Abstract
Lactic acid (LA) is an important organic acid with broad industrial applications. Considered as an environmentally friendly alternative to petroleum-based plastic with a wide range of applications, polylactic acid has generated a great deal of interest and therefore the demand for optically pure l- or d-lactic acid has increased accordingly. Microbial fermentation is the industrial route for LA production. LA bacteria and certain genetic engineering bacteria are widely used for LA production. Although some fungi, such as Saccharomyces cerevisiae, are not natural LA producers, they have recently received increased attention for LA production because of their acid tolerance. The main challenge for LA bioproduction is the high cost of substrates. The development of LA production from cost-effective biomasses is a potential solution to reduce the cost of LA production. This review examined and discussed recent progress in optically pure l-lactic acid and optically pure d-lactic acid fermentation. The utilization of inexpensive substrates is also focused on. Additionally, for PLA production, a complete biological process by one-step fermentation from renewable resources is also currently being developed by metabolically engineered bacteria. We also summarize the strategies and procedures for metabolically engineering microorganisms producing PLA. In addition, there exists some challenges to efficiently produce PLA, therefore strategies to overcome these challenges through metabolic engineering combined with enzyme engineering are also discussed.
Keywords: lactic acid, polylactic acid, microbial production, renewable resource, clean fermentation, metabolic engineering
1. Introduction
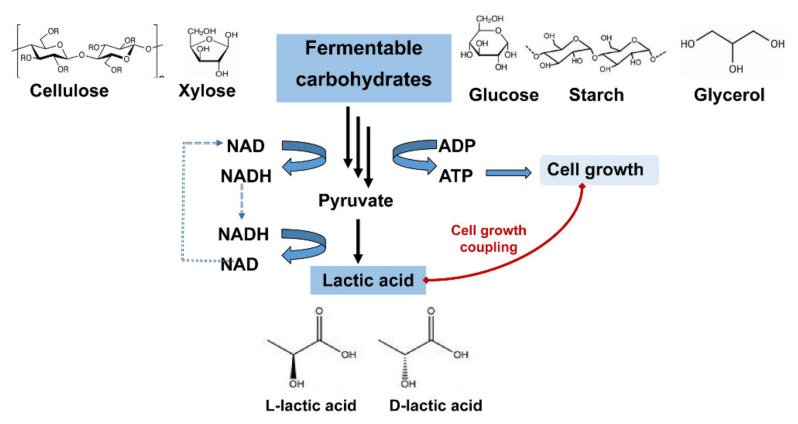
Lactic acid (CH3-CHOHCOOH, LA) is one of the most important building-block chemicals in the world. It contains a hydroxyl group and a carboxyl group. Because of its functional groups, LA could be used as a starting material for the synthesis of various chemicals, such as acrylic acid, 1,2-propanediol, 2,3-pentanedione [1]. The most widely applied use for LA is in the production of biodegradable polymers, polylactic acid (PLA) [2]. PLA is a biodegradable polymer, which has recently increased in global demand due to its increasing application as a bioplastic. Global plastics production totalled 368 million metric tons in 2020. At an estimated 10% replacement of fossil-fuel-based plastics, the overall demand for PLA is envisioned to reach 30 million tons per year. As the precursor of PLA, the demand for LA is estimated to reach 1960 kilotons by 2025 [3]. There are three forms of LA: l-lactic acid, d-lactic acid and racemic mixtures (dl-lactic acid) [4]. LA exists in two enantiomeric forms of l-lactic acid and d-lactic acid (Figure 1), and optically pure l-lactic acid and optically pure d-lactic acid are considered to have more value than racemic mixtures. l-lactic acid is widely used in the food industry. Optically pure l-lactic acid (≥99%) is the main precursor of PLA, and the addition of optically pure d-lactic acid may change the mechanical properties of PLA. Therefore, two isomers of LA have recently received increasing focus [5]. LA can be generated either by chemical synthesis from hydrocarbon-based sources or by microbial fermentation routes. A racemic form of d-/l-lactic acid is obtained through chemical routes, which have limited applications, whereas optical pure d- or l-lactic acid can be obtained by microbial fermentation [6]. Microbial production of LA has more benefits, including cheap raw materials and mild production conditions. At present, microbial fermentation is the main industrial route for LA production [3].
Figure 1.
LA exists in two enantiomeric forms of l-lactic acid and d-lactic acid.
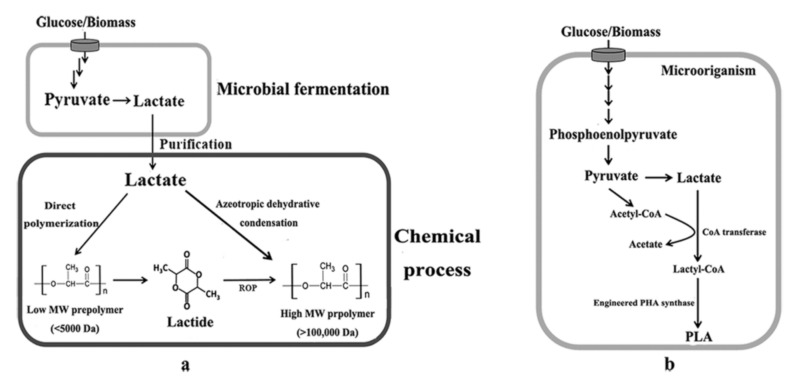
On the other hand, as the most widely applied use of LA and one of the most promising biodegradable plastics, PLA possesses much more outstanding advantages compared to other polymers, since it has biodegradability, biocompatibility, clarity, and superior barrier properties, along with suitable material properties for general performance plastics [7,8,9]. PLA is traditionally synthesized by bio-chemical hybrid process (Figure 2a), in which optical pure l-lactic acid or d-lactic acid as the monomer of PLA firstly is produced by microbial fermentation from renewable resources such as wheat, straw, corn, and sorghum [10], and then PLA is synthesized via the ring opening polymerization (ROP) of lactide, a cyclic dimer of lactate, or by the direct solvent-based azeotropic dehydrative condensation of LA [11,12,13]. PLA synthesis process usually requires catalysts under rigorously controlled conditions (temperature, pressure, and pH) and long polymerization times, which leads to high energy consumption [14]. In addition, the toxicity caused by the use of metal catalysts seriously affects the applications of PLA as biomedical and food-packaging materials [11]. As an alternative to the traditional production process, one-step fermentative production of PLA has recently been developed by employing metabolically engineered microorganisms (Figure 2b), such as Escherichia coli [15,16,17,18,19], Sinorhizobium meliloti and Pseudomonas putida [20].
Figure 2.
The process of PLA synthesis. (a) bio-chemical hybrid process; (b) one-step fermentative production by metabolically engineered microorganisms.
Although there are some reviews on LA production, few reviews focus on the production of optically pure LA, which is the precursor for biodegradable plasmics, PLA. LA production will increase significantly over the coming years, mainly to provide PLA manufactures. A racemic mixture of dl-lactic acid is the common product for most LA-producing strains. dl-lactic acid cannot be utilized in specific industrial applications where only optically pure LA is desired. In this paper, we have gathered our accumulated knowledge on the main achievements in LA bio-production, especially in optically pure l-lactic acid and optically pure d-lactic acid fermentation. In addition, the detailed procedure and the latest research progresses of one-step fermentative production of PLA, including the exploration of key enzymes for polymerization of lactate, designing of microorganism chassis cell by metabolic engineering are also reviewed and discussed.
