Highlights
-
•
BMI is used to characterize nutritional status but may not accurately depict body composition in CF.
-
•
DXA and bioelectrical impedance are the most commonly used methods for assessing BC.
-
•
Lower fat-free mass associates with worse pulmonary function and greater CF disease severity.
-
•
Fat-free mass associates with greater bone mineral density in individuals with CF.
Keywords: Cystic fibrosis, Body composition, Dual-energy X-ray absorptiometry, Bioelectrical impedance, Lean body mass, Fat mass
Abbreviations: CF, Cystic fibrosis; CFTR, Cystic fibrosis transmembrane conductance regulator; BMI, Body mass index; FM, Fat mass; LBM, Lean body mass; FFM, Fat-free mass; BC, Body composition; %BF, Percentage body fat; DXA, Dual-energy X-ray absorptiometry; BIA, Bioelectrical impedance; MRI, Magnetic resonance imaging; CT, Computed tomography; FFMI, Fat-free mass index; FMI, Fat mass index; HEMT, Highly Effective Modulator Therapy
Abstract
Because nutritional status is intimately linked with pulmonary function and survival, nutrition has been at the mainstay of cystic fibrosis (CF) care. Body Mass Index (BMI) is traditionally used to define nutritional status because of the ease with which it can be calculated, but it has a number of limitations including its inability to differentiate fat mass (FM) from lean body mass (LBM), the latter thought to confer health advantage. A number of tools are available to quantify body composition including dual-energy x-ray absorptiometry (DXA), bioelectrical impedance, MRI, CT, air displacement plethysmography, and stable isotopes, and these have been used to varying degrees in studies of CF. In CF, LBM tends to be lower for a given BMI, particularly at lower BMI. In adults, lower fat-free mass (FFM) correlates with greater CF disease severity, lower pulmonary function and higher inflammatory markers. FFM is also positively associated with greater bone mineral density, while greater FM is associated with greater loss of lumbar spine bone mineral density over 2 years. In youth, LBM is positively associated with pulmonary function. The predictive value of body composition for functional and clinical outcomes and the role of improving LBM on these outcomes remain undefined. With improvements in BMI accompanying highly-effective modulator therapy, closer evaluations of body composition may inform risk for more traditional, non-CF adult outcomes in CF.
Introduction
Cystic fibrosis (CF) arises from recessive mutations in the gene encoding the cystic fibrosis transmembrane conductance regulator (CFTR) protein, a channel for chloride, bicarbonate, and other molecules. Loss of CFTR function leads to thickened mucus secretions that affect multiple organs including the lungs, pancreas, intestine, and liver. Maldigestion and malabsorption, especially of fat and fat-soluble vitamins, poor appetite, and increased energy expenditure contribute to the frequent occurrence of undernutrition in the affected population [1].
A seminal study by Corey et al in 1988 found that individuals with CF with higher weight and height had better survival, a finding that was independent of pulmonary function. This finding translated into a focus on nutrition in people with CF [2]. The emphasis of nutritional counseling shifted from a low-fat, high protein diet designed to control symptoms of steatorrhea and abdominal pain, to a high-energy, high fat diet with the goal of promoting weight gain [1].
Body mass index (BMI = weight in kilograms divided by the square of height in meters) has traditionally been used to define nutritional status because of its association with multiple health outcomes. BMI is a composite of multiple tissue types (fat mass (FM) and fat-free mass (FFM), the latter composed of bone mass and lean body mass (LBM)), and this surrogate for healthy body composition serves as a screening tool. Sex-, age-, developmental stage, and racial differences in body composition challenge the use of BMI in this capacity. Additionally, BMI, fat-free mass (FFM)/LBM, and FM are not always aligned, and the differences can have health implications [3], [4].
Assessment of body composition is considered an important part of the nutrition status evaluation in CF and is recommended in professional guidelines [1]. This review is intended to provide a greater understanding of the tools used to measure body composition and the relationship of BMI to body composition. It will also review what is known about body composition in the CF population.
Body composition assessment tools
Body composition assessment considers the functional role in energy metabolism played by muscle, bone, and fat. It is often described by five different levels – atomic, molecular, cellular, tissue, and whole body. In general, body composition is subdivided into 2-compartment (FM + FFM), 3-compartment (FM, LBM + bone mass) or multicompartment (FM + ≥3 components of FFM) [5].
In the general population, BMI has largely been used as a surrogate for FM and has been focused on defining overweight/obesity and capturing cardiometabolic risk. With this risk in mind, quantification of total FM has been extended to include relative (percent) FM (%BF) and distribution of body fat. Excess “central” and/or visceral adiposity in adults is typically associated with increased risk for insulin resistance and associated comorbidities – such as Type 2 diabetes mellitus, Polycystic Ovary Syndrome, hypertension, dyslipidemia, and cardiovascular disease. Conversely, increased subcutaneous adipose tissue in the hip and thigh area is not associated with increased risk for cardiovascular disease – and in fact, may be protective [6]. In contrast, in the general population, FFM is inversely associated with mortality [3]. Adjusting skeletal mass for adiposity improves its correlation with physical performance and prediction of incident disability [7]. Moreover, the location of LBM may have implications for health. For example, appendicular LBM is most associated with disability in patients with rheumatoid arthritis [8]. Adjusting appendicular LBM for adiposity may further improve the correlation with physical functioning [9]. Weber et al have developed a practical framework for this adjustment to define deficits in appendicular LBM relative to FM [10].
