Abstract
Background: Periodontitis is characterized by excessive osteoclastic activity, which is closely associated with inflammation. It is well established that MAPK/NF-kB axis is a key signaling pathway engaged in osteoclast differentiation. It is stated that that biphasic calcium phosphate (BCP) and platelet-rich fibrin (PRF) have significant antiostoeclastogenic effects in chronic periodontitis. Objective: We aimed to elucidate the synergetic effect of PRF/BCP involvement of the nuclear factor kappa–light–chain–enhancer of activated B cells (NF-kB) and the mitogen-activated protein kinase (MAPK) signaling pathway in osteoclast differentiation in chronic periodontitis. Methods: We induced osteoclast differentiation in vitro using peripheral blood mononuclear cells (PBMCs) derived from patients with chronic periodontitis. We assessed osteoclast generation by tartrate-resistant acid phosphatase (TRAP) activity, proinflammatory cytokines were investigated by ELISA and NF-κB, and IKB by immunoblot, respectively. MAPK proteins and osteoclast transcription factors were studied by Western blot analysis and osteoclast transcriptional genes were assessed by RT-PCR. Results: The results showed that the potent inhibitory effect of PRF/BCP on osteoclastogenesis was evidenced by decreased TRAP activity and the expression of transcription factors, NFATc1, c-Fos, and the osteoclast marker genes, TRAP, MMP-9, and cathepsin-K were found to be reduced. Further, the protective effect of PRF/BCP on inflammation-mediated osteoclastogenesis in chronic periodontitis was shown by decreased levels of proinflammatory cytokines, NF-kB, IKB, and MAPK proteins. Conclusions: PRF/BCP may promote a synergetic combination that could be used as a strong inhibitor of inflammation-induced osteoclastogenesis in chronic periodontitis.
Keywords: osteoclastogenesis, MAPK, NF-kB, periodontitis, platelet-rich fibrin, biphasic calcium phosphate
1. Introduction
Periodontitis is an infectious chronic inflammatory disease which is caused by periodontal pathogenic bacteria affecting the periodontal tissues leading to tooth loss [1]. Despite eliciting an immune response, periodontal tissues also primarily provoke the host inflammatory response to Porphyromonas gingivalis, involving the recruitment of inflammatory cells, production of cytokines, osteoclast activation, and bone resorption [2].
The inflamed periodontium consists of various immune and nonimmune cells that interact with bacterial constituents such as LPS to generate interleukins, such as IL-1β, IL-6, and TNF-α which act as an autocrine factor to cause chronic periodontal inflammation. In periodontal inflammation, elevated levels of inflammatory mediators are found in tissues leading to alveolar bone resorption [3].
Inflammatory gene expression is induced by intracellular signal transduction mechanisms. Inflammatory signaling pathways are controlled by mitogen-activated protein kinases (MAPK) from the cell surface to the nucleus [4]. Three well-characterized subfamilies of MAPK pathways include: (1) The c-Jun N-terminal kinase (JNK) pathway; (2) the extracellular signal-related kinase (ERK1/2) pathway; and (3) the p38 MAPK pathway.
MAPK activates various transcription factors such as activator protein-1 (AP-1) and NF-kB, substrate proteins for phosphorylation, including the “downstream” cytoskeletal elements, nuclear receptors, cell death receptors, and serine/threonine kinases [5]
Cell differentiation and movement, gene expression, embryogenesis, mitosis, metabolism, programmed death, and many other cellular activities are regulated by MAPK [6]. The p38 MAPK pathway is vital to signal stress, inflammatory, and infectious stimuli; and it is also involved in the control of essential processes including cell proliferation, differentiation, and migration [7]. The c-Jun N-terminal kinases (JNKs) belong to a family of stress-activated protein kinases that are associated with the transactivation of c-Jun by phosphorylating the N-terminal serine residues [8]. Growth factors and inflammatory cytokines also activate JNKs [9]. JNKs and ERKs regulate cell proliferation and cell death.
MAPK are implicated in the regulation of osteoclast activation along with the expression of inflammatory mediators. Osteoclast differentiation is mainly regulated by the interplay of RANKL/RANK [10]. Rapid phosphorylation and activation of MAPK are mainly regulated by RANKL, and this activates transcription factors such as a nuclear factor of activated T cell 1 (NFATc1), c-Fos, OPG and tumor necrosis factor receptor-associated factor-6 (TRAF 6), thereby regulating the gene expression for osteoclast differentiation [11].
The application of regenerative materials has shown a promising result on osteoclast differentiation [12]. However, the role of PEF/BCP in inhibiting the MAPK signaling pathway has not been evaluated thus far.
Platelet concentrate, most widely known as platelet-rich fibrin (PRF) is derived from autologous blood which is rich in growth factors. PRF is extensively used in the management of periodontal intrabony defects, furcation defects, maxillofacial surgical procedures, and regenerative procedures [12]. Zhang et al. demonstrated the favorable effects of PRF membranes in alveolar ridge preservation [13]. PRF facilitates angiogenesis, cell proliferation, and differentiation, which in turn leads to new bone and tissue regeneration [14]. In addition, a previous study reported an impact of leukocyte and platelet-rich fibrin (L-PRF) exudate on bone regeneration when incorporated with the poly(Lactide-co-glycolide) (PLGA) [15]. Recent studies also stated that the formation of osteoclasts can be inhibited with PRF membranes from hematopoietic progenitors in bone marrow cultures [16,17].
In the era of tissue engineering and regeneration, biphasic calcium phosphate has emerged and contributed immensely. According to Silva et al., BCP has shown a great impact on the migration of macrophages and secretion [18]. Osteointegration and bone formation in periodontal defects were noted when biphasic calcium phosphate was used synthetically as fabricated bone graft [19]. One of the advantages of calcium phosphate is its osteoinductive and osteoconductive property which aids in the osteogenic differentiation of mesenchymal stem cells [20,21]. Balaguer et al. demonstrated that plasma clotted around BCP microparticles showed an osteogenic property that could be used for the treatment of bony defects [22].