2. l-Lactic Acid Biosynthesis
Many microorganisms have been reported to have the ability to produce l-lactic acid, such as fungi, Lactobacillus species, Bacillus coagulans, and various genetically modified strains. LA is produced from carbohydrates in a microorganism. There are two pathways for LA production: homolactic fermentation and heterolactic fermentation. In heterolactic fermentation, ethanol, acetic acid and CO2 are formed in addition to LA as the end product. The theoretical yield is only 0.5 g LA/g glucose [2]. In homolactic fermentation, LA is produced from carbohydrates with the regeneration of NADH (Figure 1). Pyruvate produced by the glycolytic breakdown of carbohydrates is converted into l-lactic acid and d-lactic acid by NAD-dependent l-lactate dehydrogenanse (EC 1.1.1.27) and NAD-dependent d-lactate dehydrogenanse (EC 1.1.1.28), respectively [21]. For homofermentative microorganisms, LA production is growth coupling. LA is obtained as the sole product in homolactic fermentation. Therefore, most industrial LA-producing strains are homofermentative microorganisms.
2.1. l-Lactic Acid Producing Strains
l-lactic acid can be produced by several microorganisms classified into bacteria, fungi, cyanobacteria, and algae (Table 1). The filamentous fungus Rhizopus oryzae is a natural l-lactic acid producer. The amylolytic characteristic enables R. oryzae to utilize starchy biomasses without prior saccharification. A concentration of 162 g/L l-lactic acid, with a productivity of 6.23 g/L·h, was obtained using fed-batch strategy [22]. Different renewable resources, including molasses, raw starch materials, and lignocellulosic biomass, have been reported to be used to produce l-lactic acid using Rhizopus strains. However, low conversion rate and undesirable by-products production limits the industrial uses of Rhizopus strains [23]. During LA production, the end-product LA lowers the pH of the medium, and thus impedes LA-producers’ growth. Neutral agents, such as calcium hydroxide (Ca(OH)2), sodium hydroxide (NaOH), and ammonia, are needed for LA production, which increases the costs of downstream process [3]. There have been various trials to develop the strains with tolerance to acid pH and improve the capacity to produce LA at high yields and productivity. Saccharomyces cerevisiae is not a natural LA-producer. However, it has received increasing attentions for industrial LA production because of its acid tolerance. Acid-tolerant S. cerevisiae was engineered to produce LA by expressing heterologous lactate dehydrogenase genes and several key pathway genes, including glycerol-3-phosphate dehydrogenase, cytochrome-C oxidoreductase, etc. A concentration of 142 g/L, with production yield of 0.89 g/g and productivity of 3.55 g/L·h, was obtained in fed-batch fermentation [24]. Although fungi have the merits of acid-tolerance and direct-utilization of renewable biomasses, acetic acid and ethanol are produced along with LA, therefore leaving a negative impact on the fermentation. To date, LA bacteria, including Lactobacillus strains and Bacillus strains, account for 90% of l-lactic acid large-scale production [1].
Table 1.
Lactic acid production in microorganisms.
| Microorganisms | Substrates | Fermentation Mode | Yield (g/L) | Productivity (g/L·h) | Reference |
|---|---|---|---|---|---|
| l-lactic acid producers | |||||
| Rhizopus oryzae | Glucose | One-step fermentation with fed-batch strategy | 162 | 6.23 | [22] |
| Saccharomyces cerevisiae | Glucose | fed-batch fermentation | 142 | 3.55 | [24] |
| Lactobacillus rhamnosus | Starchy biomass | One step simultaneous liquefaction, saccharification and fermentation | 108 | 3.40 | [25] |
| Lactobacillus paracasei | Glucose | Non-sterilized fermentation | 221 | 7.50 | [26] |
| Bacillus sp. 2–6 | Glucose | Non-sterilized repeated batch fermentation | 107 | 3.06 | [27] |
| Bacillus sp. strain XZL9 | Corncob molasses | Fed-batch fermentation | 75 | 0.38 | [28] |
| Bacillus sp. strain P38 | Cellulosic hydrolysate | Fed-batch fermentation | 180 | 2.40 | [29] |
| Lactobacillus paracasei | Non-detoxified wood hydrolysate | Fed-batch fermentation | 99 | 2.25~3.23 | [30] |
| Lactobacillus paracasei | Rice straw hydrolysate | Batch fermentation | 67 | 5.27 | [30] |
|
Bacillus coagulans+ Lactobacillus rhamnosus |
Cassava bagasse | Simultaneous saccharification and co-fermentation | 113 | 2.74 | [31] |
| Bacillus coagulans | Bakery waste and lucerne green juice | Batch fermentation | 62 | 2.59 | [32] |
|
Lactobacillus paracasei subsp. paracasei2 |
Food waste | Batch fermentation | 34 | 0.55 | [33] |
| Indigenous microbiota | Food waste and waste activated sludge | Batch fermentation | 30 | 0.63 | [34] |
| Lactobacillus plantarum | Raw corn starch | Batch fermentation | 50 | — | [35] |
| Lactobacillus rhamnosus | Cassava powder | Simultaneous saccharification and fermentation | 175 | 3.40 | [36] |
| Bacillus coagulans | Jerusalem artichoke powder | Fed-batch fermentation | 134 | 2.50 | [37] |
| d-lactic acid producers | |||||
| Bacillus coagulans | Glucose | Fed-batch fermentation | 145 | 1.50 | [5] |
| Sporolactobacillus sp. CASD | Glucose | Fed-batch fermentation | 207 | 3.80 | [38] |
| Saccharomyces cerevisiae | Glucose | Fed-batch fermentation | 40 | 0.83 | [39] |
| Saccharomyces cerevisiae | Glucose | Semi-neutralizing fermentation | 52 | 2.17 | [40] |
| Corynebacterium glutamicum | Glucose | Fed-batch fermentation | 264 | 3.30 | [41] |
| Escherichia coli | Glucose | Shake flask experiment | 123 | 4.39 | [42] |
| Escherichia coli | Glycerol | Batch fermentation | 115 | 3.29 | [43] |
|
Lactobacillus delbrueckii ssp. bulgaricus |
Orange peel waste | Separate hydrolysis and fermentation | 45 | 0.63 | [44] |
|
Lactobacillus coryniformis subsp. torquens |
Dried distiller’s grains with solubles hydrolysate | Simultaneous saccharification and fermentation | 38 | 0.80 | [45] |
| Lactobacillus delbrueckii | Molasses and corn steep liquor | Fed-batch fermentation | 162 | 3.37 | [46] |
| Lactobacillus delbrueckii | Sugarcane molasses and soybean meal | Fed-batch fermentation | 112 | 2.40 | [47] |
| Lactobacillus delbrueckii + engineered Lactococcus lactis | Whey permeate | Fed-batch co-culture process | ~45 | 0.63 | [48] |
Lactobacillus strains have a long history of industrial LA production. They have great commercial importance due to high acid tolerance, high yield and high productivity of l-lactic acid. Most Lactobacillus strains are mesophiles. The low fermentation temperature not only increases contamination risks, but also hampers the uses of lignocellulosic biomass [23]. Thermotolerant strains may minimize contamination problems during LA production. L. rhamnosus is a l-lactic acid producer with a thermotolerant temperature up to 42 °C. Aging paddy rice was used as alternative carbon and nitrogen sources for l-lactic acid production by L. rhamnosus DUT1908. In one step simultaneous liquefaction, saccharification and fermentation process, 108 g/L l-lactic acid was obtained with a productivity of 3.4 g/L·h and a yield of 0.89 g/g [25]. By combining metabolic engineering and adaptive evolution, a l-lactic acid producer, L. paracasei (NCBIO01-M2-ldhL1-HT), was obtained. L. paracasei (NCBIO01-M2-ldhL1-HT) produced 221 g/L l-lactic acid in non-sterilized fermentation [26]. Bacillus is a thermophilic LA producer. It can produce l-lactic acid at above 50 °C, which reduces energy consumption and contamination risks during production. A thermophilic Bacillus sp. strain 2–6 was used in completely open repeated batch fermentation for producing l-lactic acid. Up to 107 g/L l-lactic acid of optical purity 99.8% was obtained with NaOH as pH regulator [27].