A number of clinical and research methods are available to assess body composition including dual-energy X-ray absorptiometry (DXA), bioelectrical impedance (BIA), magnetic resonance imaging (MRI), computed tomography (CT), air displacement plethysmography, and stable isotopes. Each modality has advantages and disadvantages (Table 1), and no single method is considered the gold standard for body composition assessment in CF [4], [11], [12], [13], [14]. In 2019, Calella et al published a systemic review of body composition analysis in individuals with CF that included 124 studies. They reported that 34% of the studies utilized DXA as the only method of body composition assessment, 10% of studies used only BIA for body composition analysis, and 31% of studies utilized mixed methods of body composition assessment. In terms of validating body composition assessment methods in individuals with CF, this same paper nicely summarized 10 studies drawing discordant conclusions about the agreement between the various methods [13].
Table 1.
Advantages and disadvantages of commonly utilized body composition assessment tools.
| BC Assessment Tool | Advantages | Disadvantages |
|---|---|---|
| Dual-energy X-ray absorptiometry |
|
|
| Bioelectrical impedance |
|
|
| CT/MRI |
|
|
| Air displacement plethysmography |
|
|
| Stable isotopes |
|
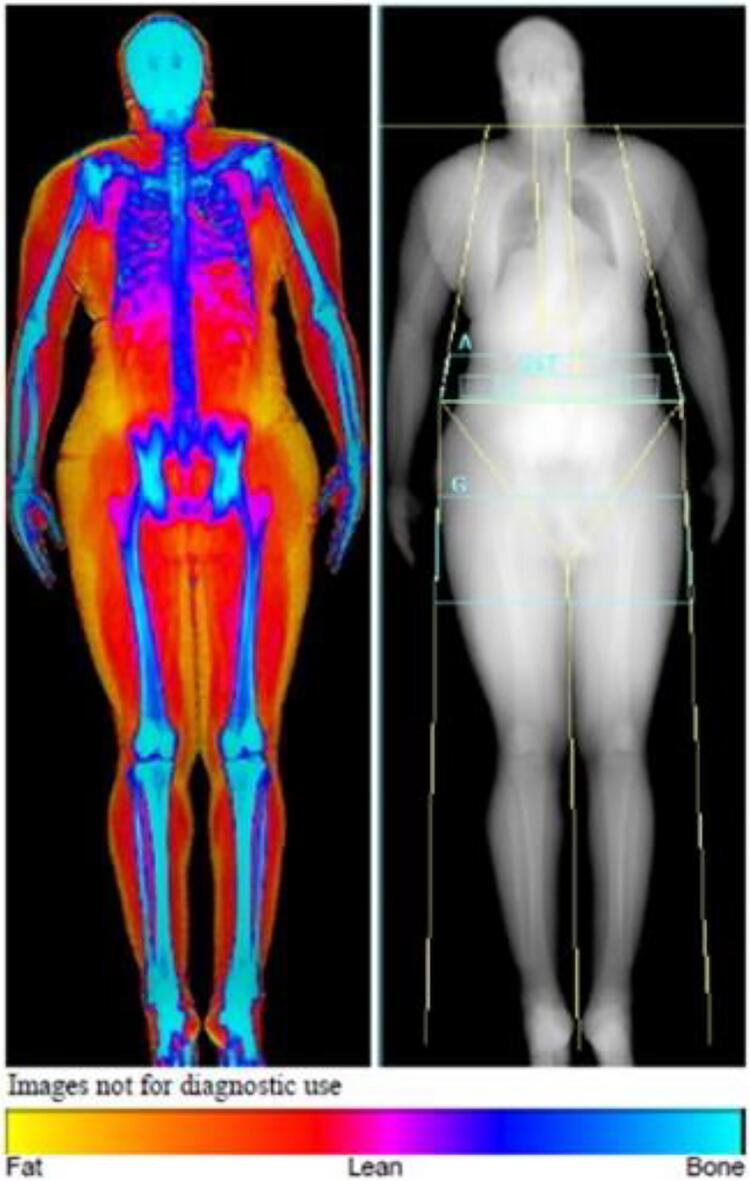
An advantage of using DXA in individuals with CF is that it is already recommended for routine monitoring of bone density in the CF population starting in childhood [13]. The latest densitometers allow body composition assessment with a single whole-body scan [14]. Visceral adipose area can also be determined [15]. Fig. 1 is an example of a postprocessed DXA image [16]. Reference data for LBM, FM, and appendicular LBM in DXA are becoming increasingly available [7], [9], [10], [17], [18], [19].
Fig. 1.
An example of a post-processed whole body DXA scan. Fat is represented in yellow, bone in blue, and muscle in red [16]. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
BIA is a quick, inexpensive, portable and simple-to-use technology that is commonly used in both research and clinical practice [4], [14]. BIA directly measures the impedance of the human body, whereas the parameters related to body composition (water content, FFM and FM) are calculated [13]. A study using a CF-specific equation for BIA was validated by Charatsi et al using DXA as a reference method [20].
CT and MRI are currently considered the gold standard for determining the muscle mass quality and quantity. They allow for quantification of total adipose tissue as well as further division into subcutaneous, intramuscular and visceral adipose tissue. Peripheral quantitative computed tomography is cheaper, easier to obtain, and requires less radiation than traditional CT. However, it has lower accuracy, only analyzes peripheral anatomical sites, and provides only limited data on muscle and fat content [13]. The quantitative magnetic resonance (QMR) EchoMRI system is a newer body composition acquisition system that has been validated in healthy adults and children. The measurement acquisition time is only 2–4 min and does not require the participant to lie completely still [4].
