Abstract
目的
观察间充质干细胞(BMSCs)归巢调节哮喘Th1/Th“ 2漂移”与Notch1/Jagged1通路的关系。
方法
20只SD大鼠用随机数字表法均分为4组:正常对照组(NC)、模型对照组(MC)、BMSCs移植组(BMSCs)、BMSC+Notch抑制剂组。采用卵清蛋白致敏和激发建立哮喘模型,尾静脉注射BMSCs。HE染色观察肺组织病理形态学,酶联免疫吸附法检测肺组织白介素(IL)-5、IL-13、IFN-γ,RT-PCR法检测肺组织Notch1、Jagged1基因表达,免疫荧光染色检测支气管上皮细胞中CXCR4蛋白表达,免疫印迹法检测肺组织T-bet、GATA-3、Notch1、Jagged1蛋白表达。
结果
与NC组比较,MC组IFN-γ、T-bet蛋白表达降低,IL-5、IL-13、GATA-3蛋白表达升高,Notch1、Jagged1mRNA和蛋白表达升高(P<0.05或P<0.01)。与MC组比较,BMSCs组、BMSCs+Notch抑制剂组CXCR4、IFN-γ、T-bet蛋白表达量显著升高,BMSCs组Notch1、Jagged1蛋白表达降低,BMSCs+Notch抑制剂组IL-5、IL-13、Notch1、Jagged1mRNA和蛋白降低(P<0.05或P<0.01)。与BMSCs组比较,BMSCs+Notch抑制剂组CXCR4、IFN-γ表达升高,IL-13、Notch1mRNA表达降低(P<0.05)。
结论
BMSCs向哮喘肺组织归巢对Th1/Th2 “漂移”产生调节作用,Notch1/ Jagged1通路可能参与其过程。
Keywords: 哮喘, 骨髓间充质干细胞, 归巢, Th1/Th“2漂移”, Notch1/Jagged1信号通路
Abstract
Objective
To explore the association of the Notch1/Jagged1 pathway with the homing of mesenchymal stem cells (BMSCs) to regulate Th1/Th2 drift in asthma.
Methods
Twenty SD rats were randomly divided into normal control group, model group, BMSC transplantation group, and BMSC+Notch inhibitor group. Ovalbumin sensitization was used to establish rat models of asthma, and BMSCs were transplanted via the tail vein. The pathology of the lung tissue was examined with HE staining, and the contents of interleukin (IL)-5, IL-13, and interferon-γ (IFN-γ) in lung tissue homogenate were determined with enzyme-linked immunosorbent assay. The expressions of Notch1 and Jagged1 mRNA were detected with RT-PCR, and CXCR4 expression in the bronchial epithelial cells was examined using immunofluorescence staining; Western blotting was used to detect the protein expressions of T-bet, GATA-3, Notch1, and Jagged1 in the lung tissue.
Results
Compared with those in the control group, the expressions of IFN-γ and T-bet proteins decreased significantly and the pulmonary expressions of IL-5, IL-13, and GATA-3 proteins as well as Notch1 and Jagged1 mRNA and protein expressions all increased significantly in the model group (P < 0.05 or 0.01). Compared with those in the model group, CXCR4, IFN-γ, and T-bet protein expressions in BMSC group and BMSCs+Notch inhibitor group all increased significantly, and Notch1 and Jagged1 protein expressions in BMSCs group and IL-5, IL-13, Notch1, and Jagged1 mRNA and protein expressions in BMSCs + Notch inhibitor group all decreased significantly (P < 0.05 or 0.01). The expressions of CXCR4 and IFN-γ were significantly higher and the expressions of IL-13 and Notch1 mRNA were significantly lower in BMSCs+Notch inhibitor group than in BMSC group (P < 0.05).
Conclusion
In asthmatic rats, the homing of the BMSCs to the lung tissue has a regulatory effect on Th1/Th2 drift, and the Notch1/Jagged1 pathway may participate in the homing of the BMSCs.
Keywords: asthma, bone mesenchymal stem cells, homing, Th1/Th2 drift, Notch1/Jagged1 signaling pathway
