Abstract
Treatment of acute leukemia with intensive chemotherapy leads to an increased risk of myelosuppression. Luteinizing hormone (LH) blockade improves hematopoietic recovery in mice after radiation or chemotherapy, through protection of the hematopoietic stem cells which express the LH receptor. We hypothesized that LH blockade improves hematopoietic recovery following intensive chemotherapy in patients with leukemia. We conducted a retrospective analysis on pre-menopausal women with acute myeloid leukemia (AML) or acute lymphoblastic leukemia (ALL) who received intensive chemotherapy and leuprolide given for abnormal uterine bleeding prevention or treatment. Given that leuprolide is more commonly administered in younger patients, we performed propensity score matching between the leuprolide (AML n=64; ALL n=49) and control groups (AML n=128; ALL n=98 patients). Patients with AML who received leuprolide had an additional increase of 13.8x109/L/year in their platelet count, and a 0.19x 109/L/year increase in their lymphocyte count after chemotherapy compared to control (P=0.02; P=0.03 respectively). Those with ALL who received leuprolide had an additional increase of 0.37x109/L/year in their absolute neutrophil count (P=0.02). In AML, leuprolide was associated with higher long-term hemoglobin levels (P<0.001) and less blood transfusions (mean, 23.9 vs. 34.7 units; P=0.002) compared to control. In a multivariate analysis, leuprolide was identified as an independent factor predicting improved hemoglobin levels, lymphocyte and platelet counts in AML. In conclusion, leuprolide use in leukemia patients receiving intensive chemotherapy was associated with improved long-term blood count recovery and with decreased transfusion requirements in AML.
Introduction
Hematopoiesis is an uninterrupted process of self-renewal, proliferation and differentiation of hematopoietic stem cells (HSC) in order to produce mature blood cells.1 Maintenance of HSC is essential for regeneration of all bone marrow elements particularly following injuries such as ionizing radiation and/or chemotherapy. Treatment of acute leukemia with intensive cytotoxic chemotherapy results in acute hematopoietic suppression, leading to increased risks of infection and bleeding, especially in older patients where the induction mortality can reach up to 20-30%.2-4 In addition to decreased blood counts, chemotherapy induces HSC senescence causing long-term bone marrow damage and, in some instances, secondary myeloid malignancies.5,6 There is an unmet need to develop strategies aimed at selectively protecting HSC from the damaging effects of chemotherapy, and maintaining the HSC pool especially for cancer survivors. Growing evidence suggests that the luteinizing hormone/choriogonadotropin receptor (LHCGR) is expressed on human HSC and that LH is implicated in HSC self-renewal.7,8 In preclinical murine models, LH suppression using an LH-releasing hormone (LHRH) antagonist improved hematopoietic recovery after chemotherapy or lethal dose radiation.9-11 Leuprolide, a gonadotropin-releasing hormone (GnRH) agonist, has been widely used in cancer patients during intensive chemotherapy and allogeneic stem cell transplantation in order to reduce the incidence of abnormal uterine bleeding and for fertility preservation.12-14 In this study, we conducted a retrospective analysis aimed at evaluating the effect of leuprolide on hematopoietic recovery following intensive cytotoxic chemotherapy in acute leukemia.
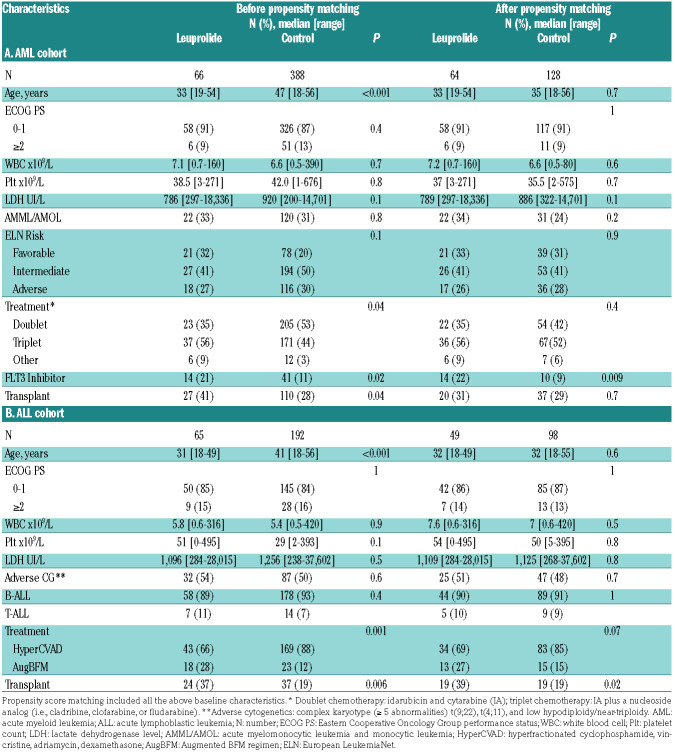
Table 1.
Baseline characteristics.
