Key Points
Question
Is there an association between bariatric surgery and major adverse liver outcomes or major adverse cardiovascular events in patients with nonalcoholic steatohepatitis and obesity in long-term follow-up?
Findings
In this retrospective cohort study of 1158 patients (including 650 patients who underwent bariatric surgery and 508 patients in the nonsurgical control group) with biopsy-proven fibrotic nonalcoholic steatohepatitis without cirrhosis, bariatric surgery was significantly associated with a lower risk of major adverse liver outcomes (adjusted absolute 10-year risk difference of 12.4%) and major adverse cardiovascular events (adjusted absolute 10-year risk difference of 13.9%).
Meaning
Among patients with nonalcoholic steatohepatitis and obesity, bariatric surgery, compared with nonsurgical management, was associated with a significantly lower risk of incident major adverse liver outcomes and major adverse cardiovascular events.
Abstract
Importance
No therapy has been shown to reduce the risk of serious adverse outcomes in patients with nonalcoholic steatohepatitis (NASH).
Objective
To investigate the long-term relationship between bariatric surgery and incident major adverse liver outcomes and major adverse cardiovascular events (MACE) in patients with obesity and biopsy-proven fibrotic NASH without cirrhosis.
Design, Setting, and Participants
In the SPLENDOR (Surgical Procedures and Long-term Effectiveness in NASH Disease and Obesity Risk) study, of 25 828 liver biopsies performed at a US health system between 2004 and 2016, 1158 adult patients with obesity were identified who fulfilled enrollment criteria, including confirmed histological diagnosis of NASH and presence of liver fibrosis (histological stages 1-3). Baseline clinical characteristics, histological disease activity, and fibrosis stage of patients who underwent simultaneous liver biopsy at the time of bariatric surgery were balanced with a nonsurgical control group using overlap weighting methods. Follow-up ended in March 2021.
Exposures
Bariatric surgery (Roux-en-Y gastric bypass, sleeve gastrectomy) vs nonsurgical care.
Main Outcomes and Measures
The primary outcomes were the incidence of major adverse liver outcomes (progression to clinical or histological cirrhosis, development of hepatocellular carcinoma, liver transplantation, or liver-related mortality) and MACE (a composite of coronary artery events, cerebrovascular events, heart failure, or cardiovascular death), estimated using the Firth penalized method in a multivariable-adjusted Cox regression analysis framework.
Results
A total of 1158 patients (740 [63.9%] women; median age, 49.8 years [IQR, 40.9-57.9 years], median body mass index, 44.1 [IQR, 39.4-51.4]), including 650 patients who underwent bariatric surgery and 508 patients in the nonsurgical control group, with a median follow-up of 7 years (IQR, 4-10 years) were analyzed. Distribution of baseline covariates, including histological severity of liver injury, was well-balanced after overlap weighting. At the end of the study period in the unweighted data set, 5 patients in the bariatric surgery group and 40 patients in the nonsurgical control group experienced major adverse liver outcomes, and 39 patients in the bariatric surgery group and 60 patients in the nonsurgical group experienced MACE. Among the patients analyzed with overlap weighting methods, the cumulative incidence of major adverse liver outcomes at 10 years was 2.3% (95% CI, 0%-4.6%) in the bariatric surgery group and 9.6% (95% CI, 6.1%-12.9%) in the nonsurgical group (adjusted absolute risk difference, 12.4% [95% CI, 5.7%-19.7%]; adjusted hazard ratio, 0.12 [95% CI, 0.02-0.63]; P = .01). The cumulative incidence of MACE at 10 years was 8.5% (95% CI, 5.5%-11.4%) in the bariatric surgery group and 15.7% (95% CI, 11.3%-19.8%) in the nonsurgical group (adjusted absolute risk difference, 13.9% [95% CI, 5.9%-21.9%]; adjusted hazard ratio, 0.30 [95% CI, 0.12-0.72]; P = .007). Within the first year after bariatric surgery, 4 patients (0.6%) died from surgical complications, including gastrointestinal leak (n = 2) and respiratory failure (n = 2).
Conclusions and Relevance
Among patients with NASH and obesity, bariatric surgery, compared with nonsurgical management, was associated with a significantly lower risk of incident major adverse liver outcomes and MACE.
This cohort study investigates the association between bariatric surgery and incident major adverse liver outcomes and major adverse cardiovascular events in patients with obesity and biopsy-proven fibrotic NASH without cirrhosis.
Introduction
Nonalcoholic steatohepatitis (NASH), the hepatic manifestation of the metabolic syndrome, is a leading cause of cirrhosis and hepatocellular carcinoma, and is also significantly linked to cardiovascular disease. Diagnosis and management of NASH are challenging. Liver biopsy is required for accurate diagnosis and assessment of disease severity. There are currently no drug therapies for NASH approved by the US Food and Drug Administration or the European Medicines Agency. Furthermore, no therapy has been shown to be effective in reducing the risk of major adverse liver outcomes or major adverse cardiovascular events (MACE) in patients with NASH.1,2,3,4,5
In patients with obesity and metabolic disease, bariatric surgery (defined as a procedure that influences metabolism by inducing weight loss and altering gastrointestinal physiology) has substantial and sustained effects on the reduction of excess body weight and the improvement of hyperglycemia, hypertension, and dyslipidemia.6,7,8,9 Small observational studies using liver biopsy before and after bariatric surgery suggested that surgically induced weight loss was associated with improvement in some histological features of NASH (eg, inflammation, fibrosis).10,11,12 Large observational studies have consistently shown bariatric surgery to be associated with lower risk of MACE and mortality in patients with obesity.13,14,15
To address the current knowledge gap, the Surgical Procedures and Long-term Effectiveness in NASH Disease and Obesity Risk (SPLENDOR) study was designed to include a large number of patients with biopsy-proven fibrotic NASH without cirrhosis using overlap weighting statistical methods to accurately balance baseline histological severity of liver injury in patients who underwent bariatric surgery compared with patients who were medically managed. The goal of this analysis was to investigate the relationship between bariatric surgical procedures and development of both major adverse liver outcomes and MACE during long-term follow-up.
Methods
This was a retrospective cohort study that considered all patients who underwent liver biopsy at the Cleveland Clinic health system in the US between 2004 and 2016. Follow-up ended in March 2021. The Cleveland Clinic institutional review board approved the study as minimal risk research using data collected for routine clinical practice and the requirement for informed consent was waived. The original study protocol and amendments to the study protocol appear in Supplement 1. Diagnosis and procedure codes appear in eTables 1-5 in Supplement 2.