Occasionally, studies on patients with CF have assessed body composition using other less commonly used methods including air displacement plethysmography, stable isotopes, and skin fold measurements. Non-CF studies have also utilized ultrasonography, NMR spectroscopy, PET/CT and PET/MR to quantify body composition.
Body composition differences across adult populations
Body composition naturally differs amongst populations. For example, men and women have different body composition. Women have more FM while men have more LBM. Men more often accumulate fat around the trunk and abdomen, while women tend to accumulate it around the hips and thighs [21]. Body composition also varies significantly between individuals of different races [22], [23]. For instance, African Americans have more LBM, specifically appendicular muscle mass, for a given BMI compared to Caucasians [24]. In addition, body composition in an individual naturally changes over time. Normal aging in adults is characterized by an increase in FM and decrease in LBM [4].
Pediatrics/Growth/Puberty and changes in body composition
Interpretation of body composition analysis in the pediatric population is inherently more challenging due to normal physiologic changes occurring throughout various developmental stages (i.e., infancy, early childhood, prepubertal, pubertal, and post-pubertal stages). The normal onset of puberty has a wide range of 5 years; it varies from age 8–13 years old in females, and 9–14 years old in males. The tempo of progression in secondary sexual characteristics as well as peak growth velocity also vary amongst individuals [25]
The normal BMI range fans out during infancy and is highest in late infancy, then narrows by 5–6 years of age. Starting at age 6–8 years, growth rates and weight/BMI gains increase, with a widening of the normal age-specific BMI range over time [5]. Prior to puberty, sex-specific differences in FM and fat distribution as well as paraspinous musculature are already operative. Arfai et al. performed quantitative CT on 31 pairs of age-, height-, and weight-matched healthy white prepubertal boys and girls 5–10 years old and found that girls had 28% more total body fat and 30% more subcutaneous fat than males. Girls had 10% lower amount of paraspinous musculature and 15% lower vertebral cross-sectional measurements [26].
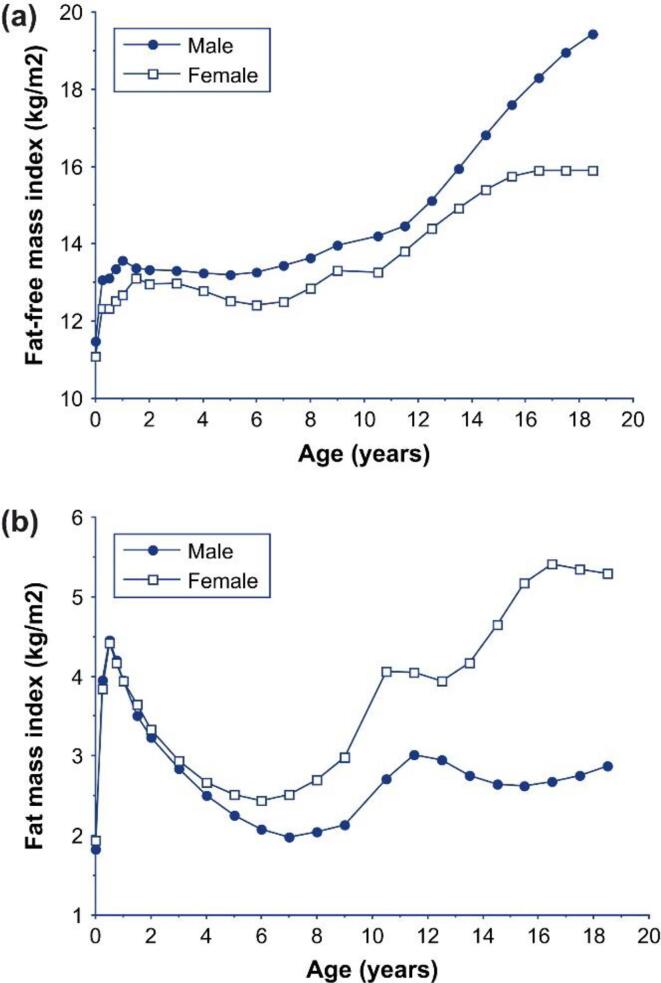
During puberty, both males and females have acceleration in linear growth as well as body weight gain. Sex-specific differences in body composition become more apparent. Just prior to the onset of puberty, males tend to have increases in FM. During puberty, they have a rapid increase in growth velocity along with increases in FFM and lower adipose tissue in the extremities. Females, however, experience greater increases in FM than LBM. Ultimately, a sexually dimorphic adult pattern in fat distribution emerges with central/visceral adiposity more typically observed in males whereas greater subcutaneous hip/leg adipose tissue is more typically observed in females. These generalizations cannot be reliably made across pediatric populations given normal physiologic differences in body composition at different developmental stages driven by hormonal and environmental factors [5]. Further, maximal bone density accrual (both cortical and trabecular bone) also occurs during puberty [27]. Fig. 2 by Wells illustrates changes in body composition adjusted for height in each sex over time using reference data [28].
Fig. 2.
