Abstract
Background
Novel anticancer agents are initially evaluated in a palliative setting in phase I studies. The benefit–risk applying the selected dose from these phase I studies can be considered acceptable at time of registration, however, it is unknown if the optimal dose has been selected during drug development.
Methods
The European Medicines Agency (EMA) European Public Assessment Reports (EPARs) overview was used to select anticancer agents evaluated between 2015 and 2020. The dose selection and tolerability data of EMA assessed anticancer agents was analysed to evaluate dose selection.
Results
Sixty EPARs were included for analysis. A dose–response relation was identified in five dossiers (8%). The maximum tolerated dose (MTD) was the selected dose for 15 anticancer agents (25%). The MTD was not determined in 27 out of 60 cases (59%). When the MTD was determined but not applied as final dose, the most frequently used dose selection criteria were the combination of toxicity, exposure response, pharmacokinetic data and pharmacodynamic data (in 7 out of 18 cases). Data on tolerability were analysed separately for protein kinase inhibitors and monoclonal antibodies as the dosing interval and mitigation of adverse events (AEs) differs. The median discontinuation, dose reduction and dose interruption rates due to AEs of protein kinase inhibitors were 10%, 26% and 45% for monotherapy and 13%, 47% and 55% for combination therapy, respectively. The median discontinuation rates due to AEs for monoclonal antibodies were 8% for monotherapy and 26% for combination therapy.
Conclusion
The dose–response relationship has not been established for the majority of the registered anticancer agents. The selected posology is often poorly tolerable as reflected by the high discontinuation and dose reduction rates. Due to the absence of dose–response data, it is often unknown if the optimal dose has been selected for anticancer agents.
Key words: dose selection, tolerability, anticancer agents
Highlights
-
•
The dose–response relationship has not been established for the majority of the anticancer agents.
-
•
The posology of anticancer agents is often poorly tolerable, reflected by the high discontinuation and dose reduction rates.
-
•
Due to the absence of dose–response data it is often unknown if the optimal dose has been selected for anticancer agents.
Introduction
Novel anticancer agents are mostly evaluated first in men and/or phase I dose escalation studies in a palliative setting.1 Promising results often lead to opening of expansion cohorts during the phase I studies. The used posology of the expansion cohorts is mainly based on the safety evaluation of the dose escalation phase. Due to the last line setting and the aim of the phase I studies to assess the toxicity, often no control arm is included in such oncology studies. Multiple dose level safety evaluations at the expansion phase are often omitted. Therefore, the recommended dose for anticancer agents is frequently based on the maximum tolerated dose (MTD) determined at the dose escalation phase. Current general drug development guidance dictates that dose–response evaluation should be an integral part of drug development, encompassing phase I studies with further dose optimisation in phase II studies.2 The phase II dose optimisation step, however, is often lacking in the oncology setting. Instead, in phase I studies, pharmacokinetic (PK) data, pharmacodynamic (PD) data, exposure response and exposure safety relationships may be used to support the posology used in the expansion cohorts. The supportive data based on exposure can be difficult to interpret due to confounders such as cachexia.3,4 Cachectic patients with a low body weight are part of the study population in oncology. Cachectic patients are known to have a higher protein catabolism.4,5 Monoclonal antibodies are cleared at a higher rate in these patients which results in a lower exposure.4 Due to their fragile state these patients also have a poor prognosis and are vulnerable to adverse events (AEs). Time-varying clearance of monoclonal antibodies with disease dynamics further complicates exposure response analysis in oncology.6 For these reasons, the supportive data based on exposure to anticancer agents can be difficult to interpret, as confounding factors cannot be eliminated in the absence of dose–response evaluation data. In this study the presence of dose–response evaluation is analysed to assess if the selected dose is based on the dose response.
Parameters to assess if the selected dose is tolerable for patients are treatment interruptions due to AEs, dose reduction due to AEs and discontinuations due to AEs.7 In the absence of dose–response data it is unknown where the plateau of efficacy on the dose–response curve starts.8 Exceeding the efficacy plateau while escalating the dose further will lead to more off-target toxicity without providing additional antitumour effect.9 In many cases, the PD effect can be measured to assess the saturation of the PD effect of the drug target effect.10 PD markers applied, however, are often not fully clinically validated. In addition, in the case of protein kinase inhibitors, multiple kinases can be inhibited, while only one or a few are used to demonstrate target engagement. At the time of dose escalation of first-in-class anticancer agents the clinical validity of PD biomarkers often has not been established. Therefore, PD evaluation has also intrinsic limitations for dose finding.
Seamless study designs, in which the traditional separate study phases are integrated, are emergent in early-phase studies in oncology drug development.11 Despite the above described intrinsic limitations and non-comparative setting of phase I clinical oncology studies for dose selection, these studies are submitted to the regulatory agencies as part of the set of studies in support of regulatory approval.1 The benefit–risk applying the selected dose from these phase I studies can be considered positive at the time of registration, however, it is unknown if the optimal dose has been selected during drug development.