Study Cohorts and Enrollment Criteria
Available liver biopsy reports were reviewed to identify eligible patients with fibrotic NASH without cirrhosis for possible inclusion in the study. The histological nonalcoholic fatty liver disease (NAFLD) activity score was calculated for each patient based on the cumulative scores of liver steatosis (grade of 0 to 3), hepatocyte ballooning (grade of 0 to 2), and lobular inflammation (grade of 0 to 3). Diagnosis of NASH was confirmed by experienced pathologists and required having at least 1 point for each of steatosis, hepatocellular ballooning, and lobular inflammation. Liver fibrosis was staged from F0 (no fibrosis) to F4 (cirrhosis). Grading and staging of biopsies were done based on definitions from the Nonalcoholic Steatohepatitis Clinical Research Network.16
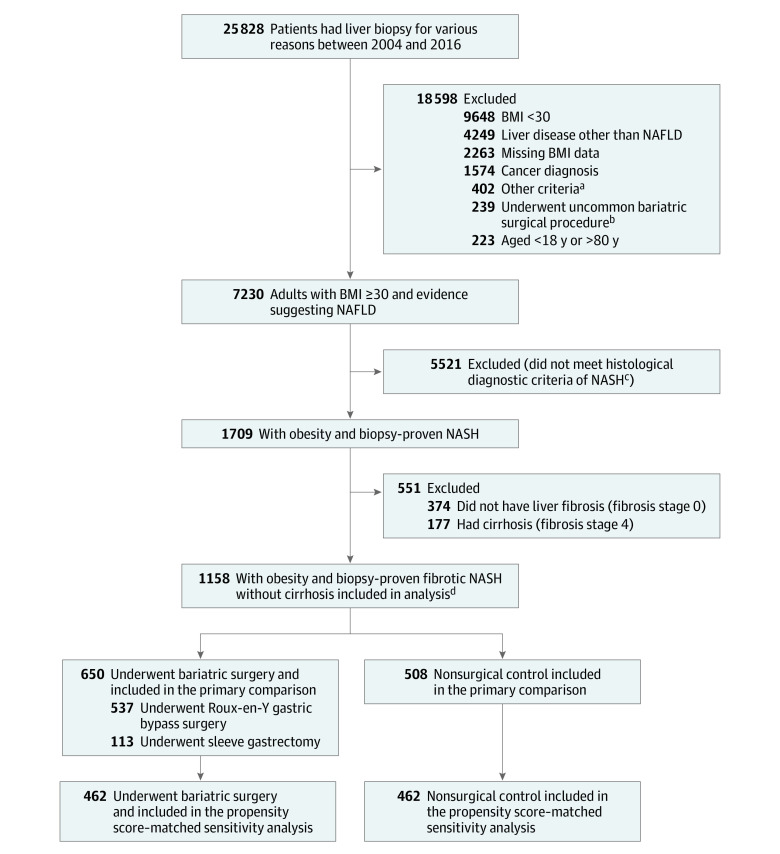
Among all patients with a liver biopsy (including percutaneous, transjugular, or surgical liver biopsy) performed during the study period, patients were included only if they met the following criteria: (1) were aged 18 years to 80 years, (2) with obesity (defined as a body mass index [BMI; calculated as weight in kilograms divided by height in meters squared] ≥30), (3) had a confirmed histological diagnosis of NASH (based on the Nonalcoholic Steatohepatitis Clinical Research Network criteria), and (4) had presence of fibrosis on the baseline liver biopsy (stages F1-F3) (Figure 1).
Figure 1. Identification of Eligible Patients and Development of Cohorts in the Study.
Diagnoses and procedure codes appear in eTables 1-5 in Supplement 2. BMI indicates body mass index (calculated as weight in kilograms divided by height in meters squared); NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis.
aHistory of excessive alcohol use or any medical conditions related to alcohol use disorder, organ transplantation, dialysis, HIV infection, severe heart failure (ejection fraction <20%), and received total parenteral nutrition prior to liver biopsy.
bGastric banding, gastric plication, or biliopancreatic diversion.
cAccording to criteria from the Nonalcoholic Steatohepatitis Clinical Research Network,16 which awards at least 1 point for each of the following to establish a diagnosis: steatosis, hepatocellular ballooning, and lobular inflammation.
dSelection criteria resulted in a total of 650 patients who underwent bariatric surgery and 508 patients in the nonsurgical control group with biopsy-proven fibrotic (fibrosis stages 1, 2, or 3) NASH without cirrhosis and BMI of 30 or greater for primary comparison using overlap weighting. The date of bariatric surgery and liver biopsy was considered as the index date for patients in the bariatric surgery group. The date of first liver biopsy on which all selection criteria were met served as the index date for patients in the nonsurgical control group.
Patients were excluded if they met any of the following criteria: (1) had evidence of histological (F4) or clinical diagnosis of cirrhosis (eg, presence of esophageal varices or ascites), (2) had a cause of chronic liver disease other than NASH, including drug-induced, viral, autoimmune, and genetic diseases (the diagnosis codes appear in eTable 1 in Supplement 2), (3) had a history of excessive alcohol use or any medical conditions related to alcohol use disorder (the diagnosis codes appear in eTable 1 in Supplement 2), (4) had hepatocellular carcinoma, (5) had received an organ transplantation, (6) had HIV infection, (7) were undergoing dialysis treatment prior to the liver biopsy, (8) had a history of severe heart failure (ejection fraction <20%) at any time before the liver biopsy, (9) had a diagnosis of any type of cancer within 1 year prior to the liver biopsy, or (10) had received total parenteral nutrition within the 6 months prior to the liver biopsy.
After application of the inclusion and exclusion criteria, eligible patients were categorized by whether they underwent bariatric surgery (Roux-en-Y gastric bypass or sleeve gastrectomy) or received nonsurgical care (the control group). Patients who underwent less common bariatric surgical procedures (the procedure codes appear in eTable 2 in Supplement 2) were excluded. As a routine practice, simultaneous core needle biopsy from the left lobe of liver under direct laparoscopic visualization was performed for all patients who underwent bariatric surgery and that was used as a baseline liver biopsy in the bariatric surgery group. The date of bariatric surgery and liver biopsy was considered as the index date for patients in the bariatric surgery group. The date of first liver biopsy on which all selection criteria were met served as the index date for patients in the nonsurgical control group.
Race data were obtained from the electronic health records and were based on patient self-report selected from fixed categories. Race was collected because it could be associated with both exposure and study end points.
Primary End Points
The prespecified primary end points were the incidence of major adverse liver outcomes and MACE. Major adverse liver outcomes as a composite end point was defined as first occurrence of progression to clinical (eg, development of esophageal varices, ascites, or hepatic encephalopathy) or histological (F4 on repeat liver biopsy) cirrhosis, development of hepatocellular carcinoma, liver transplantation, or liver-related mortality after the index date (definitions and diagnosis codes appear in eTable 4 in Supplement 2).
MACE as a composite end point was defined as first occurrence of coronary artery events (unstable angina, myocardial infarction, or coronary intervention or surgery), cerebrovascular events (ischemic or hemorrhagic stroke, transient ischemic attack, or carotid intervention or surgery), heart failure, or cardiovascular death after the index date (definitions and diagnosis codes appear in eTable 5 in Supplement 2). Any conditions or events that a patient had before liver biopsy were omitted from the analysis of MACE during follow-up. For example, if a patient had a history of heart failure before the liver biopsy, any hospitalization for heart failure after the liver biopsy was not considered an event for MACE. However, occurrence of stroke after the liver biopsy would be included in the composite end point.
Other End Points
Changes from baseline in body weight and hemoglobin A1c (only for patients with diabetes at baseline) were compared between patients in the bariatric surgery group and patients in the nonsurgical control group. Major complications of bariatric surgery were recorded.
Statistical Analysis
Continuous measures were summarized as median (IQR) for all samples. Categorical measures were reported as frequency and percentage for crude and matched samples and as percentage only for the weighted samples.
To minimize the effects of confounding factors, doubly robust estimation combining the overlap weighting and outcome regression was used to compare outcomes in the bariatric surgery group and in the nonsurgical control group. Overlap weighting is a propensity score method that attempts to mimic important features of randomized clinical trials. Weights are assigned to each patient that are proportional to the probability of that patient belonging to the opposite treatment group, resulting in inclusion of all available patients and exact balance for the mean of all covariates included in the model. Overlap weighting also has been shown in simulations to have improved precision relative to other weighting options.17,18 Six a priori–identified potential confounders (age at index date, sex, smoking status, presence of type 2 diabetes, histological NAFLD activity score, and histological liver fibrosis stage) were used for overlap weighting.
Subsequently, confounding factors also were addressed during the analytic phase by using statistical adjustment of the weighted samples. The Firth penalized method in the fully adjusted Cox proportional hazard framework19 was used by adjusting the models for the index date and for the following variables at baseline: BMI; race; annual zip code income; Cleveland Clinic location (Ohio vs Florida); Charlson Comorbidity Index score; presence of hypertension, dyslipidemia, heart failure, coronary artery disease, or cerebrovascular disease; levels of serum bilirubin, albumin, international normalized ratio, and creatinine; and use of insulin and noninsulin diabetes medication. The proportional hazards assumptions for the treatment variable were assessed visually through evaluations of the survival plots and the log minus log survival plots and through assessing the correlation between the Schoenfeld residuals and time.
For major adverse liver outcomes and MACE, cumulative incidence estimates (Kaplan-Meier method) and unadjusted absolute risk differences were calculated for 10 years after the index date. The adjusted absolute risk differences at 10 years were estimated for the composite end points using adjusted Cox models. The 95% CIs for the difference in 10-year risk were obtained by the percentile method from 2000 bootstrap iterations.