The relationship between age and (a) FFMI and (b) fat mass index (FMI; FM divided by height in m2) during the life course, using data from the reference child and adolescent. During early life, boys and girls have similar fatness, but boys have more LBM and are therefore heavier. During childhood, both sexes lose fatness, but boys gain relatively more LBM. During adolescence, boys gained primarily LBM and little FM, whereas girls gain little LBM but substantial FM. In adulthood, men show substantially less FM and greater relative LBM than women in all human populations [28].
Given these normal physiologic changes throughout childhood, body composition in pediatric patients needs to be compared to age- and sex-matched references [29]. BMI is thus interpreted as percentile (or Z-score) relative to chronologic age and sex. In many patients, the BMI percentile may be an accurate marker of their body habitus. However, the clinical utility may be more limited in patients with predominantly abdominal obesity (such as children who were born with low birth weight / small for gestational age), patients with elevated BMI due to a large amount of muscle tissue, and patients with untreated endocrinopathies (growth hormone deficiency, hypothyroidism, cortisol excess) [30], [31]. Despite these limitations, Taylor et al found that higher BMI values correlate with higher %BF determined via DXA. Amongst males, those just prior to the onset of puberty were found to have a higher %BF (34–36%) as opposed to younger prepubertal males (24–30%) and older pubertal/post-pubertal males (27–30%). In females, increasing chronologic age was associated with increasing %BF. For example, an 18 year-old male with BMI 30 kg/m2 was predicted to have 27% BF, while a female of the same age and BMI was predicted to have a significantly higher %BF of 42% [32]. Ultimately, BMI is an efficient and cost-effective tool to use in the clinical setting: 1) Low BMI values are associated with lower adipose tissue in most patients, 2) Elevated BMI relative to chronologic age corresponds to increased risk of obesity-associated comorbidities, and 3) the trend in BMI percentiles remains useful for assessing an individual patient’s nutritional progress and risk factors [32], [33].
Many of the body composition studies in the pediatric population include white children. Additional studies in other ethnic groups are needed to determine the generalizability of the findings.
Body composition in individuals with CF
Most studies have reported that adults with CF have lower FFM, LBM, and skeletal muscle mass compared to the general population [13], [34], [35], [36]. As a notable exception, a recent study by Bellisimo et al using DXA to assess body composition did not find a significant difference in LBM between adult subjects with CF and controls [37], the extent to which this difference reflects the overall better health status of adults with CF is not clear. Studies are more conflicted with respect to FM in patients with CF. Some studies have reported lower FM in patients with CF, while some found similar FM [34], [35], [36], [37], [38], [39]. Most, but not all, of the studies reporting lower FM are in children. Some studies have reported comparable FM but higher central or visceral fat [37], [40].
Body composition vs. BMI in the CF population
In the CF population, evaluating only BMI as a marker of nutrition status may overlook other complex factors that contribute to poor nutritional status and ultimately poorer lung function. King et al reported that using a BMI cutoff of < 18.5 kg/m2 to identify malnutrition missed 58% of FFM depletion [41]. Similarly, Bolton et al assessed body composition in 51 adult CF patients vs. 18 normal controls by DXA and found that using a cutoff of BMI < 19.9 kg/m2 missed 43% of patients with a low fat-free mass index (FFMI; FFM divided by height in m2). Interestingly, of the 28 patients with a low BMI, 7 had a normal FFMI [36]. BMI measurements alone may also miss CF patients with normal-weight obesity, which is defined as a normal BMI but elevated %BF. This “normal weight obesity” condition may increase the risk for metabolic complications. Alvarez et al assessed body composition via air displacement plethysmography and found that 31% of participants had normal-weight obesity, defined as a BMI under 25 kg/m2 with a %BF > 30% in women and > 23% in men [42].
Body composition and CF outcomes
Pulmonary function
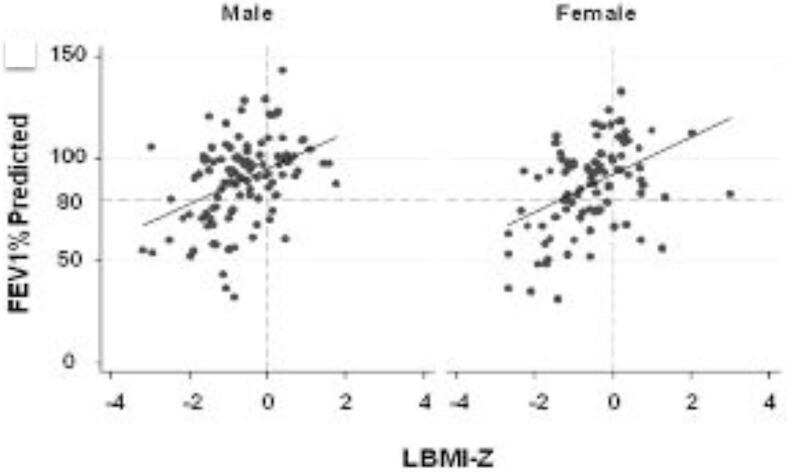
Excess fat has been inversely associated with pulmonary function in CF [42], while LBM is hypothesized to positively impact respiratory strength and pulmonary function [37], [43]. Fig. 3 illustrates the finding from Sheikh et al that in CF youth, LBM adjusted for height and age is positively associated with FEV1% predicted [43]. A study assessing FFM, diaphragm parameters, and spirometry in 40 adults with CF found that individuals with CF and low FFM have lower respiratory markers, with the lower FFM associated with lower diaphragm muscle mass [44]. Lower LBM has also been associated with worse lung function in individuals with cystic fibrosis [40]. A study published this year in 114 pediatric CF patients found that patients with a FFMI < 10th percentile had 9.5% lower FEV1% than patients with FFMI > 10th percentile [45]. In a study of 85 young adults with CF, male patients with frequent exacerbations (>2 per year) were found to have lower whole body and appendicular FFMI compared to patients with infrequent exacerbations [46].
