Abstract
In the tyrosine kinase inhibitor era, the blast phase of chronic myeloid leukaemia (BP-CML) renders an uncommon presentation and has a poor prognosis with an estimated overall survival below 20%. Mixed-phenotype blast phase is even more infrequent, presenting in 3.3% of these patients. Blast phase manifests along haematological sarcomas, with extramedullary activity in lymph nodes, skin and bone. We report the case of a patient with an ovarian sarcoma as an extramedullary presentation of mixed-phenotype BP-CML refractory to conventional treatment which responded to immunotherapy against CD33 and CD19.
Keywords: cancer - see oncology, chronic myeloid leukaemia, haematology (drugs and medicines), haematology (incl blood transfusion)
Background
When treated with tyrosine kinase inhibitors (TKIs), chronic myeloid leukaemia (CML) usually has a good prognosis and an overall survival akin to that of the general population. CML may manifest as chronic, accelerated or blast phase. Blast phase is defined by greater than 20% blasts in peripheral blood or bone marrow, or the presence of an extramedullary proliferation.1 Even with TKI treatment, 1%–1.5% of patients will develop BP-CML, worsening the prognosis.2 The overall survival of BP-CML drops from 72.5% to 2% at 48 months compared with chronic phase patients treated with a third-generation TKI after failing the first-generation or second-generation therapy.3 Seventy percent of the blast crises are myeloid. However, sequential lymphoblastic and myeloblastic crises are common. Blasts with primitive or heterogeneous expression, or antigens of ambiguous lineage have been reported in these patients. Mixed-phenotype BP-CML represents approximately 3% of all blast crisis.1 Extramedullary crises are reported in 4%–15% of CML, with lymph nodes, skin and central nervous system being the most frequently affected sites. Nevertheless, mixed-phenotype blast crises have been described in fewer than 20 cases in the last 25 years.1 4 The treatment for blast crisis is similar to that of acute leukaemia, with intensive chemotherapy regimens. In relapsed and/or refractory acute leukaemia of lymphoid and myeloid lineages, immunotherapy with blinatumomab and gemtuzumab-ozogamicin is used accordingly.5–7
Case presentation
A 28-year-old woman without relevant medical history presented at our clinic in November 2015 with leucocytosis (245.300 × 109/L; 7% blasts in peripheral blood). She was diagnosed with CML. The lactate dehydrogenase level was 2060 U/L. Bone marrow was reported as hypercellular without blast infiltration. Her karyotype was complex with 46 XX,+ins(15;3)+iso(17). BCR/ABL was positive in 129%.
The patient started imatinib 400 mg/day with BCR/ABL maximum response of 40% after 6 months of treatment. Therapy was switched to dasatinib with no response after 6 months. Nilotinib 400 mg/day was then started. In October 2017, she developed neutropenia and thrombocytopenia, with 25% blasts in bone marrow. Immunophenotype demonstrated two blast populations. The first one was positive for CD19, CD10, CD34, and TdT and negative for CD20, MPO and CD3; the second population was positive for CD13, CD33, CD34, CD117 and MPO. BCR/ABL was 91.540%. Complex karyotype persisted: 46 XX, add(3)(q12), ins(15;?)(q11.2;?)[17] 49,ídem,+8,+14,+22[3]; the origin of the insertion on chromosome 15 could not be identified. A diagnosis of mixed-phenotype BP-CML was made.
Following the Augmented Berlin-Frankfurt-Münster regimen, intensive chemotherapy induction was started in November 2017 with nilotinib 400 mg/day as TKI. After the induction phase, haematological response was achieved with no blasts found in the bone marrow aspirate. Bone marrow biopsy revealed persistence of BCR/ABL in 99.68% and complex karyotype with 46 XX, add(3)(q12), ins(15;?)(q11.2;?)[12] 49,ídem,+8,+14,+22[7] 46, XX[1]. Mutational panel of ABL and BCR was negative. Nilotinib was discontinued and the patient was started on third-generation TKI with ponatinib at 45 mg/day.