Overall, there were limited data missing. Data on demographics, comorbidities, medications, and liver biopsies were complete (Table 1), whereas annual zip code income and most laboratory values were available for more than 90% of patients. Only the lipid panel and the international normalized ratio had missing data for more than 10% of patients. To address missing values, 5 imputation data sets were created using all variables from Table 1 and the outcome variables. A regression-based imputation model with predictive mean matching was used. Imputation-corrected SEs of model estimates and comparisons were obtained using the Rubin formula.20,21
Table 1. Baseline Characteristics at Time of First Liver Biopsy.
| Unweighted | Weighteda | |||||
|---|---|---|---|---|---|---|
| Bariatric surgery | Nonsurgical control | Standardized differenceb |
Bariatric surgery | Nonsurgical control | Standardized differenceb |
|
| No. of patients | 650 | 508 | ||||
| Liver biopsy | ||||||
| Median date | 7/27/2011 | 6/15/2011 | 0.13 | 7/08/2011 | 5/26/2011 | 0.20 |
| IQR | 12/22/2009-12/18/2013 | 9/20/2008-8/02/2014 | 12/01/2009-12/03/2013 | 8/22/2008-7/08/2014 | ||
| Demographics | ||||||
| Sex, No. (%) or % | ||||||
| Female | 436 (67.1) | 304 (59.8) | 0.15 | 62.9 | 62.9 | 0c |
| Male | 214 (32.9) | 204 (40.2) | 37.1 | 37.1 | ||
| Age, median (IQR), y | 49.0 (40.5 to 57.0) | 50.8 (41.0 to 58.3) | −0.06 | 49.0 (41.0 to 57.0) | 50.2 (40.5 to 58.1) | 0c |
| BMI, median (IQR)d | 45.8 (41.2 to 53.1) | 35.7 (32.8 to 39.7) | 1.41 | 45.7 (41.2 to 52.8) | 36.0 (32.9 to 39.9) | 2.09 |
| Weight, median (IQR), kg | 128.8 (112.2 to 152.4) | 103.7 (91.7 to 115.5) | 1.09 | 129.0 (112.3 to 153.4) | 103.9 (91.6 to 115.4) | 1.67 |
| Race, No. (%) or % | ||||||
| American Indian | 3 (0.5) | 0 | 0.20 | 0.3 | 0 | 0.20 |
| Asian | 0 | 2 (0.4) | 0 | 0.4 | ||
| Black | 59 (9.1) | 21 (4.1) | 9.3 | 4.0 | ||
| Multiracial | 11 (1.7) | 6 (1.2) | 1.7 | 1.2 | ||
| White | 577 (88.8) | 479 (94.3) | 88.7 | 94.4 | ||
| Annual zip code income, median (IQR), $e | 54 821 (46 110 to 69 125) | 60 230 (46 873 to 73 697) | −0.23 | 55 182 (46 110 to 69 125) | 60 095 (46 873 to 72 122) | −0.31 |
| Smoking status, No. (%) or % | ||||||
| Never | 346 (53.2) | 263 (51.8) | 0.11 | 52.5 | 52.5 | 0c |
| Former | 260 (40.0) | 194 (38.2) | 39.1 | 39.1 | ||
| Current | 44 (6.8) | 51 (10.0) | 8.4 | 8.4 | ||
| Cleveland Clinic location, No. (%) or % | ||||||
| Florida | 6 (0.9) | 25 (4.9) | 0.24 | 1.0 | 4.9 | 0.23 |
| Ohio | 644 (99.1) | 483 (95.1) | 99.0 | 95.1 | ||
| Medical history, No. (%) or %f | ||||||
| CCI, median (IQR)g | 3.0 (2.0 to 4.0) | 2.0 (1.0 to 4.0) | 0.23 | 3.0 (2.0 to 4.0) | 2.0 (1.0 to 4.0) | 0.32 |
| Hypertension | 535 (82.3) | 241 (47.4) | 0.78 | 83.0 | 46.9 | 0.82 |
| Dyslipidemia | 479 (73.7) | 241 (47.4) | 0.56 | 73.9 | 46.3 | 0.59 |
| Type 2 diabetes | 330 (50.8) | 169 (33.3) | 0.36 | 40.6 | 40.6 | 0c |
| Heart failure | 39 (6.0) | 9 (1.8) | 0.22 | 6.1 | 1.7 | 0.23 |
| Coronary artery disease | 35 (5.4) | 21 (4.1) | 0.06 | 6.1 | 4.2 | 0.08 |
| Cerebrovascular disease | 13 (2.0) | 9 (1.8) | 0.02 | 1.9 | 1.4 | 0.04 |
| Medication history, No. (%) or % | ||||||
| Antihypertensive | 511 (78.6) | 310 (61.0) | 0.39 | 79.0 | 60.2 | 0.42 |
| Lipid-lowering | 354 (54.5) | 179 (35.2) | 0.39 | 54.4 | 34.9 | 0.40 |
| For diabetes | ||||||
| Not insulin | 343 (52.8) | 150 (29.5) | 0.49 | 49.4 | 30.8 | 0.39 |
| Insulin | 178 (27.4) | 43 (8.5) | 0.51 | 27.6 | 8.2 | 0.52 |
| Vitamin E | 39 (6.0) | 37 (7.3) | −0.05 | 6.2 | 7.3 | −0.04 |
| Clinical and laboratory data, median (IQR) h | ||||||
| Systolic BP, mm Hge | 136 (123 to 148) | 132 (121 to 142) | 0.18 | 136 (123 to 148) | 131 (121 to 143) | 0.24 |
| Diastolic BP, mm Hge | 73 (65 to 83) | 77 (68 to 84) | −0.19 | 73 (65 to 83) | 77 (68 to 84) | −0.27 |
| Albumin, g/dLe | 4.3 (4.1 to 4.5) | 4.4 (4.1 to 4.6) | −0.08 | 4.3 (4.1 to 4.5) | 4.4 (4.1 to 4.6) | −0.11 |
| Bilirubin, mg/dLe | 0.5 (0.4 to 0.6) | 0.5 (0.4 to 0.7) | −0.05 | 0.5 (0.4 to 0.6) | 0.5 (0.3 to 0.7) | −0.04 |
| Creatinine, mg/dLe | 0.8 (0.7 to 1.0) | 0.8 (0.7 to 0.9) | 0.15 | 0.8 (0.7 to 1.0) | 0.8 (0.7 to 0.9) | 0.35 |
| Hemoglobin A1c, %e,i | 7.3 (6.5 to 8.6) | 6.8 (6.0 to 7.6) | 0.39 | 7.4 (6.5 to 8.6) | 6.8 (6.0 to 7.5) | 0.68 |
| International normalized ratioe | 1.0 (1.0 to 1.1) | 1.0 (1.0 to 1.0) | 0.23 | 1.0 (1.0 to 1.1) | 1.0 (1.0 to 1.0) | 0.36 |
| Platelet count, × 109/Le | 253 (210 to 297) | 240 (199 to 289) | 0.13 | 248 (208 to 292) | 243 (201 to 290) | 0.07 |
| HDL cholesterol, mg/dLe | 41 (35 to 49) | 43 (36 to 50) | −0.12 | 41 (35 to 49) | 43 (36 to 51) | −0.25 |
| LDL cholesterol, mg/dLe | 98 (77 to 123) | 111 (89 to 141) | −0.45 | 98 (77 to 123) | 114 (89 to 142) | −0.67 |
| Triglycerides, mg/dLe | 162 (119 to 225) | 165 (121 to 231) | 0.02 | 159 (118 to 228) | 167 (122 to 232) | 0.05 |
| Liver biopsy, No. (%) or % | ||||||
| Steatosis score | ||||||
| 1 | 263 (40.5) | 159 (31.3) | 0.19 | 38.7 | 33.3 | 0.14 |
| 2 | 253 (38.9) | 227 (44.7) | 38.1 | 44.1 | ||
| 3 | 134 (20.6) | 122 (24.0) | 23.2 | 22.6 | ||
| Lobular inflammation score | ||||||
| 1 | 325 (50.0) | 298 (58.7) | 0.20 | 47.3 | 61.6 | 0.31 |
| 2 | 303 (46.6) | 186 (36.6) | 48.6 | 34.5 | ||
| 3 | 22 (3.4) | 24 (4.7) | 4.0 | 3.9 | ||
| Hepatocyte ballooning score | ||||||
| 1 | 544 (83.7) | 351 (69.1) | 0.35 | 81.6 | 72.0 | 0.23 |
| 2 | 106 (16.3) | 157 (30.9) | 18.4 | 28.0 | ||
| Histological NAFLD activity scorej | ||||||
| 3 | 129 (19.8) | 85 (16.7) | 0.16 | 18.5 | 18.5 | 0c |
| 4 | 209 (32.2) | 150 (29.5) | 30.7 | 30.7 | ||
| 5 | 200 (30.8) | 140 (27.6) | 29.2 | 29.2 | ||
| 6 | 86 (13.2) | 102 (20.1) | 16.5 | 16.5 | ||
| 7 | 23 (3.5) | 29 (5.7) | 4.8 | 4.8 | ||
| 8 | 3 (0.46) | 2 (0.39) | 0.4 | 0.4 | ||
| Histological liver fibrosis stagek | ||||||
| 1 | 380 (58.5) | 226 (44.5) | 0.28 | 50.8 | 50.8 | 0c |
| 2 | 158 (24.3) | 160 (31.5) | 29.2 | 29.2 | ||
| 3 | 112 (17.2) | 122 (24.0) | 20.0 | 20.0 | ||
Abbreviations: BMI, body mass index; BP, blood pressure; CCI, Charlson Comorbidity Index; HDL, high-density lipoprotein; LDL, low-density lipoprotein; NAFLD, nonalcoholic fatty liver disease.