To reveal the dose selection criteria of European Medicines Agency (EMA) approved, refused and withdrawn anticancer agents from 2015 to 2020, the EMA European Public Assessment Reports (EPARs) are analysed. Dose selection criteria based on dose response, toxicity, PK, PD, exposure-efficacy and exposure-safety evaluations are included. To evaluate the tolerability of the selected dose, data on discontinuations due to AEs, dose reduction due to AEs and dose interruptions due to AEs are also presented in this study. Further, an overview of the dose selection criteria and the regulatory decision are presented. Finally, since revision of the dose after approval may also indicate the shortcomings of the dose selection approach and dose optimisation, the occurrence of such post-approval change was investigated.
Methods
The EMA EPARs overview (an Excel table) was used to select anticancer agents evaluated between January 2015 and August 2020.12 The relevant EPARs were selected based on categories ‘human’, ‘marketing authorisation date’, ‘date of refusal’, ‘Anatomical Therapeutic Chemical (ATC)-code’, ‘generics’ and ‘biosimilars’ to include the relevant agents (Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2021.100301). We excluded generics and biosimilars, as the dose for these agents has been established by the innovator. The EPARs were obtained via the EMA website.12 The initial marketing authorisation EPARs documents were used for analysis. Occasionally, information about dose selection appeared to be omitted in the EPARs when no particularities were observed during the assessment procedure. In this case, the internal clinical assessment reports (ARs) of the EMA Committee for Medicinal Products for Human Use (CHMP) reports were used to obtain the missing information.
The clinical aspects of the EPARs and ARs were analysed to determine the dose selection methods and objectives carried out. To obtain an overview of the dose selection, multiple factors discussed in the EPARs/ARs were included. From the PD section, exposure efficacy and exposure safety evaluations were included. Secondly, we evaluated if the MTD was determined and if the MTD was applied as the selected dose. The available dose response, exposure response and other data used in the dose finding, including receptor occupancy data or other relevant in vitro/in vivo data and exposure biomarker data were evaluated as well. Dose-finding data collected included toxicology data, efficacy data, PK data and PD data. Additionally, we determined if the dose–response relationship had been established and if dose optimisation in phase II studies was carried out.
To evaluate the tolerability of the final selected dose, data on discontinuation, dose reduction and dose interruptions due to AEs were collected. Monotherapy and combination therapy were shown separately to characterise the tolerability as a single agent as well as in combination.
To determine further regulatory requirements on dose selection, the conditional approvals or post-authorisation measures (PAMs) were screened to assess whether these were related to the dose selection. For the refused or withdrawn agents, we obtained the reason for refusal/withdrawal to indicate the presence of a relationship with the selected dose. To determine whether the initial approved posology was changed after approval, the document ‘Procedural steps taken and scientific information after authorisation’ from the EMA EPARs website was reviewed for each agent.
Results
The full list of EPARs, used as starting point for selection of relevant EPARs, contained 1722 EPARs. The selection process of the agents from the EMA Excel database is shown in Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2021.100301. After filtering on human products, generics, marketing authorisation date/date of refusal, ATC codes for anticancer agents, and biosimilars, 76 agents evaluated by the EMA during the study period from 2015 to August 2020 were selected. Of these initially selected 76 agents, 16 agents were excluded due to various reasons explained in Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2021.100301, yielding 60 products for further analysis.