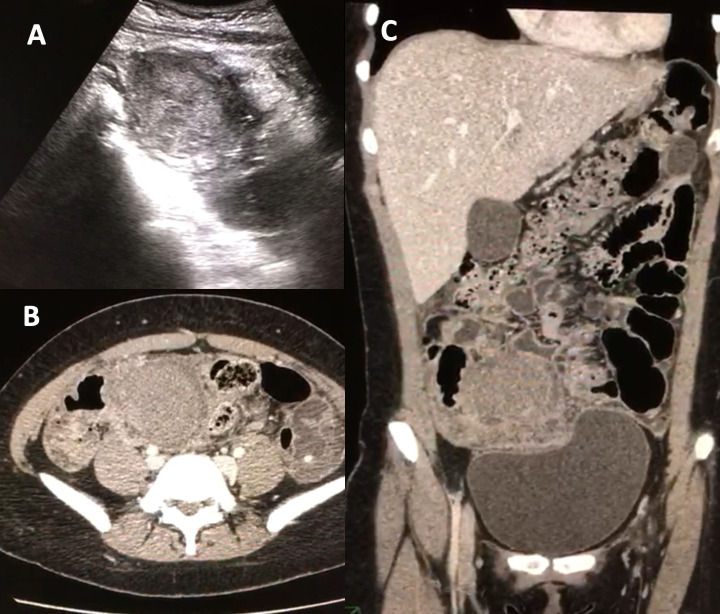
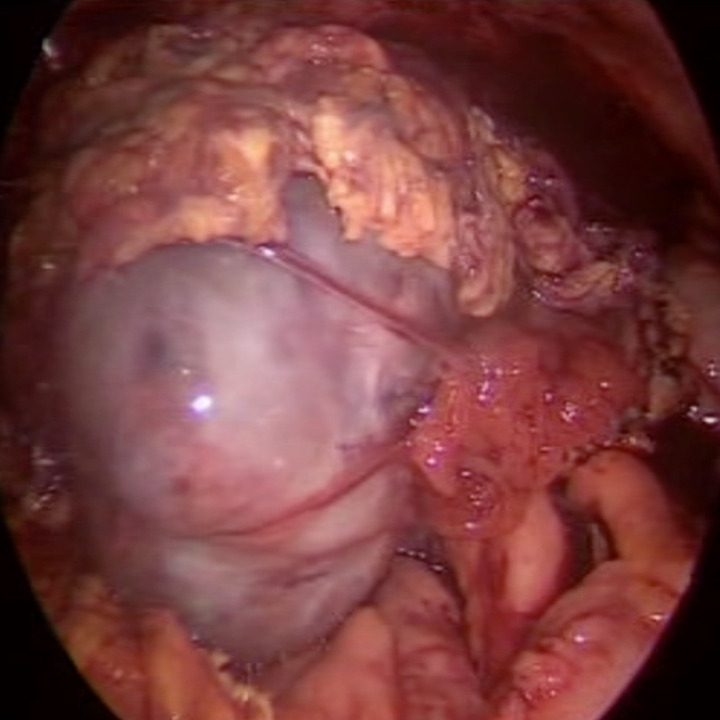
Due to several episodes of infections, including influenza pneumonia that required inpatient management and oseltamivir, and a second hospitalisation due to Clostridium difficile infection requiring vassopresor therapy, intense chemotherapy could not be restarted and allogeneic haematopoietic cell transplantation could not be performed during the first semester of 2018. In April 2018, her BCR/ABL was 60.45%. In June 2018, she arrived at the emergency department with abdominal pain, haemoglobin of 103 g/L, while blood cells (WBC) of 88 500 × 109/L with left shift and platelets of 474 000 × 109/L. CT scan showed an adnexal mass on her right iliac fossa, which showed inflammatory changes of the mesentery towards the hypogastrium and both iliac fossae (figure 1). Laparoscopic biopsy revealed findings consistent with a granulocytic sarcoma in the right ovary, with positive expression of MPO and CD117 (figure 2). Fluorodeoxyglucose-positron emission tomography (PET)-CT scan showed a left adnexal ovarian mass with metabolic activity and an SUVmax of 5.52. Bone marrow smear displayed 25% blasts of myeloid lineage.