2.2. Substrates for l-Lactic Acid Production
Nowadays, microbial production of l-lactic acid is performed with pure sugars. The substrate costs constitute approximately 40~70% of the entire costs. The utilization of renewable, lost-cost, and non-food substrates as an alternative for pure sugars is an economical way for LA production [6,49].
2.2.1. Lignocellulosic Biomass
Lignocellulosic biomass has gained increasing attention due to its abundance, non-food sugar constituents, renewability and cost efficiency. Pre-treatment is essential to lignocellulosic biomasses for releasing fermentable sugars. However, the inhibitor compounds, such as hydroxymethylfurfural, furfural, and phenolic acid, are also released [49]. Furthermore, mixed sugars (xylose, glucose, and arabinose) derived from lignocellulosic biomass cannot be efficiently used by most LA-producers.
Bacillus strain is one of the widely reported strains for l-lactic acid production using lignocellulosic biomass (Table 1). Wang et al. [28] isolated a Bacillus sp. strain XZL9. It could metabolize glucose and xylose simultaneously into only l-lactic acid by the homofermentative pathway. In microorganisms, xylose is firstly converted into xylulose-5-phosphate (X5P). This metabolite is further metabolized through two pathways: the pentose phosphate pathway (PPP) and the phosphoketolase pathway (PKP). For the PKP, xylose is converted to LA and acetic acid. While in the PPP, X5P is converted into LA through the Embden–Meyerhof pathway (EMP). The theoretical value of xylose conversion is 1.0. Xylose is metabolized through the PPP pathway in Bacillus sp. strain XZL9. The concentration of l-lactic acid (75 g/L) was produced from corncob molasses. Pre-treatment of lignocellulosic biomass inevitably produced toxic compounds, such as 2-furfural, that inhibit microbial growth. To fully utilize lignocellulosic feedstocks, considerable interest has been focused on the study of tolerance to inhibitory compounds in lignocellulosic hydrolysates. A novel Bacillus sp. strain P38 was isolated from the sludge of a sewage treatment plant by using a high concentration of cellulosic hydrolysate as sole carbon source. It had extraordinary tolerance to 10 g/L 2-furfural. The l-lactic acid concentration of 180 g/L was obtained from corn stover hydrolysate, with a high volumetric productivity of 2.4 g/L·h and a yield of 0.96 g/g total reducing sugars [29].
In addition to Bacillus strain, strains of other species have been recently reported to have the ability to produce l-lactic acid from a lignocellulosic biomass. A novel strain, Lactobacillus paracasei, which has a tolerance to inhibitors derived from lignocellulosic biomass, was isolated. Deletion of the intrinsic d-lactate dehydrogenase enabled the production of 215 g/L l-lactic acid with glucose as carbon resource. A concentration of 99 g/L L-lactic acid was obtained using non-detoxified wood hydrolysate. Rice straw hydrolysate without detoxification was also tested and 67 g/L l-lactic acid was obtained, with a productivity of 5.27 g/L·h [30]. The mixed culture of B. coagulans and L. rhamnosus was used to produce l-lactic acid from co-saccharified cassava bagasse. The l-lactic acid concentration and productivity of 113 g/L and 2.74 g/L·h was achieved [31]. Nowadays, cassava bagasse, beechwood hydrolysate, sugarcane bagasse, corn stover, and wood hydrolysate have been used as substrates for l-lactic acid production. Pre-treatment of lignocellulosic feedstocks are critical for high-quality LA production. LA production from lignocellulosic biomass is quite challenging with an associated high pre-treatment costs [3].
2.2.2. Food Waste
Food waste has more potential due to its high carbohydrate content and does not require expensive pre-treatment. Kitchen residues, tea leaves, and vegetable leaves have been reported to be feasible for LA production [32]. A pilot-scale study on l-lactic acid production from food waste was reported by Gao et al. [33]. l-Lactic acid production was carried out under sterilized and non-sterilized conditions. A concentration of 34 g/L l-lactic acid was produced, with a productivity of 0.55 g/L·h. Compared with dl-lactic acid, optically pure LA is far more valuable. It attracts industry interests as a precursor for the promising biodegradable plasmics, PLA. Therefore, researchers try to develop reliable and cost-effective approaches to produce optically pure LA. The challenges with l-lactic acid production from food waste are low l-lactic acid yield due to slow hydrolysis rate, consumption of l-lactic acid by other microorganisms, and decreased economic value due to generation of racemic dl-lactic acid [50]. It has been reported that salt addition could enhance the optical purity of l-lactic acid. The mixed substrate of food waste and waste activated sludge were used for l-lactic acid. The optical pure l-lactic acid with a yield of 30 g/L was obtained at 30 g/L NaCl. The reason for the enhanced optical purity of l-lactic acid is perhaps that the activity of d-lactic acid producing enzymes are sensitive to high concentration of salt. Furthermore, high salt concentration resulted in the changes of microbial community and decreased diversity of indigenous microbiota [34,51].
2.2.3. Starchy Materials
Starchy feedstocks have more potential due to their high carbohydrate content and do not require expensive pre-treatment. An engineered L. plantarum NCIMB 8826 strain was constructed by deleting d-lactate dehydrogenase gene and lactate racemase gene. The engineered strain could produce optically pure l-lactic acid from raw starch with a concentration of 50 g/L [35]. Cassava is one of the most efficient crops in terms of carbohydrate production. It is a tropical perennial plant that grows on poor or depleted soils where the yields of other crops are very low. Cassava powder, produced by grinding cassava to powder, was used for l-lactic acid production by Wang et al. [36]. The high l-lactic acid concentration (175 g/L) was obtained in simultaneous saccharification and fermentation. This is the highest l-lactic acid concentration reported, from cassava source. Jerusalem artichoke is a low-requirement crop with high sugar content. l-Lactic acid was produced from the hydrolysates of Jerusalem artichoke powder by a thermophilic bacterium, B. coagulans XZL4. High l-lactate production (134 g/L) was obtained using Jerusalem artichoke powder and corn steep powder in fed-batch fermentation, with an average productivity of 2.5 g/L·h and an optical purity of 99.5% [37]. Despite huge potentials and recently growing interest in commercial LA production from starchy biomass, literature lacks studies that evaluate the techno-economic feasibility of its commercial production. Manandhar and Shah [52] estimated the resources required including equipment, chemicals, consumables, utilities, and labor for commercial scale LA production based on three fermentation pathways using either LA producing bacteria, fungi or yeast. Results showed that LA production costs were highly sensitive to sugar-to-LA conversion rates, materials’ price, plant size, annual operation hours, and potential use of gypsum. Improvements in process efficiencies and lower equipment and chemical costs would further reduce the cost of LA production.
3. d-Lactic Acid Biosynthesis
Both l-lactic acid and d-lactic acid are the precursors for PLA production. Compared with the extensive investigation of l-lactic acid production, there are relatively few studies on d-lactic acid fermentation. Approximately 70% of LA is used in the food industry because of its role in the production of yogurt and cheese. Because d-lactic acid cannot be metabolized by the human body, studies on d-lactic acid fermentation are limited [2]. Recently, the increasing use of PLA has led to a surge in the demand for d-lactic acid [3]. A few wild-type strains, such as L. delbrueckii, B. laevolacticus, L. coryniformis, Corynebacterium glutamicum, and L. bulgaricus, have been reported to be homofermentative d-lactic acid producers. Furthermore, metabolically engineered S. cerevisiae and E. coli have been reported for the production of optical pure d-lactic acid. The titer of d-lactic acid production is generally much lower than that of l-lactic acid [38].