We recently demonstrated in vitro that in chronic periodontitis patients, the ostoeoclastic effect was inhibited through the promotion of the proteolytic cascade of apoptosis when treated with PRF/BCP [23]. In this study, we hypothesized that inhibiting the MAPK/NF-kB signaling strategy would provide a novel antiosteoclastogenic target in periodontitis progression. Earlier studies described the role of several natural MAPK pathway inhibitors such as myricetin, luteolin, morin, fisetin, and panduratin A from medicinal plants on osteoclastic differentiation and periodontal inflammation [24,25]. No past studies have reported the molecular effect of PRF and BCP on influencing the MAPK/NF-Kbaxis, IL-6, IL-1β, TNF-α, transcription factors, and osteoclastic marker genes
Hence, in the present study, we aimed to elucidate the synergistic effect of PRF/BCP on the markers for inflammation and osteoclast differentiation by suppressing the MAPK/NF-kB axis in chronic periodontitis in vitro.
2. Materials and Methods
2.1. Materials and Reagents
Bone BCP (SigmaGraft, CA, USA) is comprised of 40% beta-tricalcium phosphate (β-TCP) and 60% hydroxyapatite (HA). The reagents included were 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), Dulbecco’s Modified Eagle’s Medium (DMEM), TRI reagent, trypan blue. All other chemicals of research-grade were obtained from Sigma-Aldrich, Inc (St. Louis, MO, USA). The heat-inactivated fetal bovine serum (FBS) was procured from GIBCO Grand Island, New York, USA. RANKL was obtained from Sigma-Aldrich (St. Louis, MO, USA) and M-CSF from BioVision, (Milpitas, CA, USA). The bicinchoninic acid (BCA) protein assay kit was purchased from Thermo Fisher Scientific (Rockford, IL, USA) and the primary antibodies (1:1000 dilutions) NF-κB, NFATc1, c-Fos, TRAF 6, ERK, p-ERK, JNK, p-JNK, P38, and p-P38 were supplied by Santa Cruz, CA, USA. Secondary antibody, β-actin was purchased from Santa Cruz (USA).
2.2. Study Design
The patients were recruited from the Department of Periodontology during the period from July 2015 until February 2016. Fifteen generalized chronic periodontitis individuals, both male and female, within the age range of 35–45 years were selected according to the criteria mentioned below. The human subject’s ethics board of MAHER, Chennai, India approved the study (“Institutional Review Board”, Protocol No: MU-128-IEC-2015). The research was conducted at par with the 1975 Declaration of Helsinki 1975, as revised in 2013. The written consent was obtained from the subjects who were willing to participate in the study.
2.3. Inclusion and Exclusion Criteria
According to the criteria, both male and female patients aged 35–45 years with more than 5 mm of clinical attachment loss in 30% or more sites with at least 20 teeth remaining in the oral cavity were included as generalized chronic periodontitis (AAP 1999 classification) patients and became part of our investigation [26]. These patients were nonsmokers and had no previous history of other systemic diseases. Moreover, they were also not treated for chronic periodontitis for the past 6 months. Patients under antibiotic and anti-inflammatory therapy were excluded from the study. From the selected chronic periodontitis (CP) patients, the PBMCs were isolated and divided into four groups based on the treatment with PRF/BCP. The first group (CP) remained untreated, the second group (CP+BCP) was treated with BCP alone, the third group (CP+PRF) was treated with PRF alone, and the fourth group (CP+PRF/BCP) was treated with PRF/BCP in combination.
2.4. Preparation of PRF and BCP
Five ml venous blood from chronic periodontitis subjects was collected and transferred to 10 mL sterile glass centrifuge tubes with centrifugation at 3000 rpm for 12 min (R-4C, REMI, Maharashtra, India). In the middle of the tube, a fibrin clot was formed between the red corpuscles and acellular plasma. The serum was squeezed out from the clot using sterile gauze, and a resistant autologous platelet-rich fibrin membrane was obtained. The membrane was then cut into fragments. PRF membranes were then minced to a size of 1 × 1 cm for the further analyses. The BCP grafting particles, composed of 60% hydroxyapatite and 40% β-tricalcium phosphate were purchased commercially (SigmaGraft, CA, USA).
The minced PRF threads were added to BCP and the PRF/BCP mixture was used for treating the cells. The PRF/BCP mixture was prepared as 1 mL/well using the DMEM medium and added to the wells. BCP (60 µg/mL) was dissolved in stored acellular plasma and the culture medium was used to adjust the concentration of the drug.
2.5. Monocytes Isolation from Whole Blood
Five ml of peripheral blood was collected from the antecubital vein for analysis from chronic periodontitis patients. Mononuclear cells were obtained from diluted peripheral blood (1:2 in phosphate-buffered saline (PBS)), which was layered over Ficoll–Paque (Sigma-Aldrich, Inc., USA), and it was further centrifuged at 1700 rpm for 30 min at room temperature. The solution was then washed and resuspended in Dulbecco’s Modified Eagle Medium (DMEM) (Sigma-Aldrich Inc., USA) containing 10% fetal bovine serum (FBS) (GIBCO Grand Island, New York, NY, USA). Consequently, these cells were calculated in a hemocytometer using trypan blue.
2.6. Osteoclasts Generation and Differentiation
PBMCs were plated in 24-well plates at a density of 1 × 105 cells per well in 2 mL of DMEM medium, containing 10% FBS (FBS; HyClone), penicillin (100 µg/mL), and streptomycin (100 µg/mL) and allowed to adhere overnight. The next day, the medium was semidepleted and replaced with a fresh osteoclastogenic differentiation medium containing 25 ng/mL M-CSF (BioVision, Milpitas, CA, USA), 40 ng/mL RANKL (Sigma-Aldrich Inc., USA), 1 μM dexamethasone, and 2 mm L-glutamine. The cells were allowed to be re-fed twice a week by withdrawing half of the medium and replacing it with fresh. The cells were harvested, separated, and then divided into four different groups where BCP, PRF, and PRF/BCP mixtures were added in six parallel wells, the CP group was the control and they were cultured for 21 days and stained for tartate resistant acid phosphatase (TRAP) activity. All the experiments were performed in triplicate for error elimination and authenticity of the results.
2.7. TRAP Activity Assay
Ten μL of cell lysate was added and incubated at 37 °C for 30 min to the 50 μL TRAP reaction buffer (2.5 naphthol ASBI phosphate HCl in 100 mM sodium acetate and 50 mM disodium tartrate pH 6.1). To stop the enzymatic reaction finally, 150 μL of 0.1 M NaOH was added. A multifunction microplate reader was used to measure the fluorescence with the wavelength of 405/520 nm. In all four groups, calibrator solutions with different TRAP concentrations (0.6 to 12 U/L) (Bone TRAP, Medac, Wedel, Germany), were used to correlate the fluorescence intensity with TRAP activity.