SI conversion factors: To convert bilirubin to μmol/L, multiply by 17.104; creatinine to μmol/L, multiply by 76.25; hemoglobin A1c to mmol/mol, subtract 2.152 from the percentage value and divide by 0.0915; HDL and LDL cholesterol to mmol/L, multiply by 0.0259; triglycerides to mmol/L, multiply by 0.0113.
After overlap weighting, a single individual no longer represents a single data entity and thus raw counts are not reported after overlap weighting.
Absolute value of the between-group difference in means or proportions (bariatric surgery − nonsurgical control) divided by the pooled SD.
Overlap weighting provided precise balance for these important variables.
Calculated as weight in kilograms divided by height in meters squared.
There were missing data for this variable. Missing values were handled with multiple imputation. For albumin, there were missing data for 82 patients; annual zip code income, 17 patients; bilirubin, 81 patients; creatinine, 85 patients; HDL cholesterol, 350 patients; hemoglobin A1c, 154 patients; international normalized ratio, 523 patients; LDL cholesterol, 365 patients; platelet count, 59 patients; systolic and diastolic BP, 31 patients; and triglycerides, 347 patients.
The diagnosis codes appear in eTable 3 in Supplement 2.
A method of predicting the risk of mortality based on the International Classification of Diseases diagnosis codes for 17 comorbidities. Presence of each comorbidity is awarded a point from 1 to 6. The final score is calculated by the summation of applicable points and scores range from 0 (no disease burden) to 29 (maximal disease burden).
The normal value range is 3.9 g/dL to 4.9 g/dL for albumin; 0.2 mg/dL to 1.3 mg/dL for bilirubin; 0.58 mg/dL to 0.96 mg/dL for creatinine; >39 mg/dL for HDL cholesterol; 4.3% to 5.6% for hemoglobin A1c; 0.9 to 1.3 for international normalized ratio; <100 mg/dL for LDL cholesterol; 150 to 400 × 109/L for platelet count; and <150 mg/dL for triglycerides.
Only measured in patients with type 2 diabetes at baseline.
Higher scores indicate more severe histological changes. Calculated based on the cumulative scores of liver steatosis (grade of 0 to 3), hepatocyte ballooning (grade of 0 to 2), and lobular inflammation (grade of 0 to 3).
Higher stages represent more severe fibrosis. Stage 0, no fibrosis; stage 1, perisinusoidal or periportal; stage 2, perisinusoidal and portal or periportal; stage 3, bridging fibrosis; and stage 4, cirrhosis.
A 4-knot restricted cubic spline for time × treatment interaction within a linear mixed-effects model with random intercept for patients was used for comparing mean changes in weight and hemoglobin A1c over time.
A significance level of .05 for 2-sided comparisons was considered statistically significant. Hazard ratios (HRs) with 95% CIs are reported. Because of the nature of the study and the potential for type I error due to multiple comparisons, all findings should be interpreted as exploratory. All analyses were performed using SAS version 9.4 (SAS Institute Inc) and R version 4.0 (R Foundation for Statistical Reporting).
Sensitivity Analysis
To adjust for baseline differences, matching with a conventional propensity score method was used for the sensitivity analysis instead of overlap weighting. To create matched samples, a nearest-neighbor propensity score–matching method was performed based on the 6 baseline variables that were used for the overlap weighting (described above) with the MatchIt program in the R software using a caliper width equal to 0.2 of the SD of the propensity score logit.22 After matching, multivariable-adjusted Cox regression analysis was used to investigate time-to-incident major adverse liver outcomes and MACE using the Firth penalized method.
Furthermore, to assess the robustness of the identified association between bariatric surgery and the primary end points to potential unmeasured confounders, E-values were calculated using the methods of VanderWeele and Ding.23
Results
Among 25 828 patients with liver biopsy data, a total of 1158 adult patients (740 [63.9%] women; median age, 49.8 years [IQR, 40.9-57.9 years]; median BMI, 44.1 [IQR, 39.4-51.4]), including 650 patients in the bariatric surgery group and 508 patients in the nonsurgical control group, were analyzed (Figure 1). Bariatric surgical procedures included Roux-en-Y gastric bypass (n = 537; 83%) and sleeve gastrectomy (n = 113; 17%). Among the patients in the overlap-weighted analysis, the median follow-up time for the entire cohort was 7 years (IQR, 4-10 years), including 7 years (IQR, 3-10 years) for patients in the bariatric surgery group and 7 years (IQR, 4-11 years) for patients in the nonsurgical control group.
Distribution of baseline covariates between the study groups was well-balanced after overlap weighting for age, sex, smoking status, presence of diabetes, histological NAFLD activity score, and histological liver fibrosis stage (Table 1). Among the patients in the overlap-weighted analysis, the mean age was 49 years, 62.9% were women, 40.6% had type 2 diabetes, and 8.4% were current smokers in both groups. Of the patients at baseline, 18.5% had a NAFLD activity score of 3, 30.7% had a score of 4, 29.2% had a score of 5, 16.5% had a score of 6, 4.8% had a score of 7, and 0.4% had a score of 8. Of the patients at baseline, 50.8% were categorized as histological liver fibrosis stage 1, 29.2% as stage 2, and 20.0% as stage 3. These frequencies were precisely similar after overlap weighting for the bariatric surgery group and the nonsurgical control group. For other covariates, patients in the bariatric surgery group had a higher risk profile at baseline than patients in the nonsurgical control group, including a higher BMI (45.7 vs 36.0, respectively) and more comorbidities.
Major Adverse Liver Outcomes
At the end of the study period in the unweighted data set, 5 patients in the bariatric surgery group and 40 patients in the nonsurgical control group experienced major adverse liver outcomes. The frequency of major adverse liver outcomes components in the crude (unweighted) data set appears in eTable 6 in Supplement 2.
Among the patients in the overlap-weighted analysis, the cumulative incidence of major adverse liver outcomes at 10 years was 2.3% (95% CI, 0%-4.6%) in the bariatric surgery group and 9.6% (95% CI, 6.1%-12.9%) in the nonsurgical group (unadjusted absolute risk difference, 7.3% [95% CI, 3.2%-11.4%]; adjusted absolute risk difference, 12.4% [95% CI, 5.7%-19.7%]; adjusted HR, 0.12 [95% CI, 0.02-0.63], P = .01) (Figure 2A and Table 2).