Figure 1.
Images of adnexal mass on right iliac fossa. Detection of adnexal mass on right iliac fossa by pelvic ultrasound (A) and CT scan with inflammatory changes of the mesentery towards hypogastrium and both iliac fossae with the presence of free fluid in the posterior cul-de-sac (B, C).
Figure 2.
Right ovarian adnexal tumour prior to laparoscopic removal.
In the setting of a second blast crisis with myeloid predominance, the patient underwent intensive chemotherapy with a 7+3 regimen (cytarabine 100 mg/m2/day and daunorubicin 45 mg/m2/day). Failure to treatment was documented, with 12% persisting blasts with positivity for CD10, CD19, HLA-DR and TdT. BCR/ABL was 67.35%. There was no response on PET-CT.
Blinatumomab and hydroxyurea were started in August 2018. At the time, the patient had 89 000 leucocytes× 109/L with myeloid blasts and young myeloid forms in the peripheral blood smear. After the first week of blinatumomab, WBC count dropped to 4000 × 109/L and azacytidine and venetoclax were added to the regimen. Bone marrow aspirate at the end of the first cycle showed response on lymphoblastic lineage with 28% of myeloid blasts by immunophenotype. PET-CT showed increased activity in left iliac fossa with an SUVmax of 6.7 and diffuse activity in the axial skeleton. Molecular myeloid mutations assessed by next-generation sequencing, which was not available previously, revealed a Runt-related transcription factor 1 (RUNX1) somatic-haematopoietic mutation.
Treatment
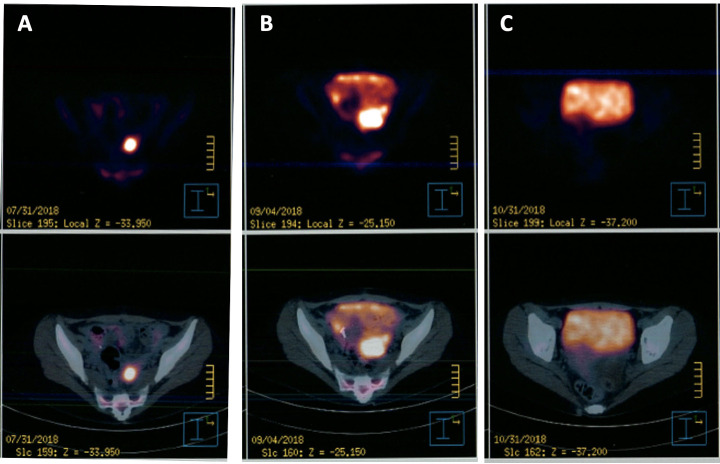
In September 2018, the importation of gemtuzumab-ozogamicin, until then unavailable in Mexico, was achieved. The patient was started on second cycle of blinatumomab in combination with gemtuzumab 4.5 mg/day on the first, fourth and seventh day, followed by cytarabine 3 g/m2 two times a day for six doses starting at day 8. The patient had haematological toxicity with neutropenic fever at the seventh day of the cycle secondary to extended-spectrum beta-lactamases (ESBLs)-producing Escherichia coli bacteraemia. On the 14th day, she developed an upper airway infection due to parainfluenza virus. At the end of the chemotherapy regimen, response was documented with a bone marrow smear and biopsy which showed no evidence of blasts and a repeat PET-CT negative for activity (shown in figure 3).
Figure 3.
Evolution of extramedullary relapse of blastic-chronic myeloid leukaemia activity by positron emission tomography (PET)-CT. (A) Tumour observed on PET-CT on July 2018 after treatment with cytarabine and daunorubicin (7+3). (B) Tumour observed after first cycle of azacytidine 75 mg/m2 for 7 days plus venetoclax ramped up to 400 mg/day. (C) Cessation of tumour activity after treatment with blinatumomab plus gemtuzumab plus high doses of cytarabine.