Dose selection criteria (all products combined)
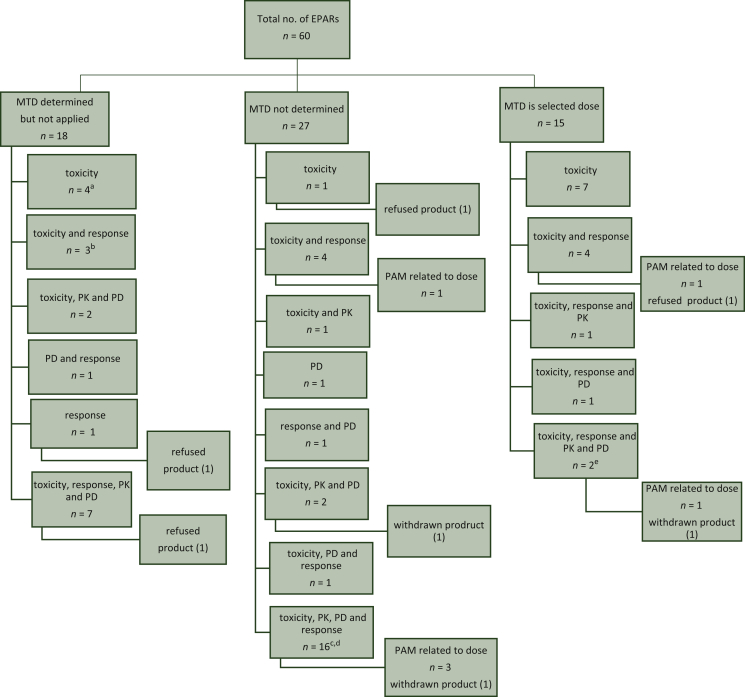
In the set of 60 EPARs that were included for analysis, some agents were included multiple times in case of separate initial drug product applications for the same agent. This concerns lenvatinib (with two brand names Kisplyx® and Lenvima®) and nivolumab (with brand names Opdivo® and Nivolumab BMS®). An overview of the observed dose selection criteria for the 60 agents is given in Figure 1. A dose–response relation was identified in 5 of the 60 dossiers (8%), i.e. for lenvatinib, daratumumab, panobinostat, venetoclax and blinatumomab. The MTD was the selected dose for 15/60 (25%) agents. The MTD was determined but not applied for 18/60 (30%) agents. The most frequently used dose selection criterion for the latter set of agents was the combination of toxicity, response, PK data and PD data (7/18, 39%). The MTD was not determined for 27/60 agents. The most frequently used dose selection criterion in these cases was also the combination of toxicity, response, PK data and PD data (16/27, 59%).
Figure 1.
Overview of used dose selection criteria.
aFor pabinostat the dose response was determined. bFor blinatumomab the efficacy was dose dependent. cFor daratumumab, the 16 mg/kg dose level had an acceptable safety profile and resulted in higher and deeper response rates as compared to the 8 mg/kg dose level. dFor venetoclax doses from 150 to 1200 mg led to the selection of 400 mg. The observed response rate was not different when comparing results for subjects treated at 400 mg versus those treated at higher doses. eFor Lenvima® a clear dose response trend was observed.
EPARs, European Public Assessment Reports; MTD, maximum tolerated dose; PAM, post-authorisation measure; PD, pharmacodynamics; PK, pharmacokinetics.
Overall, four products were refused and three products were withdrawn after initial registration. Six PAMs were related to the posology. Phase II studies were carried out for 42/60 applications, however, these studies were not always dedicated to dose–response analysis for the applied indication. Sixteen phase II studies included two dose levels. Five phase II studies were dedicated to dose–response analysis. Two phase II dose–response studies established a flat dose response and two phase II studies were in other tumour types. One conditional approval was related to the dose selection.
Post-approval posology changes
The registered posology was changed post-approval for nine agents. Dose alterations after market approval were carried out for 6 out of 20 monoclonal antibodies (30%). For three of these six agents, the mg/kg regimen was modified to a flat dose regimen.
Dose selection of subclasses of the selected agents
Three drug categories were evaluated separately, i.e. ‘protein kinase inhibitors’, ‘monoclonal antibodies’ and ‘other agents’. The flow charts of the protein kinase inhibitors (Figure 2) and monoclonal antibodies (Figure 3) are shown separately, as these drug classes are less heterogeneous compared with the ‘other agents’ (Supplementary Figure S2, available at https://doi.org/10.1016/j.esmoop.2021.100301) category which included both targeted agents and cytotoxic chemotherapy.
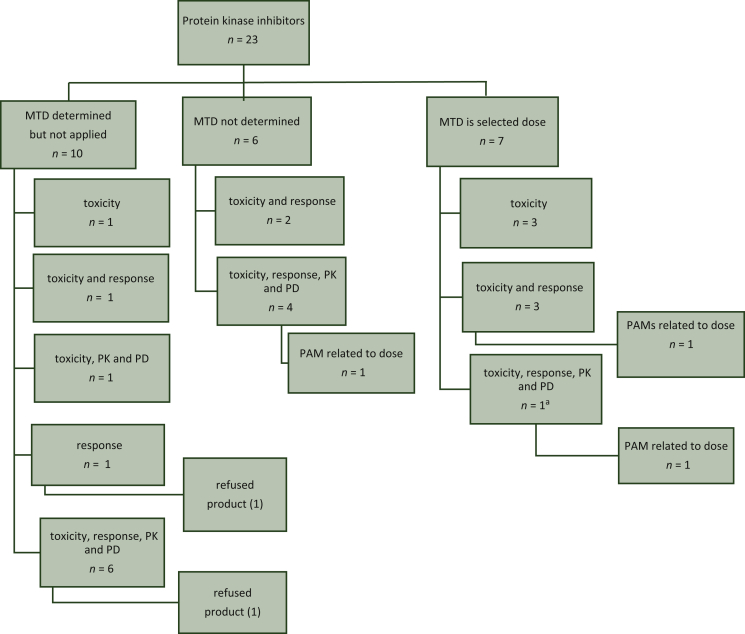
Figure 2.
