Abstract
A middle-aged woman was diagnosed with postural orthostatic tachycardia syndrome based on her clinical symptoms, elevated norepinephrine levels and positive tilt-table test. The patient was refractory to conventional treatment and improved only after she was treated with methylated B vitamins for her heterozygous catechol-O-methyltransferase Val158Met polymorphism.
Keywords: cardiovascular medicine, cardiovascular system
Background
This is the first case report to link the catechol-O-methyltransferase (COMT) Val158 single-nucleotide polymorphism (SNP) to hyperadrenergic postural orthostatic tachycardia syndrome (POTS). COMT is an enzyme that primarily catalyses the methylation of catechol substrates in the process of catecholamine metabolism. COMT has been found to have a role in acting on other substrates such as catechol oestrogen and polyphenols.1 Individuals who are homozygous for the COMT Val158Met SNP have a threefold to fourfold reduction in enzyme activity.2 Given that the alleles are codominant, heterozygous individuals are not as severely impacted.3
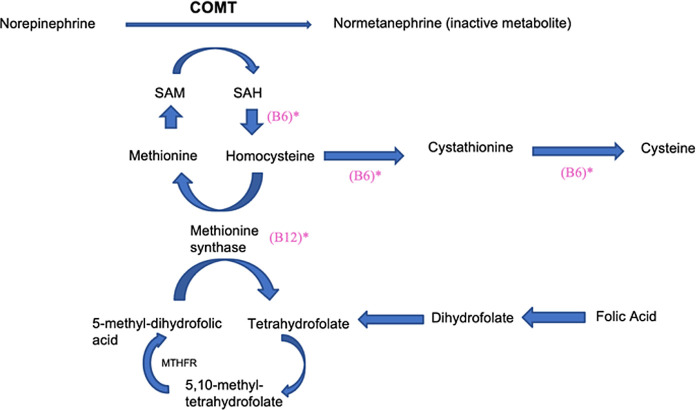
Thus far, the implications of the COMT SNP have been known predominantly in the context of neuropsychiatric disorders and neurobiology of cognition. However, In the most recent study on COMT published in 2017, the COMT gene was discussed for the first time as a potential contributor in cardiovascular diseases though its exact role was not formally established.1 In this report, our objective is to further elaborate on the role COMT may have in cardiovascular diseases, specifically hyperadrenergic POTS. Particularly, we hypothesise that a mutation in the gene, specifically the COMT Val158 polymorphism which leads to reduced COMT enzyme activity and impaired metabolism of norepinephrine (NE), can result in higher levels of NE in the blood contributing to the pathophysiology of hyperadrenergic POTS (figure 1).
Figure 1.
The role of the catechol-O-methyltransferase (COMT) enzyme in norepinephrine inactivation. Based on this pathway, we suggest that impaired function of the COMT enzyme can lead to increased levels of norepinephrine. This pathway is further dependent on adequate levels of cofactors including vitamins B6, B9 and B12. *Low levels of product. SAM, S-adenosyl-L-methionine; SAH, S-adenosyl-L-homocysteine.
Abnormal overactivation of the sympathetic nervous system has been implicated as a key mechanism in patients with hyperadrenergic POTS.4 Most cases of this subtype of disease have been found to be due to a loss-of-function mutation that leads to a norepinephrine transporter (NET) deficiency. Silencing NET causes a defect in the reuptake of NE leading to NE levels in the blood resulting in symptoms such as tachycardia on standing, palpitations and anxiety. Patients also often experience lightheadedness, dyspnoea, headaches, fatigue and near syncope.1 Due to the heterogeneity of symptoms, the diagnosis of POTS is usually delayed and patients are often dismissed as having psychiatric illnesses such as anxiety and depression.
Case presentation
A woman in her fourth decade was followed over the course of 2 years in primary care and specialty clinics for multiple medical issues. She initially presented with a constellation of symptoms including tachycardia, brain fog and fatigue 5 years prior to receiving a formal diagnosis POTS in 2018. On presentation to cardiology in 2018, her physical examination was notable for a heart rate (HR) of 71 beats per minute (bpm) and blood pressure (BP) of 116/77 mm Hg, which was taken 3 min after laying supine. She was then asked to stand for 3 min, and at the end of the 3 min her BP was 108/80 mm Hg with a heart rate of 94 bpm.
Medical history included Crohn’s disease in remission, hypermobile Ehlers-Danlos syndrome, mast cell activation and insomnia. Due to the patient’s complex history of autoimmune disease and her genetic predisposition to conditions overlapping with symptoms of POTS, the diagnosis of POTS was initially discounted and her symptoms were mistakenly attributed to her underlying conditions.