支气管哮喘是由多种细胞及细胞组分参与的气道免疫炎症性疾病[1-2],其中辅助T细胞Th1/Th“ 2漂移”在哮喘炎症的发生发展中起重要作用[3-4]。Th1/Th2间维持动态平衡是机体应对外界刺激的基本免疫调节方式[5]。哮喘作为一种免疫疾病,在过敏原刺激下其发病以Th1/Th2间平衡向Th“ 2漂移”,形成炎症反应为主要特征[6]。积极有效地调控Th1/Th“ 2漂移”是治疗哮喘的重要手段。骨髓间充质干细胞(BMSCs)是骨髓中的成体干细胞[7]。既往研究发现[8-9],BMSCs驱动的免疫调节可重新调控Th1/Th2平衡,减少Th2细胞因子,抑制嗜酸性粒细胞在肺组织的聚集,降低哮喘炎症损伤。这一过程的前提是“归巢”:组织发生炎症损伤时,BMSCs向受损部位迁移。归巢能在靶向部位发挥免疫效应。BMSCs归巢对哮喘中的免疫失衡虽有调节作用,但具体作用机制尚不明确。Notch信号通路广泛参与细胞发育、分化及免疫调节等功能活动[10]。Notch受体(Notch1等)和配体(Jagged1等)结合后,其胞内段易位至细胞核,与DNA结合转录阻遏物结合并作用,将其转化为诱导靶基因转录的转录激活复合物,活化下游靶基因的表达[11]。Notch通路中的受体Notch1和配体Jagged1是哮喘Th1/Th2平衡向Th2 “漂移”的关键因素。研究表明[12-13],Jagged1蛋白参与了Th2细胞的发育,而Notch1通过促进Th2特异性转录因子GATA-3的表达,诱导Th2细胞分化。BMSCs归巢与Notch通路均与哮喘Th1/Th“ 2漂移”关系密切,其中可能存在更深入的关联。为进一步观察Notch通路是否参与哮喘BMSCs归巢过程,本文拟通过阻断Notch信号通路研究BMSCs归巢对哮喘Th2型炎症反应的调节及归巢与Notch1/Jagged1通路的关联性。旨在推测Notch信号通路可能参与了Th1/Th“ 2漂移”和BMSCs归巢免疫调节,从而改善哮喘炎症反应。
1. 材料和方法
1.1. 材料
1.1.1. 实验动物
清洁级雄性SD大鼠(20只,体质量180~200 g),用于实验分组。4周龄清洁级雄性SD大鼠(10只,体质量80~100 g)用于骨髓间充质干细胞及哮喘支气管上皮细胞提取。以上大鼠均由安徽医科大学实验动物管理中心提供。动物伦理由安徽中医药大学动物伦理委员会批准(批号:AHUCM-rats-2019005)。
1.1.2. 主要设备和试剂
显微镜(OLYMPUS);超声雾化器(上海鱼跃医疗设备股份有限公司);荧光定量PCR仪(Thermo)。流式细胞仪(贝克曼)。卵清蛋白(OVA):V级(Sigma);酶联免疫吸附法(ELISA)试剂盒(Invitrogen);角蛋白8(keratin 8)抗体(Abcam)。CD29-异硫氰基荧光素(FITC)、CD45-FITC、CD44-藻红蛋白(PE)、CD11b/c-PE单克隆抗体(eBioscience)。PCR试剂盒(赛默飞);趋化因子受体4抗体(Proteintech);Cy3标记山羊抗兔IgG(H+L)(Beyotime);T-bet、GATA-3、Notch1、Jagged1抗体(Santa Cruz)。
1.2. 方法
1.2.1. 分组
将20只大鼠用随机数字表法分为正常对照组(NC)、模型对照组(MC)、BMSCs移植组(BMSCs)、BMSC+Notch抑制剂组,5只/组。
1.2.2. 间充质干细胞的分离、培养、鉴定
4周龄大鼠断颈处死后,取股骨并剪去两端,吸取胎牛血清+双抗溶液多次冲出骨髓细胞后吹打,1000 r/min离心5 min,弃上清,用PBS重悬细胞,以2×106/cm2密度接种,置于37 ℃、5% CO2恒温培养箱中培养。每3~4 d更换1次培养基。原代细胞融合至80%~90%左右开始传代培养,调整细胞浓度至1×106/mL,倒置显微镜下观察细胞形态。取融合达90%的第4代BMSCs,分别加入荧光标记的抗体(Anti-CD29-FITC、Anti-CD44-PE、AntiCD45-FITC、Anti-CD11b/c-PE),混匀,4 ℃避光孵育30 min,PBS漂洗2遍以去除未结合的抗体,流式细胞仪鉴定其表面标志物。
1.2.3. CFSE标记BMSCs
将荧光染料羧基荧光素二醋酸盐琥珀酰亚胺(CFSE)以DMSO溶解,配成使用液,贮存于4 ℃条件下,避免反复冻融。移植前将5×106 BMSCs细胞悬浮于2 mL 0.1% BSA/PBS内,加入2 μL CFSE工作液,置37 ℃ 10 min;4倍体积4 ℃的胎牛血清终止反应,室温5 min;PBS室温洗涤3次,第2次结束后37 ℃ 5 min;将1×106BMSCs细胞悬浮于1 mL PBS内,备用于移植。
1.2.4. 哮喘模型建立[14]及细胞移植
除正常对照组外的各组大鼠,在第0和7天腹腔注射致敏混合液(含1 mg 10%卵清蛋白和100 mg氢氧化铝凝胶),第14天将大鼠置于密闭容器内,予1%卵清蛋白雾化激发,30 min/次,1次/d,连续7 d。正常组予生理盐水替代完成上述操作。BMSCs组及BMSCs+Notch抑制剂组在激发最后一天经尾静脉将CFSE标记的1×106/mL BMSCs悬液植入体内,其余2组以PBS替代注射。BMSCs+Notch抑制剂组在激发前1 h按1 mg/kg体质量腹腔注射抑制剂,隔日1次,连续7 d。
1.2.5. 哮喘支气管上皮细胞的分离及鉴定
大鼠麻醉后在无菌环境下取出气管,清除肺组织及表面其他软组织后,灌入1%链酶蛋白酶,结扎上端,4 ℃过夜,置于90 mm培养皿中,剪开气管, 予培养基冲洗,细胞刷充分刷洗,收集培养皿中的刷洗液,离心洗涤收集细胞。1000 r/min离心5 min,弃上清,用原代细胞培养液吹打细胞后置于无菌培养瓶,置于37 ℃、5% CO2恒温培养箱中培养,备用于实验。各组分离培养获得的支气管上皮细胞与角蛋白单克隆抗体作用后,经细胞核荧光染料4', 6-二脒基-2-苯基吲哚(DAPI)染色,磷酸缓冲盐溶液(PBS)漂洗,置于荧光显微镜下观察。
1.3. 观察指标
1.3.1. 间充质干细胞归巢至肺组织观察
在BMSCs移植后24、72、144 h取大鼠肺组织,将组织剪成1~2 mm3小块,用消化酶消化,不锈钢过滤网过滤消化液,调整各组全肺单个细胞悬液浓度,采用流式细胞术和荧光显微镜分析CFSE-BMSCs(绿色)荧光强度。
1.3.2. 支气管上皮细胞CXCR4表达检测
采用免疫荧光检测支气管上皮细胞CXCR4表达。将分离培养获得的支气管上皮细胞传代,待长至80%~90%经PBS洗涤、胰酶消化,充分吹打成单细胞悬液,接种于已置玻片的6孔板。爬片以4%多聚甲醛避光固定,滴加5% BSA均匀覆盖。经CXCR4抗体(1∶100),Cy3标记山羊抗兔IgG(H+L)(1∶500)作用后,予以DAPI染色,封片,置于荧光显微镜下观察并采集图像。(DAPI紫外激发波长330~380 nm,发射波长420 nm,发蓝光;CY3激发波长510~560 nm,发射波长590 nm,发红光)。
1.3.3. 肺组织病理形态学观察
取大鼠右下肺组织,石蜡包埋,HE染色,光学显微镜下观察肺组织病理形态学变化。
1.3.4. 哮喘大鼠肺组织Th1/Th2细胞表达
检测各组大鼠肺组织IFN-γ、IL-5、IL-13的蛋白水平,操作方法按照美国Invitrogen的ELISA试剂盒操作步骤,用酶标仪测定并计算各组蛋白含量。
1.3.5. 肺组织Notch1/Jagged1通路相关基因表达
提取大鼠肺组织总mRNA,Notch1、Jagged1引物设计由上海生工合成(Notch1、Jagged1引物序列见表 1)。反转录试剂盒反转录第一链互补DNA(cDNA),PCR扩增,扩增产物进行1.5%琼脂糖电泳,UVP凝胶显像仪扫描,图像分析。以β-actin作为内源参照,使用2-ΔΔCT方法计算Notch1、Jagged1 mRNA的相对表达量。
1.