Methods
Patient selection
We screened adult female patients younger than 55 years at The University of Texas MD Anderson Cancer Center (Houston, TX) with newly diagnosed acute myeloid leukemia (AML) or acute lymphoblastic leukemia (ALL), treated with intensive chemotherapy between January 2000 and December 2017. We identified those who received at least one leuprolide injection for prevention or treatment of abnormal uterine bleeding with intensive chemotherapy (leuprolide received between day -7 and day 90 of induction chemotherapy start). The control group consisted of patients who had never received leuprolide (Figure 1). Baseline variables including age, types of treatment, laboratory parameters as well as clinical outcomes were collected and analyzed. All peripheral blood counts, performed between the start of treatment with induction chemotherapy until the date of last follow-up, were extracted from the electronic medical records and analyzed. We compared short and long-term count recovery, transfusion requirements and survival between the leuprolide and control groups.
This study was performed in accordance with the Declaration of Helsinki and was approved by the MD Anderson Institutional Review Board.
Statistical analysis
Patient characteristics were summarized using median (range) for continuous variables and frequency (percentage) for categorical variables. Fisher’s exact test and Wilcoxon rank-sum test were used to assess differences in categorical and continuous variables. Given that leuprolide was more commonly given in younger patients, propensity score matching was used to adjust for covariate imbalances including the age difference between the respective case and control groups (Figure 1). Using the nearest-neighbor algorithm, patients from the leuprolide groups were matched to control at a 1:2 ratio.15 A logistic regression model was used to estimate propensity scores. All subsequent analyses including count recovery, transfusion requirements and survival were performed on matched cohorts. Absolute neutrophil count (ANC) and platelet recovery were defined as achievement of an ANC ≥1x109/L, and a platelet count ≥100x109/L after first induction chemotherapy. Scatterplots of all peripheral blood cell counts for each patient, collected between induction chemotherapy (day 0) and last follow-up date, were extracted from health records, plotted and compared between leuprolide and control matched groups. Lowess smooth curves were used for indicating longitudinal trajectories of counts and differences were assessed using the generalized estimation equation model.16,17 The probabilities of recovery were estimated using the Kaplan-Meier method. Overall survival (OS) was calculated as the time interval from treatment start date to the date of death, and was censored at the last followup date for patients who were alive. Event free survival (EFS) was defined as the time interval between the date of response and the date of relapse or death, whichever was first and was censored at last follow-up in patients alive and in remission. The Kaplan- Meier method was used to estimate the probability of OS and EFS, and log-rank test was used to compare survival between matched cohorts. Univariate and multivariate analyses were performed to determine the differential effect of leuprolide on count recovery and the interaction with baseline characteristics, treatment type, and relapse status. All computations were done in R version 3.4.4.
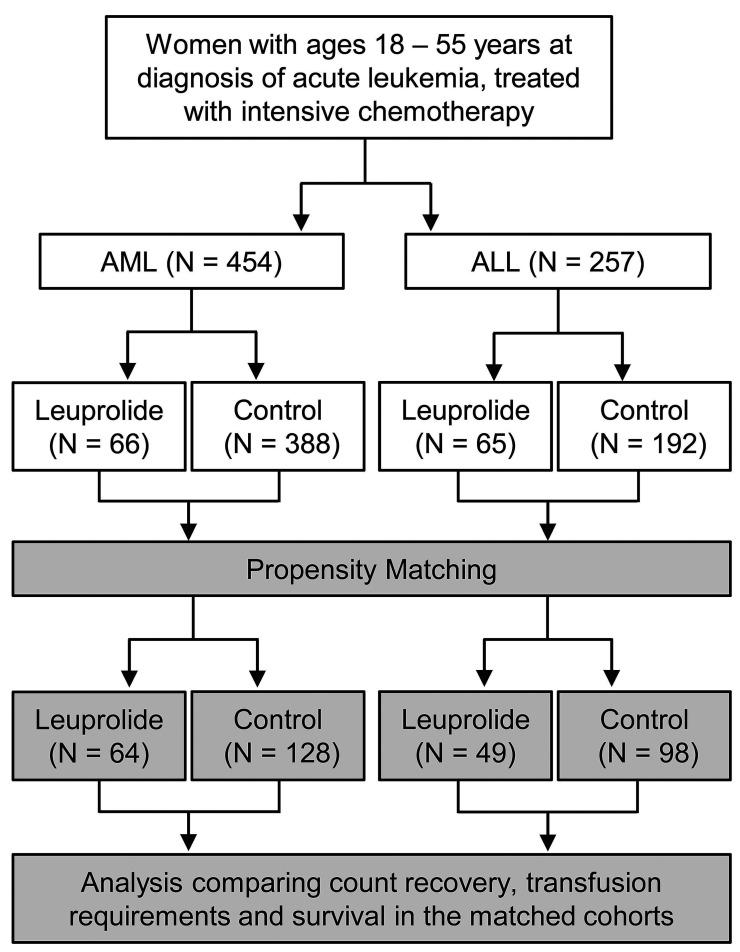
Figure 1.
Patient selection. Control cohorts consisted of patients who had never received leuprolide. Propensity matching was done to adjust for covariate imbalances including the age difference between the respective case and control groups. All subsequent analyses were done comparing matched cohorts. AML: acute myeloid leukemia; ALL: acute lymphoblastic leukemia.