Overview of dose selection criteria protein kinase inhibitors.
aFor Lenvima® a clear dose response trend was observed.
MTD, maximum tolerated dose; PAM, post-authorisation measure; PD, pharmacodynamics; PK, pharmacokinetics.
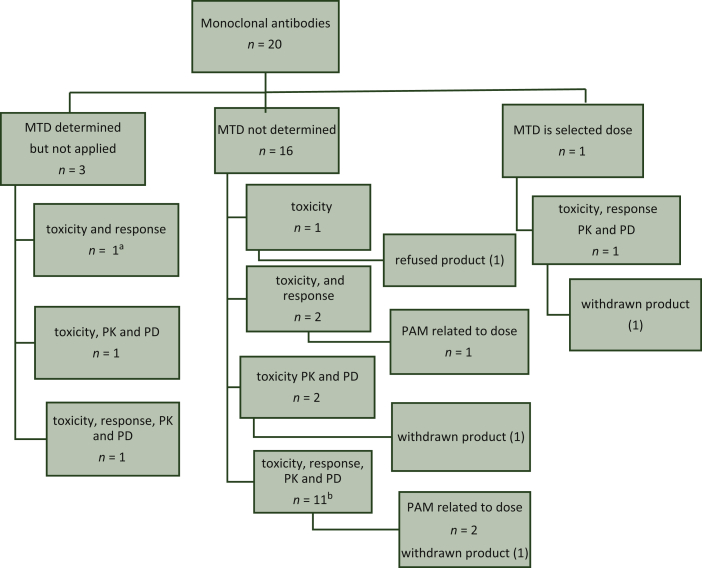
Figure 3.
Overview of dose selection criteria monoclonal antibodies.
aFor blinatumomab the efficacy was dose dependent. bFor daratumumab, the 16 mg/kg dose level had an acceptable safety profile and resulted in higher and deeper response rates as compared to the 8 mg/kg dose level.
MTD, maximum tolerated dose; PAM, post-authorisation measure; PD, pharmacodynamics; PK, pharmacokinetics.
Protein kinase inhibitors
A total of 23 of the 60 selected EPARs concern protein kinase inhibitors. Midostaurin was approved for two indications at the time of marketing authorisation. Midostaurin has both a monotherapy registration and a combination therapy registration. The used dose selection criteria for midostaurin were similar for both the monotherapy and combination therapy dose selection.
Dose selection criteria
The MTD was determined but not applied for 10/27 protein kinase inhibitors (37%). In these cases, the most frequently used dose selection criterion was the combination of toxicity, response, PK data and PD data (6/10, 60%). The MTD was not determined for 6/23 agents (26%). In these cases, the most frequently used dose selection criterion was the combination of toxicity, response, PK data and PD data [4/6 (67%)]. The MTD was the selected dose for 7/23 protein kinase inhibitors (30%). Two protein kinase inhibitors were refused. Three PAMs were related to the posology.
Discontinuation, dose reduction and dose interruptions due to AEs
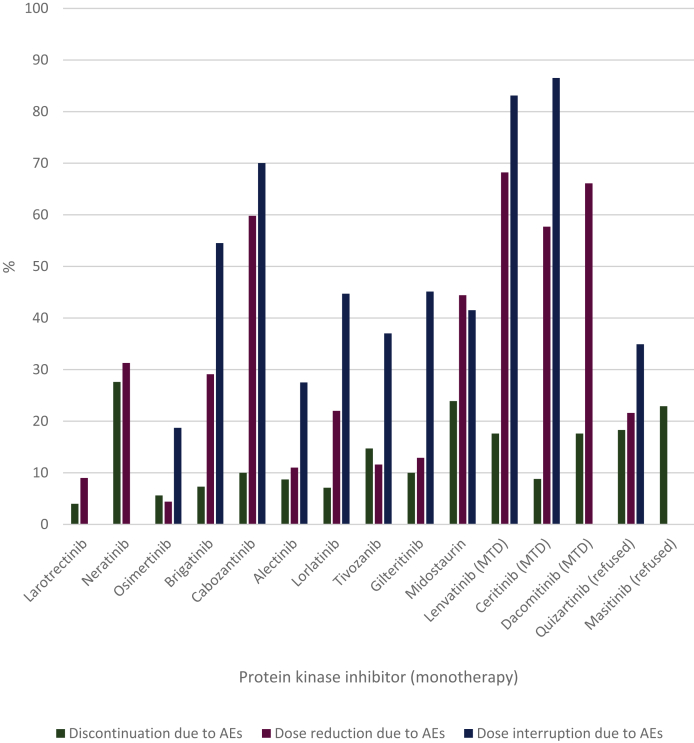
The discontinuation, dose reduction and dose interruption rates due to AEs of the individual protein kinase inhibitors monotherapy are shown in Figure 4. The rates for combination therapy are shown in Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2021.100301. For some therapies, only the combined term dose reduction and/or interruption due to AEs was reported. This group was included in the dose interruptions due to the AEs group for calculation of the median. The median discontinuation, dose reduction and dose interruption rates for monotherapy were 10%, 26% and 45%, respectively. For combination therapy, the medians for these rates were 13%, 47% and 55%, respectively.