Notch1、Jagged1引物序列
Primer sequences of Notch1 and Jagged1
| Gene | Sequence | Length (bp) |
| Notch1 | 148 | |
| Sense primer | 5'-AGGCTCTGCCGACATCA-3' | |
| Antisense primer | 5'-AGGAAGGGGTGCTCTGG-3' | |
| Jagged1 | 140 | |
| Sense primer | 5'-CAGGGATTGCCCACTTT-3' | |
| Antisense primer | 5'-GCAGGTTTTGTTGCCATT-3' | |
| β-actin | 120 | |
| Sense primer | 5'-CCTCACTGTCCACCTTCCA-3' | |
| Antisense primer | 5'-GGGTGTAAAACGCAGCTCA-3' |
1.3.6. 肺组织T-bet、GATA-3、Notch1、Jagged1蛋白表达
将肺组织在冷的组织裂解缓冲液中均匀化、溶解,提取肺组织总蛋白。将样品放入标准蛋白样品缓冲液中煮沸,用10%三甘氨酸凝胶进行蛋白电泳,然后转移至聚偏二氟乙烯膜膜。将膜依次加入T- bet、GATA- 3、Notch1、Jagged1一抗(稀释浓度分别为1∶1000、1∶1000,1∶800、1∶1000)、二抗(山羊抗兔IgG,上海碧云天生物技术有限公司,A0516)孵育,稀释浓度为1∶5000,按照标准规程对膜进行显影。使用发光试剂盒检测试剂对这些条带进行曝光、显影。Image J量化各个条带的强度,并以相对于内参β-actin的百分比表示T-bet、GATA-3、Notch1、Jagged1蛋白表达量。
1.4. 统计学分析
采用SPSS19.0统计软件对数据进行统计分析,结果用均数±标准差表示,各组间比较采用单因素方差分析,两组间比较采用独立样本t检验,P<0.05表示差异有统计学意义。
2. 结果
2.1. 骨髓间充质干细胞、支气管上皮细胞鉴定骨髓间充质干细胞观察及鉴定
初次接种2 h后开始贴壁,细胞呈类圆形,密集分布,排列无序。原代培养7 d后,细胞开始增殖,形态呈锤形或梭形。第3代未分化间充质干细胞,形态均一呈成纤维细胞样,漩涡状分布,排列整齐有序。流式细胞仪分析发现,BMSCs表面标志物CD29、CD44呈阳性,表达量分别为97.4%、96.5%。CD45、CD11b呈阴性,表达量分别为1.62%、1.08%(图 1)。支气管上皮细胞鉴定:经DAPI染色后细胞核呈蓝色荧光,支气管上皮细胞特异性目的蛋白keratin 8的抗体表达于胞浆,呈红色荧光,鉴定为支气管上皮细胞(图 2)。
1.
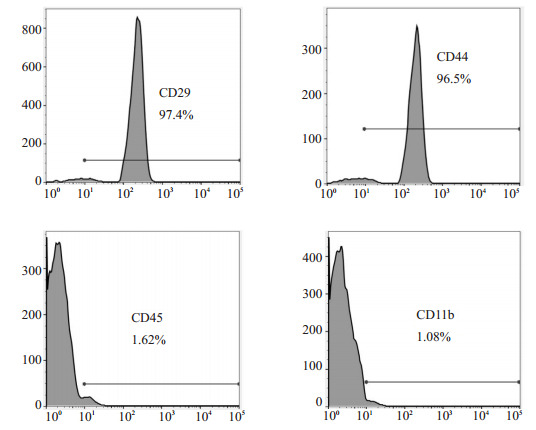
BMSCs表面标志物CD29、CD44、CD45、CD11b表达
Expression of BMSC surface markers CD29, CD44, CD45 and CD11b.
2.
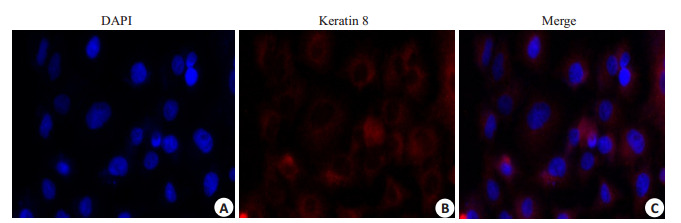
支气管上皮细胞鉴定
Identification of bronchial epithelial cells (Immunofluorescence staining, original magnification: ×200). A: The nuclei of bronchial epithelial cells were blue after DAPI staining; B: The cytoplasm showed red fluorescence; C: Merged image.
2.2. 骨髓间充质干细胞归巢至肺组织观察
通过将CFSE标记BMSCs移植至哮喘大鼠体内,追踪CFSE表达量来反映BMSCs在肺组织中的归巢程度。流式细胞术观察肺组织CFSE表达量发现,BMSCs体内移植后,24 h CFSE表达量为(4.89±1.65)%,72 h CFSE表达量为(14.78±4.65)%,144 h CFSE表达量为(3.07±1.06)%,CFSE在肺组织表达量随着时间推移逐渐升高,在72 h达到高峰,后逐渐降低,证明移植后BMSCs可向肺组织归巢,表明同源异体BMSCs经尾静脉注射入哮喘大鼠体内后可向肺组织迁移(图 3)。
3.
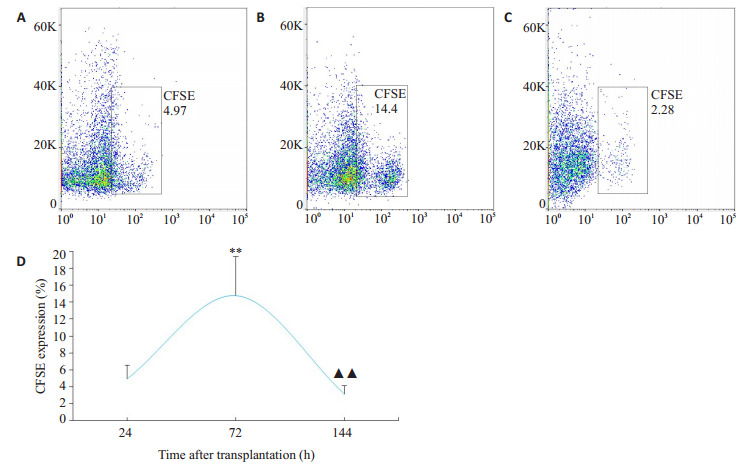
CFSE标记的BMSCs在哮喘大鼠肺组织中表达
Expression of CFSE-labeled BMSCs in lung tissues of asthmatic rats. A: Homing amount of CFSE+ cells at 24 h. B: Homing amount of CFSE +cells at 72 h. C: Homing amount of CFSE +cells at 144 h. D: Comparison of CFSE expression across the time points. **P < 0.01 vs 24 h; ▲▲P < 0.01 vs 72 h.