Results
Patient population
We identified 454 pre-menopausal women with AML and 257 with ALL, newly diagnosed and treated with intensive chemotherapy. Among those patients, 66 with AML and 65 with ALL had received leuprolide (Figure 1). Those who never received leuprolide were used as control cohorts and included 388 patients with AML and 192 patients with ALL (Figure 1; Table 1). Among patients with AML who received leuprolide, 33 (52%) received it between day -7 and day 15 of induction chemotherapy start, compared to 22 patients (45%) with ALL who received leuprolide during this time interval (Online Supplementary Table S2). Leuprolide was given in various dosage forms either through subcutaneous or intramuscular injections depending on the platelet count at time of administration. The median cumulative dose of leuprolide per patient, given throughout cycles of chemotherapy was 22.5 mg (range, 3–78.75 mg) for those with AML and 22.5 mg (range, 11.25–135 mg) for those with ALL (Online Supplementary Table S2). Patients who received leuprolide were significantly younger than the control cohorts (AML: 33 years vs. 47 years, P<0.001; ALL: 31 years vs. 41 years, P<0.001). Baseline characteristics were well balanced after propensity score matching (Table 1). For patients with AML, the most commonly given treatment regimens consisted of triplets including the backbone of idarubicin and cytarabine in addition to a nucleoside analog (cladribine, clofarabine or fludarabine).18-20 The rest of the patients received a combination of idarubicin and cytarabine. Targeted therapy was added by the treating physicians when indicated (Online Supplementary Table S1 includes mutations identified in the AML matched cohorts). Patients in the ALL cohorts received either HyperCVAD (alternating cycles of hyperfractionated cyclophosphamide, vincristine, doxorubicin, dexamethasone and methotrexate and cytarabine) or Augmented Berlin– Frankfurt–Münster (AugBFM) regimens.21
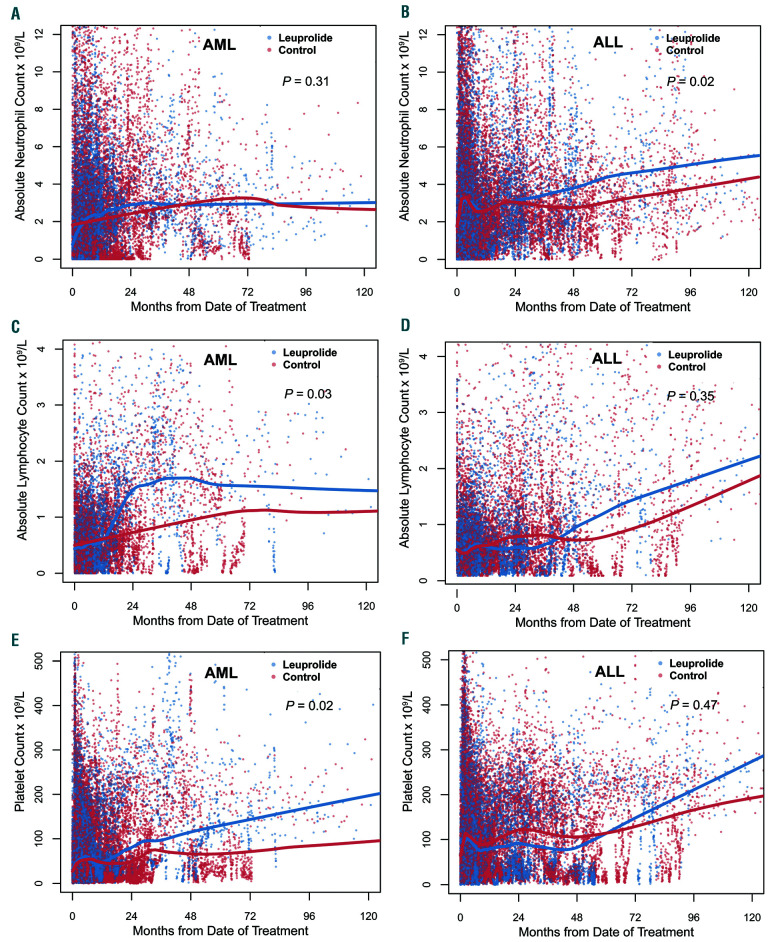
Figure 2.
Long-term peripheral blood count recovery following chemotherapy with and without leuprolide. Scatterplots of all corresponding peripheral blood laboratory data extracted from health records, collected after induction chemotherapy (day 0) where each dot represents a single value (blue for leuprolide, red for control). (A-B) Change in absolute neutrophil count. (C-D) Change in absolute lymphocyte count. (E-F) Change in platelet count. Lowess smooth curves were used for indicating longitudinal trajectories of counts and differences were assessed using the generalized estimation equation model. AML: acute myeloid leukemia; ALL: acute lymphoblastic leukemia.