Figure 2. Cumulative Incidence Estimates (Kaplan-Meier) for 2 Composite End Points in the Overlap-Weighted Analysis.
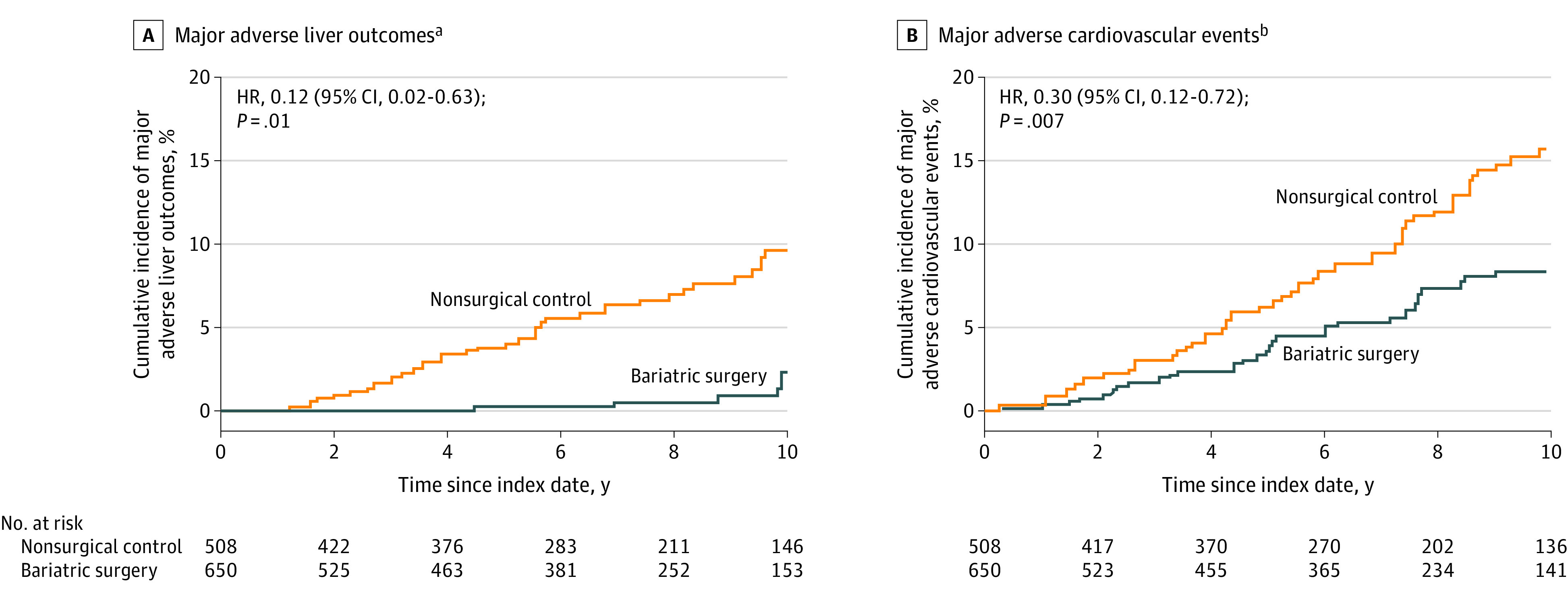
HR indicates hazard ratio. The date of bariatric surgery and liver biopsy was considered as the index date for patients in the bariatric surgery group. The date of first liver biopsy on which all selection criteria were met served as the index date for patients in the nonsurgical control group.
aComposite end point that was defined as the first occurrence of progression to clinical or histological cirrhosis, development of hepatocellular carcinoma, liver transplantation, or liver-related mortality after the index date. The median observation time was 84.6 months (IQR, 37.1-118.5 months) in the bariatric surgery group and 85.3 months (IQR, 48.5-127.4 months) in the nonsurgical control group.
bComposite end point that was defined as the first occurrence of coronary artery events, cerebrovascular events, heart failure, or cardiovascular mortality after the index date. The median observation time was 81.6 months (IQR, 34.9-116.0 months) in the bariatric surgery group and 80.6 months (IQR, 46.2-126.1 months) in the nonsurgical control group.
Table 2. Major Adverse Liver Outcomes and Major Adverse Cardiovascular Events Among Patients Who Underwent Bariatric Surgery vs a Nonsurgical Control Group at 10 Years.
| Bariatric surgery | Nonsurgical control | 10-Year absolute risk difference, % (95% CI)b,c | Hazard ratio (95% CI)b |
P valueb | |||
|---|---|---|---|---|---|---|---|
| No. at risk | Cumulative incidence at 10 years, % (95% CI)a | No. at risk | Cumulative incidence at 10 years, % (95% CI)a | ||||
| Major adverse liver outcomes d | |||||||
| Primary comparisone | 650 | 2.3 (0-4.6) | 508 | 9.6 (6.1-12.9) | 12.4 (5.7-19.7) | 0.12 (0.02-0.63) | .01 |
| Sensitivity analysisf | 462 | 2.2 (0-4.7) | 462 | 10.7 (6.8-14.5) | 13.5 (6.0-22.4) | 0.09 (0.02-0.35) | <.001 |
| Major adverse cardiovascular events g | |||||||
| Primary comparisone | 650 | 8.5 (5.5-11.4) | 508 | 15.7 (11.3-19.8) | 13.9 (5.9-21.9) | 0.30 (0.12-0.72) | .007 |
| Sensitivity analysisf | 462 | 7.9 (4.4-11.2) | 462 | 16.3 (11.6-20.8) | 14.6 (6.6-23.4) | 0.25 (0.12-0.49) | <.001 |
Unadjusted estimates. Analysis treating death as a competing risk was also performed and provided similar estimates. The date of bariatric surgery and liver biopsy was considered as the index date for patients in the bariatric surgery group. The date of first liver biopsy on which all selection criteria were met served as the index date for patients in the nonsurgical control group.
From fully adjusted Firth penalized Cox regression models (using the variables of liver biopsy index date, baseline body mass index, race, annual zip code income, location [Florida or Ohio], and Charlson Comorbidity Index score; presence of hypertension, dyslipidemia, heart failure, coronary artery disease, or cerebrovascular disease; baseline levels of serum bilirubin, albumin, creatinine, and international normalized ratio; and use of insulin and noninsulin diabetes medication).
The 95% CIs for the difference in 10-year absolute risk (nonsurgical control group − bariatric surgery group) were obtained by the percentile method from 2000 bootstrap iterations.
A composite end point that was defined as occurrence of progression to clinical or histological cirrhosis, development of hepatocellular carcinoma, liver transplantation, or liver-related mortality. The frequency of events in the crude (unweighted) data set appears in eTable 6 in Supplement 2.
Balancing the bariatric surgery and nonsurgical control groups with overlap weighting.
Balancing the bariatric surgery and nonsurgical control groups with propensity score matching in the sensitivity analysis.
A composite end point that was defined as occurrence of coronary artery events, cerebrovascular events, heart failure, or cardiovascular mortality. The frequency of events in the crude (unweighted) data set appears in eTable 6 in Supplement 2.
MACE
At the end of the study period in the unweighted data set, 39 patients in the bariatric surgery group and 60 patients in the nonsurgical control group experienced MACE. The frequency of MACE components in the crude (unweighted) data set appears in eTable 6 in Supplement 2.
Among the patients in the overlap-weighted analysis, the cumulative incidence of MACE at 10 years was 8.5% (95% CI, 5.5%-11.4%) in the bariatric surgery group and 15.7% (95% CI, 11.3%-19.8%) in the nonsurgical control group (unadjusted absolute risk difference, 7.2% [95% CI, 2.0%-12.3%]; adjusted absolute risk difference, 13.9% [95% CI, 5.9%-21.9%]; adjusted HR, 0.30 [95% CI, 0.12-0.72], P = .007) (Figure 2B and Table 2). The proportional hazards assumption was satisfied for the composite outcomes.