Fig. 3.
The association between FEV1%-predicted and LBMI-Z adjusted for age among males and female youth with CF. LBMI-Z is positively associated with FEV1%-predicted in males (p < 0.0001) and females (p < 0.0001) [43].
Bone mineral density
In a study of 64 adults with CF, greater FFMI was associated with greater BMD. In contrast, greater FMI was associated with greater lumbar spine BMD loss over 2 years [47]. The recent study in 114 pediatric patients with CF referenced above also found that individuals with a FFMI < 10th percentile had a lower BMD Z-score compared to patients with FFMI > 10th percentile. In this study both FMI and FFMI correlated with BMD [45].
Inflammatory markers
Studies in adults with CF have found higher concentrations of the inflammatory markers IL-6 and CRP in patients with lower FFM/LBM [48], [49]. IL-6 levels have been associated with FFM loss in adults with CF over time [50]. It is hypothesized that chronic inflammation and pulmonary infections are a contributory factor to the altered body composition seen in CF patients, specifically the reduction in LBM and BMD [46], [48].
Glucose
A study of 24 adults with CF found that visceral adipose tissue was positively associated with fasting blood glucose [37]. A 2021 study examining 59 adolescents and young adults with CF found that FMI z-score correlated positively with higher insulin resistance and lower insulin sensitivity [39].
Impact of sex steroids in men
In a cross-sectional study of 40 men with CF compared to healthy controls, men with CF were noted to have lower serum testosterone. However, these lower testosterone concentrations were not correlated with weight, total or regional LBM, or FM. This same study noted that men with CF did show a shift from FM to LBM when compared to healthy controls. Since adipose is a site of conversion of testosterone to estradiol, lower FM may compensate for the lower testosterone to favor LBM over FM [51].
Impact of highly effective modulator therapy (HEMT) on body composition
The introduction of CFTR modulation therapy has revolutionized cystic fibrosis care, and these therapies are expected to result in longer lifespan, less-severe symptoms, and less risk for undernutrition for many patients. A few studies have reported on the impact of HEMT upon anthropometric outcomes i.e. height, weight and BMI, but little is known about their effects on body composition.
King et al recently reported on body composition changes in 20 adults with CF from a single-center double-blind, placebo-controlled 28-day crossover study of ivacaftor followed by an open-label extension for 5 months. Eleven of the patients on ivacaftor underwent body composition measurements 2 years later. Weight, BMI and FFM all increased significantly during the 28-day intervention, but were notably not different in the ivacaftor vs placebo groups. After the 5-month open-label extension period in ivacaftor users, weight and FM increased from baseline, but not FFM. For the 11 patients who were measured after an additional 2 years, no further changes in weight, BMI or body composition were observed as compared to measurements at 6 months [52].
Another recently published manuscript by King et al examined body composition in 24 adults with severe CF who were treated with clinically prescribed lumacaftor/ivacaftor for at least 1 year. No changes in body composition were observed during the first month of therapy. By 6 months, patients had gained significant FM which plateaued by 1 year. In the group as a whole no significant change in FFM or FFMI was found over the year. However, 20% of patients gained > 5% of baseline FFM in 1 year of treatment. Baseline BMI was inversely correlated with change in FFM, indicating that those with the poorest baseline nutritional status experienced the greatest gains in FFM. Changes in FFM by 1 year correlated with greater reduction in the number of both hospitalization days and IV antibiotic days in the year after lumacaftor/ivacaftor started compared with the year prior [53].
While elexacaftor/tezacaftor/ivacaftor has been shown to significantly increase weight and BMI in the “short-term,” body composition outcomes have not been published [54]. The CFF Promise study is examining body composition by DXA at 0, 12–18 months, and 24–30 months following clinically prescribed therapy in individuals aged ≥ 12 years.
Knowledge gaps
The predictive value of body composition for functional and clinical outcomes remains undefined, and current evidence is insufficient to recommend one method of accessing body composition over others in clinical care. While the landscape of CF is changing with HEMT, consideration of nutritional status and LBM deficits remains crucial for the subset of patients with established severe disease or CFTR mutations unresponsive to current therapies. With increasing rates of overweight and obesity in the CF population, more traditional adult outcomes including cardiometabolic risk and vascular disease may need to be considered in the context of body composition.