Figure 4.
Discontinuation, dose reduction and dose interruptions due to adverse events of protein kinase inhibitors (monotherapy).
AEs, adverse events; MTD, maximum tolerated dose.
Monoclonal antibodies
A total of 20 of the 60 selected EPARs concerned monoclonal antibodies. The MTD was determined, but not applied for 3/20 agents (15%). The MTD was not determined for 16/20 agents (80%); the most frequently used dose selection criterion in this case was the combination of toxicity, response, PK data and PD data (11/16, 69%). The MTD was the selected dose for 1/20 monoclonal antibodies (5%). One monoclonal antibody was refused. Three PAMs were related to the posology.
Discontinuation, dose reduction and dose interruptions due to AEs
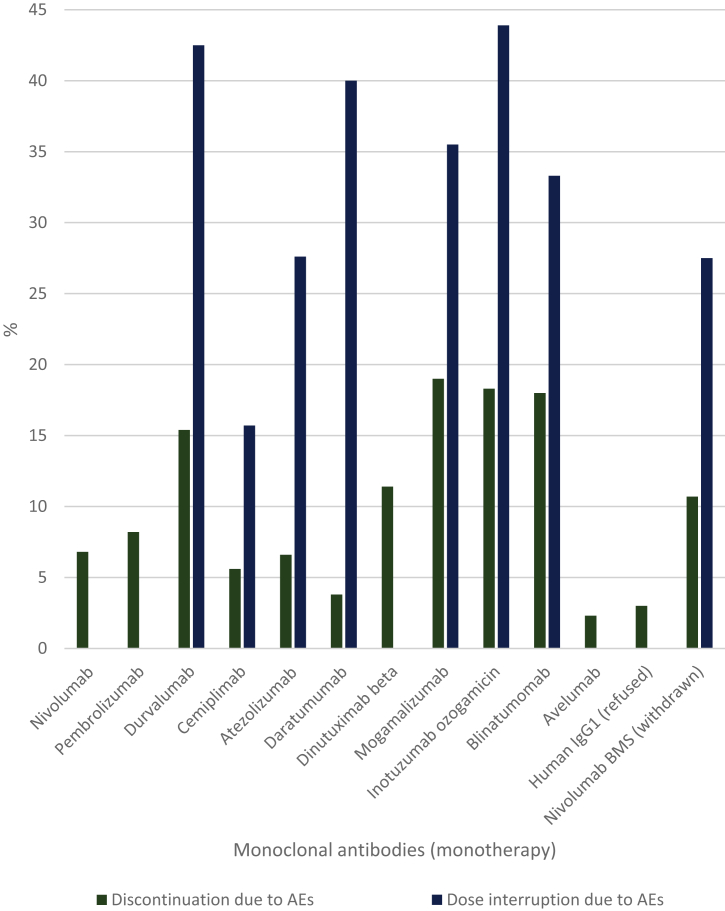
The discontinuation and dose interruption rates due to AEs of the individual monoclonal antibodies monotherapy are shown in Figure 5. The medians for the discontinuation and dose interruption rates were 8% and 34%, respectively. Dose reduction instead of dose interruption as a strategy to mitigate AEs was used for two agents and occurred in 1.2% and 3% of the patients. The median discontinuation rate due to AEs in the case of monoclonal antibody-containing combination therapy was 26%. The rates for combination therapy are shown in Supplementary Table S4, available at https://doi.org/10.1016/j.esmoop.2021.100301.
Figure 5.
Discontinuation, dose reduction and dose interruptions due to adverse events of monoclonal antibodies (monotherapy).
AEs, adverse events; IgG1, immunoglobulin G1.
Other agents
A total of 17 of the 60 selected EPARs concerned agents other than protein kinase inhibitors or monoclonal antibodies. The MTD was determined, but not applied for 5/17 agents (29%). The MTD was not determined for 6/17 agents (35%). The MTD was the selected dose for 6/17 agents (35%). One agent was refused. No PAMs related to posology were required for the other agents.