Status of Obesity and Diabetes Over Time
At 10 years, mean body weight was reduced by 22.4% (95% CI, 21.7%-23.0%) in patients in the bariatric surgery group and by 4.6% (95% CI, 3.9%-5.4%) in patients in the nonsurgical control group (mean between-group difference, 17.7% [95% CI, 16.7%-18.7%], P < .001). Bariatric surgery also was associated with a significant reduction in hemoglobin A1c level in patients with diabetes (mean between-group difference for change from baseline to 10 years, 1.6% [95% CI, 1.3%-1.9%], P < .001) (Figure 3 and eTable 7 in Supplement 2).
Figure 3. Trend Curves of Mean Change in Body Weight and Hemoglobin A1c Level Over 10 Years of Follow-up in the Overlap-Weighted Analysis.
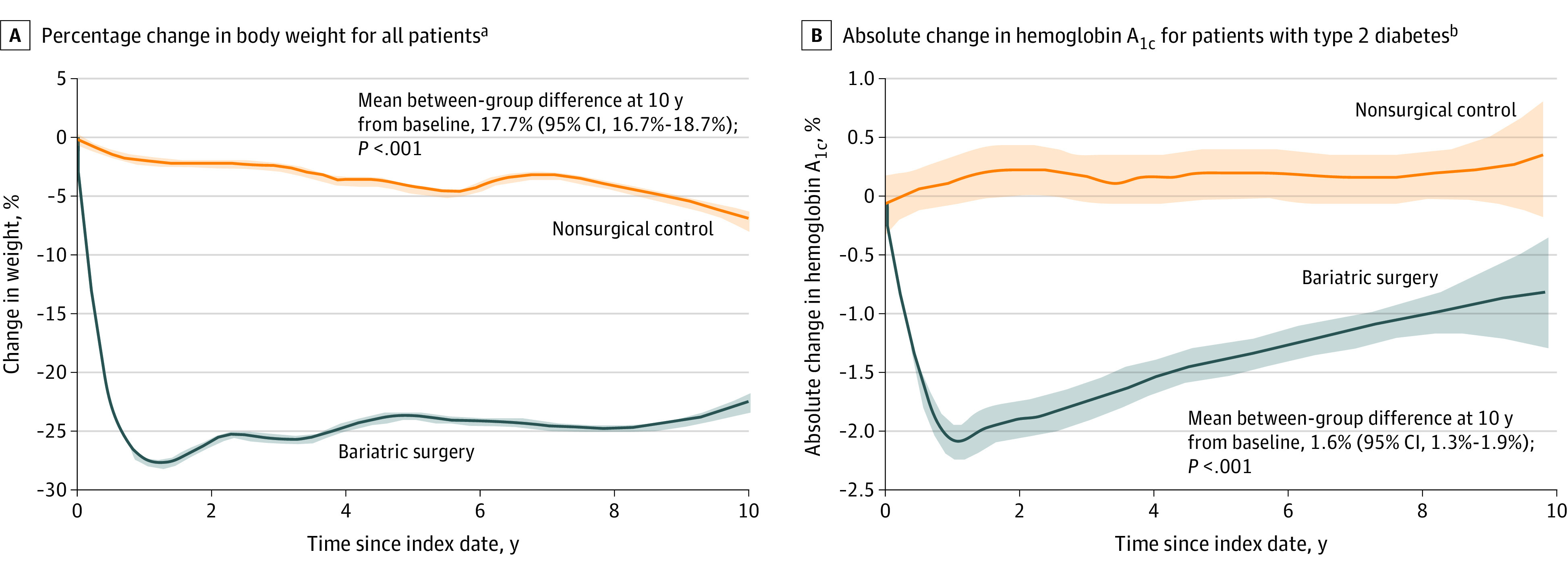
Data are illustrated as smoothed mean trends from baseline to follow-up. The shaded areas indicate 95% CIs. The mean between-group differences at 10 years from baseline were estimated from a flexible regression model with a 4-knot restricted cubic spline for time × treatment interaction because the time from index date interacted with the treatment group. The date of bariatric surgery and liver biopsy was considered as the index date for patients in the bariatric surgery group. The date of first liver biopsy on which all selection criteria were met served as the index date for patients in the nonsurgical control group. The sample sizes at different time points appear in eTable 7 in Supplement 2.
aThe median observation time was 74.9 months (IQR, 27.6-110.2 months) in the bariatric surgery group and 77.3 months (IQR, 38.4-123.1 months) in the nonsurgical control group.
bThe median observation time was 41.2 months (IQR, 7.1-85.3 months) in the bariatric surgery group and 81.9 months (IQR, 43.7-120.0 months) in the nonsurgical control group. The shorter observation time in the bariatric surgery group is likely due to less need for regular testing in patients with improved diabetes after surgery.
Major Adverse Events After Bariatric Surgery
Within 30 days after bariatric surgery, 62 patients (9.5%) developed major adverse events, including postoperative sepsis (n = 23; 3.5%), gastrointestinal leak (n = 14; 2.2%), pulmonary adverse events (n = 14; 2.2%), bleeding (n = 11; 1.7%), venous thromboembolism (n = 9; 1.4%), acute kidney injury (n = 8; 1.2%), small bowel obstruction (n = 4; 0.6%), and cardiac adverse events (n = 3; 0.5%).
Within the first year after bariatric surgery, 4 patients (0.6%) died from surgical complications, including gastrointestinal leak (n = 2) and respiratory failure (n = 2). Subsequently, 3 additional patients died during long-term follow-up from conditions that could be attributed to their history of bariatric surgery, including intestinal obstruction and perforation (n = 1), possible suicide (n = 1), and a death possibly related to alcohol use disorder (n = 1). All 7 patients had undergone gastric bypass.
Sensitivity Analysis
Using conventional propensity score matching, 462 patients in each group were used for the analysis (eTable 8 in Supplement 2). Overall, the differences in HRs comparing the risk of major adverse liver outcomes and MACE in patients in the bariatric surgery group vs patients in the nonsurgical control group and the adjusted absolute risk differences were negligible between the overlap weighting (primary comparison) and conventional matching (sensitivity analysis) (Table 2 and eFigure in Supplement 2).
Examining the E-values for major adverse liver outcomes and MACE and comparing these E-values with the HR estimates of known risk factors for these end points (eTable 9 in Supplement 2) indicates that it would be unlikely that an unmeasured confounder exists that could account for the observed association between bariatric surgery and risk of major adverse liver outcomes and MACE.
Discussion
In this observational study, bariatric surgery, compared with nonsurgical management, was associated with a significantly lower risk of incident major adverse liver outcomes and MACE among patients with NASH and obesity.
NASH in patients with obesity is associated with major adverse liver and cardiovascular outcomes. Ideal management requires a comprehensive approach to reverse liver injury and minimize the risk of both major adverse liver outcomes and MACE. To our knowledge, this is the first study reporting a treatment modality that is associated with decreased risk of major clinical end points in patients with biopsy-proven NASH. Given the large sample size, accurate histological diagnosis of NASH, statistical adjustments based on histological severity of liver disease by using overlap weighting statistical methods, and long duration of follow-up (median, 7 years), the current study provides evidence that bariatric surgery is associated with lower risk of major adverse liver outcomes and MACE in patients with NASH and obesity. The findings from this observational study suggest that bariatric surgery can be considered as a therapeutic option for patients with NASH and obesity.