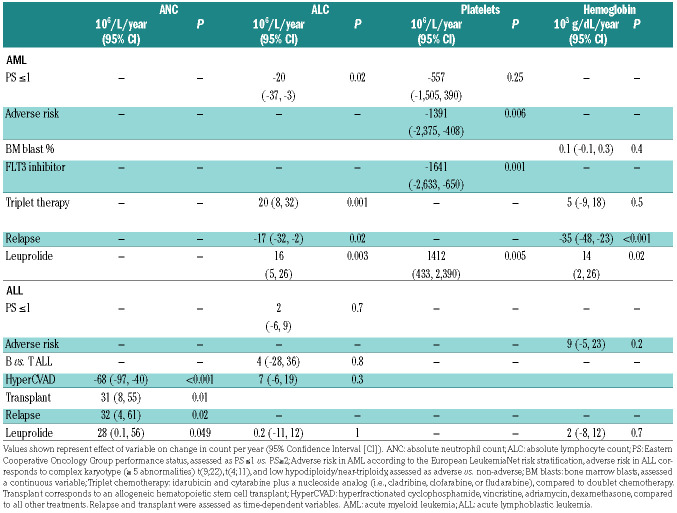
Table 2.
Multivariate analysis of factors predicting long-term count recovery.
Hematopoietic recovery
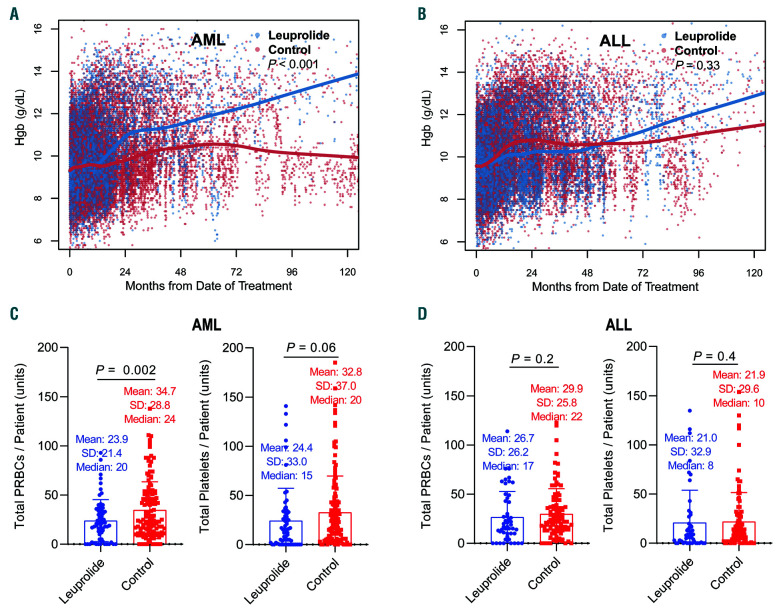
We evaluated whether leuprolide administration was associated with an improved blood count recovery following intensive chemotherapy in propensity matched cohorts. There was no difference in immediate count recovery following initial cycles of treatment when comparing those who received leuprolide to those who did not from the respective matched cohorts (Online Supplementary Figure S1). The median times to ANC and platelet count recovery were 25 days (95% Confidence Interval [CI]: 23-27) and 22 days (95% CI: 21-24) for AML matched patients who received leuprolide versus 25 days (95% CI: 24-28, P=0.47) and 23 days (95% CI: 22-24, P=0.2) for those who didn’t receive leuprolide respectively. Matched patients with ALL who received leuprolide had a median time to ANC and platelet count recovery of 19 days (95% CI: 17-21) and 20 days (95% CI: 18-22) compared to 17 days (95% CI: 16-18, P=0.32) and 19 days (95% CI: 18-20, P=0.25) respectively. Given that preclinical data indicated an impact of LH suppression on the earliest hematopoietic stem cell progenitors, we evaluated the effect of leuprolide on long-term count recovery. Patients with ALL from matched cohorts who received leuprolide had an additional increase of their ANC of 0.37x109/L/year compared to those who did not receive leuprolide (P=0.02) (Figure 2A-B). We also found that lymphocyte count recovery significantly differed between the two matched groups in the AML cohort with an additional increase in the ALC of 0.19x109/L/year in patients who received leuprolide compared to control (P=0.03) (Figure 2C-D). Similarly, for platelet count recovery, patients with AML from matched cohorts who received leuprolide had an additional increase of 13.8x109/L/year following chemotherapy compared with those in the control group (P=0.02) (Figure 2E-F). We found an association between long-term improvement of red blood cell parameters including hemoglobin and hematocrit levels in addition to red blood cell count and leuprolide in AML (P<0.001 and P=0.004, respectively) (Online Supplementary Figure S2). Patients with AML who received leuprolide had an additional increase in their hemoglobin level of 0.03 g/dL/year (P<0.001) (Figure 3A-B). These differences were most evident 2-4 years after the initial date of chemotherapy.