3.1. d-Lactic Acid Producing Strains
A highly efficient d-lactic acid producer, Sporolactobacillus sp. CASD, was reported to produce 207 g/L d-lactic acid, with the average productivity of 3.8 g/L h and optical purity of 99.3% (Table 1) [38]. To our knowledge, this is the highest d-lactic acid production. These values are comparable to those obtained in l-lactic acid. Thermophilic B. coagulans can utilize a broad range of inexpensive carbon resources and produce optically pure l-lactic acid at 50~55 °C, which is expected to minimize contamination during fermentation in industrial scale. To obtaining a thermophilic d-lactic acid producer, an optically pure l-lactic acid producer, B. coagulans DSM1, was chosen for genetic engineering. By replacing the key gene for l-lactic acid production with LdhD from L. delbrueckii subsp. bulgaricus DSM 20081, the genetically engineered strain produced high optical purity of d-lactic acid under non-sterilized condition [5]. More recently, S. cerevisiae was systematically engineered to produce d-lactic acid by overexpressing d-lactic acid producing genes and deleting glycerol pathway genes. A concentration of 40 g/L d-lactic acid was achieved, with a yield of 0.81 g/g [39]. LA production requires the addition of neutralizing agent in the medium. Acid-tolerant S. cerevisiae was constructed by using the CRISPR-Cas-mediated genome evolution method. Approximately 34 g/L d-lactic acid was produced in non-neutralized condition, and 52 g/L d-lactic acid was obtained in a semi-neutralized condition [40].
C. glutamicum is a Gram-positive soil bacterium, which has been widely used for the industrial production of amino acid. Its genome is sequenced, and genetic engineering tools are available. C. glutamicum was developed to produce LA through intensive metabolic engineering including the introduction of the Entner–Doudoroff (ED) pathway genes, overexpression of glycolytic genes, and modulation of redox balance. Finally, the production of 264 g/L d-lactic acid was obtained, with an optical purity of 99.9% [41]. E. coli strains have simple nutritional requirements and are easily genetically manipulated. They are ideal cell factories for the production of metabolic products. Several studies reported the use of engineered E. coli strains for LA production from glucose, xylose, sucrose, and glycerol. However, the final concentration (≤63 g/L) and fermentation temperature (~37 °C) by engineered E. coli strains were much lower than that achieved with many LA bacteria and Bacillus species [23]. The PR-PL promoters were exploited as a genetic switch to regulate the expression of lactate dehydrogenase and the subsequent production of LA in E. coli. The d-lactate dehydrogenase promoter, PldhA, was replaced by PR-PL promoters (as a genetic switch), resulting in the thermo-controllable strain B0013-070B. A concentration of 123 g/L d-lactic acid was obtained at 42 °C [42]. Recently, an E. coli strain was manipulated for its glycerol dissimilation and d-lactic acid synthesis pathways. Combining adaptive evolution under high crude glycerol, a titer of 115 g/L d-lactic acid was obtained in batch fermentation, with a productivity of 3.29 g/L·h [43].
3.2. d-Lactic Acid Production from Renewable Resources
Manufacturing commercially viable d-lactic acid is desirable compared to petrochemical resources. The production of d-lactic acid from corn stover, brown rice, and hardwood pulp hydrolysate has been studied. Orange peel waste was used as raw materials for d-lactic acid production using L. delbrueckii ssp. bulgaricus CECT 286. The concentration of 45 g/L d-lactic acid was produced, with an optical purity of 99.5% [44]. Dried Distiller’s Grains with Soluble (DDGS) hydrolysate was used as a substrate for d-lactic acid production by Lactobacillus coryniformis subsp. torquens. Two strategies of separate hydrolysis and fermentation (SHF) and simultaneous saccharification and fermentation (SSF) were used, and a concentration of 38 g/L was obtained in SSF, with an optical purity of 99.9% [45]. Molasses and corn steep liquor were used for d-lactic acid production by L. delbrueckii. A high titer of d-lactic acid (162 g/L) was achieved after 48 h of fermentation with a productivity of 3.37 g/L·h [46]. During the utilization of sugarcane molasses and soybean meal, adaptive evolution was used in L. delbrueckii S-NL31 in order to improve d-lactic acid concentration. Finally, fed-batch simultaneous enzymatic hydrolysis of soybean meal and fermentation process by evolved strain resulted in d-lactic acid levels of 112 g/L, with an average production efficiency of 2.4 g/L·h and optical purity of 99.6% [47]. In another study, the co-culture batch process of L. delbrueckii and engineered L. lactis was carried out to efficiently produce d-lactic acid from lactose or whey-derived lactose. d-Lactate dehydrogenase gene together with galactose permease gene was over-expressed in L. lactis. The recombinant L. lactis could convert galactose into d-lactic acid. By co-culturing L. delbrueckii and engineered L. lactis, approximately 45 g/L d-lactic acid was achieved from whey permeate [48]. l-Lactic acid production has been extensively studied using several strains and substrates including lignocellulosic hydrolysates, food wastes and starchy materials. Some companies, such as Corbion (Amsterdam, The Netherlands), Galactic (Celles, Belgium), and NatureWorks LLC (Minnetonka, MN, USA), are operating on l-lactic acid production using renewable resources [53]. d-Lactic acid production using renewable resources is quite limited. Due to the significance in the production of PLA, d-lactic acid production from renewable resources is currently in the spotlight. There is still need for research of efficient d-lactic acid production process, especially in terms of utilization of cheap resources.
4. Strategies for Clean Fermentation Technology of Lactic Acid
The downstream recovery of LA is one of the biggest challenges in LA production. The cost of LA purification accounts for approximately 50% of the total cost. Moreover, a large amount of solid waste (calcium sulfate) is produced during the operation, which makes them environmentally unfriendly. Therefore, it is necessary to search for other economical, efficient and environmentally friendly techniques for LA production [49]. The utilization of sodium hydroxide (NaOH) instead of calcium carbonate (CaCO3) as a neutralizer of LA fermentation can solve the problem of environmental pollution caused by the traditional addition of CaCO3 [1]. Alkaliphiles grow optimally at a pH above 9. They are also tolerant to salt, especially those of monovalent ions, such as sodium ions. Alkaliphilic strains may be promising producers of organic acids and their tolerance to high levels of salt and a high pH could also minimize contamination. An alkaliphilic strain Bacillus sp. WL-S20 was isolated from a marine environment. In multi-pulse fed-batch fermentation, a l-lactic acid concentration of 225 g/L with a yield of 99.3% was obtained [54]. The high concentration of optically pure l-lactic acid produced by an alkaliphilic strain using environment-friendly NaOH-based process provides a potentially novel way for LA production at an industrial scale. Another alkaliphilic strain, Enterococcus hirae BoM 1–2, was isolated to production LA using NaOH as neutralizer. The LA concentration of 181 g/L was achieved in a multi-pulse fed batch strategy with volumetric productivity of 0.65 g/L·h [55]. Alkalophilic microorganisms are the crucial sources for LA fermentation. The concentration of LA produced by alkaliphilic strains was higher than that produced by the non-alkaliphilic counterparts. Alkalophilic microorganisms enriched the LA fermentation strain resources and provided a new idea for the development of new LA fermentation and separation coupling fermentation process [56].
Except for alkaliphilic strains, membrane-based hybrid reactor systems could also be used for clean production of lactic acid. Membrane based hybrid reactor system successfully stands in that objective without creating any negative environmental impacts. More than 95% removals of impurities were achieved in the hybrid reactor system, with a purity of 95% [57]. Membrane separation technology has the advantages of high separation efficiency, mild operating conditions, non-toxic separation medium, environmentally friendly, and reliable process amplification [1]. A two-step electrodialysis system was established to purify LA. Firstly, the fermentation broth was clarified by microfiltration membrane to remove bacteria and macromolecular proteins. Due to the calcium salt regulation process adopted in the fermentation, the divalent metal ions in the fermentation broth are easy to cause membrane pollution in the process of bipolar membrane operation. The fermentation broth is again removed by the nanofiltration process. The clarified fermentation broth is concentrated by electrodialysis in the first step, and then transformed into LA and corresponding alkali by secondary electrodialysis of bipolar membrane. The alkali is returned to the fermentation tank and used as neutralizer again [58]. The use of NaOH as neutralizer can avoid the pollution of divalent metal ions to the membrane. Therefore, the development of new LA producing strains, such as alkaliphilic strains, can significantly improve the economy of membrane separation.