2.8. Measurement of Proinflammatory Cytokines by ELISA
ELISA kits {R&D Systems, Inc (Minneapolis, MN, USA)} were used to analyze the levels of IL-1β, IL-6, and TNF-α, and they were determined in the osteoclast culture medium based on the manufacturer’s directions.
2.9. Western Blot Analysis
The cells from four different experimental groups were plated in 24-well plates at a density of 5 × 104 cells per well in 1 mL of DMEM containing 10% FBS overnight. After 24 h, nonadherent cells were removed by gentle washing. The biomaterials, namely BCP, PRF, and the combination of PRF and BCP, were added to the cells. After 24 h of treatment, cell lysis was conducted by adding a cold RIPA buffer (150 mM NaCl, 50 mM Tris HCL, 0.1% SDS, 1% Triton X-100, 1 mM PMSF, 2 mM NaF, Na3VO4, β-glycerophosphate, 2 mM EDTA, and fresh protease inhibitor cocktail), and the cell lysate was centrifuged at 14,000 rpm at 4 °C for 20 min. The BCA method was used to analyze the protein content of the supernatant. Protein was denatured in a sample buffer, separated on 12% SDS-PAGE, and was then transferred to a polyvinylidene difluoride membrane. The blots were blocked for 2 h at room temperature with Tris-buffered saline (TBS, 50 mM Tris-HCl, pH 7.5, 150 mM NaCl) containing 5% nonfat milk. The blots were then washed three times with TBST (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, and 0.02% Tween 20) and incubated with a specific primary antibody (1:1000 dilutions) of NF-κB, IKB, NFATc1, c-Fos, ERK, p-ERK, JNK, p-JNK, P38, and p-P38 (Santa Cruz, CA, USA) at 4 °C and kept overnight. The blots were then incubated for 1 h at room temperature with secondary antibody (1:5000 dilutions), and detected by an enhanced chemiluminescence (ECL) (Thermo Fisher Scientific) detection reagent. β-actin was used as an internal control to ensure that equal amounts of sample proteins were applied for electrophoresis. Densitometric analysis was performed using Image Lab software (Bio-Rad Laboratories, Hercules, CA, USA).
2.10. RNA Isolation and Reverse Transcription-Polymerase Chain Reaction
According to the manufacturer’s instructions, in all four groups, total RNA was isolated from cells by a TRIzol reagent (Invitrogen, Carlsbad, CA, USA). cDNA was synthesized from 1 µg of RNA using an iScript cDNA Synthesis Kit. (Bio-Rad Laboratories, Hercules, CA, USA). PCR amplifications were performed as follows: 30 cycles for TRAP (95 °C for 60 s, 55 °C for 30 s, and 60 °C for 60 s), 30 cycles for CAT-K (94 °C for 1 min, 60 °C for 1 min, and 72 °C for 1 min) (ENST00000678337.1), 40 cycles for MMP-9 (94 °C for 60 s, 60 °C for 60 s, and 68 °C for 120 s), and 30 cycles for β-actin (94 °C for 35 s, 64 °C for 45 s, and 72 °C for 1 min) (ENSG00000075624). β-actin was used as an endogenous control. Ethidium bromide was used to stain the PCR products and then analyzed in a 1% agarose gel. A 100 bp ladder was used to confirm the size of the amplification products. Relative mRNA expression levels were obtained by normalizing to the β-actin expression. All the experiments were repeated three times. Primers for the study are represented in Table 1.
Table 1.
Primer sequences used in real-time PCR.
| Gene | Forward Primer Sequence (5′ to 3′) | Reverse Primer Sequence (5′ to 3′) | Size (bp) |
|---|---|---|---|
| TRAP | CCAATGCCAAAGAGATCGCC | TCTGTGCAGAGACGTTGCCAAG | 216 |
| CAT-K | CCGCAGTAATGACACCCTTT | AAGGCATTGGTCATGTAGCC | 258 |
| MMP-9 | CTCTGGAGGTTCGACGTG | GTCCACCTGGTTCAACTCAC | 183 |
| β-actin | TCACCCACACTGTGCCCATCTACGA | CAGCGGAACCGCTCATTGCCAATGG | 141 |
TRAP: tartrate resistant acid phosphatase; CAT-K: cathepsin K; MMP-9: matrix metalloproteinase 9; β-actin: beta-actin.
2.11. Statistical Analysis
Statistical package social sciences version (SPSS) 23.0 was used to perform the statistical analysis. All the data in this study was presented as mean ± standard deviation (SD). Differences among groups were assessed using one way ANOVA and Dunnett’s multiple comparison tests for intergroup comparison was conducted using Graphpad Prism 6.0 software package for windows. p < 0.05 was considered to be statistically significant. * p < 0.05, ** p < 0.01, *** p < 0.001.
3. Results
3.1. PRF/BCP Decreases TRAP Activity
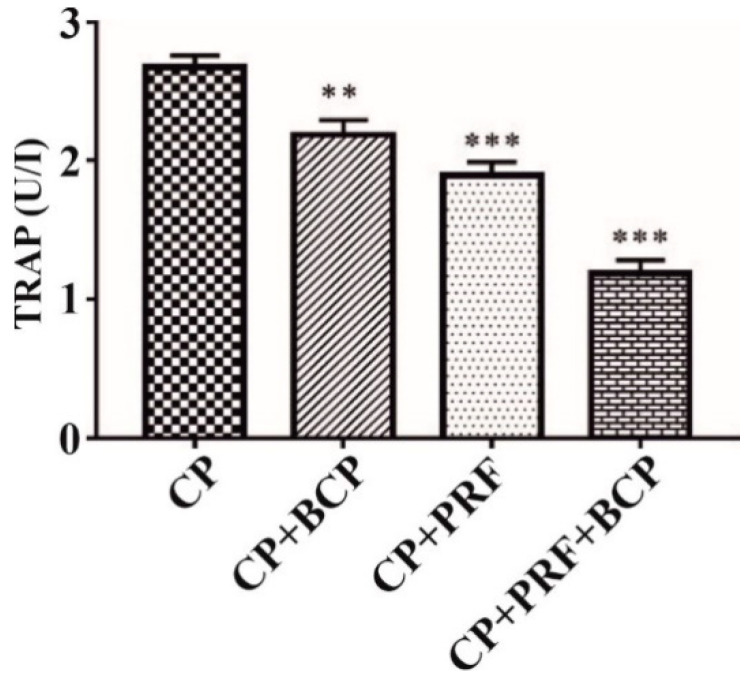
The activity of TRAP was significantly suppressed by PRF/BCP as compared with other groups (PRF, BCP and CP) (Figure 1). Hence, PRF/BCP might efficiently prevent osteoclastogenesis by inhibiting TRAP.