Discontinuation, dose reduction and dose interruptions due to AEs
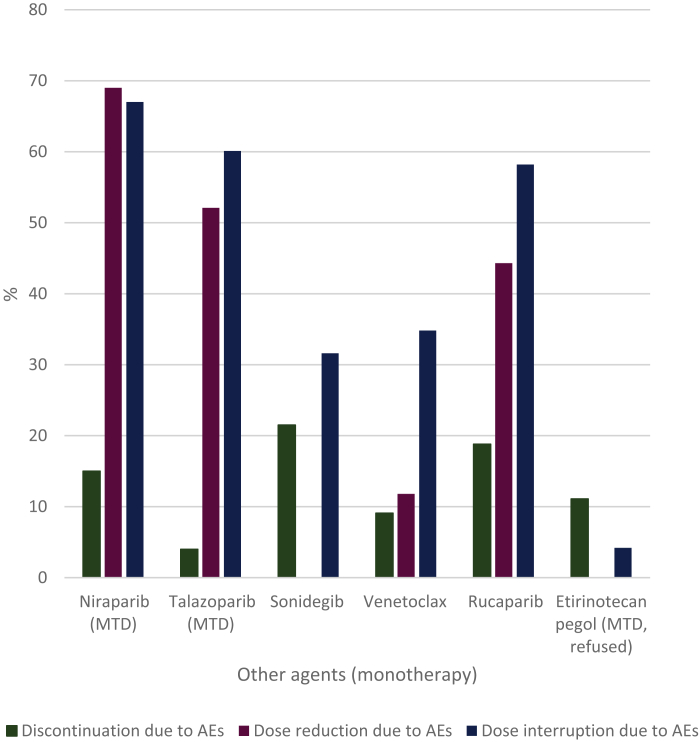
The discontinuation, dose reduction and dose interruption rates due to AEs of the other agents as monotherapy are shown in Figure 6. The medians for these rates were 13%, 48% and 47%, respectively. The discontinuation, dose reduction and dose interruption rates due to AEs for combination therapy are shown in Supplementary Table S5, available at https://doi.org/10.1016/j.esmoop.2021.100301. The medians for the discontinuation, dose reduction and dose interruption rates were 16%, 31% and 66%, respectively.
Figure 6.
Discontinuation, dose reduction and dose interruptions due to adverse events of other anticancer agents (monotherapy).
AEs, adverse events; MTD, maximum tolerated dose.
Discussion
Despite the development of targeted agents in oncology to enable precision medicine, the tolerability of these agents often remains poor as reflected in the high percentage of discontinuations and dose reductions due to AEs for the anticancer agents analysed in our study. The reported median dose reduction rates are even an underestimation, as for some therapies only the combined term dose reduction and/or interruption due to AEs was reported. The rates of the combined term were included in the dose interruptions due to the AEs group for calculation of the median. For future studies, reporting dose reduction and/or interruption due to AEs as a combined measure should be avoided, as the interpretation and analysis of the tolerability of a dose level is hampered. The characterisation of the dose–response curve was only carried out for 5 out of 60 anticancer agents assessed in this study. This is considered an undesirable low number, since to improve tolerability of anticancer therapy, it is essential to know where the plateau on the dose efficacy response curve starts to avoid unnecessary overdosing.9 The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) E4 guideline states that if development of dose–response information is built into the development process, it can usually be accomplished with no loss of time and minimal extra effort compared with development plans that ignore dose response.2 Relatively high doses deprive some patients of the potential benefit of a drug by inducing toxicity that leads to cessation of therapy. Within oncology, dose–response analysis can be carried out using, for instance, RECIST for the target tumour lesions, to determine the response rate and to identify the plateau on the dose efficacy response curve. In phase II studies where the beneficial effect of therapy is not known, tumour re-evaluation every 6-8 weeks is considered reasonable.13
As mentioned in the introduction, exposure response analysis based on limited phase I data is susceptible to confounding.3,4,6 Other analyses, however, including the dose response analysis, particularly those based on limited data, are also susceptible to confounding, including variability in exposure. Further, in the case of monoclonal antibodies, target saturation is expected due to the high affinity of the monoclonal antibody for its target. Off-target toxicity can still occur, however, and depends on expression of the target by tissue other than the tumour.9 Protein kinase inhibitors often have a broader inhibition affinity profile due to conserved kinase domains within cell cycle signalling. Therefore, off-target toxicity may occur more frequently, which may result in dose-limiting toxicity and poor tolerability of protein kinase inhibitors. The MTD was determined for 17 out of 23 protein kinase inhibitors, whereas the MTD was determined in 4 out of 20 monoclonal antibodies. With respect to off-target toxicity, the selectivity of protein kinase inhibitors is relative, as conserved kinase binding domains are presented in several cell types. More kinases are inhibited as the relative selectivity for the kinases will diminish at higher concentrations, which results in more off-target toxicity, whereas monoclonal antibodies will only bind to tissues which express the target protein.