Conclusion
The majority of studies have identified differences in body composition in adults with CF compared to the general population. Utilizing only BMI to quantify nutritional status may miss a significant portion of CF patients with abnormal body composition who are at risk for adverse health effects. These considerations are highlighted by associations of low FFM/LBM with impaired respiratory function and decreased bone mineral density in CF. The impact of modulators upon body composition and downstream implications for health remain undefined. Looking forward, assessing body composition changes over time may become an important mechanism to monitor therapy and guide treatment decisions in the CF population.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the Cystic Fibrosis Foundation Envision CF Program [grant number SOLTMA19GE0]
References
- 1.McDonald C.M., Alvarez J.A., Bailey J., Bowser E.K., Farnham K., Mangus M., et al. Cystic fibrosis evidence analysis center evidence-based nutrition practice guideline. J Acad Nutrit Diet. 2021;121(8):1591–1636.e3. doi: 10.1016/j.jand.2020.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Corey M., McLaughlin F.J., Williams M., Levison H. A comparison of survival, growth, and pulmonary function in patients with cystic fibrosis in Boston and Toronto. J Clin Epidemiol. 1988;41(6):583–591. doi: 10.1016/0895-4356(88)90063-7. [DOI] [PubMed] [Google Scholar]
- 3.Abramowitz M.K., Hall C.B., Amodu A., Sharma D., Androga L., Hawkins M., et al. Muscle mass, BMI, and mortality among adults in the United States: A population-based cohort study. PLoS ONE. 2018;13(4):e0194697. doi: 10.1371/journal.pone.0194697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lemos T., Gallagher D. Current body composition measurement techniques. Curr Opin Endocrinol Diabetes Obesity. 2017;24 doi: 10.1097/MED.0000000000000360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weber D.R., Leonard M.B., Zemel B.S. Body composition analysis in the pediatric population. Pediatric Endrocrinol Rev. 2012;10:130–139. [PMC free article] [PubMed] [Google Scholar]
- 6.Slyper A.H. Childhood obesity, adipose tissue distribution, and the pediatric practitioner. Pediatrics. 1998;102 doi: 10.1542/peds.102.1.e4. [DOI] [PubMed] [Google Scholar]
- 7.Baker J.F., Long J., Leonard M.B., Harris T., Delmonico M.J., Santanasto A., et al. Estimation of skeletal muscle mass relative to adiposity improves prediction of physical performance and incident disability. J Gerontol: Series A. 2018;73 doi: 10.1093/gerona/glx064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giles J.T., Bartlett S.J., Andersen R.E., Fontaine K.R., Bathon J.M. Association of body composition with disability in rheumatoid arthritis: Impact of appendicular fat and lean tissue mass. Arthritis Rheum. 2008;59(10):1407–1415. doi: 10.1002/art.v59:1010.1002/art.24109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baker J.F., Giles J.T., Weber D., Leonard M.B., Zemel B.S., Long J., et al. Assessment of muscle mass relative to fat mass and associations with physical functioning in rheumatoid arthritis. Rheumatology. 2017;56 doi: 10.1093/rheumatology/kex020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weber D., Long J., Leonard M.B., Zemel B., Baker J.F., Anderson R.M. Development of novel methods to define deficits in appendicular lean mass relative to fat mass. PLoS ONE. 2016;11(10):e0164385. doi: 10.1371/journal.pone.0164385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davies P.S.W. Stable isotopes and bioelectrical impedance for measuring body composition in infants born small for gestational age. Horm Res. 1997;48(1):50–55. doi: 10.1159/000191269. [DOI] [PubMed] [Google Scholar]
- 12.Bodamer O.A.F. Uses of stable isotopes in clinical diagnosis and research in the paediatric population. Archiv Disease Childhood. 2001;84 doi: 10.1136/adc.84.5.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calella P., Valerio G., Brodlie M., Taylor J., Donini L.M., Siervo M. Tools and methods used for the assessment of body composition in patients with cystic fibrosis: A systematic review. Nutrit Clin Practice. 2019;34(5):701–714. doi: 10.1002/ncp.v34.510.1002/ncp.10247. [DOI] [PubMed] [Google Scholar]
- 14.Ceniccola G.D., Castro M.G., Piovacari S.M.F., Horie L.M., Corrêa F.G., Barrere A.P.N., et al. Current technologies in body composition assessment: advantages and disadvantages. Nutrition. 2019;62:25–31. doi: 10.1016/j.nut.2018.11.028. [DOI] [PubMed] [Google Scholar]
- 15.Goldberg E.K., Fung E.B. Precision of the Hologic DXA in the assessment of visceral adipose tissue. J Clin Densitomet. 2020;23(4):664–672. doi: 10.1016/j.jocd.2019.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shepherd J.A., Ng B.K., Sommer M.J., Heymsfield S.B. Body composition by DXA. Bone. 2017;104:101–105. doi: 10.1016/j.bone.2017.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duran I., Martakis K., Rehberg M., Stark C., Koy A., Schoenau E. The appendicular lean mass index is a suitable surrogate for muscle mass in children with cerebral palsy. J Nutrit. 2019;149 doi: 10.1093/jn/nxz127. [DOI] [PubMed] [Google Scholar]