Because sufficiently powered randomized clinical trials using liver biopsy and adequate follow-up duration to assess rare clinical end points in this slowly progressive disease are unlikely to be conducted in the near future, carefully designed observational studies (with adjustment for patient characteristics across treatment groups) can be useful to inform treatment decisions. Overlap weighting can reproduce many aspects of randomized clinical trials and avoids some of the limitations associated with classic propensity score matching.17,18 In the current study, overlap-weighting techniques were used to adjust for differences in major risk factors between patients in the bariatric surgery group and patients in the nonsurgical control group for MACE (age, sex, smoking status, and presence of diabetes) and major adverse liver outcomes (presence of diabetes, histological NAFLD activity score, and histological liver fibrosis stage), which led to precise balance on these critical variables at baseline. Instead of relying on imaging or available clinical risk scoring systems, liver biopsy data were used to characterize and quantify histological features of steatosis, hepatocyte ballooning, lobular inflammation, and liver fibrosis to accurately diagnose NASH and statistically adjust the study groups based on severity of liver disease, particularly liver fibrosis, at baseline. Liver fibrosis is a key prognostic marker for development of major adverse liver outcomes and mortality in patients with NASH.1,2,3,4,5 During the second step of statistical adjustment, models were further adjusted based on several other covariates (eg, hypertension and dyslipidemia) by using the Firth method, which can increase the precision of estimates in Cox proportional hazards models. Comprehensive hepatology and bariatric surgery programs with liberal performance of liver biopsy for patients with NAFLD in medical and surgical settings during the last 2 decades were essential in identifying a large number of patients despite having strict clinical and histological selection criteria.
Although several drugs targeting improvement in histological features of NASH are in various stages of development, there is currently no drug therapy for NASH approved by regulatory authorities. A few small randomized clinical trials with short-term follow-up using liver biopsy before and after treatment have shown modest histological benefits for a small number of medications including vitamin E,24 pioglitazone,24 obeticholic acid,25 liraglutide,26 and semaglutide.27 Histological benefits after treatment, including resolution of NASH or improvement of liver fibrosis, are considered as surrogate markers for major clinical outcomes. Even if a medication is approved based on improvement in surrogate markers in the near future, it will be challenging to examine the effects on major clinical outcomes, including major adverse liver outcomes and MACE, that often take a decade to develop. Furthermore, some medications such as vitamin E and obeticholic acid are unlikely to improve cardiovascular risk.1,28,29
Obesity is the main pathophysiologic driver of NASH. Although losing weight, regardless of how it is achieved, is the current primary treatment of NASH,1,2,3,4 bariatric surgery is the most effective available therapy for obesity. Accumulation of ectopic fat, immune system activation, and insulin resistance are the key initial steps that trigger development of NASH in patients with obesity.1,2,3,4,5 Substantial and sustained weight loss after bariatric surgery and subsequent improvement in metabolic dysfunction and inflammatory state can potentially reverse histopathological changes and spare the liver from progressive damage. In a prospective study including 64 patients with repeat liver biopsy 5 years after bariatric surgery, NASH was resolved in 84% (95% CI, 73%-92%), fibrosis stage decreased in 70% (95% CI, 57%-82%), and fibrosis disappeared in 56% (95% CI, 42%-69%).10 It is estimated that about 20% of patients with NASH develop cirrhosis during their lifetimes,30 and the findings of the current study suggest that bariatric surgery is associated with an 88% lower risk of progression to major adverse liver outcomes during long-term follow-up.
NASH and cardiovascular diseases share numerous risk factors because both represent end-organ damage caused by metabolic derangements. In addition, NASH independently increases the risk of atherosclerosis, cardiomyopathy, and cardiac arrhythmia.5 Bariatric surgery leads to a substantial and sustained weight loss, and effectively improves cardiometabolic risk factors6,7,8,9 and quality of life.6,31 Large matched-cohort studies have shown that bariatric surgery is associated with reduction in risk of MACE in patients with type 2 diabetes and obesity, presumably by its positive effects on cardiac risk factors, metabolism, function, workload, and geometry.13,14,15 Therefore, it was not unexpected in the current study to observe a lower risk of MACE among patients with NASH in the bariatric surgery group.
Limitations
This study has several limitations. First, even though establishing a histological diagnosis of fibrotic NASH and balancing the study groups based on the histological grade and stage of liver disease were unique features of this study, challenges in interpreting liver biopsies including sampling bias and interobserver variability are well-known problems in histological assessments of NASH.1,2,3
Second, although overlap weighting created precise balance for certain critical covariates,17,18 there was imbalance on other baseline variables, albeit mostly favoring the nonsurgical control group. In the doubly robust estimation approach used in this study, the intention of the regression adjustment using the Firth penalized models was to control any imbalances that remained after the overlap-weighting process. Nevertheless, residual measured or unmeasured confounders, such as the healthy user effect (ie, patients who chose to undergo bariatric surgery could be more health conscious and partake in other healthy behaviors), can bias results in this observational study. No matching or statistical adjustment was performed based on the severity of other medical conditions at baseline. As a sensitivity analysis to assess the robustness of the observed associations in the presence of potential unmeasured confounders, E-values were calculated. The estimated E-values for major adverse liver outcomes and MACE were larger than the magnitude of the associations between these end points and their known risk factors, indicating that it is unlikely that an unmeasured confounder exists that can eliminate the favorable association between bariatric surgery and major adverse liver outcomes or MACE (eTable 9 in Supplement 2).
Third, the modest number of major adverse liver outcomes and MACE resulted in relatively wide 95% CIs. However, the statistical comparisons consistently reached significance both in the primary comparison and in the sensitivity analysis. Individual analysis of the components of the composite end points was not possible due to the small number of individual events.
Fourth, although the Firth penalized likelihood methods (instead of standard Cox models) were used to reduce the small sample bias and allow models to be fit,19 given the small number of events and the large number of predictors included in the models, the fully adjusted results should be interpreted with caution.
Fifth, there may have been coding errors, misclassification, and misdiagnosis in the electronic health records. Sixth, even though sleeve gastrectomy is currently the most common bariatric surgical procedure,32 only 17% of patients in the current study underwent sleeve gastrectomy.
Conclusions
Among patients with NASH and obesity, bariatric surgery, compared with nonsurgical management, was associated with a significantly lower risk of incident major adverse liver outcomes and MACE.
