to a substantial proportion of the variation in blood lipids. Circ Res. 2015;117(9):817–824. doi: 10.1161/CIRCRESAHA.115.306807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Haghikia A, Li XS, Liman TG, Bledau N, Schmidt D, Zimmermann F, et al. Gut microbiota–dependent trimethylamine N-oxide predicts risk of cardiovascular events in patients with stroke and is related to proinflammatory monocytes. Arterioscler Thromb Vasc Biol. 2018;38(9):2225–2235. doi: 10.1161/ATVBAHA.118.311023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roth WH, Cai A, Zhang C, Chen ML, Merkler AE, Kamel H. Gastrointestinal Disorders and Risk of First-Ever Ischemic Stroke. Stroke. 2020;51(12):3577–3583. doi: 10.1161/STROKEAHA.120.030643. [DOI] [PubMed] [Google Scholar]
- 56.Yahn GB, Abato JE, Jadavji NM. Role of vitamin B12 deficiency in ischemic stroke risk and outcome. Neural Regeneration Research. 2021;16(3):470. doi: 10.4103/1673-5374.291381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Patel AR, Ritzel R, McCullough LD, Liu F. Microglia and ischemic stroke: a double-edged sword. International journal of physiology, pathophysiology and pharmacology. 2013;5(2):73. [PMC free article] [PubMed] [Google Scholar]
- 58.Biber K, Neumann H, Inoue K, Boddeke HW. Neuronal ‘On'and ‘Off'signals control microglia. Trends Neurosci. 2007;30(11):596–602. doi: 10.1016/j.tins.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 59.Ponomarev ED, Veremeyko T, Weiner HL. MicroRNAs are universal regulators of differentiation, activation, and polarization of microglia and macrophages in normal and diseased CNS. Glia. 2013;61(1):91–103. doi: 10.1002/glia.22363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xia C-Y, Zhang S, Gao Y, Wang Z-Z, Chen N-H. Selective modulation of microglia polarization to M2 phenotype for stroke treatment. Int Immunopharmacol. 2015;25(2):377–382. doi: 10.1016/j.intimp.2015.02.019. [DOI] [PubMed] [Google Scholar]
- 61.Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541(7638):481–487. doi: 10.1038/nature21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shichita T, Sugiyama Y, Ooboshi H, Sugimori H, Nakagawa R, Takada I, et al. Pivotal role of cerebral interleukin-17–producing γδT cells in the delayed phase of ischemic brain injury. Nat Med. 2009;15(8):946–950. doi: 10.1038/nm.1999. [DOI] [PubMed] [Google Scholar]
- 63.Brea D, Poon C, Benakis C, Lubitz G, Murphy M, Iadecola C, et al. Stroke affects intestinal immune cell trafficking to the central nervous system. Brain Behav Immun. 2021 doi: 10.1016/j.bbi.2021.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thornton AM, Korty PE, Tran DQ, Wohlfert EA, Murray PE, Belkaid Y, et al. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol. 2010;184(7):3433–3441. doi: 10.4049/jimmunol.0904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ito M, Komai K, Mise-Omata S, Iizuka-Koga M, Noguchi Y, Kondo T, et al. Brain regulatory T cells suppress astrogliosis and potentiate neurological recovery. Nature. 2019;565(7738):246–250. doi: 10.1038/s41586-018-0824-5. [DOI] [PubMed] [Google Scholar]
- 66.Na S-Y, Mracsko E, Liesz A, Hünig T, Veltkamp R. Amplification of regulatory T cells using a CD28 superagonist reduces brain damage after ischemic stroke in mice. Stroke. 2015;46(1):212–220. doi: 10.1161/STROKEAHA.114.007756. [DOI] [PubMed] [Google Scholar]
- 67.Santamaría-Cadavid M, Rodríguez-Castro E, Rodríguez-Yáñez M, Arias-Rivas S, López-Dequidt I, Pérez-Mato M, et al. Regulatory T cells participate in the recovery of ischemic stroke patients. BMC neurology. 2020;20(1):1–10. doi: 10.1186/s12883-020-01648-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Winek K, Engel O, Koduah P, Heimesaat MM, Fischer A, Bereswill S, et al. Depletion of cultivatable gut microbiota by broad-spectrum antibiotic pretreatment worsens outcome after murine stroke. Stroke. 2016;47(5):1354–1363. doi: 10.1161/STROKEAHA.115.011800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Benakis C, Poon C, Lane D, Brea D, Sita G, Moore J, et al. Distinct commensal bacterial signature in the gut is associated with acute and long-term protection from ischemic stroke. Stroke. 2020;51(6):1844–1854. doi: 10.1161/STROKEAHA.120.029262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xia G-H, You C, Gao X-X, Zeng X-L, Zhu J-J, Xu K-Y, et al. Stroke dysbiosis index (SDI) in gut microbiome are associated with brain injury and prognosis of stroke. Frontiers in neurology. 2019;10:397. doi: 10.3389/fneur.2019.00397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Arya AK, Hu B. Brain–gut axis after stroke. Brain Circulat. 2018;4(4):165. doi: 10.4103/bc.bc_32_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Abdel-Haq R, Schlachetzki JC, Glass CK, Mazmanian SK. Microbiome–microglia connections via the gut–brain axis. J Exp Med. 2019;216(1):41–59. doi: 10.1084/jem.20180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sadler R, Cramer JV, Heindl S, Kostidis S, Betz D, Zuurbier KR, et al. Short-chain fatty acids improve poststroke recovery via immunological mechanisms. J Neurosci. 2020;40(5):1162–1173. doi: 10.1523/JNEUROSCI.1359-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Singh V, Roth S, Llovera G, Sadler R, Garzetti D, Stecher B, et al. Microbiota dysbiosis controls the neuroinflammatory response after stroke. J Neurosci. 2016;36(28):7428–7440. doi: 10.1523/JNEUROSCI.1114-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liesz A, Suri-Payer E, Veltkamp C, Doerr H, Sommer C, Rivest S, et al. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat Med. 2009;15(2):192–199. doi: 10.1038/nm.1927. [DOI] [PubMed] [Google Scholar]
- 76.Omenetti S, Pizarro TT. The Treg/Th17 axis: a dynamic balance regulated by the gut microbiome. Front Immunol. 2015;6:639. doi: 10.3389/fimmu.2015.00639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sanmarco LM, Wheeler MA, Gutiérrez-Vázquez C, Polonio CM, Linnerbauer M, Pinho-Ribeiro FA, et al. Gut-licensed IFNγ+ NK cells drive LAMP1+ TRAIL+ anti-inflammatory astrocytes. Nature. 2021;590(7846):473–479. doi: 10.1038/s41586-020-03116-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schirmer M, Smeekens SP, Vlamakis H, Jaeger M, Oosting M, Franzosa EA, et al. Linking the human gut microbiome to inflammatory cytokine production capacity. Cell. 2016;167(4):1125–1136. doi: 10.1016/j.cell.2016.10.020. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Candelario-Jalil E, Paul S. Impact of aging and comorbidities on ischemic stroke outcomes in preclinical animal models: a translational perspective. Exp Neurol. 2020 doi: 10.1016/j.expneurol.2020.113494. [DOI] [PMC free article] [PubMed] [Google Scholar]