Figure 1.
TRAP activity images. All data are presented by mean ± SD. Results are expressed as the mean ± SD. The comparisons were made among CP and BCP, CP and PRF, and CP and PRF/BCP. (** p< 0.01; *** p < 0.001).
3.2. PRF/BCP Attenuates Proinflammatory Cytokines
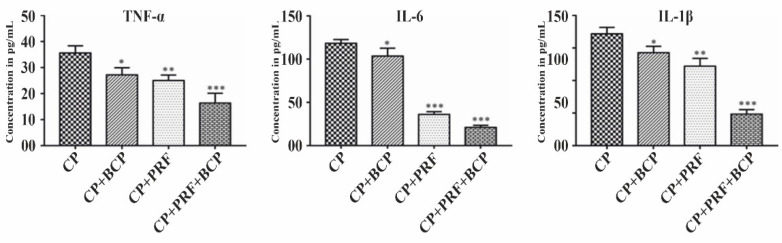
IL-1β, IL-6, and TNF-α were significantly decreased when treated with PRF/BCP as compared with PRF and BCP alone (Figure 2). In addition, as compared with other cytokines, the level of IL-6 expression was markedly decreased.
Figure 2.
Effect of PRF/BCP on TNF-α, IL-6 and IL-1β. Results are expressed as the mean ± SD. The comparisons were made among CP, CP + BCP, CP + PRF, and CP + PRF/BCP combination. (* p < 0.05; ** p< 0.01; *** p< 0.001).
3.3. PRF/BCP Regulates Periodontal Inflammation and Osteoclastogenesis by Inhibiting MAPK Signaling and NF-κB Pathways
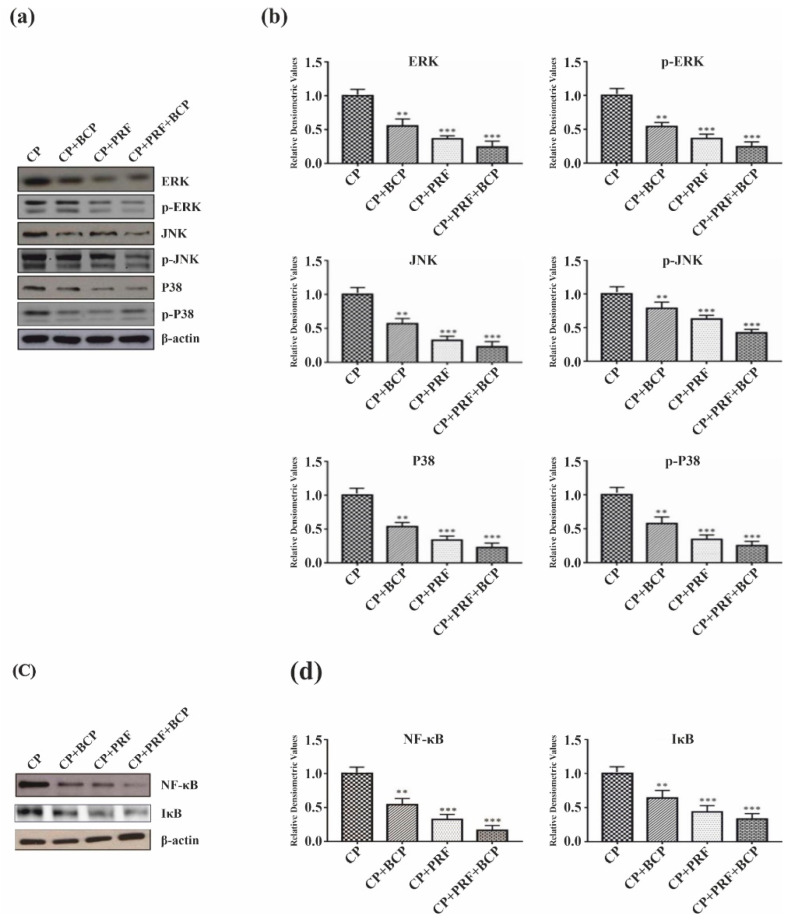
MAPK signaling and NF-kB pathways were examined at the protein level on osteoclasts. In the chronic periodontitis group (CP), MAPK signaling proteins and their phosphorylated forms were highly expressed (Figure 3a,b). PRF/BCP markedly inhibited the expression of ERK, p-ERK, JNK, p-JNK, p-38, and p-p38 and also diminished the expression of NF-kB and IKB (Figure 3c,d). These results indicate that PRF/BCP has antiosteoclastogenic and anti-inflammatory effects which are regulated via the inhibition of MAPK signaling proteins.
Figure 3.
Effect of PRF/BCP on MAPK/NF-kB pathway. Western blot analysis of MAPK molecules (ERK, p-ERK, JNK, p-JNK and P38, p-P38) were blotted and normalized with β-actin. (a) Representative blot image. (b) Relative densitometric analysis in histograms. NF-kB and IKB molecules were blotted and normalized with β-actin. (c) Representative blot image. (d) Relative densitometric analysis in histograms. Results are presented by mean ± SD. Comparisons were made among CP and BCP, CP and PRF, and CP and PRF/BCP combination. (** p< 0.01; *** p< 0.001).