Trial protocol
eTable 1. Diagnosis and procedure codes to assist in identifying pre-existing conditions that could disqualify patients for study eligibility
eTable 2. Procedure codes to assist in identifying different types of bariatric and metabolic surgical interventions
eTable 3. Diagnosis and procedure codes to assist in identifying baseline medical conditions
eTable 4. Diagnosis and procedure codes to assist in identifying conditions that could qualify as a major adverse liver outcome
eTable 5. Diagnosis and procedure codes to assist in identifying conditions that could qualify as a major adverse cardiovascular event
eTable 6. Number of events in metabolic surgery patients and nonsurgical control patients in unadjusted dataset before overlap weighting
eTable 7. Total number of observations and number of distinct patients with available measurements at and after each time-point following the index date for HbA1c and weight values by treatment groups
eTable 8. Baseline characteristics of metabolic surgery patients and nonsurgical control patients at time of first liver biopsy before and after propensity score matching
eTable 9. E-value for the effect of metabolic surgery on MALO and MACE (and its upper limit of 95% CI) in fully-adjusted Cox models and details about the E-value
eFigure. 10-year cumulative incidence estimates (Kaplan-Meier) for 2 composite endpoints in the propensity matched patients
References
- 1.Powell EE, Wong VW, Rinella M. Non-alcoholic fatty liver disease. Lancet. 2021;397(10290):2212-2224. doi: 10.1016/S0140-6736(20)32511-3 [DOI] [PubMed] [Google Scholar]
- 2.Sheka AC, Adeyi O, Thompson J, Hameed B, Crawford PA, Ikramuddin S. Nonalcoholic steatohepatitis: a review. JAMA. 2020;323(12):1175-1183. doi: 10.1001/jama.2020.2298 [DOI] [PubMed] [Google Scholar]
- 3.Diehl AM, Day C. Cause, pathogenesis, and treatment of nonalcoholic steatohepatitis. N Engl J Med. 2017;377(21):2063-2072. doi: 10.1056/NEJMra1503519 [DOI] [PubMed] [Google Scholar]
- 4.Ferguson D, Finck BN. Emerging therapeutic approaches for the treatment of NAFLD and type 2 diabetes mellitus. Nat Rev Endocrinol. 2021;17(8):484-495. doi: 10.1038/s41574-021-00507-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stahl EP, Dhindsa DS, Lee SK, Sandesara PB, Chalasani NP, Sperling LS. Nonalcoholic fatty liver disease and the heart: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73(8):948-963. doi: 10.1016/j.jacc.2018.11.050 [DOI] [PubMed] [Google Scholar]
- 6.Mingrone G, Panunzi S, De Gaetano A, et al. Metabolic surgery versus conventional medical therapy in patients with type 2 diabetes: 10-year follow-up of an open-label, single-centre, randomised controlled trial. Lancet. 2021;397(10271):293-304. doi: 10.1016/S0140-6736(20)32649-0 [DOI] [PubMed] [Google Scholar]
- 7.Schauer PR, Bhatt DL, Kirwan JP, et al. ; STAMPEDE Investigators . Bariatric surgery versus intensive medical therapy for diabetes—5-year outcomes. N Engl J Med. 2017;376(7):641-651. doi: 10.1056/NEJMoa1600869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ikramuddin S, Korner J, Lee WJ, et al. Lifestyle intervention and medical management with vs without Roux-en-Y gastric bypass and control of hemoglobin A1c, LDL cholesterol, and systolic blood pressure at 5 years in the Diabetes Surgery Study. JAMA. 2018;319(3):266-278. doi: 10.1001/jama.2017.20813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schiavon CA, Bersch-Ferreira AC, Santucci EV, et al. Effects of bariatric surgery in obese patients with hypertension: the GATEWAY randomized trial (Gastric Bypass to Treat Obese Patients With Steady Hypertension). Circulation. 2018;137(11):1132-1142. doi: 10.1161/CIRCULATIONAHA.117.032130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lassailly G, Caiazzo R, Ntandja-Wandji LC, et al. Bariatric surgery provides long-term resolution of nonalcoholic steatohepatitis and regression of fibrosis. Gastroenterology. 2020;159(4):1290-1301.e5. doi: 10.1053/j.gastro.2020.06.006 [DOI] [PubMed] [Google Scholar]
- 11.Manco M, Mosca A, De Peppo F, et al. The benefit of sleeve gastrectomy in obese adolescents on nonalcoholic steatohepatitis and hepatic fibrosis. J Pediatr. 2017;180:31-37.e2. doi: 10.1016/j.jpeds.2016.08.101 [DOI] [PubMed] [Google Scholar]
- 12.Mattar SG, Velcu LM, Rabinovitz M, et al. Surgically-induced weight loss significantly improves nonalcoholic fatty liver disease and the metabolic syndrome. Ann Surg. 2005;242(4):610-617. doi: 10.1097/01.sla.0000179652.07502.3f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aminian A, Zajichek A, Arterburn DE, et al. Association of metabolic surgery with major adverse cardiovascular outcomes in patients with type 2 diabetes and obesity. JAMA. 2019;322(13):1271-1282. doi: 10.1001/jama.2019.14231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sjöström L, Peltonen M, Jacobson P, et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA. 2014;311(22):2297-2304. doi: 10.1001/jama.2014.5988 [DOI] [PubMed] [Google Scholar]
- 15.Fisher DP, Johnson E, Haneuse S, et al. Association between bariatric surgery and macrovascular disease outcomes in patients with type 2 diabetes and severe obesity. JAMA. 2018;320(15):1570-1582. doi: 10.1001/jama.2018.14619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kleiner DE, Brunt EM, Van Natta M, et al. ; Nonalcoholic Steatohepatitis Clinical Research Network . Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313-1321. doi: 10.1002/hep.20701 [DOI] [PubMed] [Google Scholar]
- 17.Thomas LE, Li F, Pencina MJ. Overlap weighting: a propensity score method that mimics attributes of a randomized clinical trial. JAMA. 2020;323(23):2417-2418. doi: 10.1001/jama.2020.7819 [DOI] [PubMed] [Google Scholar]
- 18.Li F, Morgan KL, Zaslavsky AM. Balancing covariates via propensity score weighting. J Am Stat Assoc. 2018;113(521):390-400. doi: 10.1080/01621459.2016.1260466 [DOI] [Google Scholar]
- 19.Heinze G, Schemper M. A solution to the problem of monotone likelihood in Cox regression. Biometrics. 2001;57(1):114-119. doi: 10.1111/j.0006-341X.2001.00114.x [DOI] [PubMed] [Google Scholar]
- 20.van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res. 2007;16(3):219-242. doi: 10.1177/0962280206074463 [DOI] [PubMed] [Google Scholar]
- 21.Rubin DB. Inference and missing data. Biometrika. 1976;63(3):581-592. doi: 10.1093/biomet/63.3.581 [DOI] [Google Scholar]
- 22.Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011;10(2):150-161. doi: 10.1002/pst.433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med. 2017;167(4):268-274. doi: 10.7326/M16-2607 [DOI] [PubMed] [Google Scholar]
- 24.Sanyal AJ, Chalasani N, Kowdley KV, et al. ; NASH CRN . Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362(18):1675-1685. doi: 10.1056/NEJMoa0907929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neuschwander-Tetri BA, Loomba R, Sanyal AJ, et al. ; NASH Clinical Research Network . Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385(9972):956-965. doi: 10.1016/S0140-6736(14)61933-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Armstrong MJ, Gaunt P, Aithal GP, et al. ; LEAN Trial Team . Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387(10019):679-690. doi: 10.1016/S0140-6736(15)00803-X [DOI] [PubMed] [Google Scholar]
- 27.Newsome PN, Buchholtz K, Cusi K, et al. ; NN9931-4296 Investigators . A placebo-controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. N Engl J Med. 2021;384(12):1113-1124. doi: 10.1056/NEJMoa2028395 [DOI] [PubMed] [Google Scholar]
- 28.Heart Outcomes Prevention Evaluation Study Investigators . Vitamin E supplementation and cardiovascular events in high-risk patients. N Engl J Med. 2000;342:154-160. doi: 10.1056/NEJM200001203420302 [DOI] [PubMed] [Google Scholar]
- 29.Eslam M, Alvani R, Shiha G. Obeticholic acid: towards first approval for NASH. Lancet. 2019;394(10215):2131-2133. doi: 10.1016/S0140-6736(19)32963-0 [DOI] [PubMed] [Google Scholar]
- 30.Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116(6):1413-1419. doi: 10.1016/S0016-5085(99)70506-8 [DOI] [PubMed] [Google Scholar]
- 31.Aminian A, Kashyap SR, Wolski KE, et al. Patient-reported outcomes after metabolic surgery versus medical therapy for diabetes: insights from the STAMPEDE randomized trial. Ann Surg. 2021;274(3):524-532. doi: 10.1097/SLA.0000000000005003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.English WJ, DeMaria EJ, Hutter MM, et al. American Society for Metabolic and Bariatric Surgery 2018 estimate of metabolic and bariatric procedures performed in the United States. Surg Obes Relat Dis. 2020;16(4):457-463. doi: 10.1016/j.soard.2019.12.022 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
eTable 1. Diagnosis and procedure codes to assist in identifying pre-existing conditions that could disqualify patients for study eligibility
eTable 2. Procedure codes to assist in identifying different types of bariatric and metabolic surgical interventions
eTable 3. Diagnosis and procedure codes to assist in identifying baseline medical conditions
eTable 4. Diagnosis and procedure codes to assist in identifying conditions that could qualify as a major adverse liver outcome
eTable 5. Diagnosis and procedure codes to assist in identifying conditions that could qualify as a major adverse cardiovascular event
eTable 6. Number of events in metabolic surgery patients and nonsurgical control patients in unadjusted dataset before overlap weighting
eTable 7. Total number of observations and number of distinct patients with available measurements at and after each time-point following the index date for HbA1c and weight values by treatment groups
eTable 8. Baseline characteristics of metabolic surgery patients and nonsurgical control patients at time of first liver biopsy before and after propensity score matching
eTable 9. E-value for the effect of metabolic surgery on MALO and MACE (and its upper limit of 95% CI) in fully-adjusted Cox models and details about the E-value
eFigure. 10-year cumulative incidence estimates (Kaplan-Meier) for 2 composite endpoints in the propensity matched patients