Investigations
The first time POTS was considered in our patient was in 2015 when the patient was 38 years old and presented with symptoms of persistent fatigue, brain fog and palpitations. A tilt-table test was done, which showed a 21-point increase in her HR (baseline: HR 70 bpm with BP 133/63 mm Hg, end of tilt: HR 91 bpm with BP 108/64 mm Hg) within 10 min of head-up tilt (HUT). Since a diagnosis of POTS requires a heart rate increase of 30 bpm or more within the first 10 min of HUT,5 POTS was initially excluded in our patient based on her negative tilt-table test.
It was not until 2018 that the diagnosis of POTS was reconsidered. Focused laboratory testing for hyperadrenergic POTS was conducted, and the patient had testing of her NE levels for the first time. Her values were found to be elevated with a laying NE level of 901 pg/mL and standing level of 1676 pg/mL (reference range 80–520 pg/mL). These findings were in line with the diagnosis of hyperadrenergic subtype of POTS, which requires NE levels to be greater than 600 pg/mL on standing.5 Her diagnosis was then confirmed by a repeat tilt-table test (baseline: HR was 66 bpm with a BP 113/76 mm Hg, end of tilt: HR 105 bpm with a BP of 120/83 mm Hg), which this time, was positive.
Additional testing to rule out other diagnoses was conducted. We found normal urine metanephrine levels and performed an MRI of the abdomen and pelvis, which was negative for any intra-abdominal or pelvic abnormality as well as for pheochromocytoma. Her echocardiogram was normal and her event monitor did not reveal any arrhythmias.
Laboratory testing included checking her complete blood count, complete metabolic panel, thyroid functional panel and HIV testing. At the time of evaluation, her B12 level was normal at 638 pg/mL (range 232–1245 pg/mL) in the setting of B12 injections administered by her naturopathic physician. Her urinary Methylmalonic acid (MMA) levels measured normal at 0.8 mmol/mol creatine (ref range ≤1.9 mmol/mol creatine) while she was receiving injections. Of note, she was previously diagnosed with vitamin B12 deficiency in 2014 and reported some improvement in her fatigue after receiving injections, but MMA levels were not reported at that time.
Differential diagnosis
The aetiology for this patient’s palpitations, fatigue and brain fog was initially unclear. Broad differential diagnosis for her non-specific symptoms included chronic fatigue syndrome, adrenal malignancy and POTS.
We also considered underlying arrhythmias, intra-abdominal or pelvic abnormalities, and pheochromocytoma as potential causes for her symptoms.
Treatment
Shortly after diagnosis, the patient was started on ivabradine 5 mg two times per day for POTS. Two months after starting ivabradine, she did not notice any significant improvement in her symptoms and her NE levels continued to be elevated with a laying level of 901 pg/mL and a standing level of 1676 pg/mL. The patient subsequently discontinued ivabradine due to lack of symptomatic improvement.
Over the course of the 2 years, the patient was prescribed several medications including atenolol, ivabradine (intermittently) and guanfacine for POTS with minimal improvement.
In 2020, the patient’s integrative medicine team obtained a nutrigenomic and micronutrient panel to assess for impaired methylation and possible nutritional insufficiencies given her persistent anxiety and fatigue. Of note, the patient was considered to be in symptomatic and biochemical remission from Crohn’s disease at the time of evaluation. Salivary testing revealed that the patient is heterozygous for the COMT SNP which is associated with moderate reduction in COMT enzyme activity. Her Genova NutrEval revealed a high need for vitamins B3, B6, B9, as well as moderate need for vitamin B1 and B12. The patient was subsequently started on methylated B vitamin complex (vitamin B2 1.6 mg, vitamin B6 25 mg, vitamin B9 as calcium L-5-methyltetrahydrofolate 1330 µg, vitamin B12 as methylcobalamin 1000 µg). Less than 1 month after starting this supplement, she noted significant improvement in fatigue, insomnia, ‘epinephrine surges’ and flushing.