Outcome and follow-up
With a third chronic phase achieved, an haploidentical stem cell transplant from her brother was performed using cyclophosphamide, fludarabine and total body irradiation as conditioning regimen and cyclophosphamide and tacrolimus as graft-versus-host disease prophylaxis. At day +28 of transplant, BCR/ABL levels were 0.001% and with a negative minimal residual disease of 0.009%. Karyotype was 46, XY[26] and the PET-CT was negative for tumour activity. Five months after transplant, the patient had extramedullary relapse which responded to donor lymphocyte infusion, resulting in graft-versus-host disease. The patient died 3 months later due to complications of pneumonia in the setting of immunosuppression.
Discussion
This publication features a rare case of BP-CML with mixed phenotype and gonadal extramedullary activity, refractory to all the TKIs currently approved for clinical use as well as intensive chemotherapy. To our knowledge, this is the first case of treatment with anti-CD19 and anti-CD33 combination therapy in an adult. Recently, a paediatric case report of mixed-phenotype acute leukaemia with this scheme was also published.8
The atypical presentation of CML at a young age with multiple cytogenetic mutations as poor prognosis factors is remarkable in this case. Additional mutations to Philadelphia chromosome have been identified in 80% of patients, which progress to blast crisis. These additional cytogenetic aberrations are described as major or minor routes. Major routes include a second Philadelphia chromosome, trisomy 8, isochromosome 17q or trisomy 19, which have worse prognosis with an estimated progression=free survival of 50% compared with 90% in patients with t(9;22) mutation alone.9 A study of patients with clonal evolution after a second-generation TKI demonstrated that those with complex karyotype mutations have an estimated overall survival of 8 months compared with 43 months in those without a complex karyotype mutation.10 Our patient presented with one major route aberration (trisomy 8) in a complex karyotype scenario, leading to poor prognosis since diagnosis. Later in the clinical course of our patient, the presence of RUNX1 somatic mutation was revealed; this mutation is commonly found in haematological malignancies linked to disease progression and shorter survival in CML and is strongly linked to its progression to blast phase.11–13 We believe that next-generation sequencing can help with early detection of this mutation, allowing for a change in the treatment or the surveillance in chronic or blast phase.
Extramedullary manifestations in CML are not rare; however, our patient presented with an atypical site as a primary manifestation of relapse. Gonadal or genital tract activity in CML has only been reported in two other cases in literature. Although gonadal or genital activity is unusual, the differential diagnosis must be suspected in patients with myeloproliferative neoplasms of extramedullary disease.14–16 Extramedullary disease in both ovaries increased its complexity because of the abdominal surgical emergency and the refractoriness of the remaining disease to intensive chemotherapy and targeted therapy with a BCL2 inhibitor plus an hypomethylating agent.
As the patient was refractory to multiple treatments, it prompted us to look for a new high-efficiency therapeutic strategy. Blinatumomab is a bispecific CD19‐directed CD3 T‐cell engager antibody approved for relapsed/refractory acute lymphoblastic leukaemia, which has been successfully used in mixed-phenotype acute leukaemia under the reasoning that mixed-phenotype blasts express CD19.17 In vitro studies show remission in cells of patients with CML with myeloid blast phase and RUNX1 mutation, due to the existence of aberrant CD19 expression in the RUNX1 mutation. In this case, the response to an anti-CD19 was confirmed with CD19-chimeric antigen receptor cells (CAR-T cells).12 Thus, the use of an anti-CD19 antibody was promising; however, the response was not optimal, so a broader approach was required aimed at the myeloid lineage.