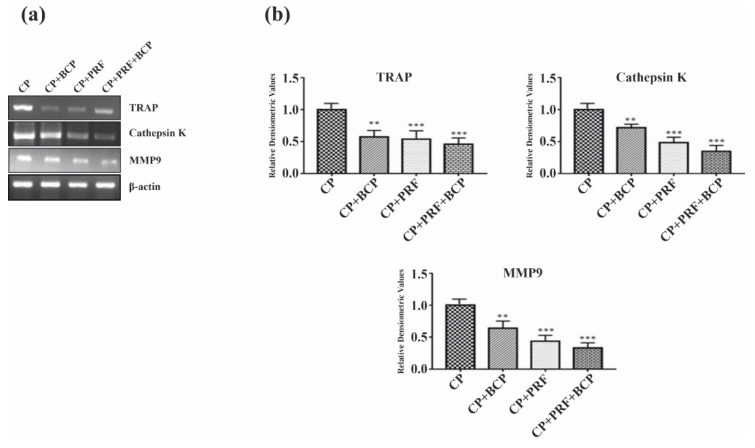
3.4. PRF/BCP Inhibits Osteoclastogenic Transcription Factors and Osteoclast Marker Genes
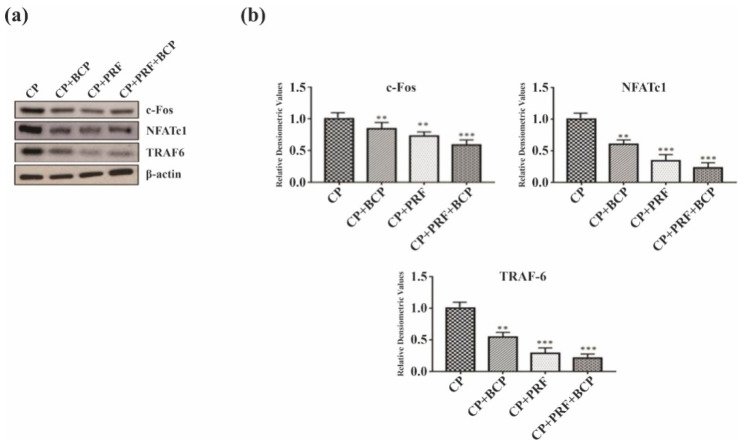
Osteoclastic transcription factors, c-Fos, NFATc1, and TRAF 6 and bone resorptive enzymes, cathepsin K, TRAP, and MMP-9 were significantly decreased when treated with PRF/BCP (Figure 4a,b and Figure 5a,b). The decrease was greater in the PRF/BCP combined group than the PRF and BCP group alone. These results indicated the antiosteoclastic effect of PRF/BCP was established through inhibition of osteoclast-related marker genes.
Figure 4.
PRF/BCP inhibits osteoclastogenic transcription factors. Results are expressed as the mean ± SD. Western blot analysis of osteoclast pathway molecules (c-Fos, NFATc1 and TRAF 6) was blotted and normalized with β-actin. (a) Representative blot image. (b) Relative densitometric analysis in histograms. Results are expressed as mean ± SD. Comparisons were made among CP and BCP, CP and PRF, and CP and PRF/BCP combination. (** p < 0.01; *** p < 0.001).
Figure 5.
PRF/BCP inhibits osteoclast marker genes. RT-PCR analysis of osteoclastogenic genes TRAP, cathepsin K and MMP-9. (a) Representative blot image. (b) Relative densitometric analysis in histograms. Results are expressed as mean ± SD. Comparisons were made among CP and BCP, CP and PRF, and CP and PRF/BCP combination. (** p < 0.01; *** p < 0.001).
4. Discussion
In the existing study, PRF along with BCP, was identified as an important negative modulator of markers for inflammation and osteoclast differentiation in chronic periodontitis [23]. This has led to the foundation for further research to explore the antiosteoclastic effect of PRF/BCP via NF-kB and MAPK signaling pathways.
The monocytes were derived from the peripheral blood and differentiated to form multinucleated osteoclasts [27]. According to Kumar et al., TRAP staining showed that there was a relative decrease in the number of TRAP positive multinucleated osteoclasts in PRF+BCP combination (p < 0.001) as compared with other groups [23]. In the present study, TRAP activity on the osteoclastic cells also found a significant suppression of osteoclastic cells when treated with PRF/BCP, which is further confirmation of our previous study (Figure 1).
In general, it is hypothesized that IL-1β, IL-6, and TNF-α play a key role in activating osteoclasts that terminate in net bone resorption [28]. In the present study, IL-1β, IL-6, and TNF-α were increased in the CP group, suggesting an inflammatory response of the inflammatory cells during the progression of the disease (Figure 2). There were consistent results in different studies demonstrating the increased levels of proinflammatory cytokines in inflamed periodontal tissue [29,30]. It is observed that inflammatory responses are inhibited by the antagonist of IL-1 and TNF-α, thereby decreasing the amount of bone destruction in experimentally induced periodontitis [31].
NF-kB is one of the most common inflammatory cytokines and plays a key role in RANKL-induced osteoclast formation [32]. In the current study, NF-kB level was significantly increased in the CP group (Figure 3c,d), which can be explained as NF-kB binds to the promoter regions of proinflammatory cytokine genes and activates their transcription, thereby regulating the inflammatory process which is mainly mediated by MAPK pathway. The results also showed that PRF/BCP suppressed the osteoclast differentiation through the inhibition of IKB phosphorylation (Figure 3c,d) in the NF-kB signaling pathway.
MAPK, extracellular signal-regulated kinase (ERK1/2), c-Jun N-terminal kinase (JNK), and p38 mainly activate NF-kB, resulting in the expression of proinflammatory cytokines. However, PRF/BCP significantly decreased the level of proinflammatory cytokines (Figure 2) and NF-kB (Figure 3c,d), thus protecting against periodontal inflammation.
Moreover, considering that periodontal inflammation accelerates osteoclastogenesis, the function of MAPK signaling molecules in regulating osteoclast differentiation in periodontitis was further studied. RANKL is overexpressed by the inflammatory responses, which result in RANK receptor binding on an osteoclast, thereby causing osteoclastogenesis [33,34]. We observed an increased level of p38, ERK1/2, and JNK in the CP group where PRF/BCP was not introduced (Figure 3a,b). On the other hand, in PRF/BCP group, osteoclastogenesis was inhibited by antagonizing the MAPK signaling pathway (Figure 3a,b). Our study was in agreement with a study conducted by Lee et al. who concluded that osteoclastogenesis is mainly influenced by the MAPK pathway [35]. Another study also concluded that pharmacological inhibitors and gene silencing can also inhibit the MAPK pathway [36]. Previous studies also reported the importance of p38 in the regulation of IL-6 in periodontal ligament fibroblasts and osteoblasts [37,38]. This implies that the activation of the MAPK pathway might exist in periodontitis-induced osteoclastogenesis. Kim et al. showed the effect of panduratin A on inhibiting the MAPK signaling pathway in periodontitis [24]. The study also demonstrated the key aspects of p38, JNK, and ERK signals, which play a pivotal role in the differentiation of osteoclasts and intracellular signaling transduction.