2.3. 支气管上皮细胞中CXCR4表达
NC组与MC组之间比较,MC组CXCR4表达量较NC组升高(t=2.8271、P=0.0222)。BMSCs移植及Notch抑制剂干预后发现,与NC组、MC组比较,BMSCs组、BMSCs+Notch抑制剂组CXCR4表达量均显著升高(t=2.6021、P=0.0315,t=5.8649、P=0.0004)。与BMSCs组比较,BMSCs+Notch抑制剂组CXCR4表达量升高(t=3.254、P=0.0116,图 4)。
4.
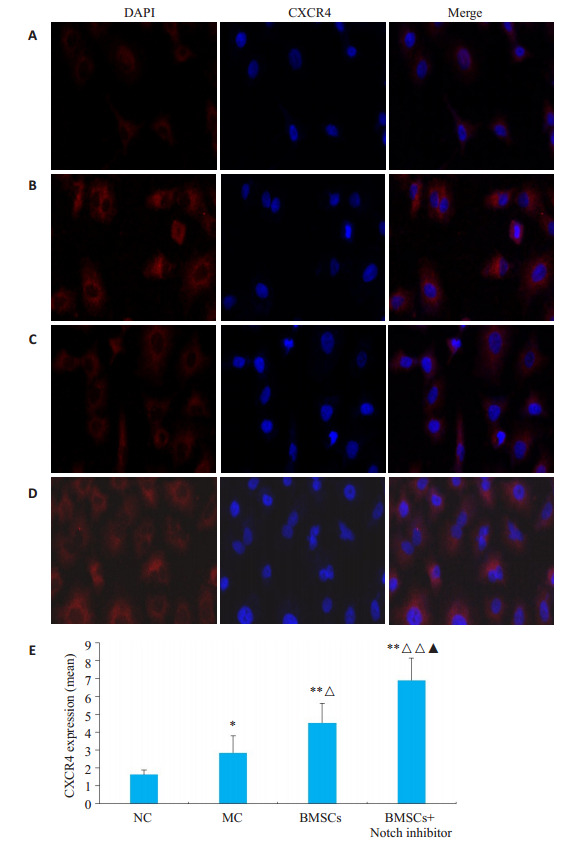
支气管上皮细胞中CXCR4表达
CXCR4 expression in bronchial epithelial cells (immunofluorescence staining, ×200). A: NC group; B: MC group; C: BMSCs group; D: BMSCs + Notch inhibitor group; E: Comparison of relative expression of CXCR4 among the groups. *P < 0.05, **P < 0.01 vs NC group; ΔP < 0.05, ΔΔP < 0.01 vs MC group; ▲P < 0.05 vs BMSCs.
2.4. 肺组织病理形态学观察
NC组大鼠肺组织结构完整,支气管管腔无狭窄,管腔壁无增厚紊乱,周围无明显炎症细胞浸润。MC组大鼠肺组织结构受损明显,支气管管腔狭窄,平滑肌增生明显,周围大量炎症细胞浸润,以嗜酸性粒细胞为主,成团聚集。BMSCs组及BMSCs+Notch抑制剂组肺组织受损、管腔狭窄及炎症细胞浸润情况均较模型组有不同程度减轻(图 5)。
5.
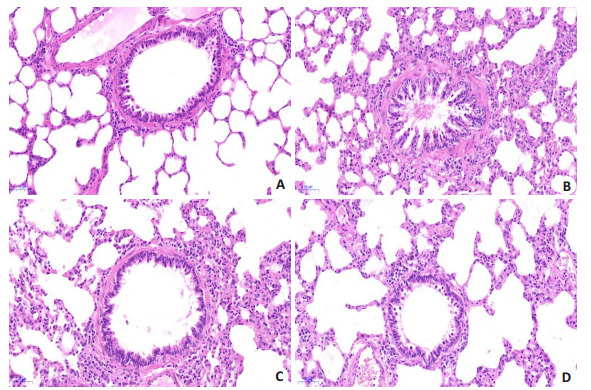
各组大鼠肺组织病理形态学观察
Observation of pathologies of rat lung tissue in each group (HE staining, ×200). A: NC group; B: MC group; C: BMSCs group; D: BMSCs+Notch inhibitor group.
2.5. 肺组织Th1、Th2细胞因子表达
ELISA结果显示,与NC组比较,MC组IFN-γ表达降低(t=5.2001、P=0.0008),IL-5、IL-13表达升高(t=4.2173、P=0.0029,t=3.7989、P=0.0052)。
与MC组比较,BMSCs组、BMSCs+Notch抑制剂组IFN- γ表达升高(t=2.653、P=0.0291,t=5.4014、P= 0.0006);BMSCs+Notch抑制剂组IL-5、IL-13降低(t= 2.5386、P=0.0348,t=3.7295、P=0.0058)。BMSCs组IL-5(t=1.8189、P=0.1064)、IL-13(t=1.1298、P=0.2913)表达降低,但差异无统计学意义(P>0. 05)。
与BMSCs组比较,BMSCs+Notch抑制剂组IFN-γ表达升高(t=2.7269、P=0.0260),IL-13降低(t=2.9032、P=0.0198,表 2)。
2.
肺组织Th1/Th2细胞表达
IFN-γ, IL-5 and IL-3 expressions in the lung tissue (Mean±SD, pg/mL, n=5)
| Group | IFN-γ | IL-5 | IL-13 |
| *P<0.05, **P<0.01 vs NC; ΔP<0.05, ΔΔP<0.01 vs MC; ▲P<0.05 vs BMSCs. | |||
| NC | 1397.348±175.864 | 48.374±9.615 | 24.342±5.965 |
| MC | 924.137±102.359** | 79.305±13.286** | 42.252±8.692** |
| BMSCs | 1108.365±116.759*Δ | 65.432±10.694* | 36.627±6.957* |
| BMSCs+Notch inhibitor | 1319.296±127.608ΔΔ▲ | 60.189±10.344Δ | 25.267±5.306ΔΔ▲ |
2.6. 肺组织Th1、Th2细胞特异性转录因子蛋白表达
免疫印迹结果显示,与NC组比较,MC组肺组织Th1细胞特异性转录因子T- bet蛋白表达降低(t= 5.3118、P=0.0007),Th2细胞特异性转录因子GATA-3蛋白表达升高(t=5.9062、P=0.0004)。经BMSCs移植及Notch1通路阻滞剂后,与MC组比较,BMSCs组、BMSCs+N抑制剂组T-bet蛋白表达升高(t=4.2132、P= 0.0029,t=5.9316、P=0.0003),BMSCs组、BMSCs+N抑制剂组GATA-3蛋白表达降低,但无统计学意义(P>0.05,表4,图 6)。
6.