Outcome and follow-up
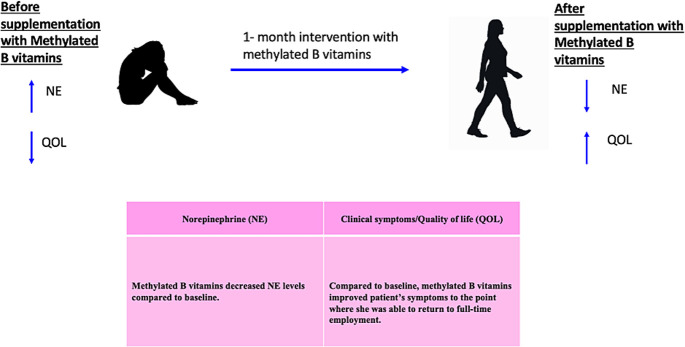
The patient was re-evaluated 3 and 7 months after supplementation with B vitamins and reported sustained near resolution of her POTSs and improved overall quality of life (figure 2). Her laboratory values at 3 months supported her clinical improvement (2221) revealing a supine NE level of 384 pg/mL and a standing level of 824 pg/mL compared with 901 pg/mL and 1676 pg/mL pre treatment (reference range 80–520 pg/mL)(table 1). Additionally, B6 level was reassessed due to high-dose supplementation and found to be 123.8 nm/L compared with 26 nm/L prior to supplementation. We rechecked her B12 level (1207 pg/mL compared with 677 pg/mL (table 1) due to her personal history of B12 deficiency. During this time, the patient sustained employment for the first time in over 5 years and was able to tolerate high-intensity aerobic exercise. Symptomatic improvement was largely sustained 6 months after discontinuation of supplementation, thus B vitamins and NE level were not re-evaluated at that time. She did, however, endorse minimal subjective fatigue prompting a complete blood count, which revealed an increase in her mean corpuscular volume and mean corpuscular haemoglobin. To further investigate this finding, B9 and B12 levels were obtained, which were within the normal range (10.2 ng/mL and 673 pg/mL, respectively).
Figure 2.
In our patient, who is a heterozygote for COMTVal158Met polymorphism, she was found to have low levels of vitamin B6, B9 and B12. Post supplementation of these methylated vitamins, her NE levels and quality of life improved.
Table 1.
Analysis of methylated B vitamins on patient lab values in POTS
| Before methylated B vitamins | After methylated B vitamins (7 months post supplementation) | |
| Norepinephrine (80–520 pg/mL) | Supine: 901 pg/mL Standing: 1676 pg/mL |
Supine: 384 pg/mL Standing: 824 pg/mL |
| Vitamin B6 (20–125 nm/L) | 26 nm/L | 124 nm/mL |
| Vitamin B12 (211–946 pg/mL) | 677 pg/mL | 1207 pg/mL |
Discussion
Hyperadrenergic POTS is a debilitating multifactorial clinical disorder with complex pathophysiology that significantly impairs quality of life. This case report suggests that the COMT Val158MET genetic polymorphism could be a potential therapeutic target with methylated B vitamins for patients with hyperadrenergic POTS.
Though the patient did not meet the criteria for POTS based on her initial orthostatic vitals, her symptoms (palpitations, tremor, tachycardia, ‘epinephrine surges’ and anxiety) were suggestive of a hyperadrenergic state. Further workup revealed a positive tilt-table test and elevated NE levels which confirmed her diagnosis of hyperarenergic POTS. This report shows that patients with POTS can have dynamic fluctuations in their symptoms daily, making it critical to frequently reassess these patients while keeping their overall clinical picture in mind.
Despite years of pharmacologic and non-pharmacological interventions for the patient’s symptoms of fatigue, brain fog, palpitations and tachycardia, it was not until the patient was started on a methylated B vitamin complex that she experienced almost complete resolution of her symptoms. In this patient, repletion of these methylated vitamins likely circumvented the impaired methyltransferase reaction and allowed for adequate substrates for downstream pathways. This linkage is further supported by the fact that the patient saw rapid resolution of symptoms after she was given these specific supplements. She also had corresponding decrease in her NE levels after taking methylated B vitamin complex.
Based on this patient’s symptom progression and improvement with specific supplements, we propose that the symptoms of hyperadrenergic POTS were related to her underlying COMT Val158MET genetic polymorphism and further compounded by B vitamin insufficiencies. The underlying pathophysiology is centred around the polymorphism leading to impaired function of the COMT enzyme, which plays an essential role in NE degradation.6 IThis is why elevated levels of NE characterize hyperadrenergic POTSd. This case illustrates the potential role of methylated B vitamins in supporting the downstream COMT pathways, resulting in clinical improvement. Of note, correcting B12 deficiencies has previously shown benefit in patients with dysautonomia due to a similar pathophysiological mechanism.7 B1 repletion has also displayed benefit; 6% of patients with POTS were found to be deficient and 25% of them noted significant improvement in symptoms post oral B1 supplementation.8
To date, this is the first case report to investigate the potential link between the COMT SNP and hyperadrenergic POTS.
Patient’s perspective.