In the present study, PRF/BCP affectively reduced the expression of transcription factors such as NFATc1 and c-Fos in osteoclast differentiation in the PRF/BCP group as compared with PRF and BCP alone (Figure 4a,b). Our findings were in accordance with Choi et al., who described the role of fisetin as a potential inhibitor of RANKL-induced osteoclast differentiation [39]. Another study by Boyce et al. demonstrated that a decrease in signaling by p38/c-Fos/NFATc1 could subsequently lead to a reduction in the expression of genes required for bone resorption, which is similar to our study [40]. It is assumed that during RANKL and RANK interaction, the transcription factors combine to the target gene within the nucleus of the osteoclastic cells. Further, phosphorylated MAPK signaling proteins activate NFATc1 and c-Fos. Thus, the phosphorylation of NFATc1 through the MAPK/NF-kB axis is critical for the master osteoclast transcription factor, NFATc1, to translocate into the nucleus of the osteoclast progenitor cells and activate the osteoclastogenic mechanism. In our study, the synergistic effect of PRF/BCP showed to attenuate the action of the transcription factors, thereby influencing the MAPK signaling proteins and reducing the osteoclastic activity.
We also observed that in response to the transcriptional signal, the expression of mRNA in osteoclastic specific genes such as TRAP, cathepsin K, and MMP-9 were significantly inhibited by PRF/BCP (Figure 5a,b). Our findings were in agreement with Franco et al., who described the inhibitory effect of doxycycline on the functional expression of osteoclast markers genes, such as TRAP activity, MMP9 enzyme activity, and cathepsin K on RANKL-induced osteoclastogenesis [41]. Hence, keeping the aforementioned concepts in mind, it can be concluded that PRF/BCP can reduce the manifestation and regulation of osteoclastic transcription factors by regulating the MAPK signaling pathway proteins, whereby blocking the activation of TRAP, cathepsin K, and MMP-9. One of the most important factors that exhibits the bioactive feature of BCP is the degradation and release of calcium ions which also regulate the formation and resorption of osteoclasts [21,42]. On the other hand, phosphates and the osteoblastic lineage via the IGF-1 and ERK1/2 pathways regulate the growth and differentiation of osteoblasts, thereby increasing the expression of BMPs [43,44]. Hence, BCP has a dual role to play as a regenerative material.
Phosphate mainly plays an important role in the regulation of the RANK ligand: OPG ratio to inhibit osteoclast differentiation and bone resorption [45]. The expression of osteoblastic differentiation markers such as ALP, BMPs, OPN, OCN, BSP, ON, and RunX2 is also affected by calcium and phosphate ions [46,47]. However, the mechanism by which PRF/BCP induces osteogenesis in favor of bone formation remains to be established. The findings of this study can also pave the way to understanding new treatment modalities to manage periodontal diseases. Further research should be undertaken to realize the importance of signaling mechanisms in inflammatory mediators, their interactions with the MAPK/NF-kB signaling pathway, and also to obtain more predictable clinical results.
Limitations of the Current Study
This study has certain limitations. First, the study identified osteoclasts using TRAP activity; however, pit assay can be used to determine the bone resorption activity of osteoclasts. Secondly, there was a lack of healthy groups which could provide more insight concerning the TRAP activity, MAPK pathway, transcription factors, and osteoclast marker genes when treated with PRF and BCP in comparison with the other groups. Furthermore, the investigations were restricted to only one time point. Various study designs at varying time points would enable better inference of the study. In the future, a large number of studies with more samples may ensure better significant findings with the best precise outcome in estimating the clinical effects of PRF/BCP combination in regenerative periodontics.
5. Conclusions
Overall, this study demonstrated the inhibitory effect of PRF and BCP on the activation of NF-kB and MAPK signaling pathways, as well as on the expression of inflammatory and osteoclastogenic genes. The study results provide vital evidence substantiating the potential role of a PRF and BCP combination as a regenerative medicine in the therapeutic approach to periodontitis. As the present study showed the involvement of multiple signaling pathways and the crosstalk between PRF/BCP and signaling modulators, future studies on the biologic roles of other signaling pathways/modulators activated by PRF/BCP might provide further understanding of biomaterials as novel therapeutic agents in the treatment of chronic periodontitis.
Acknowledgments
The authors acknowledge The Central Research Lab, Meenakshi Ammal Dental College, Chennai, India for the Preliminary work carried out in the laboratory.
Author Contributions
Conceptualization, A.K., J.M. and L.M.; methodology, H.H.A. and M.S.; software, A.K., H.H.F. and S.P.; validation, L.M., H.H.A. and M.S.; formal analysis, J.M., H.H.F. and S.V.; investigation, Z.H.A., L.M. and H.A.B.; resources, A.K.B., A.K. and T.M.B.; data curation, M.H.M., S.V. and W.O.A.; writing—original draft preparation, A.K., J.M., L.M., H.H.A., M.S., H.H.F. and Z.H.A.; writing—review and editing, A.K.B., S.V., M.H.M., T.M.B., H.A.B., W.O.A. and S.P.; visualization, T.M.B., M.H.M. and Z.H.A.; supervision, A.K.B., W.O.A. and H.A.B.; project administration, J.M. and S.P. All authors have read and agreed to the published version of the manuscript.