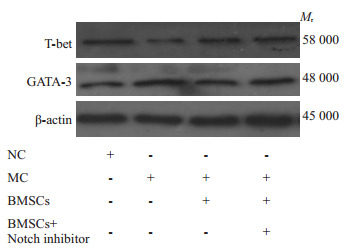
肺组织Th1、Th2细胞特异性转录因子蛋白表达
Th1 and Th2 cell specific transcription factor protein expression in lung tissue.
3.
肺组织Th1、Th2细胞特异性转录因子蛋白表达
Expression of Th1 and Th2 cell-specific transcription factor proteins in the lung (Mean±SD, n=5)
| Group | T-bet | GATA-3 |
| *P<0.05, **P<0.01 vs NC; ΔP<0.05, ΔΔP<0.01 vs MC. | ||
| NC | 1.057±0.316 | 0.342±0.095 |
| MC | 0.302±0.034** | 1.138±0.286** |
| BMSCs | 0.714±0.216**ΔΔ | 0.876±0.147** |
| BMSCs+Notch inhibitor | 0.924±0.232**ΔΔ | 0.897±0.156** |
2.7. 肺组织Notch1/Jagged1表达
RT- PCR检测结果显示,与NC组比较,MC组Notch1、Jagged1 mRNA和蛋白表达升高(P<0.01)。与MC组比较,BMSCs组Notch1、Jagged1蛋白表达降低,BMSCs+Notch抑制剂组Notch1、Jagged1mRNA和蛋白表达降低(P<0.05或P<0.01)。与BMSCs组比较,BMSCs+ Notch抑制剂组Notch1mRNA表达降低(P<0.05,表 5,图 7)。
5.
肺组织Notch1/Jagged1表达
Expression of Notch1/Jagged1 in lung tissue (Mean±SD, n=5)
| Group | mRNA | Protein | |||
| Notch1 | Jagged1 | Notch1 | Jagged1 | ||
| *P<0.05, **P<0.01 vs NC; ΔP<0.05, ΔΔP<0.01 vs MC; ▲P<0.05 vs BMSCs. | |||||
| NC | 1.042±0.125 | 1.078±0.236 | 0.597±0.157 | 0.586±0.142 | |
| MC | 2.374±0.787** | 2.355±0.964** | 1.036±0.305** | 2.427±0.597** | |
| BMSCs | 1.996±0.414* | 1.732±0.343* | 0.645±0.249Δ | 1.146±0.376**ΔΔ | |
| BMSCs+Notch inhibitor | 1.142±0.475*Δ▲ | 1.684±0.354*Δ | 0.648±0.186Δ | 1.542±0.413**Δ | |
7.
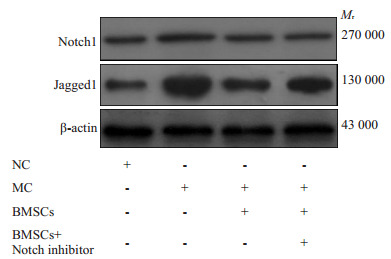
肺组织Notch1、Jagged1蛋白表达
Expression of Notch1 and Jagged1 protein in lung tissue.
3. 讨论
哮喘作为免疫相关的疾病,是以Th1/Th2失衡诱发的Th2型免疫炎症占主导[15]。骨髓反应机制参与了哮喘炎症的发生发展。在过敏原刺激下,Eos/B祖细胞自骨髓释放量增多,经外周循环周转至肺,在Th2细胞因子IL-4、IL-5等调控下分化成熟,出现以嗜酸性粒细胞气道浸润为主的哮喘病理变化[16-17]。哮喘气道炎症反应是多种炎症细胞及细胞组分相互作用的结果[18]。过敏原刺激下,细胞免疫调节功能下降,导致Th1/Th2平衡向Th“ 2漂移”,Th1细胞因子(IFN-γ等)分化受抑制,Th2炎症因子(IL-5、IL-13等)大量产生,通过促进肥大细胞募集,IgE合成,嗜酸性粒细胞浸润等方式,促进哮喘炎症反应的发生发展[19-20]。本研究结果显示,与NC组比较,MC组IFN-γ表达降低,IL-5、IL-13表达升高。而Th1、Th2转录因子亦随之变化,同时大量Th2型炎性因子分化并对相关炎症介质的调控作用,致使慢性气道炎症发生及加剧。此研究结果与Abdolreza等[21]的研究结果相似。
源于骨髓的间充质干细胞具有多向分化能力,增殖性高且具有良好的免疫调节性和免疫相容性。BMSCs通过调节免疫炎症可有效改善哮喘[22]。哮喘发作时,BMSCs通过调节树突状细胞和T细胞功能,抑制相关炎症细胞的分泌,发挥免疫调节剂的作用[23]。本次研究发现,将间充质干细胞注射入哮喘大鼠体内后,支气管上皮细胞中CXCR4表达随之升高,且肺组织病理形态学改善。说明BMSCs可有效缓解哮喘炎症损伤。而改善的前提是“归巢”,“归巢”使得骨髓中休眠的BMSCs被炎症损伤部位分泌的趋化因子“唤醒”,迁移至受伤的肺组织,免疫调节炎症环境和修复受损肺组织,从而改善哮喘症状。Th1/Th“ 2漂移”即Th2型反应模式是哮喘气道炎症的重要特征,也是哮喘炎症形成的始动和维持因素。BMSCs具有免疫相容性和免疫调节的特征[24]。免疫相容性使得BMSCs不易被机体的免疫细胞识别,有效逃脱免疫系统的攻击排斥,为异体应用提供可能[25]。本研究发现,经BMSCs干预后,BMSCs组、BMSCs + Notch抑制剂组IFN-γ表达升高,IL-5、IL-13表达降低。提示在机体出现炎症反应时,受损部位释放趋化因子与BMSCs表面的趋化因子受体(CXCR4)结合,沿着趋化轴,BMSCs归巢至炎症损伤部位,调节免疫内环境,发挥抑制炎症和组织修复作用,从而改善哮喘大鼠肺组织病理形态学变化。研究表明[26],在哮喘的炎症反应中,BMSCs可通过分泌和调节生长因子、细胞因子、抗纤维化因子等实现旁分泌效应,抑制T细胞的过度活化,改变Th1/Th2平衡,缓解哮喘气道炎症。
Notch信号通路广泛参与多种组织细胞的信号识别、增殖、分化和凋亡等功能活动[27]。在哺乳动物中,Notch通路由Notch配体和Notch受体组成,它们都是I型跨膜蛋白[28]。受体与配体结合,在受体Jagged1触发后由γ-分泌酶介导的蛋白水解机制释放Notch胞内域(NICD),通过与DNA结合蛋白(DNA结合转录阻遏物)关联介导了Notch信号的传导[29]。这种蛋白质-蛋白质间的相互作用可激活下游的基因[30]。Notch信号通路参与了对BMSCs的调控。有研究发现[31],Notch信号通路可抑制间充质干细胞向成骨细胞转化。用Jagged1处理BMSCs后,BMSCs中下游的基因mRNA水平显着增加,BMSCs中的白蛋白表达被阻断。研究还发现[32],通过γ-促分泌酶抑制剂阻断Notch信号传导或敲除BMSCs中Notch信号的转录因子RBP- J可导致CXCR4表达上调,并促进BMSCs在体外和小鼠肝脏缺血/ 再灌注模型体内肝脏组织中对SDF-1的反应,说明通过阻断Notch信号传导可上调BMSCs中的CXCR4表达以达到增强BMSCs归巢能力的目的。本次研究结果显示,与NC组比较,MC组Notch1mRNA、Jagged1mRNA表达升高,Notch1、Jagged1蛋白表达升高。说明哮喘发生时存在Notch信号通路受体和配体表达的变化。而通过BMSCs植入归巢后发现,BMSCs组、BMSCs+ Notch抑制剂组Notch1mRNA、Jagged1mRNA表达降低,且BMSCs+Notch抑制剂组Jagged1蛋白降低更明显,说明抑制Notch通路过激活可促进BMSCs归巢,从而影响哮喘的进展。
综上所述,哮喘存在Th1/Th2平衡向Th2 “漂移”,且BMSCs向哮喘肺组织归巢对Th1/Th“ 2漂移”产生调节作用,而Notch1/Jagged1通路可能参与其过程,通过抑制Notch1/Jagged1通路过激活可改善哮喘炎症的发作。
Biography
王坤,医学博士,E-mail: yxwangk@163.com
Funding Statement
国家自然科学基金(81373600),安徽省教育厅高校自然科学研究项目(KJ2020A0426)
Supported by National Natural Science Foundation of China (81373600)
Contributor Information
王 坤 (Kun WANG), Email: yxwangk@163.com.
朱 慧志 (Huizhi ZHU), Email: huizhizhu87@163.com.
References
- 1.Peebles RS. Prostaglandins in asthma and allergic diseases. Pharmacol Ther. 2019;193:1–19. doi: 10.1016/j.pharmthera.2018.08.001. [Peebles RS. Prostaglandins in asthma and allergic diseases[J]. Pharmacol Ther, 2019, 193: 1-19.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalmarzi RN, Rajabinejad M, Lotfi R. Immune semaphorins: Crucial regulatory signals and novel therapeutic targets in asthma and allergic diseases. Eur J Pharmacol. 2020;881:173209. doi: 10.1016/j.ejphar.2020.173209. [Kalmarzi RN, Rajabinejad M, Lotfi R. Immune semaphorins: Crucial regulatory signals and novel therapeutic targets in asthma and allergic diseases[J]. Eur J Pharmacol, 2020, 881: 173209.] [DOI] [PubMed] [Google Scholar]