I would just like to share from a patient’s perspective that the journey to finding my way back to ‘health’ with all my overwhelmingly complicated conditions was a very long and arduous journey. Trying to verbally explain my many strange and multisystemic bodily reactions was like speaking another language and just praying that one of the dozens of specialists I was passed around to would finally be the one to provide me with a proper diagnosis so that I could start some type of regimen back to health. I needed to find myself again but felt so loss as a former athlete and successful businesswomen, who now found herself unable to work and unable to get out of bed.
Some of the most disruptive and unbearable symptoms that I had been experiencing for long term was the complete and utter exhaustion but at the same time being physically unable to sleep, at times for 3 days at a time, all due to an overwhelmingly high epinephrine such as surge. A restless surge that felt like it was constantly rushing through my blood. I felt constantly wired, and jumpy, and easily startled, unable to sit still or sleep, heart racing and at times palpitating, and the brain fog, unable to find my words were all frustrating and embarrassing. Did anyone believe me? Could anyone help? I must admit at times I thought about giving up on this pursuit. However, something changed when I started this regimen. After being on disability for over 5 years, I am successfully working full time again as a senior network manager in the healthcare arena. I have a new lease on life, and I know there are others out there like me, I know, I communicate with them all the time on self-help websites. If I can be helped, I know others can as well. I not only was helped, but I also feel I got my life back- which is the greatest gift a doctor can provide their patient. Thank you.
Learning points.
The catechol-O-methyltransferase enzyme is an essential enzyme that regulates levels of dopamine and norepinephrine (NE). Its methyltransferase activity is tightly linked to B vitamin levels and its polymorphisms compromises the functioning of the enzyme and its methyltransferase activity.
The hallmark of hyperadrenergic POTS is elevated levels of NE (greater than 600 pg/mL on standing), leading to overactivation of the sympathetic nervous system resulting in symptoms of tachycardia, brain fog and fatigue.
Patients with symptoms suggestive of POTS need to be continually re-evaluated with multiple parameters as their symptoms are dynamic and a relying on a single parameter, such as the tilt-table test or orthostatic vitals, could lead to a delay or missed diagnosis.
Footnotes
Contributors: NM, AP and PT contributed to the design and implementation of the research, to the analysis of the results and to the writing of the case report.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: PT: Amgen (consultant) Novo-Nordisk (consultant) Sanofi (consultant) Boehringer-Ingelheim (consultant) Epirium Bio (shareholder) Esperion therapeutics (consultant).
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s)
References
- 1.Bastos P, Gomes T, Ribeiro L. Catechol-O-Methyltransferase (COMT): an update on its role in cancer, neurological and cardiovascular diseases. Rev Physiol Biochem Pharmacol 2017;173:1–39. 10.1007/112_2017_2 [DOI] [PubMed] [Google Scholar]
- 2.Lachman HM, Papolos DF, Saito T, et al. Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics 1996;6:243–50. 10.1097/00008571-199606000-00007 [DOI] [PubMed] [Google Scholar]
- 3.Weinshilboum RM, Otterness DM, Szumlanski CL. Methylation pharmacogenetics: catechol O-methyltransferase, thiopurine methyltransferase, and histamine N-methyltransferase. Annu Rev Pharmacol Toxicol 1999;39:19–52. 10.1146/annurev.pharmtox.39.1.19 [DOI] [PubMed] [Google Scholar]
- 4.Sheldon RS, Grubb BP, Olshansky B, et al. 2015 heart rhythm Society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm 2015;12:e41–63. 10.1016/j.hrthm.2015.03.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taub PR, Zadourian A, Lo HC, et al. Randomized trial of ivabradine in patients with Hyperadrenergic postural orthostatic tachycardia syndrome. J Am Coll Cardiol 2021;77:861–71. 10.1016/j.jacc.2020.12.029 [DOI] [PubMed] [Google Scholar]
- 6.Mitchell ES, Conus N, Kaput J. B vitamin polymorphisms and behavior: evidence of associations with neurodevelopment, depression, schizophrenia, bipolar disorder and cognitive decline. Neurosci Biobehav Rev 2014;47:307–20. 10.1016/j.neubiorev.2014.08.006 [DOI] [PubMed] [Google Scholar]
- 7.Öner T, Guven B, Tavli V, et al. Postural orthostatic tachycardia syndrome (POTS) and vitamin B12 deficiency in adolescents. Pediatrics 2014;133:e138–42. 10.1542/peds.2012-3427 [DOI] [PubMed] [Google Scholar]
- 8.Blitshteyn S. Vitamin B1 deficiency in patients with postural tachycardia syndrome (POTS). Neurol Res 2017;39:685–8. 10.1080/01616412.2017.1331895 [DOI] [PubMed] [Google Scholar]