Transfusion requirements
Patients with AML treated with leuprolide received less packed red blood cells (pRBC) transfusions compared to the matched control group with an average of 23.9 units versus 34.7 units (P=0.002), given at any time following start of chemotherapy, which was concordant with the count recovery analysis for this group (Figure 3C). These patients also had less platelet transfusions with an average of 24.4 units compared to 32.8 units in the matched control group (P=0.06, respectively) (Figure 3C). This difference in transfusion requirements was less pronounced in the ALL matched cohorts where patients in the leuprolide group had on average 26.7 units of pRBC compared to 29.9 units in the matched control group (P=0.24), and an average of 21.0 units of pRBC compared to 21.9 units in the same respective groups (P=0.44) (Figure 3D).
Figure 3.
Long-term changes in hemoglobin levels and transfusion requirements with and without leuprolide. (A-B) Scatterplots of all corresponding peripheral blood hemoglobin (Hgb) levels extracted from health records, collected between induction chemotherapy (day 0) and last follow-up date where each dot represents a single value (blue for leuprolide, red for control). (A) Acute myeloid leukemia (AML) matched cohorts and (B) acute lymphoblastic leukemia (ALL) matched cohorts. Lowess smooth curves were used for indicating longitudinal trajectories of counts and differences were assessed using the generalized estimation equation model. (C-D) Transfusion requirements: differences in the mean number of blood and platelet units given between induction chemotherapy date (day 0) and last follow-up date in AML (C) and ALL (D) comparing matched leuprolide and control cohorts. Each value represents the total number of transfusion units given during this time period for each patient.
Multivariate analysis
Given that numerous factors such dose of leuprolide, age or relapse status for example could affect count recovery and the possible association with leuprolide, we performed a univariate analysis followed by a multivariate analysis for significant factors impacting long-term count recovery. We examined whether leuprolide dosing (higher cumulative leuprolide dose) or timing of administration of leuprolide (between day -7 and day 15 of induction chemotherapy compared to leuprolide given later) correlated with long-term count recovery and found no statistically significant associations in the univariate analysis (Online Supplementary Table S3). Patients with AML who received FMS-like tyrosine kinase 3 (FLT3) inhibitors in addition to their chemotherapy had lower platelet recovery (P<0.001). Relapse status, investigated as a time dependent variable, was associated with lower long-term lymphocyte count and hemoglobin levels in AML (P=0.04 and P=0.01 respectively) and higher neutrophil count recovery in ALL (P=0.001). The full results of the univariate analysis are included in Online Supplementary Table S3. We next sought to assess the differential impact of leuprolide on count recovery when all co-factors are considered. We found that leuprolide administration was independently associated with long-term hemoglobin levels, lymphocyte and platelet counts in AML (P=0.02, P=0.003, and P=0.005, respectively) and ANC levels in ALL (P=0.049) (Table 2). Additional independent co-factors identified in this analysis for AML included performance status, adverse risk according to the European LeukemiaNet classification, relapse status, and receipt of a FLT3 inhibitor or triplet chemotherapy. For ALL, independent factors included relapse or whether patients had an allogeneic stem cell transplant or were treated with HyperCVAD.
Impact on survival
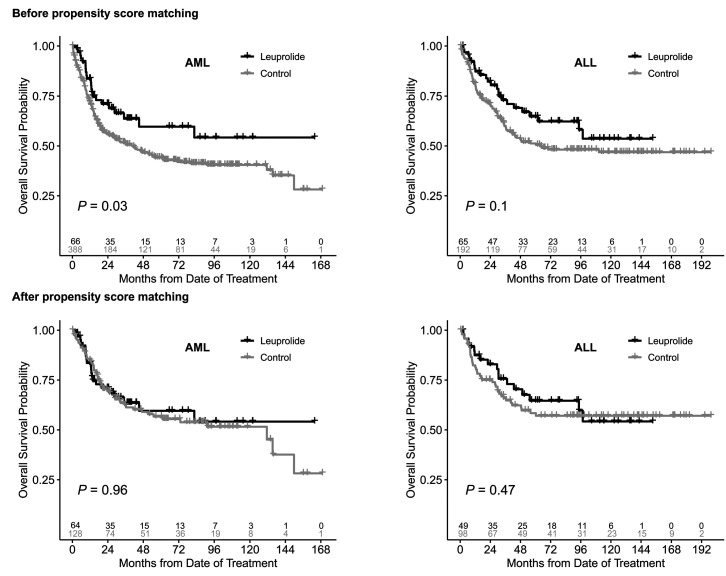
We found that patients in the leuprolide groups were significantly younger than the respective control groups (Table 1). This was also associated with an improved OS in the leuprolide groups compared to the control groups prior to propensity score matching (Figure 4A-B). However, after propensity score matching there was no association between leuprolide administration and survival outcomes. The 5-year OS for patients with AML who had received leuprolide was 60% (95% CI: 47-76) compared to 57% (95% CI: 48-67) in the matched control cohort (P=0.96) (Figure 4C). The 5-year OS for patients with ALL who had received leuprolide was 65% (95% CI: 52-81) compared to 58% (95% CI: 49-70) in the matched control cohort (P=0.47) (Figure 4D). Similarly, there was no difference in EFS comparing patients with AML with leuprolide and the matched control group (P=0.29) (Online Supplementary Figure S3) and no difference in ALL patients with leuprolide and their matched control group (P=0.85) (Online Supplementary Figure S3).