5. Polylactic Acid (PLA) Biosynthesis
Relative to LA, the most of which have been produced by microbial fermentation, PLA are mainly synthesized by the chemical polymerization process of LA (ROP of lactide) to date. Recently, the direct one-step fermentative processes for the production of PLA and several LA-containing polyesters have been developed by employing metabolically engineered microorganisms [59]. In this biosynthesis process, the most critical is to develop two key catalytic enzymes, propionyl-CoA transferase and PHA synthase. Firstly, LA is converted into lactyl-CoA by propionyl-CoA transferase, and then lactyl-CoA is polymerized by PHA synthase (Figure 2b).
5.1. Development of Enzyme for Converting LA into Lactyl-CoA
Although there is no enzyme that specifically catalyzes LA to produce lactyl-CoA in nature, previous reports have shown that propionyl-CoA transferase (Pct), found from several microorganisms including C. propionicum, Megasphaera elsdenii, Bacteroides ruminicola, and C. homopropionicum in alanine fermentation pathway, can transfer CoA from propionyl-CoA or acetyl-CoA to LA to form lactyl-CoA [16,17,60]. However, the two propionyl-CoA transferases from C. propionicum (PctCp) and M. elsdenii (PctMe) were also found to strongly inhibit the growth of the cell when expressed in E. coli. To solve this issue, random mutagenesis was performed to create several PctCp mutants harboring the enhanced ability to supply lactyl-CoA in E. coli without severe growth inhibition. Among these positive PctCp mutants, two beneficial PctCp mutants, Pct532Cp (A243T, and one silent nucleotide mutation of A1200G) and Pct540Cp (V193A, and four silent nucleotide mutations of T78C, T669C, A1125G, and T1158C), which led to an increase in both the polymer content and lactate mole fraction in the PLA copolymer [17]. Although, the positive PctCp mutants had no inhibition on cell growth, the catalytic activity of the enzyme still cannot meet the requirements of efficient synthesis of lactyl-CoA. Recently, it has been found that CoA transfers from Roseburia sp., Eubacterium hallii, Faecalibacterium prausnitzii, and Anaerostipes caccae can also convert LA into LA-CoA [61]. Furthermore, isocaprenoyl-CoA:2-hydroxyisocaproate (2HIC) CoA-transferase (HadA) from Clostridium difficile was also found to be capable of activating LA into lactyl-CoA in addition to its original substrate, 2HIC [62,63]. All these CoA transferases above can activate LA to produce LA-CoA, which provides abundant enzyme resources for the biosynthesis of PLA. Some of the CoA-transferases capable of production of lactyl-CoA are summarized in Table 2.
Table 2.
Some of the CoA-transferases capable of production of lactyl-CoA.
| Enzyme Type | Source | Reference |
|---|---|---|
| Butyryl-CoA transferase (Bct) | Roseburia sp. | [61] |
| Eubacterium hallii | ||
| Faecalibacterium prausnitzii | ||
| Anaerostipes caccae | ||
| Isocaprenoyl-CoA:2HIC CoA-transferase (HadA) | Clostridium difficile | [62,63] |
| Propionyl-CoA transferase (Pct) | Clostridium propionicum | [17,64,65,66,67] |
| Megasphaera elsdenii | [16,68] | |
| Clostridium perfringens | [69] | |
| Cupriavidus necator H16 | [70,71] |
5.2. Development of Enzyme for Polymerization of Lactyl-CoA into PLA
Apart from CoA-transferase, another key enzyme involved in PLA synthesis is PHA synthase, which generally used as the main enzyme of polyhydroxyalkanoates (PHAs) biosynthesis. Depending on the carbon numbers PHAs are classified into major two groups with different material properties: short-chain-length (SCL) and medium-chain-length (MCL)-PHAs. SCL-PHAs are composed of monomers having 3 to 5 carbon atoms and display thermoplastic material properties such as polypropylene. MCL-PHAs are composed of monomers with 6 to 14 carbon atoms and have elastic material properties similar to rubber and elastomer. In microorganisms, many bacteria naturally accumulate PHAs in their cytoplasm as carbon and energy storage materials when they encounter limited growth conditions in the presence of excess carbon sources [61]. However, the natural PHA synthases generally accept 3-hydroxyacyl-CoAs as the most favorable substrates, and 4-, 5- and 6-hydroxyacyl-CoAs can also be used as substrates but showed no or only slight activities on lactyl-CoA [15,72].
Depending on the subunit compositions and substrate specificities of the PHA enzymes, they are generally classified into four groups: class I, II, III, and IV (Table 3) [73]. Class I and II PHA synthases are composed of a single one subunit enzyme, PhaC. Class I PHA synthases such as Ralstonia eutropha and Alcaligenes latus PHA synthase accept short-chain-length-HA-CoAs for polymerization [74], while class II PHA synthases mainly from Pseudomonads display substrate specificity towards medium-chain-length-HA-CoAs [75]. Some class II PHA synthases from Pseudomonas sp. 61-3 [76] accept both SCL- and MCL-monomers, with much weak activity towards SCL-monomers. Class III PHA synthases are composed of two different subunits, PhaC and PhaE [77]. These subunits have much low sequence homology to class I and II PHA synthases; for example, the PhaC subunits display only 20–30% homology with each other. Class III PHA synthases are highly specific for SCL-HA-CoAs, but also accept MCL-HA-CoAs as substrates when expressed in some Pseudomonads [78]. Class IV PHA synthases are composed of two different subunits, PhaC and PhaR, which are usually found in Bacillus strains producing P(3HB) [79,80]. All the PHA synthases except for Pseudomonas sp. MBEL 6–19 PhaC1 showed poor activity to the substrate.
Table 3.
The classification of PHA synthases.
| Class | Subunit Composition | Species | Substrate |
|---|---|---|---|
| I | PhaC+ PhaC | Ralstonia eutropha | C3–C5 |
| II | PhaC+ PhaC | Pseudomonads sp. | ≤C6(or C4) |
| III | PhaC+ PhaE | Allochromatium vinosum | C3–C6 |
| IV | PhaC+ PhaR | Bacillus sp. | C3–C5 |
In order to obtain the PHA synthase that efficiently catalyzes lactyl-CoA, PHA synthases from Pseudomonas MBEL sp. 6-19 and Pseudomonas sp. 61-3 were selected to perform site-directed mutagenesis and resulting variants that had amino acid residues substitutions of Glu130Asp, Ser325Thr, Ser477Arg/His/Phe/Gly, as well as Gln481Lys/Met were effective for in vivo catalysis of lactyl-CoA [16,17,64]. Similarly, other PHA synthases from different Pseudomonas strains were also engineered through site-directed mutagenesis and the resulting variants showed enhanced substrate specificity toward lactyl-CoA [81].
5.3. Metabolic Engineering for Production of PLA
By employing the two key enzymes of CoA transferase and engineered PHA synthase, the microorganisms assembled with both LA biosynthetic and LA polymerizing pathways have been further developed for the cell factory, which efficiently converts the cheap carbohydrates such as glucose into PLA in vivo. PLA firstly was produced in recombinant E. coli with a significantly low content, 0.5 wt%, of dry cell weight [17]. Even though such a system is suitable for a proof-of-concept study, it is not preferred for the industrial-level production of polymers. Accordingly, many studies focused on the engineering metabolic pathways of host strains to provide more precursors LA [82,83].