A limitation of this study is the inclusion of submitted dossiers only which hamper extrapolation to general dose finding. This results in selection bias, as applicants would only submit a registration dossier to the EMA in case a marketing approval is considered feasible in the opinion of the applicant. Registered products, however, are most relevant in clinical practice. A positive benefit–risk balance is required for marketing authorisation, and multiple factors contribute to the benefit–risk balance of drug therapy. Further, the results of the dose-finding methodology in relation to regulatory approval and refusal by the EMA should be interpreted with caution due to the limited number of refusals. Only 4 agents of the selected 60 agents were refused during the registration procedure and only 3 were withdrawn following their initial registration. For this reason, no conclusions can be drawn on potential differences in dose selection between approved, refused and withdrawn agents. All EMA EPARs were included based on submission for initial marketing authorisation. Therefore, some duplication of the data occurred for nivolumab (Nivolumab BMS® and Opdivo®) and for lenvatinib (Kisplyx® and Lenvima®). Different indications are discussed in these EPARs.
The results obtained in this study demonstrate that MTD is still a relatively common dose selection strategy for anticancer agents, and in the period 2015-2020, 25% of all selected anticancer drugs were registered applying the MTD as approved dose. For the subclass of the protein kinase inhibitors, this was even 30%. In a study of drugs registered by the United States Food and Drug Administration (FDA) in the earlier period 2010-2015, 85% of the protein kinase inhibitors had the MTD as the registered dose,14 which indicates a reduction in the use of the MTD, and an increased use of alternative dose-finding methodologies than MTD for protein kinase inhibitors in more recent years. For the EMA 2015-2020 approved protein kinase inhibitors, the MTD as a dose-finding strategy led to a PAM request related to dose for 29% (2 out of 7) agents, where there was only one PAM request for the remaining 16 protein kinase inhibitors, for which the dose was selected by alternative methods to MTD. The study of FDA-approved anticancer agents also demonstrated that a high level, i.e. 64% of the protein kinase inhibitors with the MTD as selected dose, were approved with post-marketing requirements (PMR)/post-marketing commitments (PMC) (equivalent to the PAM at the EMA) related to dose optimisation in 2010-2015. Quantitative comparison of the EMA and FDA results is not feasible due to the different period of the evaluation of those agents, and the differences in the requests of PAMs/PMRs/PMCs of the FDA and the EMA.
For one of the protein kinase inhibitors registered by the EMA based on the MTD, ceritinib, no dose-related PAM was requested. The dose was modified after approval, however, due to the observed decreased occurrence of gastrointestinal disorders at a lower dose, under fed conditions, instead of the initially registered fasting conditions.15 This provides a specific example of an agent for which dose selection has not been optimised before marketing approval, and illustrates a recognised problem in oncology dose finding.7,16 One of the agents with the MTD not applied as the selected dose, quizartinib, was refused by the EMA, partly due to the uncertainty of the safety observed at the selected dose. According to information obtained from the EPARs, the relatively high percentages of discontinuations due to AEs support the EMA's assumption that the rationale of the selected dose was not completely justified and that there was a fundamental risk of toxicity, which contributed to the refusal of quizartinib.
With respect to anticancer monoclonal antibodies, in the study of FDA-approved anticancer agents evaluated between 2010 and 2015, a total of nine monoclonal antibodies were evaluated, of which only three had an identified MTD, and only one agent was approved with the MTD as the selected dose. This indicates that for the monoclonal antibodies, a relatively lower number of agents was approved by the FDA based on MTD than for protein kinase inhibitors in the same time period.14 Although numerically different, our EMA-based study (with 20 monoclonal antibodies included, 4 with determined MTD and 1 approved but withdrawn with the MTD as selected dose), is in line with the FDA-based findings. Additionally, in the US study from 2010 to 2015, two antibody-drug conjugates (ADCs) were approved by the FDA, both with the MTD as the selected dose.14 In our study, three ADCs were evaluated, of which one had an identified MTD, but not applied as a registered dose. Because a cytotoxic agent is linked to a monoclonal antibody in ADCs, toxicity is challenging, and dose-limiting toxicity is often observed for these agents. Although only one of the ADCs had an identified MTD in our study, the discontinuation rates were relatively high for all three of these agents (18%, 27%, 31%) compared with the median discontinuation rate of 8% of the monoclonal antibodies, which may be correlated to this known toxicity of ADCs.
PAMs related to dose were requested for 3 out of the 20 monoclonal antibodies, all 3 monoclonal antibodies with no identified MTD. In the study with FDA-approved anticancer agents from 2010 to 2015, a PMR was requested for one out of nine monoclonal antibodies (11%, 8% including ADCs) and a PMC for one out of three ADCs, which is in line with our findings.14
Noticeably, dose alterations were carried out for 6 out of 20 monoclonal antibodies (30%) after market approval. For three of these six agents, the mg/kg regimen was modified to a flat dose regimen. These modifications, however, were not related to better efficacy or safety, but to practical reasons.