Gemtuzumab-ozogamicin is a monoclonal antibody directed to CD33, a myeloid lineage marker, linked to calicheamicin. It was the first antibody approved for myeloid leukaemia and was initially used in refractory/relapsed acute myeloid leukaemia. It was removed from market in 2010 and posteriorly reapproved in 2017 for acute myeloid leukaemia as first-line or second-line therapy with dose adjustment. Studies in relapsed/refractory population showed an overall response rate from 26% to 33%. We also found clinical reports of response in relapse of acute myeloid leukaemia manifested as extramedullary activity.18–20 The use of two antibodies directed at myeloid and lymphoid lineage in this setting was proposed to the patient, which led to a successful therapeutic response allowing a subsequent haematopoietic stem cell transplantation. The haematological toxicity with successful recovery observed with the synergic use of blinatumomab and gemtuzumab was similar to that expected from the use of gemtuzumab ozogamicin alone. To our knowledge, this is the first report of the synergic use of blinatumomab and gemtuzumab for mixed-phenotype leukaemia and acute or blast phase CML in an adult.
A report by Nicolini et al compared the overall survival of patients with CML under allogeneic haematopoietic cell transplantation versus third-generation TKI. They found that when a chronic or accelerated phase was present, the prognosis was better with TKI. Nevertheless, when the patient was in blast crisis, the overall survival was noticeably better with transplantation.3 This supports the idea of early transplantation when blast crisis is identified so that the general conditions and accumulated mutations are favourable.
Learning points.
Correct staging of chronic myeloid leukaemia is vital to establish aggressive therapy in young patients with poor prognosis.
The use of new prognostic tools, such as next-generation sequencing, is very useful for detecting additional mutations to BCR/ABL that confer poor prognosis and that could require changes in treatment, including early allogenic transplant.
The importance of early allogenic bone marrow transplantation in blast crisis is reaffirmed to avoid deterioration in patients and to prolong overall survival.
Although rare, extramedullary chronic myeloid leukaemia relapse can be present as gonadal sarcomas.
Blinatumomab and gemtuzumab-ozogamicin appear to be safe and effective in patients with mixed-phenotype leukaemia refractory to multiple lines of treatment even in extramedullary activity.
Acknowledgments
We want to thank the relatives of the patient for allowing us to share the clinical case with the subsequent medical knowledge to other clinicians.
Footnotes
Contributors: RO-M, XC-R and PEB-I were responsible for the hemato-oncological treatment of the patient. LAWS was responsible for the surgical treatment of the patient. The original idea was conceived by RO-M. The text was written by PEB-I. The images were provided by LAWS. All authors contributed to the investigation and discussion of the case.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Obtained.
References
- 1.World Health Organization classification of tumours . WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. In: Swerdlow SH, Campo E, Harris NL, eds. 4th edn. Lyon, 2017. [Google Scholar]
- 2.Patel AB, Wilds BW, Deininger MW. Treating the chronic-phase chronic myeloid leukemia patient: which TKI, when to switch and when to stop? Expert Rev Hematol 2017;10:659–74. 10.1080/17474086.2017.1330144 [DOI] [PubMed] [Google Scholar]