Figure 4.
Overall survival in acute myeloid leukemia and acute lymphoblastic leukemia patients with and without leuprolide before and after propensity score matching. AML: acute myeloid leukemia; ALL: acute lymphoblastic leukemia.
Discussion
In this study, we show that use of leuprolide in premenopausal women with acute leukemia receiving chemotherapy was associated with less transfusions and better long-term count recovery. Bone marrow suppression caused by cytotoxic chemotherapy is a common dose limiting adverse event in cancer treatment, especially in hematologic malignancies. It leads to increased morbidity and mortality because of the higher risk of infection and bleeding. Myelosuppression is caused by apoptosis of highly proliferative multipotent and hematopoietic progenitors. 22,23 Moreover, use of cytotoxic chemotherapy can lead to long-term bone marrow damage by various mechanisms including induction of apoptosis, senescence of HSC, or damage to bone marrow stromal cells.6,24 After chemotherapy insult, dormant HSC transiently proliferate to replenish blood cells and sustain hematopoietic homeostasis. 25 An unbalanced HSC proliferation and exit from dormancy could lead to long-term bone marrow suppression, and an increased risk of DNA damage.26,27 Therefore, there is a critical need to limit the damaging effects of cytotoxic chemotherapy on HSC and preserve the HSC pool.
There is growing evidence that several pituitary hormone receptors including LH, follicle-stimulating hormone, prolactin, and growth hormone receptors, are expressed by human HSC and are directly implicated in HSC self-renewal, proliferation and differentiation.7,8,28 Notably, patients with history of germ cell tumors have an increased risk of developing myeloid neoplasms.29-31While this had been attributed in some cases to therapy-related leukemogenesis, recent genomic analysis demonstrated that these neoplasms could be clonally related, thus indicating shared ancestry between the corresponding tissues of origin.32 Use of leuprolide was found to enhance T-cell recovery following allogeneic bone marrow transplantation in a mouse model through enhanced thymic reconstitution. 33 Velardi and colleagues demonstrated that LH blockade protects HSC from the damaging effects of chemotherapy or radiation in mice. LHRH antagonism led to quiescence in early hematopoietic progenitors. The HSC pool was maintained by preventing early progenitors from entering the cell cycle thus protecting them from chemotherapy or radiation damage.9 This mechanism is very similar to the fertility preserving effect of leuprolide.34 LHRH blockade in preclinical models halted recruitment of primordial quiescent follicles after treatment with chemotherapy thereby preserving the functional potential of the ovary. This has been validated in clinical studies and is now one of the strategies to preserve fertility in women receiving chemotherapy.35,36 It is plausible that this observed protective effect on HSC seen with leuprolide is mediated through a downstream effect on estrogen or other sex hormones.7,37 In a mouse model, estrogen increased hematopoietic stem-cell self-renewal in females and during pregnancy.37 However, levels of these hormones were not assessed in patients included in our analysis.
We report the first clinical evidence indicating a correlation between use of leuprolide and improved transfusion requirements in addition to improved long-term blood count recovery in women with acute leukemia receiving intensive chemotherapy. We observed an improvement in neutrophil, lymphocyte and platelet counts mostly when the corresponding lineage was not affected by leukemia. Some of the improvement in transfusion requirements could be related to a decrease in uterine bleeding through the hormonal suppression induced by LH blockade. However, we were unable to accurately quantify bleeding episodes from corresponding medical records. We also could not determine whether patients prone to bleeding preferentially received leuprolide which could affect the interpretation of ours results, however we tried to correct for selection bias through propensity score matching and the multivariate analysis. We found in our analysis that patients who received leuprolide and a FLT3 inhibitor added to their induction chemotherapy had a reduced platelet count recovery. Given that the FLT3 receptor is expressed by immature hematopoietic cells, and is restricted in normal bone marrow to early progenitors, targeting FLT3 could affect normal hematopoiesis leading to thrombocytopenia and delayed count recovery after chemotherapy.38 Thrombocytopenia is reported in 12-46% of patients receiving sorafenib, the FLT3 inhibitor most commonly used in this cohort. Sorafenib is a multikinase inhibitor that could affect numerous other pathways important for normal hematopoiesis and platelet generation from long-term HSC, therefore explaining this observation.39 There was no effect of LH blockade on rates of leukemia relapse or death in both AML and ALL cohorts, indicating that the theoretical risk of leuprolide protecting leukemia stem cells potentially expressing the LH receptor was not clinically meaningful in our study. Prospective studies are needed in order to confirm our findings and evaluate the effect of LH blockade on safety and count recovery following intensive chemotherapy in all hematologic malignancies. There are several ongoing clinical trials evaluating the effect of leuprolide on immune function following bone marrow transplantation (clinicaltrials gov. Identifier: 00275262, 01746849 and 01338987).