Conclusion
Characterisation of the dose-response curve was only carried out for 5 out of 60 assessed anticancer agents registered by the EMA in the time period 2015-2020. This low number of anticancer products with extensive dose-finding investigations may explain that, despite the development of targeted agents in oncology to enable precision medicine, the tolerability of these anticancer agents often remains poor. This poor tolerability is reflected in the high percentage of discontinuations and dose reductions due to AEs for these agents. To improve tolerability of anticancer therapy, it is essential to know where the plateau on the dose–response curve starts to avoid unnecessary overdosing to improve dose selection.
Acknowledgements
We thank Dr M. Pasmooij for her help with the supervision of our students.
Funding
None declared.
Disclosure
The authors have declared no conflicts of interest.
Supplementary data
References
- 1.Tenhunen O., Lasch F., Schiel A., Turpeinen M. Single-arm clinical trials as pivotal evidence for cancer drug approval: a retrospective cohort study of centralized European marketing authorizations between 2010 and 2019. Clin Pharmacol Ther. 2020;108(3):653–660. doi: 10.1002/cpt.1965. [DOI] [PubMed] [Google Scholar]
- 2.European Medicines Agency (EMA) ICH Topic E4 Dose Response Information to Support Drug Registration. https://www.ema.europa.eu/en/documents/scientific-guideline/ich-e-4-dose-response-information-support-drug-registration-step-5_en.pdf Available at.
- 3.Chen S.C., Quartino A., Polhamus D., et al. Population pharmacokinetics and exposure-response of trastuzumab emtansine in advanced breast cancer previously treated with ≥2 HER2-targeted regimens. Br J Clin Pharmacol. 2017;83(12):2767–2777. doi: 10.1111/bcp.13381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turner D.C., Kondic A.G., Anderson K.M., et al. Pembrolizumab exposure-response assessments challenged by association of cancer cachexia and catabolic clearance. Clin Cancer Res. 2018;24(23):5841–5849. doi: 10.1158/1078-0432.CCR-18-0415. [DOI] [PubMed] [Google Scholar]
- 5.Porporato P.E. Understanding cachexia as a cancer metabolism syndrome. Oncogenesis. 2016;5:e200. doi: 10.1038/oncsis.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu C., Yu J., Li H., et al. Association of time-varying clearance of nivolumab with disease dynamics and its implications on exposure response analysis. Clin Pharmacol Ther. 2017;101(5):657–666. doi: 10.1002/cpt.656. [DOI] [PubMed] [Google Scholar]
- 7.Janne P.A., Kim G., Shaw A.T., Sridhara R., Pazdur R., McKee A.E. Dose finding of small-molecule oncology drugs: optimization throughout the development life cycle. Clin Cancer Res. 2016;22(11):2613–2617. doi: 10.1158/1078-0432.CCR-15-2643. [DOI] [PubMed] [Google Scholar]
- 8.European Medicines Agency (EMA) Guideline on strategies to identify and mitigate risks for first-in-human and early clinical trials with investigational medicinal products. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-strategies-identify-mitigate-risks-first-human-early-clinical-trials-investigational_en.pdf Available at. [DOI] [PMC free article] [PubMed]
- 9.European Medicines Agency (EMA) Guideline on the evaluation of anticancer medicinal products in man. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-evaluation-anticancer-medicinal-products-man-revision-5_en.pdf Available at.
- 10.Hantschel O. Unexpected off-targets and paradoxical pathway activation by kinase inhibitors. ACS Chem Biol. 2015;10(1):234–245. doi: 10.1021/cb500886n. [DOI] [PubMed] [Google Scholar]
- 11.Hobbs B.P., Barata P.C., Kanjanapan Y., et al. Seamless designs: current practice and considerations for early-phase drug development in oncology. J Natl Cancer Inst. 2019;111(2):118–128. doi: 10.1093/jnci/djy196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.EMA European assessment reports overview. https://www.ema.europa.eu/sites/default/files/Medicines_output_european_public_assessment_reports.xlsx Available at.
- 13.Eisenhauer E.A., Therasse P., Bogaerts J., et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 14.Lu D., Lu T., Stroh M., et al. A survey of new oncology drug approvals in the USA from 2010 to 2015: a focus on optimal dose and related postmarketing activities. Cancer Chemother Pharmacol. 2016;77(3):459–476. doi: 10.1007/s00280-015-2931-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Procedural steps taken and scientific information after the authorisation: Zykadia. https://www.ema.europa.eu/en/documents/procedural-steps-after/zykadia-epar-procedural-steps-taken-scientific-information-after-authorisation_en.pdf Available at.
- 16.Zykadia-epar-product-information. https://www.ema.europa.eu/documents/product-information/zykadia-epar-product-information_en.pdf Available at.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.