- 3.Anatriello E, Cunha M, Nogueira J, et al. Oral feeding of Lactobacillus bulgaricus N45.10 inhibits the lung inflammation and airway remodeling in murine allergic asthma: Relevance to the Th1/ Th2 cytokines and STAT6/T-bet. Cell Immunol. 2019;341:103928. doi: 10.1016/j.cellimm.2019.103928. [Anatriello E, Cunha M, Nogueira J, et al. Oral feeding of Lactobacillus bulgaricus N45.10 inhibits the lung inflammation and airway remodeling in murine allergic asthma: Relevance to the Th1/ Th2 cytokines and STAT6/T-bet[J]. Cell Immunol, 2019, 341: 103928.] [DOI] [PubMed] [Google Scholar]
- 4.Gandhi GR, Neta MTSL, Sathiyabama RG, et al. Flavonoids as Th1/Th2 cytokines immunomodulators: a systematic review of studies on animal models. Phytomedicine. 2018;44:74–84. doi: 10.1016/j.phymed.2018.03.057. [Gandhi GR, Neta MTSL, Sathiyabama RG, et al. Flavonoids as Th1/Th2 cytokines immunomodulators: a systematic review of studies on animal models[J]. Phytomedicine, 2018, 44: 74-84.] [DOI] [PubMed] [Google Scholar]
- 5.Yu N, Weng Y, Liu W, et al. TLRs induce Th1/Th2 responses by affecting the secretion of CCL2 at the maternal-foetal interface. Int Immunopharmacol. 2021;100:108070. doi: 10.1016/j.intimp.2021.108070. [Yu N, Weng Y, Liu W, et al. TLRs induce Th1/Th2 responses by affecting the secretion of CCL2 at the maternal-foetal interface[J]. Int Immunopharmacol, 2021, 100: 108070.] [DOI] [PubMed] [Google Scholar]
- 6.Abdelaziz MH, Ji X, Wan J, et al. Mycobacterium-induced Th1, helminths-induced Th2 cells and the potential vaccine candidates for allergic asthma: imitation of natural infection. Front Immunol. 2021;12:696734. doi: 10.3389/fimmu.2021.696734. [Abdelaziz MH, Ji X, Wan J, et al. Mycobacterium-induced Th1, helminths-induced Th2 cells and the potential vaccine candidates for allergic asthma: imitation of natural infection[J]. Front Immunol, 2021, 12: 696734.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao Y, Huang Y, Jia L, et al. A novel tension machine promotes bone marrow mesenchymal stem cell osteoblastic and fibroblastic differentiation by applying cyclic tension. https://pubmed.ncbi.nlm.nih.gov/34422062/ Stem Cells Int. 2021;2021:6647651. doi: 10.1155/2021/6647651. [Zhao Y, Huang Y, Jia L, et al. A novel tension machine promotes bone marrow mesenchymal stem cell osteoblastic and fibroblastic differentiation by applying cyclic tension[J]. Stem Cells Int, 2021, 2021: 6647651.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang LB, He M. Effect of mesenchymal stromal (stem) cell (MSC) transplantation in asthmatic animal models: a systematic review and meta-analysis. Pulm Pharmacol Ther. 2019;54:39–52. doi: 10.1016/j.pupt.2018.11.007. [Zhang LB, He M. Effect of mesenchymal stromal (stem) cell (MSC) transplantation in asthmatic animal models: a systematic review and meta-analysis[J]. Pulm Pharmacol Ther, 2019, 54: 39-52.] [DOI] [PubMed] [Google Scholar]
- 9.Du YM, Zhuansun YX, Chen R, et al. Mesenchymal stem cell exosomes promote immunosuppression of regulatory T cells in asthma. Exp Cell Res. 2018;363(1):114–20. doi: 10.1016/j.yexcr.2017.12.021. [Du YM, Zhuansun YX, Chen R, et al. Mesenchymal stem cell exosomes promote immunosuppression of regulatory T cells in asthma[J]. Exp Cell Res, 2018, 363(1): 114-20.] [DOI] [PubMed] [Google Scholar]
- 10.Zheng ZK, Zhang B, Yu HX, et al. UBE3A activates the NOTCH pathway and promotes esophageal cancer progression by degradation of ZNF185. Int J Biol Sci. 2021;17(12):3024–35. doi: 10.7150/ijbs.61117. [Zheng ZK, Zhang B, Yu HX, et al. UBE3A activates the NOTCH pathway and promotes esophageal cancer progression by degradation of ZNF185[J]. Int J Biol Sci, 2021, 17(12): 3024-35.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsuno Y, Kiwamoto T, Morishima Y, et al. Notch Signaling Regulates Cell Density-dependent Apoptosis of NIH 3T3 through an IL-6/STAT3 Dependent Mechanism. Eur J Cell Biol. 2018;97(7):512–22. doi: 10.1016/j.ejcb.2018.09.001. [Matsuno Y, Kiwamoto T, Morishima Y, et al. Notch Signaling Regulates Cell Density-dependent Apoptosis of NIH 3T3 through an IL-6/STAT3 Dependent Mechanism[J]. Eur J Cell Biol, 2018, 97 (7): 512-22.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dua B, Upadhyay R, Natrajan M, et al. Notch signaling induces lymphoproliferation, T helper cell activation and Th1/Th2 differentiation in leprosy. Immunol Lett. 2019;207:6–16. doi: 10.1016/j.imlet.2019.01.003. [Dua B, Upadhyay R, Natrajan M, et al. Notch signaling induces lymphoproliferation, T helper cell activation and Th1/Th2 differentiation in leprosy[J]. Immunol Lett, 2019, 207: 6-16.] [DOI] [PubMed] [Google Scholar]