With metabolic pathways engineered by knocking out the ackA, ppc, and adhE genes encoding acetic acid kinase, phosphoenolpyruvate carboxylase and aldehyde dehydrogenase, respectively, and by replacing the promoters of the ldhA and acs genes with the strong trc promoter, the resulting strain E. coli JLX10 equipped with evolved class II PhaC1 from Pseudomonas sp. MBEL 61-9 and C. propionicum Pct can produce P(14mol%3HB-co-86 mol%LA) with high LA fraction, and PLA could be produced up to 11 wt% of dry cell weight when 20g/L glucose supplied [84]. Recently, except for E. coli, Sinorhizobium meliloti as the native polymer producer also can produce PLA up to 3.2 wt% dried cell weight when expression of C. propionicum propionate CoA transferase (Pct532Cp) and an evolved Pseudomonas sp. MBEL 6-19 PHA synthase 1 (PhaC1Ps6-19, PhaC1400).
So far, even though PHA containing high fraction of lactate and even PLA homopolymer has been produced employing recombinant E. coli expressing evolved PHA synthase, it is almost impossible to generate a truly 100% PLA homopolymer because PHA synthase evolved to accept lactyl-CoA as substrate still has significantly greater substrate specificity towards 3HB-CoA than lactyl-CoA [85]. Conversely, it seems to be possible to obtain PLA homopolymer if the monomer molecule for PLA synthesis such as 3HB-CoA is present in a quantity that is too low to be detected [73]. Therefore, future research should focus more on improving the substrate specificity of PHA synthase and increasing the yield of PLA.
5.4. Metabolic Engineering for Production of LA-Containing Copolymers
As mentioned above, PHA synthase is well known for its broad substrate availability towards various hydroxycarboxylic acids (HAs). Therefore, it is relatively easy to incorporating several other HAs monomers with LA to produce various LA-containing copolymers by employing the engineered PHA synthase. Originally, LA-containing copolymers generally consisted of natural monomers, including 3-hydroxybutyrate (3HB) [16,84], 3-hydroxypropionate (3HP) [81] and 4-hydroxybutyrate (4HB) [65]. Recently, some novel LA-containing copolymers have been synthetized by employing monomers of 2-hydroxybutyrate (2HB), 2-hydroxyisovalerate (2HIV), 2-hydroxyisocaproate (2HIC) and 2-hydroxy-3-methylvalerate (2H3MV) [62], phenyllactate (PhLA), mandelate (MA) and 4-hydroxyphenyllactate (4HPhLA). Poly(lactate-co-glycolate) and poly(lactate-co-glycolate-co-2-hydroxybutyrate) were produced from xylose as a sole carbon source by using five different synthetic promoters for the expression of Caulobacter crescentus XylBC in E. coli [86]. P(2HIV-co-2HB-co-3HB-co-LA) was produced in E. coli by metabolic engineering including overexpression of feedback resistant ilvBN mut genes encoding acetohydroxyacid synthase and ilvCD genes encoding ketol-acid reductoisomerase and dihydroxyacid dehydratase, respectively, and panE gene encoding (D)-2-hydroxyacid dehydrogenase, and pct540 gene encoding evolved propionyl-CoA transferase and phaC1437 gene encoding evolved PHA synthase were also overexpressed, along with ilvBN mut, ilvCD, and panE genes [87]. Additionally, the aromatic polyesters poly(3HB-co-D-phenyllactate) can be produced from glucose as a sole carbon source by additional expression of Ralstonia eutropha ketothiolase (phaA) and reductase (phaB) genes [63]. As another novel biopolymer, the quaterpolymer P(3HB-co-LA-co-3HHx-co-3HO) was produced in P. putida with polymer content of 42% dry cell weight when cultured in defined media with the addition of sodium octanoate [20].
6. Concluding Remarks and Outlook
LA is a versatile green platform compound. LA and its derivatives have been widely used in food, medicine, environmental protection, biodegradable polymer production and other industrial fields. The demand for LA and its derivatives is increasing. With the increasing cost of glucose and other raw materials, the production cost of LA is also increasing, which has seriously affected the profit margin of the LA industry. The economic and technical analysis showed that the concentration of LA should be higher than 180 g/L and the conversion rate could exceed 95% at an industrial scale in the future [1]. At present, the reported strains showed low utilization efficiency for cheap raw materials. It is necessary to optimize the utilization rate of substrates and improve the industrial adaptability of strains through biological engineering. Furthermore, the development for extremophilic LA producers, including thermophiles, acidophiles, and alkaliphiles, can minimize contamination problems during processing. This will be a direction for low-cost LA fermentation.
Although it is now possible to produce PLA by the one-step fermentation of engineered microorganisms, this complete biosynthesis process still faces many challenges. Firstly, the PLA yield and productivity is still low; the contents were below 11 wt%, and the titer and productivity were about 0.6 g/L and 0.02 g/L·h, respectively, which cannot reach the requirements of industrial application and has totally no price advantage compared with chemical synthesis. Thus, the production capability of this microbial PLA synthesis system needs to be much improved for future commercialization. For instance, to enhance the conversion of pyruvate to lactic acid by designing a novel multi-substrate co-utilization pathway will release the inhibition of acetic acid on host strains and improve the production capability of PLA, according to reported strategy [88]. Besides, the average molecular weight of PLA synthesized by engineered PHA synthases was found to be lower than 50,000 Da, which is not acceptable for many polymer applications. It has been suggested that the expression level of PHA synthase is one of the major factors determining the molecular weight of PHA [89]. Thus, PHA synthase needs to be further engineered to accept lactyl-CoA more efficiently and consequently to increase the molecular weights of PLA and lactate-containing polyesters. Considering many successful cases of microbial production systems that have greatly increased compound production through employing systematic metabolic engineering strategies involved in synthetic biology, protein engineering, and evolutionary engineering, it is expected that the microorganism can be a versatile and powerful platform for production of PLA, LA-copolymers, and other non-natural polymers.
Author Contributions
Conceptualization, S.H., L.W., C.Z. and Y.M.; methodology, S.H., Y.X., B.Y. and L.W.; formal analysis, S.H., B.Y., L.W. and C.Z.; investigation, S.H., B.Y., L.W. and C.Z.; writing-original draft preparation, S.H., L.W. and C.Z.; writing-review and editing, S.H., Y.X., B.Y., L.W., C.Z. and Y.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by National Key R&D Program of China (No. 2020YFA0906800), Major Science and Technology Innovation Program of Shandong Province (2019JZZY011004) and the Strategic Priority Research Program of the Chinese Academy of Sciences (Grant No. XDA28030000).