Repurposing old approved drugs in the field of cancer is gaining more importance given the high global burden of cancer and its substantial costs.40 LH antagonism, which allows for a reversible ablation of this hormonal axis, has a strong safety profile in other cancers such as breast and prostate cancer. Our findings potentially add another indication for use of leuprolide in women receiving high doses of chemotherapy by (i) minimizing uterine bleeding, (ii) decreasing the risk of ovarian failure and preserving fertility and (iii) protecting HSC from damage and improving long-term count recovery. Another hypothesis worth testing is whether LH blockade would reduce expansion of clonal hematopoiesis of indeterminate potential (CHIP) cells following chemotherapy or radiation, thus reducing the risk of therapy-related myeloid malignancies (assuming CHIP clones express the LHCGR).41
In summary, use of leuprolide in patients with newly diagnosed acute leukemia receiving intensive chemotherapy was associated with decreased transfusion requirements and improved long-term blood count recovery. Further studies are needed to validate these findings.
Supplementary Material
Funding Statement
Funding: The study was supported by Leukemia Texas (PI – Issa GC), the National Cancer Institute K12 Paul Calabresi Clinical Scholarship Award (NIH/NCI K12 CA088084 to Issa GC) and the Cancer Center Support Grant (NCI Grant P30 CA016672).
References
- 1.Orkin SH, Zon LI. Hematopoiesis: an evolving paradigm for stem cell biology. Cell. 2008;132(4):631-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tilly H, Castaigne S, Bordessoule D, et al. Low-dose cytarabine versus intensive chemotherapy in the treatment of acute nonlymphocytic leukemia in the elderly. J Clin Oncol. 1990;8(2):272-279. [DOI] [PubMed] [Google Scholar]
- 3.Menzin J, Lang K, Earle CC, et al. The outcomes and costs of acute myeloid leukemia among the elderly. Arch Intern Med. 2002;162(14):1597-1603. [DOI] [PubMed] [Google Scholar]
- 4.Kantarjian H, O'Brien S, Cortes J, et al. Results of intensive chemotherapy in 998 patients age 65 years or older with acute myeloid leukemia or high-risk myelodysplastic syndrome: predictive prognostic models for outcome. Cancer. 2006; 106(5):1090-1098. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Probin V, Zhou D. Cancer therapy- induced residual bone marrow injury- Mechanisms of induction and implication for therapy. Curr Cancer Ther Rev. 2006;2(3):271-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shao L, Wang Y, Chang J, et al. Hematopoietic stem cell senescence and cancer therapy-induced long-term bone marrow injury. Transl Cancer Res. 2013;2(5):397-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mierzejewska K, Borkowska S, Suszynska E, et al. Hematopoietic stem/progenitor cells express several functional sex hormone receptors-novel evidence for a potential developmental link between hematopoiesis and primordial germ cells. Stem Cells Dev. 2015;24(8):927-937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abdelbaset-Ismail A, Suszynska M, Borkowska S, et al. Human haematopoietic stem/progenitor cells express several functional sex hormone receptors. J Cell Mol Med. 2016;20(1):134-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Velardi E, Tsai JJ, Radtke S, et al. Suppression of luteinizing hormone enhances HSC recovery after hematopoietic injury. Nat Med. 2018;24(2):239-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khong DM, Dudakov JA, Hammett MV, et al. Enhanced hematopoietic stem cell function mediates immune regeneration following sex steroid blockade. Stem Cell Reports. 2015;4(3):445-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dudakov JA, Goldberg GL, Reiseger JJ, et al. Sex steroid ablation enhances hematopoietic recovery following cytotoxic antineoplastic therapy in aged mice. J Immunol. 2009;183(11):7084-7094. [DOI] [PubMed] [Google Scholar]
- 12.Quaas AM, Ginsburg ES. Prevention and treatment of uterine bleeding in hematologic malignancy. Eur J Obstet Gynecol Reprod Biol. 2007;134(1):3-8. [DOI] [PubMed] [Google Scholar]
- 13.Jadoul P, Kim SS, Committee IP. Fertility considerations in young women with hematological malignancies. J Assist Reprod Genet. 2012;29(6):479-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poorvu PD, Barton SE, Duncan CN, et al. Use and effectiveness of gonadotropinreleasing hormone agonists for prophylactic menstrual suppression in postmenarchal women who undergo hematopoietic cell transplantation. J Pediatr Adolesc Gynecol. 2016;29(3):265-268. [DOI] [PubMed] [Google Scholar]
- 15.Ho DE, Imai K, King G, Stuart EA. MatchIt: nonparametric preprocessing for parametric causal inference. J Stat Software. 2011; 42(8):1-28. [Google Scholar]
- 16.Cleveland WS. Robust locally weighted regression and smoothing scatterplots. J Am Stat Association. 1979;74(368):829-836. [Google Scholar]
- 17.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73(1):13-22. [Google Scholar]