- 13.Xu S, Kong YG, Jiao WE, et al. Tangeretin promotes regulatory T cell differentiation by inhibiting Notch1/Jagged1 signaling in allergic rhinitis. Int Immunopharmacol. 2019;72:402–12. doi: 10.1016/j.intimp.2019.04.039. [Xu S, Kong YG, Jiao WE, et al. Tangeretin promotes regulatory T cell differentiation by inhibiting Notch1/Jagged1 signaling in allergic rhinitis[J]. Int Immunopharmacol, 2019, 72: 402-12.] [DOI] [PubMed] [Google Scholar]
- 14.Yan Y, Liu L, Dou Z, et al. Soufeng Yuchuan decoction mitigates the ovalbumin-induced lung damage in a rat model of asthma. Biomed Pharmacother. 2020;125:109933. doi: 10.1016/j.biopha.2020.109933. [Yan Y, Liu L, Dou Z, et al. Soufeng Yuchuan decoction mitigates the ovalbumin-induced lung damage in a rat model of asthma[J]. Biomed Pharmacother, 2020, 125: 109933.] [DOI] [PubMed] [Google Scholar]
- 15.Shrestha Palikhe N, Wu Y, Konrad E, et al. Th2 cell markers in peripheral blood increase during an acute asthma exacerbation. Allergy. 2021;76(1):281–90. doi: 10.1111/all.14543. [Shrestha Palikhe N, Wu Y, Konrad E, et al. Th2 cell markers in peripheral blood increase during an acute asthma exacerbation[J]. Allergy, 2021, 76(1): 281-90.] [DOI] [PubMed] [Google Scholar]
- 16.Nelson RK, Bush A, Stokes J, et al. Eosinophilic Asthma. J Allergy Clin Immunol Pract. 2020;8(2):465–473. doi: 10.1016/j.jaip.2019.11.024. [Nelson RK, Bush A, Stokes J, et al. Eosinophilic Asthma[J]. J Allergy Clin Immunol Pract, 2020, 8(2): 465-473.] [DOI] [PubMed] [Google Scholar]
- 17.Humbert M, Albers FC, Bratton DJ, et al. Effect of mepolizumab in severeeosinophilic asthma according to omalizumab eligibility. Respir Med. 2019;154:69–75. doi: 10.1016/j.rmed.2019.06.004. [Humbert M, Albers FC, Bratton DJ, et al. Effect of mepolizumab in severeeosinophilic asthma according to omalizumab eligibility[J]. Respir Med, 2019, 154: 69-75.] [DOI] [PubMed] [Google Scholar]
- 18.Finotto S. Resolution of allergic asthma. Semin Immunopathol. 2019;41(6):665–74. doi: 10.1007/s00281-019-00770-3. [Finotto S. Resolution of allergic asthma[J]. Semin Immunopathol, 2019, 41(6): 665-74.] [DOI] [PubMed] [Google Scholar]
- 19.欧阳 若芸, 胡 成平, 陈 平, et al. 哮喘中Th1/Th2类细胞因子免疫失衡对神经生长因子表达的影响. 中南大学学报: 医学版. 2007;9(1):119–23. doi: 10.3321/j.issn:1672-7347.2007.01.021. [欧阳若芸, 胡成平, 陈平, 等. 哮喘中Th1/Th2类细胞因子免疫失衡对神经生长因子表达的影响[J]. 中南大学学报: 医学版, 2007, 9(1): 119-23.] [DOI] [Google Scholar]
- 20.Kardan M, Rafiei A, Ghaffari J, et al. Effect of ginger extract on expression of GATA3, T-bet and ROR-γt in peripheral blood mononuclear cells of patients with Allergic Asthma. Allergol Immunopathol: Madr. 2019;47(4):378–85. doi: 10.1016/j.aller.2018.12.003. [Kardan M, Rafiei A, Ghaffari J, et al. Effect of ginger extract on expression of GATA3, T-bet and ROR-γt in peripheral blood mononuclear cells of patients with Allergic Asthma[J]. Allergol Immunopathol: Madr, 2019, 47(4): 378-85.] [DOI] [PubMed] [Google Scholar]
- 21.Esmaeilzadeh A, Tahmasebi S, Athari SS. Chimeric antigen receptor -T cell therapy: Applications and challenges in treatment of allergy and asthma. Biomed Pharmacother. 2020;123:109685. doi: 10.1016/j.biopha.2019.109685. [Esmaeilzadeh A, Tahmasebi S, Athari SS. Chimeric antigen receptor -T cell therapy: Applications and challenges in treatment of allergy and asthma[J]. Biomed Pharmacother, 2020, 123: 109685.] [DOI] [PubMed] [Google Scholar]