Conflicts of Interest
The authors declare no conflict of interests.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Yu B., Zeng Y., Jiang X., Wang L., Ma Y. Trends in polymer-grade L-lactic acid fermentation by non-food biomass. Sheng Wu Gong Cheng Xue Bao. 2013;29:411–421. [PubMed] [Google Scholar]
- 2.Martinez F., Balciunas E., Salgado J., González J., Domínguez M., Converti A., Oliveira R. Lactic acid properties, applications and production: A review. Trends Food Sci. Technol. 2013;30:70–83. doi: 10.1016/j.tifs.2012.11.007. [DOI] [Google Scholar]
- 3.Nwamba M., Sun F., Mukasekurua M., Song G., Harindintwalia J., Boyia S., Sun H. Trends and hassles in the microbial production of lactic acid from lignocellulosic biomass. Environ. Technol. Innov. 2021;21:101337–101357. doi: 10.1016/j.eti.2020.101337. [DOI] [Google Scholar]
- 4.Tsuji H., Kondoh F. Synthesis of meso-lactide by thermal configurational inversion and depolymerization of poly(l-lactide) and thermal configurational inversion of lactides. Polym. Degrad. Stab. 2017;141:77–83. doi: 10.1016/j.polymdegradstab.2017.05.016. [DOI] [Google Scholar]
- 5.Zhang C., Zhou C., Assavasirijinda N., Yu B., Wang L., Ma Y. Non-sterilized fermentation of high optically pure d-lactic acid by a genetically modified thermophilic Bacillus coagulans strain. Microb. Cell Factories. 2017;16:213. doi: 10.1186/s12934-017-0827-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rawoof S.A.A., Kumar P.S., Vo D.-V.N., Devaraj K., Mani Y., Devaraj T., Subramanian S. Production of optically pure lactic acid by microbial fermentation: A review. Environ. Chem. Lett. 2021;19:539–556. doi: 10.1007/s10311-020-01083-w. [DOI] [Google Scholar]
- 7.Drumright R.E., Gruber P.R., Henton D.E. Polylactic Acid Technology. Adv. Mater. 2000;12:1841–1846. doi: 10.1002/1521-4095(200012)12:23<1841::AID-ADMA1841>3.0.CO;2-E. [DOI] [Google Scholar]
- 8.Erwin T.H.V., Karl R.R., David A.G., Patrick R.G. Application of life cycle assessment to Nature Works TM polylactide (PLA) production. Polym. Degrad. Stabil. 2003;80:403–419. [Google Scholar]
- 9.Lim L.-T., Auras R., Rubino M. Processing technologies for poly(lactic acid) Prog. Polym. Sci. 2008;33:820–852. doi: 10.1016/j.progpolymsci.2008.05.004. [DOI] [Google Scholar]
- 10.Li G., Zhao M., Xu F., Yang B., Li X., Meng X., Teng L., Sun F., Li Y. Synthesis and biological application of polylactic acid. Molecules. 2020;25:5023. doi: 10.3390/molecules25215023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park S.J., Lee S.Y., Kim T.W., Jung Y.K., Yang T.H. Biosynthesis of lactate-containing polyesters by metabolically engineered bacteria. Biotechnol. J. 2012;7:199–212. doi: 10.1002/biot.201100070. [DOI] [PubMed] [Google Scholar]
- 12.Erwin T.H.V., Karl R.R., David A.G., Bob S., Ryan P.O., Jeff K., Patrick R.G. The sustainability of Nature Works polylactide polymers and Ingeo polylactide fibers: An update of the future. Macromol. Biosci. 2004;4:551–564. doi: 10.1002/mabi.200400023. [DOI] [PubMed] [Google Scholar]
- 13.Maharana T., Mohanty B., Negi Y. Melt–solid polycondensation of lactic acid and its biodegradability. Prog. Polym. Sci. 2009;34:99–124. doi: 10.1016/j.progpolymsci.2008.10.001. [DOI] [Google Scholar]
- 14.Lopes M.S., Jardini A., Filho R.M. Poly (lactic acid) production for tissue engineering applications. Procedia Eng. 2012;42:1402–1413. doi: 10.1016/j.proeng.2012.07.534. [DOI] [Google Scholar]
- 15.Cho J.H., Park S.J., Lee S.Y., Jung Y.K. Cells or Plants Having a Producing Ability of Polylactate or Its Copolymers and Method for Preparing Polylactate or Its Copolymers Using the Same. MY150715A. U.S. Patent. 2014 February 28;
- 16.Taguchi S., Yamada M., Matsumoto K., Tajima K., Satoh Y., Munekata M., Ohno K., Kohda K., Shimamura T., Kambe H., et al. A microbial factory for lactate-based polyesters using a lactate-polymerizing enzyme. Proc. Natl. Acad. Sci. USA. 2008;105:17323–17327. doi: 10.1073/pnas.0805653105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang T.H., Kim T.W., Kang H.O., Lee S.-H., Lee E.J., Lim S.-C., Oh S.O., Song A.-J., Park S.J. Biosynthesis of polylactic acid and its copolymers using evolved propionate CoA transferase and PHA synthase. Biotechnol. Bioeng. 2010;105:150–160. doi: 10.1002/bit.22547. [DOI] [PubMed] [Google Scholar]
- 18.Park S.J., Kang K.H., Lee H., Park A.R., Yang J.E., Oh Y.H., Song B.K., Jegal J., Lee S.H., Lee S.Y. Propionyl-CoA de-pendent biosynthesis of 2-hydroxybutyrate containing polyhydroxyalkanoates in metabolically engineered Escherichia coli. J. Biotechnol. 2013;165:93–98. doi: 10.1016/j.jbiotec.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 19.Jung Y.K., Lee S.Y. Efficient production of polylactic acid and its copolymers by metabolically engineered Escherichia coli. J. Biotechnol. 2011;151:94–101. doi: 10.1016/j.jbiotec.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 20.Tran T., Charles T.C. Lactic acid containing polymers produced in engineered Sinorhizobium meliloti and Pseudomonas putida. PLoS ONE. 2020;15:e0218302. doi: 10.1371/journal.pone.0218302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang L., Cai Y., Zhu L., Guo H., Yu B. Major role of NAD-dependent lactate dehydrogenases in the production of l-lactic acid with high optical purity by the thermophile Bacillus coagulans. Appl. Environ. Microbiol. 2014;80:7134–7141. doi: 10.1128/AEM.01864-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fu Y., Sun X., Zhu H., Jiang R., Luo X., Yin L. An optimized fed-batch culture strategy integrated with a one-step fermentation improves l-lactic acid production by Rhizopus oryzae. World J. Microbiol. Biotechnol. 2018;34:74. doi: 10.1007/s11274-018-2455-2. [DOI] [PubMed] [Google Scholar]
- 23.Abdel-Rahman M.A., Tashiro Y., Sonomoto K. Recent advances in lactic acid production by microbial fermentation processes. Biotechnol. Adv. 2013;31:877–902. doi: 10.1016/j.biotechadv.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 24.Song J., Park J., Kang C., Cho H., Yang D., Lee S., Cho K. Introduction of a bacterial acetyl-CoA synthesis pathway im-proves lactic acid production in Saccharomyces cerevisiae. Metab. Eng. 2016;35:38–45. doi: 10.1016/j.ymben.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 25.Sun Y., Liu H., Yang Y., Zhou X., Xiu Z. High-efficient l-lactic acid production from inedible starchy biomass by one-step open fermentation using thermotolerant Lactobacillus rhamnosus DUT1908. Bioprocess Biosyst. Eng. 2021;44:1935–1941. doi: 10.1007/s00449-021-02573-z. [DOI] [PubMed] [Google Scholar]
- 26.Tian X., Liu X., Zhang Y., Chen Y., Hang H., Chu J., Zhuang Y. Metabolic engineering coupled with adaptive evolution strategies for the efficient production of high-quality l-lactic acid by Lactobacillus paracasei. Bioresour. Technol. 2021;323:124549. doi: 10.1016/j.biortech.2020.124549. [DOI] [PubMed] [Google Scholar]
- 27.Zhao B., Wang L., Ma C., Yang C., Xu P., Ma Y. Repeated open fermentative production of optically pure l-lactic acid using a thermophilic Bacillus sp. strain. Bioresour. Technol. 2010;101:6494–6498. doi: 10.1016/j.biortech.2010.03.051. [DOI] [PubMed] [Google Scholar]
- 28.Wang L., Zhao B., Liu B., Yu B., Ma C., Su F., Hua D., Li Q., Ma Y., Xu P. Efficient production of l-lactic acid from corncob molasses, a waste by-product in xylitol production, by a newly isolated xylose utilizing Bacillus sp. strain. Bioresour. Technol. 2010;101:7908–7915. doi: 10.1016/j.biortech.2010.05.031. [DOI] [PubMed] [Google Scholar]