- 18.Weber D.R., Moore R.H., Leonard M.B., Zemel B.S. Fat and lean BMI reference curves in children and adolescents and their utility in identifying excess adiposity compared with BMI and percentage body fat. The Am J Clin Nutrit. 2013;98 doi: 10.3945/ajcn.112.053611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baker J.F., Harris T., Rapoport A., Ziolkowski S.L., Leonard M.B., Long J., et al. Validation of a description of sarcopenic obesity defined as excess adiposity and low lean mass relative to adiposity. J Cachexia Sarcopenia Muscle. 2020;11(6):1580–1589. doi: 10.1002/jcsm.v11.610.1002/jcsm.12613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Charatsi A.M., Dusser P., Freund R., Maruani G., Rossin H., Boulier A., et al. Bioelectrical impedance in young patients with cystic fibrosis: Validation of a specific equation and clinical relevance. J Cyst Fibros. 2016;15(6):825–833. doi: 10.1016/j.jcf.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 21.Bredella M.A. Sex differences in body composition. Adv Exp Med Biol. 2017:1043. doi: 10.1007/978-3-319-70178-3_2. [DOI] [PubMed] [Google Scholar]
- 22.Fedewa M.V., Nickerson B.S., Tinsley G.M., Esco M.R., Dunbar E.G., Boucher A.G., et al. Examining race-related error in two-compartment models of body composition assessment: A systematic review and meta-analysis. J Clin Densit. 2021;24(1):156–168. doi: 10.1016/j.jocd.2019.10.002. [DOI] [PubMed] [Google Scholar]
- 23.Wulan S.N., Westerterp K.R., Plasqui G. Ethnic differences in body composition and the associated metabolic profile: A comparative study between Asians and Caucasians. Maturitas. 2010;65(4):315–319. doi: 10.1016/j.maturitas.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 24.Jones A., Shen W., St-Onge M.-P., Gallagher D., Heshka S., Wang Z., et al. Body-composition differences between African American and white women: relation to resting energy requirements. Am J Clin Nutrit. 2004;79 doi: 10.1093/ajcn/79.5.780. [DOI] [PubMed] [Google Scholar]
- 25.Wolf R.M., Long D. Pubertal development. Pediatrics Rev. 2016;37 doi: 10.1542/pir.2015-0065. [DOI] [PubMed] [Google Scholar]
- 26.Arfai K., Pitukcheewanont P.D., Goran M.I., Tavare C.J., Heller L., Gilsanz V. Muscle, and fat: sex-related differences in prepubertal children. Radiology. 2002;224(2):338–344. doi: 10.1148/radiol.2242011369. [DOI] [PubMed] [Google Scholar]
- 27.Bailey D.A., Martin A.D., McKay H.A., Whiting S., Mirwald R. Calcium accretion in girls and boys during puberty: A longitudinal analysis. J Bone Miner Res. 2000;15(11):2245–2250. doi: 10.1359/jbmr.2000.15.11.2245. [DOI] [PubMed] [Google Scholar]
- 28.Wells J.C.K. Sexual dimorphism of body composition. Best Practice Res Clin Endocrinol Metab. 2007;21(3):415–430. doi: 10.1016/j.beem.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 29.Fomon S.J., Haschke F., Ziegler E.E., Nelson S.E. Body composition of reference children from birth to age 10 years. Am J Clin Nutrit. 1982;35 doi: 10.1093/ajcn/35.5.1169. [DOI] [PubMed] [Google Scholar]
- 30.Wells JCK, Cole T.J. Adjustment of fat-free mass and fat mass for height in children aged 8 y. Internat J Obesity. 2002;26(7):947–952. doi: 10.1038/sj.ijo.0802027. [DOI] [PubMed] [Google Scholar]
- 31.Wells JCK. A Hattori chart analysis of body mass index in infants and children. Internat J Obesity. 2000;24(3):325–329. doi: 10.1038/sj.ijo.0801132. [DOI] [PubMed] [Google Scholar]
- 32.Taylor R.W., Jones I.E., Williams S.M., Goulding A. Body fat percentages measured by dual-energy X-ray absorptiometry corresponding to recently recommended body mass index cutoffs for overweight and obesity in children and adolescents aged 3–18 y. Am J Clin Nutrit. 2002;76 doi: 10.1093/ajcn/76.6.1416. [DOI] [PubMed] [Google Scholar]
- 33.Maynard L.M., Wisemandle W., Roche A.F., Chumlea W.C., Guo S.S., Siervogel R.M. Childhood body composition in relation to body mass index. Pediatrics. 2001;107(2):344–350. doi: 10.1542/peds.107.2.344. [DOI] [PubMed] [Google Scholar]
- 34.Calella P., Valerio G., Brodlie M., Donini L.M., Siervo M. Cystic fibrosis, body composition, and health outcomes: a systematic review. Nutrition. 2018;55-56:131–139. doi: 10.1016/j.nut.2018.03.052. [DOI] [PubMed] [Google Scholar]
- 35.Rochat T., Slosman D.O., Pichard C., Belli D.C. Body composition analysis by dual-energy X-ray absorptiometry in adults with cystic fibrosis. Chest. 1994;106(3):800–805. doi: 10.1378/chest.106.3.800. [DOI] [PubMed] [Google Scholar]
- 36.Bolton C., Ionescu A., Evans W., Pettit R., Shale D. Altered tissue distribution in adults with cystic fibrosis. Thorax. 2003;58 doi: 10.1136/thorax.58.10.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bellissimo M.P., Zhang I., Ivie E.A., Tran P.H., Tangpricha V., Hunt W.R., et al. Visceral adipose tissue is associated with poor diet quality and higher fasting glucose in adults with cystic fibrosis. J Cyst Fibros. 2019;18(3):430–435. doi: 10.1016/j.jcf.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schönenberger K.A., Reber E., Bally L., Geiser T., Lin D., Stanga Z. Nutritional assessment in adults with cystic fibrosis. Nutrition. 2019:67–68. doi: 10.1016/j.nut.2019.05.010. [DOI] [PubMed] [Google Scholar]