Funding
This Research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of Human Subject’s Ethics Board of MAHER—Deemed to be University, Chennai, India (“Institutional Review Board”, Protocol No: MU-128-IEC-2015).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hajishengallis G. Periodontitis: From microbial immune subversion to systemic inflammation. Nat. Rev. Immunol. 2015;15:30–44. doi: 10.1038/nri3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hajishengallis G., Chavakis T. Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat. Rev. Immunol. 2021;21:426–440. doi: 10.1038/s41577-020-00488-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdulkhaleq L.A., Assi M.A., Abdullah R., Zamri-Saad M., Taufiq-Yap Y.H., Hezmee M.N.M. The crucial roles of inflammatory mediators in inflammation: A review. Vet. World. 2018;11:627–635. doi: 10.14202/vetworld.2018.627-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braicu C., Buse M., Busuioc C., Drula R., Gulei D., Raduly L., Rusu A., Irimie A., Atanasov A.G., Slaby O., et al. A Comprehensive Review on MAPK: A Promising Therapeutic Target in Cancer. Cancers. 2019;11:1618. doi: 10.3390/cancers11101618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dorrington M.G., Fraser I.D.C. NF-κB Signaling in Macrophages: Dynamics, Crosstalk, and Signal Integration. Front. Immunol. 2019;10:705. doi: 10.3389/fimmu.2019.00705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Basson M.A. Signaling in Cell Differentiation and Morphogenesis. Cold Spring Harb. Perspect. Biol. 2012;4:a008151. doi: 10.1101/cshperspect.a008151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corre I., Paris F., Huot J. The p38 pathway, a major pleiotropic cascade that transduces stress and metastatic signals in endothelial cells. Oncotarget. 2017;8:55684–55714. doi: 10.18632/oncotarget.18264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dhanasekaran D.N., Reddy E.P. JNK-signaling: A multiplexing hub in programmed cell death. Genes Cancer. 2017;8:682–694. doi: 10.18632/genesandcancer.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou G., Yang J., Song P. Correlation of ERK/MAPK signaling pathway with proliferation and apoptosis of colon cancer cells. Oncol. Lett. 2019;17:2266–2270. doi: 10.3892/ol.2018.9857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park J.H., Lee N.K., Lee S.Y. Current Understanding of RANK Signaling in osteoclast differentiation and Maturation. Mol. Cells. 2017;40:706–713. doi: 10.14348/molcells.2017.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim J.H., Kim N. Regulation of NFATc1 in osteoclast differentiation. J. Bone Metab. 2014;21:233–241. doi: 10.11005/jbm.2014.21.4.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ausenda F., Rasperini G., Acunzo R., Gorbunkova A., Pagni G. New Perspectives in the Use of Biomaterials for Periodontal Regeneration. Materials. 2019;12:2197. doi: 10.3390/ma12132197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Y., Ruan Z., Shen M., Tan L., Huang W., Wang L., Huang Y. Clinical effect of platelet-rich fibrin on the preservation of the alveolar ridge following tooth extraction. Exp. Ther. Med. 2018;15:2277–2286. doi: 10.3892/etm.2018.5696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saberianpour S., Heidarzadeh M., Geranmayeh M.H., Hosseinkhani H., Rahbarghazi R., Nouri M. Tissue engineering strategies for the induction of angiogenesis using biomaterials. J. Biol. Eng. 2018;12:36. doi: 10.1186/s13036-018-0133-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Witek L., Tian H., Tovar N., Torroni A., Neiva R., Gil L.F., Coelho P.G. The effect of platelet-rich fibrin exudate addition to porous poly(lactic-co-glycolic acid) scaffold in bone healing: An in vivo study. J. Biomed. Mater. Res. B Appl. Biomater. 2020;108:1304–1310. doi: 10.1002/jbm.b.34478. [DOI] [PubMed] [Google Scholar]
- 16.Strauss F.-J., Nasirzade J., Kargarpoor Z., Stähli A., Gruber R. Effect of platelet-rich fibrin on cell proliferation, migration, differentiation, inflammation, and osteoclastogenesis: A systematic review of in vitro studies. Clin. Oral Investig. 2020;24:569–584. doi: 10.1007/s00784-019-03156-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kargarpour Z., Nasirzade J., Strauss F.J., Di Summa F., Hasannia S., Müller H.-D., Gruber R. Platelet-rich fibrin suppresses in vitro osteoclastogenesis. J. Periodontol. 2020;91:413–421. doi: 10.1002/JPER.19-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silva S.N., Pereira M.M., Goes A.M., Leite M.F. Effect of biphasic calcium phosphate on human macrophage functions in vitro. J. Biomed. Mater. Res. A. 2003;65:475–481. doi: 10.1002/jbm.a.10544. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y., Yang S., Zhou W., Fu H., Qian L., Miron R.J. Addition of a Synthetically Fabricated Osteoinductive Biphasic Calcium Phosphate Bone Graft to BMP2 Improves New Bone Formation. Clin. Implant Dent. Relat. Res. 2016;18:1238–1247. doi: 10.1111/cid.12384. [DOI] [PubMed] [Google Scholar]
- 20.Cordonnier T., Langonné A., Sohier J., Layrolle P., Rosset P., Sensébé L., Deschaseaux F. Consistent osteoblastic differentiation of human mesenchymal stem cells with bone morphogenetic protein 4 and low serum. Tissue Eng. Part C Methods. 2011;17:249–259. doi: 10.1089/ten.tec.2010.0387. [DOI] [PubMed] [Google Scholar]
- 21.Jeong J., Kim J.H., Shim J.H., Hwang N.S., Heo C.Y. Bioactive calcium phosphate materials and applications in bone regeneration. Biomater. Res. 2019;23:4. doi: 10.1186/s40824-018-0149-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balaguer T., Boukhechba F., Clavé A., Bouvet-Gerbettaz S., Trojani C., Michiels J.-F., Laugier J.-P., Bouler J.-M., Carle G.F., Scimeca J.-C., et al. Biphasic calcium phosphate microparticles for bone formation: Benefits of combination with blood clot. Tissue Eng. Part A. 2010;16:3495–3505. doi: 10.1089/ten.tea.2010.0227. [DOI] [PubMed] [Google Scholar]