- 18.Jabbour E, Short NJ, Ravandi F, et al. A randomized phase 2 study of idarubicin and cytarabine with clofarabine or fludarabine in patients with newly diagnosed acute myeloid leukemia. Cancer. 2017;123(22):4430-4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nazha A, Kantarjian H, Ravandi F, et al. Clofarabine, idarubicin, and cytarabine (CIA) as frontline therapy for patients </=60 years with newly diagnosed acute myeloid leukemia. Am J Hematol. 2013;88(11):961-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jain P, Kantarjian HM, Ravandi F, et al. Cladribine combined with idarubicin and Ara-C (CLIA) as frontline and salvage treatment for young patients (≤65 yrs) with acute myeloid leukemia. Blood. 2016; 128(22):1639-1639. [Google Scholar]
- 21.Rytting ME, Jabbour EJ, Jorgensen JL, et al. Final results of a single institution experience with a pediatric-based regimen, the augmented Berlin-Frankfurt-Munster, in adolescents and young adults with acute lymphoblastic leukemia, and comparison to the hyper-CVAD regimen. Am J Hematol. 2016;91(8):819-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shao L, Sun Y, Zhang Z, et al. Deletion of proapoptotic Puma selectively protects hematopoietic stem and progenitor cells against high-dose radiation. Blood. 2010; 115(23):4707-4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu H, Shen H, Yuan Y, et al. Deletion of Puma protects hematopoietic stem cells and confers long-term survival in response to high-dose gamma-irradiation. Blood. 2010;115(17):3472-3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mauch P, Constine L, Greenberger J, et al. Hematopoietic stem cell compartment: acute and late effects of radiation therapy and chemotherapy. Int J Radiat Oncol Biol Phys. 1995;31(5):1319-1339. [DOI] [PubMed] [Google Scholar]
- 25.Wilson A, Laurenti E, Oser G, et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell. 2008;135(6):1118-1129. [DOI] [PubMed] [Google Scholar]
- 26.Walter D, Lier A, Geiselhart A, et al. Exit from dormancy provokes DNA-damageinduced attrition in haematopoietic stem cells. Nature. 2015;520(7548):549-552. [DOI] [PubMed] [Google Scholar]
- 27.Fabiani E, Falconi G, Fianchi L, et al. Clonal evolution in therapy-related neoplasms. Oncotarget. 2017;8(7):12031-12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bujko K, Cymer M, Adamiak M, et al. An overview of novel unconventional mechanisms of hematopoietic development and regulators of hematopoiesis - a roadmap for future investigations. Stem Cell Rev Rep. 2019;15(6):785-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nichols CR, Roth BJ, Heerema N, et al. Hematologic neoplasia associated with primary mediastinal germ-cell tumors. N Engl J Med. 1990;322(20):1425-1429. [DOI] [PubMed] [Google Scholar]
- 30.Hartmann JrT, Fossa SD, Nichols CR, et al. Incidence of metachronous testicular cancer in patients with extragonadal germ cell tumors. J Natl Cancer Inst. 2001; 93(22):1733-1738. [DOI] [PubMed] [Google Scholar]
- 31.Nichols CR, Hoffman R, Einhorn LH, et al. Hematologic malignancies associated with primary mediastinal germ-cell tumors. Ann Intern Med. 1985;102(5):603-609. [DOI] [PubMed] [Google Scholar]
- 32.Taylor J, Donoghue MTA, Ho C, et al. Germ cell tumors and associated hematologic malignancies evolve from a common shared precursor. J Clin Invest. 2020;130(12):6668-6676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goldberg GL, King CG, Nejat RA, et al. Luteinizing hormone-releasing hormone enhances T cell recovery following allogeneic bone marrow transplantation. J Immunol. 2009;182(9):5846-5854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ataya KM, McKanna JA, Weintraub AM, et al. A luteinizing hormone-releasing hormone agonist for the prevention of chemotherapy-induced ovarian follicular loss in rats. Cancer Res. 1985;45(8):3651-3656. [PubMed] [Google Scholar]
- 35.Munhoz RR, Pereira AA, Sasse AD, et al. Gonadotropin-releasing hormone agonists for ovarian function preservation in premenopausal women undergoing chemotherapy for early-stage breast cancer: a systematic review and meta-analysis. JAMA Oncol. 2016;2(1):65-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oktay K, Harvey BE, Partridge AH, et al. Fertility preservation in patients with cancer: ASCO Clinical Practice Guideline update. J Clin Oncol. 2018;36(19):1994-2001. [DOI] [PubMed] [Google Scholar]
- 37.Nakada D, Oguro H, Levi BP, et al. Oestrogen increases haematopoietic stemcell self-renewal in females and during pregnancy. Nature. 2014;505(7484):555-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gilliland DG, Griffin JD. The roles of FLT3 in hematopoiesis and leukemia. Blood. 2002;100(5):1532-1542. [DOI] [PubMed] [Google Scholar]
- 39.Daver N, Schlenk RF, Russell NH, et al. Targeting FLT3 mutations in AML: review of current knowledge and evidence. Leukemia. 2019;33(2):299-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pushpakom S, Iorio F, Eyers PA, et al. Drug repurposing: progress, challenges and recommendations. Nat Rev Drug Discov. 2019;18(1):41-58. [DOI] [PubMed] [Google Scholar]
- 41.Takahashi K, Wang F, Kantarjian H, et al. Preleukaemic clonal haemopoiesis and risk of therapy-related myeloid neoplasms: a case-control study. Lancet Oncol. 2017; 18(1):100-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.