- 39.Granados A., Beach E.A., Christiansen A.J., Patterson B.W., Wallendorf M., Arbeláez A.M. The association between body composition, leptin levels and glucose dysregulation in youth with cystic fibrosis. J Cyst Fibros. 2021;20(5):796–802. doi: 10.1016/j.jcf.2021.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moriconi N., Kraenzlin M., Müller B., Keller U., Nusbaumer C.P.G., Stöhr S., et al. Body composition and adiponectin serum concentrations in adult patients with cystic fibrosis. J Clin Endocrinol Metab. 2006;91(4):1586–1590. doi: 10.1210/jc.2005-2135. [DOI] [PubMed] [Google Scholar]
- 41.King S.J., Nyulasi I.B., Strauss B.J.G., Kotsimbos T., Bailey M., Wilson J.W. Fat-free mass depletion in cystic fibrosis: Associated with lung disease severity but poorly detected by body mass index. Nutrition. 2010;26(7-8):753–759. doi: 10.1016/j.nut.2009.06.026. [DOI] [PubMed] [Google Scholar]
- 42.Alvarez J.A., Ziegler T.R., Millson E.C., Stecenko A.A. Body composition and lung function in cystic fibrosis and their association with adiposity and normal-weight obesity. Nutrition. 2016;32(4):447–452. doi: 10.1016/j.nut.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sheikh S., Zemel B.S., Stallings V.A., Rubenstein R.C., Kelly A. Body composition and pulmonary function in cystic fibrosis. Front Pediatrics. 2014;2 doi: 10.3389/fped.2014.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Enright S., Chatham K., Ionescu A.A., Unnithan V.B., Shale D.J. The influence of body composition on respiratory muscle, lung function and diaphragm thickness in adults with cystic fibrosis. J Cyst Fibros. 2007;6(6):384–390. doi: 10.1016/j.jcf.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 45.Ritchie H., Nahikian-Nelms M., Roberts K., Gemma S., Shaikhkhalil A. The prevalence of aberrations in body composition in pediatric cystic fibrosis patients and relationships with pulmonary function, bone mineral density, and hospitalizations. J Cyst Fibros. 2021;20(5):837–842. doi: 10.1016/j.jcf.2021.04.004. [DOI] [PubMed] [Google Scholar]
- 46.Alicandro G., Bisogno A., Battezzati A., Bianchi M.L., Corti F., Colombo C. Recurrent pulmonary exacerbations are associated with low fat free mass and low bone mineral density in young adults with cystic fibrosis. J Cyst Fibros. 2014;13(3):328–334. doi: 10.1016/j.jcf.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 47.Baker JF, Putman MS, Herlyn K, Tillotson AP, Finkelstein JS, Merkel PA. Body composition, lung function, and prevalent and progressive bone deficits among adults with cystic fibrosis. Joint Bone Spine 2016;83. https://doi.org/10.1016/j.jbspin.2015.04.021. [DOI] [PubMed]
- 48.Ionescu A.A., Nixon L.S., Evans W.D., Stone M.D., Lewis-Jenkins V., Chatham K., et al. Bone density, body composition, and inflammatory status in cystic fibrosis. Am J Respir Crit Care Med. 2000;162(3):789–794. doi: 10.1164/ajrccm.162.3.9910118. [DOI] [PubMed] [Google Scholar]
- 49.Ionescu A.A., Chatham K., Davies C.A., Nixon L.S., Enright S., Shale D.J. Inspiratory muscle function and body composition in cystic fibrosis. Am J Respir Crit Care Med. 1998;158(4):1271–1276. doi: 10.1164/ajrccm.158.4.9710079. [DOI] [PubMed] [Google Scholar]
- 50.King S.J., Nyulasi I.B., Bailey M., Kotsimbos T., Wilson J.W. Loss of fat-free mass over four years in adult cystic fibrosis is associated with high serum interleukin-6 levels but not tumour necrosis factor-alpha. Clin Nutrit. 2014;33(1):150–155. doi: 10.1016/j.clnu.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 51.Leifke E., Friemert M., Heilmann M., Puvogel N., Smaczny C., von zur Muhlen A., et al. Sex steroids and body composition in men with cystic fibrosis. Eur J Endocrinol. 2003 doi: 10.1530/eje.0.1480551. [DOI] [PubMed] [Google Scholar]
- 52.King S.J., Tierney A.C., Edgeworth D., Keating D., Williams E., Kotsimbos T., et al. Body composition and weight changes after ivacaftor treatment in adults with cystic fibrosis carrying the G551 D cystic fibrosis transmembrane conductance regulator mutation: A double-blind, placebo-controlled, randomized, crossover study with open-label extension. Nutrition. 2021;85:111124. doi: 10.1016/j.nut.2020.111124. [DOI] [PubMed] [Google Scholar]
- 53.King S.J., Keating D., Williams E., Paul E., Borg B.M., Finlayson F., et al. Lumacaftor/ivacaftor-associated health stabilisation in adults with severe cystic fibrosis. ERJ Open Research. 2021;7(1):00203-2020. doi: 10.1183/23120541.00203-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bailey J., Rozga M., McDonald C.M., Bowser E.K., Farnham K., Mangus M., et al. Effect of CFTR modulators on anthropometric parameters in individuals with cystic fibrosis: an evidence analysis center systematic review. J Acad Nutrit Diet. 2021;121(7):1364–1378.e2. doi: 10.1016/j.jand.2020.03.014. [DOI] [PubMed] [Google Scholar]