- 22.Özdemir RBÖ, Özdemir AT, Sarıboyacı AE, et al. The investigation of immunomodulatory effects of adipose tissue mesenchymal stem cell educated macrophages on the CD4 T cells. Immunobiology. 2019;224(4):585–94. doi: 10.1016/j.imbio.2019.04.002. [Özdemir RBÖ, Özdemir AT, Sarıboyacı AE, et al. The investigation of immunomodulatory effects of adipose tissue mesenchymal stem cell educated macrophages on the CD4 T cells[J]. Immunobiology, 2019, 224(4): 585-94.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Court AC, Le-Gatt A, Luz-Crawford P, et al. Mitochondrial transfer from MSCs to T cells induces Treg differentiation and restricts inflammatory response. http://onlinelibrary.wiley.com/doi/pdf/10.15252/embr.201948052. EMBO Rep. 2020;21(2):e48052. doi: 10.15252/embr.201948052. [Court AC, Le-Gatt A, Luz-Crawford P, et al. Mitochondrial transfer from MSCs to T cells induces Treg differentiation and restricts inflammatory response[J]. EMBO Rep, 2020, 21(2): e48052.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown C, McKee C, Bakshi S, et al. Mesenchymal stem cells: Cell therapy and regeneration potential. J Tissue Eng Regen Med. 2019;13(9):1738–55. doi: 10.1002/term.2914. [Brown C, McKee C, Bakshi S, et al. Mesenchymal stem cells: Cell therapy and regeneration potential[J]. J Tissue Eng Regen Med, 2019, 13(9): 1738-55.] [DOI] [PubMed] [Google Scholar]
- 25.Rendra E, Scaccia E, Bieback K. Recent advances in understanding mesenchymal stromal cells. https://pubmed.ncbi.nlm.nih.gov/32148780/#:~:text=Recent%20advances%20in%20understanding%20mesenchymal%20stromal%20cells%20Mesenchymal,cultivation%2C%20and%20the%20high%20%3Ci%3Eex%20vivo%3C%2Fi%3E%20expansion%20potential. F1000Res. 2020;9:F1000 Faculty Rev-156. doi: 10.12688/f1000research.21862.1. [Rendra E, Scaccia E, Bieback K. Recent advances in understanding mesenchymal stromal cells[J]. F1000Res, 2020, 9: F1000 Faculty Rev-156.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ullah M, Liu DD, Thakor AS. Mesenchymal Stromal Cell Homing: Mechanisms and Strategies for Improvement. http://www.sciencedirect.com/science/article/pii/S2589004219301415. iScience. 2019;1531:421–38. doi: 10.1016/j.isci.2019.05.004. [Ullah M, Liu DD, Thakor AS. Mesenchymal Stromal Cell Homing: Mechanisms and Strategies for Improvement[J]. iScience, 2019, 1531: 421-38.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Monticone G, Miele L. Notch pathway: a journey from notching phenotypes to cancer immunotherapy. http://link.springer.com/chapter/10.1007/978-3-030-55031-8_13. Adv Exp Med Biol. 2021;1287:201–22. doi: 10.1007/978-3-030-55031-8_13. [Monticone G, Miele L. Notch pathway: a journey from notching phenotypes to cancer immunotherapy[J]. Adv Exp Med Biol, 2021, 1287: 201-22.] [DOI] [PubMed] [Google Scholar]
- 28.Zohorsky K, Lin S, Mequanint K. Immobilization of Jagged1 Enhances Vascular Smooth Muscle Cells Maturation by Activating the Notch Pathway. Cells. 2021;10(8):2089. doi: 10.3390/cells10082089. [Zohorsky K, Lin S, Mequanint K. Immobilization of Jagged1 Enhances Vascular Smooth Muscle Cells Maturation by Activating the Notch Pathway[J]. Cells, 2021, 10(8): 2089.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Viswanathan R, Hartmann J, Pallares Cartes C, et al. Desensitisation of Notch signalling through dynamic adaptation in the nucleus. https://www.embopress.org/doi/full/10.15252/embj.2020107245. EMBO J. 2021;40(18):e107245. doi: 10.15252/embj.2020107245. [Viswanathan R, Hartmann J, Pallares Cartes C, et al. Desensitisation of Notch signalling through dynamic adaptation in the nucleus[J]. EMBO J, 2021, 40(18): e107245.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.王 知广, 姜 京植, 王 重阳, et al. Pyrin重组蛋白通过TGF-β1/SMAD及jagged1/notch1信号通路减轻慢性支气管哮喘小鼠气道炎症及气道重塑. https://www.cnki.com.cn/Article/CJFDTOTAL-XBFM202003006.htm. 细胞与分子免疫学杂志. 2020;36(3):220–7. [王知广, 姜京植, 王重阳, 等. Pyrin重组蛋白通过TGF-β1/SMAD及jagged1/notch1信号通路减轻慢性支气管哮喘小鼠气道炎症及气道重塑[J]. 细胞与分子免疫学杂志, 2020, 36(3): 220-7.] [Google Scholar]
- 31.Zhang H, Lin J, Chen J, et al. Effects of Notch/p38MAPK signaling pathway on articular cartilage defect recovery by BMSCs tissue based on the rabbit articular cartilage defect models. Saudi J Biol Sci. 2020;27(3):859–64. doi: 10.1016/j.sjbs.2019.12.021. [Zhang H, Lin J, Chen J, et al. Effects of Notch/p38MAPK signaling pathway on articular cartilage defect recovery by BMSCs tissue based on the rabbit articular cartilage defect models[J]. Saudi J Biol Sci, 2020, 27(3): 859-64.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xie J, Wang W, Si JW, et al. Notch signaling regulates CXCR4 expression and the migration of mesenchymal stem cells. Cell Immunol. 2013;281(1):68–75. doi: 10.1016/j.cellimm.2013.02.001. [Xie J, Wang W, Si JW, et al. Notch signaling regulates CXCR4 expression and the migration of mesenchymal stem cells[J]. Cell Immunol, 2013, 281(1): 68-75.] [DOI] [PubMed] [Google Scholar]