- 23.Kumar A., Mahendra J., Samuel S., Govindraj J., Loganathan T., Vashum Y., Mahendra L., Krishnamoorthy T. Platelet-rich fibrin/biphasic calcium phosphate impairs osteoclast differentiation and promotes apoptosis by the intrinsic mitochondrial pathway in chronic periodontitis. J. Periodontol. 2019;90:61–71. doi: 10.1002/JPER.17-0306. [DOI] [PubMed] [Google Scholar]
- 24.Kim H., Kim M.-B., Kim C., Hwang J.-K. Inhibitory Effects of Panduratin A on Periodontitis-Induced Inflammation and Osteoclastogenesis through Inhibition of MAPK Pathways In Vitro. J. Microbiol. Biotechnol. 2018;28:190–198. doi: 10.4014/jmb.1707.07042. [DOI] [PubMed] [Google Scholar]
- 25.Bouler J.M., Pilet P., Gauthier O., Verron E. Biphasic calcium phosphate ceramics for bone reconstruction: A review of biological response. Acta Biomater. 2017;53:1–12. doi: 10.1016/j.actbio.2017.01.076. [DOI] [PubMed] [Google Scholar]
- 26.Armitage G.C. Development of a classification system for periodontal diseases and conditions. Ann. Periodontol. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- 27.Kylmäoja E., Nakamura M., Turunen S., Patlaka C., Andersson G., Lehenkari P., Tuukkanen J. Peripheral blood monocytes show increased osteoclast differentiation potential compared to bone marrow monocytes. Heliyon. 2018;4:e00780. doi: 10.1016/j.heliyon.2018.e00780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kany S., Vollrath J.T., Relja B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 2019;20:6008. doi: 10.3390/ijms20236008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Irwin C.R., Myrillas T.T. The role of IL-6 in the pathogenesis of periodontal disease. Oral Dis. 1998;4:43–47. doi: 10.1111/j.1601-0825.1998.tb00255.x. [DOI] [PubMed] [Google Scholar]
- 30.Isola G., Lo Giudice A., Polizzi A., Alibrandi A., Murabito P., Indelicato F. Identification of the different salivary Interleukin-6 profiles in patients with periodontitis: A cross-sectional study. Arch. Oral Biol. 2021;122:104997. doi: 10.1016/j.archoralbio.2020.104997. [DOI] [PubMed] [Google Scholar]
- 31.Assuma R., Oates T., Cochran D., Amar S., Graves D.T. IL-1 and TNF antagonists inhibit the inflammatory response and bone loss in experimental periodontitis. J. Immunol. 1998;160:403–409. [PubMed] [Google Scholar]
- 32.Liu T., Zhang L., Joo D., Sun S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017;2:17023. doi: 10.1038/sigtrans.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amarasekara D.S., Yun H., Kim S., Lee N., Kim H., Rho J. Regulation of osteoclast differentiation by Cytokine Networks. Immune Netw. 2018;18:e8. doi: 10.4110/in.2018.18.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matarese G., Currò M., Isola G., Caccamo D., Vecchio M., Giunta M.L., Ramaglia L., Cordasco G., Williams R.C., Ientile R. Transglutaminase 2 up-regulation is associated with RANKL/OPG pathway in cultured HPDL cells and THP-1-differentiated macrophages. Amino Acids. 2015;47:2447–2455. doi: 10.1007/s00726-015-2039-5. [DOI] [PubMed] [Google Scholar]
- 35.Lee K., Seo I., Choi M., Jeong D. Roles of Mitogen-Activated Protein Kinases in Osteoclast Biology. Int. J. Mol. Sci. 2018;19:3004. doi: 10.3390/ijms19103004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thouverey C., Caverzasio J. Focus on the p38 MAPK signaling pathway in bone development and maintenance. Bonekey Rep. 2015;4:711. doi: 10.1038/bonekey.2015.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu R., Bal H.S., Desta T., Behl Y., Graves D.T. Tumor necrosis factor-alpha mediates diabetes-enhanced apoptosis of matrix-producing cells and impairs diabetic healing. Am. J. Pathol. 2006;168:757–764. doi: 10.2353/ajpath.2006.050907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pacios S., Andriankaja O., Kang J., Alnammary M., Bae J., de Brito Bezerra B., Schreiner H., Fine D.H., Graves D.T. Bacterial infection increases periodontal bone loss in diabetic rats through enhanced apoptosis. Am. J. Pathol. 2013;183:1928–1935. doi: 10.1016/j.ajpath.2013.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choi S.-W., Son Y.-J., Yun J.-M., Kim S.H. Fisetin Inhibits osteoclast differentiation via Downregulation of p38 and c-Fos-NFATc1 Signaling Pathways. Evid.-Based Complement. Altern. Med. 2012;2012:810563. doi: 10.1155/2012/810563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boyce B.F., Xing L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch. Biochem. Biophys. 2008;473:139–146. doi: 10.1016/j.abb.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Franco G.C.N., Kajiya M., Nakanishi T., Ohta K., Rosalen P.L., Groppo F.C., Ernst C.W.O., Boyesen J.L., Bartlett J.D., Stashenko P., et al. Inhibition of matrix metalloproteinase-9 activity by doxycycline ameliorates RANK ligand-induced osteoclast differentiation in vitro and in vivo. Exp. Cell Res. 2011;317:1454–1464. doi: 10.1016/j.yexcr.2011.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klar R.M., Duarte R., Dix-Peek T., Dickens C., Ferretti C., Ripamonti U. Calcium ions and osteoclastogenesis initiate the induction of bone formation by coral-derived macroporous constructs. J. Cell. Mol. Med. 2013;17:1444–1457. doi: 10.1111/jcmm.12125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boyce B.F., Xing L. Biology of RANK, RANKL, and osteoprotegerin. Arthritis Res. Ther. 2007;9:S1. doi: 10.1186/ar2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rodríguez-Carballo E., Gámez B., Ventura F. p38 MAPK Signaling in Osteoblast Differentiation. Front. Cell Dev. Biol. 2016;4:40. doi: 10.3389/fcell.2016.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mukherjee A., Rotwein P. Akt promotes BMP2-mediated osteoblast differentiation and bone development. J. Cell Sci. 2009;122:716–726. doi: 10.1242/jcs.042770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Katarivas Levy G., Birch M.A., Brooks R.A., Neelakantan S., Markaki A.E. Stimulation of Human Osteoblast Differentiation in Magneto-Mechanically Actuated Ferromagnetic Fiber Networks. J. Clin. Med. 2019;8:1522. doi: 10.3390/jcm8101522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Komori T. Regulation of Osteoblast and Odontoblast Differentiation by RUNX2. J. Oral Biosci. 2010;52:22–25. doi: 10.1016/S1349-0079(10)80004-0. [DOI] [Google Scholar]