- 3.Nicolini FE, Basak GW, Kim D-W, et al. Overall survival with ponatinib versus allogeneic stem cell transplantation in Philadelphia chromosome-positive leukemias with the T315I mutation. Cancer 2017;123:2875–80. 10.1002/cncr.30558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gao X, Li J, Wang L, et al. Bilineal extramedullary blast crisis as an initial presentation of chronic myeloid leukemia: a case report and literature review. Am J Case Rep 2016;17:793–8. 10.12659/AJCR.899621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Durer S, Durer C, Shafqat M, et al. Concomitant use of blinatumomab and donor lymphocyte infusion for mixed-phenotype acute leukemia: a case report with literature review. Immunotherapy 2019;11:373–8. 10.2217/imt-2018-0104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Viardot A, Locatelli F, Stieglmaier J, et al. Concepts in immuno-oncology: tackling B cell malignancies with CD19-directed bispecific T cell engager therapies. Ann Hematol 2020;99:2215–29. 10.1007/s00277-020-04221-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baron J, Wang ES. Gemtuzumab ozogamicin for the treatment of acute myeloid leukemia. Expert Rev Clin Pharmacol 2018;11:549–59. 10.1080/17512433.2018.1478725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brethon B, Lainey E, Caye-Eude A, et al. Case Report: Targeting 2 Antigens as a Promising Strategy in Mixed Phenotype Acute Leukemia: Combination of Blinatumomab With Gemtuzumab Ozogamicin in an Infant With a KMT2A-Rearranged Leukemia. Front Oncol 2021;11:637951. 10.3389/fonc.2021.637951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fabarius A, Leitner A, Hochhaus A, et al. Impact of additional cytogenetic aberrations at diagnosis on prognosis of CML: long-term observation of 1151 patients from the randomized CML study IV. Blood 2011;118:6760–8. 10.1182/blood-2011-08-373902 [DOI] [PubMed] [Google Scholar]
- 10.Verma D, Kantarjian H, Shan J, et al. Survival outcomes for clonal evolution in chronic myeloid leukemia patients on second generation tyrosine kinase inhibitor therapy. Cancer 2010;116:NA–81. 10.1002/cncr.25015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sood R, Kamikubo Y, Liu P. Role of Runx1 in hematological malignancies. Blood 2017;129:2070–82. 10.1182/blood-2016-10-687830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Awad S, Kankainen M, Dufva O, et al. Runx1 mutations identify an entity of blast phase chronic myeloid leukemia (BP-CML) patients with distinct phenotype, transcriptional profile and drug vulnerabilities. Blood 2018;132:4257. 10.1182/blood-2018-99-111602 [DOI] [Google Scholar]
- 13.Zhao L-J, Wang Y-Y, Li G, et al. Functional features of Runx1 mutants in acute transformation of chronic myeloid leukemia and their contribution to inducing murine full-blown leukemia. Blood 2012;119:2873–82. 10.1182/blood-2011-08-370981 [DOI] [PubMed] [Google Scholar]
- 14.Sahu KK, Malhotra P, Uthamalingam P, et al. Chronic myeloid leukemia with extramedullary blast crisis: two unusual sites with review of literature. Indian J Hematol Blood Transfus 2016;32:89–95. 10.1007/s12288-014-0471-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rabischong B, Larraín D, Charpy C, et al. Extramedullary hematopoiesis and myeloid metaplasia of the ovaries and tubes in a patient with myelofibrosis: case report and Concise review of the reported cases. J Clin Oncol 2010;28:e511–2. 10.1200/JCO.2010.29.6442 [DOI] [PubMed] [Google Scholar]
- 16.Palatnik A, Narayan R, Walters M. Extramedullary hematopoiesis involving uterus, fallopian tubes, and ovaries, mimicking bilateral tuboovarian abscesses. Int J Gynecol Pathol 2012;31:584–7. 10.1097/PGP.0b013e31825183ad [DOI] [PubMed] [Google Scholar]
- 17.El Chaer F, Ali OM, Sausville EA, et al. Treatment of CD19-positive mixed phenotype acute leukemia with blinatumomab. Am J Hematol 2019;94:E7–8. 10.1002/ajh.25317 [DOI] [PubMed] [Google Scholar]
- 18.Owonikoko T, Agha M, Balassanian R, et al. Gemtuzumab therapy for isolated extramedullary AML relapse following allogeneic stem-cell transplant. Nat Clin Pract Oncol 2007;4:491–5. 10.1038/ncponc0899 [DOI] [PubMed] [Google Scholar]
- 19.Piccaluga PP, Martinelli G, Rondoni M, et al. Gemtuzumab ozogamicin for relapsed and refractory acute myeloid leukemia and myeloid sarcomas. Leuk Lymphoma 2004;45:1791–5. 10.1080/1042819042000219485 [DOI] [PubMed] [Google Scholar]
- 20.McNeil MJ, Parisi MT, Hijiya N, et al. Clinical and radiographic response of extramedullary leukemia in patients treated with Gemtuzumab Ozogamicin. J Pediatr Hematol Oncol 2019;41:e174–6. 10.1097/MPH.0000000000001201 [DOI] [PubMed] [Google Scholar]