- 29.Peng L., Wang L., Che C., Yang G., Yu B., Ma Y. Bacillus sp. strain P38: An efficient producer of l-lactate from cellulosic hydrolysate, with high tolerance for 2-furfural. Bioresour. Technol. 2013;149:169–176. doi: 10.1016/j.biortech.2013.09.047. [DOI] [PubMed] [Google Scholar]
- 30.Kuo Y.-C., Yuan S.-F., Wang C.-A., Huang Y.-J., Guo G.-L., Hwang W.-S. Production of optically pure l-lactic acid from lignocellulosic hydrolysate by using a newly isolated and d-lactate dehydrogenase gene-deficient Lactobacillus paracasei strain. Bioresour. Technol. 2015;198:651–657. doi: 10.1016/j.biortech.2015.09.071. [DOI] [PubMed] [Google Scholar]
- 31.Chen H., Chen B., Su Z., Wang K., Wang B., Wang Y., Si Z., Wu Y., Cai D., Qin P. Efficient lactic acid production from cassava bagasse by mixed culture of Bacillus coagulans and lactobacillus rhamnosus using stepwise pH controlled simultaneous saccharification and co-fermentation. Ind. Crop. Prod. 2020;146:112175. doi: 10.1016/j.indcrop.2020.112175. [DOI] [Google Scholar]
- 32.Alexandria M., Blanco-Catalá J., Schneidera R., Turonbc X., Venus J. High l(+)-lactic acid productivity in continuous fer-mentations using bakery waste and lucerne green juice as renewable substrates. Bioresour. Technol. 2020;316:123949–123954. doi: 10.1016/j.biortech.2020.123949. [DOI] [PubMed] [Google Scholar]
- 33.Gao M., Ma X., Song N., Wang Q., Wu C. A newly isolated strain, Lactobacillus paracasei subsp. paracasei 2, produces l-lactic acid from pilot-scale fermentation of food waste under sterile and nonsterile conditions. J. Chem. Technol. Biotechnol. 2020;95:3193–3201. doi: 10.1002/jctb.6497. [DOI] [Google Scholar]
- 34.Li X., Sadiq S., Zhang W., Chen Y., Xu X., Abbas A., Chen S., Zhang R., Xue G., Sobotka D., et al. Salinity enhances high optically active l-lactate production from co-fermentation of food waste and waste activated sludge: Unveiling the response of microbial community shift and functional profiling. Bioresour. Technol. 2021;319:124124. doi: 10.1016/j.biortech.2020.124124. [DOI] [PubMed] [Google Scholar]
- 35.Okano K., Uematsu G., Hama S., Tanaka T., Noda H., Kondo A., Honda K. Metabolic engineering of Lactobacillus plantarum for direct l-lactic acid production from raw corn starch. Biotechnol. J. 2018;13:e1700517. doi: 10.1002/biot.201700517. [DOI] [PubMed] [Google Scholar]
- 36.Wang L., Zhao B., Liu B., Yang C., Yu B., Li Q., Ma C., Xu P., Ma Y. Efficient production of l-lactic acid from cassava powder by Lactobacillus rhamnosus. Bioresour. Technol. 2010;101:7895–7901. doi: 10.1016/j.biortech.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 37.Wang L., Xue Z., Zhao B., Yu B., Xu P., Ma Y. Jerusalem artichoke powder: A useful material in producing high-optical-purity l-lactate using an efficient sugar-utilizing thermophilic Bacillus coagulans strain. Bioresour. Technol. 2013;130:174–180. doi: 10.1016/j.biortech.2012.11.144. [DOI] [PubMed] [Google Scholar]
- 38.Wang L., Zhao B., Li F., Xu K., Ma C., Tao F., Li Q., Xu P. Highly efficient production of d-lactate by Sporolactobacillus sp. CASD with simultaneous enzymatic hydrolysis of peanut meal. Appl. Microbiol. Biotechnol. 2011;89:1009–1017. doi: 10.1007/s00253-010-2904-9. [DOI] [PubMed] [Google Scholar]
- 39.Watcharawipas A., Sae-Tang K., Sansatchanon K., Sudying P., Boonchoo K., Tanapongpipat S., Kocharin K., Runguphan W. Systematic engineering of Saccharomyces cerevisiae for d-lactic acid production with near theoretical yield. FEMS Yeast Res. 2021;21:foab024. doi: 10.1093/femsyr/foab024. [DOI] [PubMed] [Google Scholar]
- 40.Ryosuke M., Ryosuke Y., Takuya M., Shizue Y., Hayato T., Hiroyasu O. Construction of lactic acid-tolerant Saccharomyces cerevisiae by using CRISPR-Cas-mediated genome evolution for efficient d-lactic acid production. Appl. Microbiol. Biotechnol. 2020;104:9147–9158. doi: 10.1007/s00253-020-10906-3. [DOI] [PubMed] [Google Scholar]
- 41.Yota T., Naoto K., Shogo Y., Masako S., Toru J., Masayuki I. Metabolic engineering of Corynebacterium glutamicum for hy-perproduction of polymer-grade l- and d-lactic acid. Appl. Microbiol. Biotechnol. 2019;103:3381–3391. doi: 10.1007/s00253-019-09737-8. [DOI] [PubMed] [Google Scholar]
- 42.Zhou L., Niu D., Tian K., Chen X., Prior B., Shen W., Shi G., Singh S., Wang Z. Genetically switched d-lactate production in Escherichia coli. Metab. Eng. 2012;14:560–568. doi: 10.1016/j.ymben.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 43.Wang Y., Liao J., Chiang C., Chao Y. A simple strategy to effectively produce d-lactate in crude glycerol-utilizing Escherichia coli. Biotechnol. Biofuels. 2019;12:273. doi: 10.1186/s13068-019-1615-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bustamante D., Tortajada M., Ramón D., Rojas A. Production of d-lactic acid by the fermentation of orange peel waste hydrolysate by lactic acid bacteria. Fermentation. 2019;6:1. doi: 10.3390/fermentation6010001. [DOI] [Google Scholar]
- 45.Nurul A., Afroditi C., Dimitris C. Microbial production of d-lactic acid from dried distiller’s grains with solubles. Eng. Life Sci. 2019;19:21–30. doi: 10.1002/elsc.201800077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Susan M., Luciana F., Jonas C. Efficient conversion of agroindustrial waste into d(-)lactic acid by Lactobacillus delbrueckii using fed-batch fermentation. BioMed Res. Int. 2020;2020:4194052. doi: 10.1155/2020/4194052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liang S., Jiang W., Song Y., Zhou S.-F. Improvement and metabolomics-based analysis of d-lactic acid production from agro-industrial wastes by Lactobacillus delbrueckii submitted to adaptive laboratory evolution. J. Agric. Food Chem. 2020;68:7660–7669. doi: 10.1021/acs.jafc.0c00259. [DOI] [PubMed] [Google Scholar]
- 48.Sahoo T.K., Jayaraman G. Co-culture of Lactobacillus delbrueckii and engineered Lactococcus lactis enhances stoichiometric yield of d-lactic acid from whey permeate. Appl. Microbiol. Biotechnol. 2019;103:5653–5662. doi: 10.1007/s00253-019-09819-7. [DOI] [PubMed] [Google Scholar]
- 49.Ahmad A., Banat F., Taher H. A review on the lactic acid fermentation from low-cost renewable materials: Recent developments and challenges. Environ. Technol. Innov. 2020;20:101138–101154. doi: 10.1016/j.eti.2020.101138. [DOI] [Google Scholar]
- 50.Zhang W., Xu X., Yu P., Zuo P., He Y., Chen H., Liu Y., Xue G., Li X., Alvarez P.J.J. Ammonium enhances food waste fermentation to high-value optically active l-lactic acid. ACS Sustain. Chem. Eng. 2020;8:669–677. doi: 10.1021/acssuschemeng.9b06532. [DOI] [Google Scholar]
- 51.Li C., Gao M., Zhu W., Wang N., Ma X., Wu C., Wang Q. Recent advances in the separation and purification of lactic acid from fermentation broth. Process. Biochem. 2021;104:142–151. doi: 10.1016/j.procbio.2021.03.011. [DOI] [Google Scholar]
- 52.Manandhar A., Shah A. Techno-economic analysis of bio-based lactic acid production utilizing corn grain as feedstock. Processes. 2020;8:199. doi: 10.3390/pr8020199. [DOI] [Google Scholar]
- 53.Maria A., Roland S., Kerstin M., Joachim V. Recent advances in d-lactic acid production from renewable resources: Case studies on agro-industrial waste streams. Food Technol. Biotechnol. 2019;57:293–304. doi: 10.17113/ftb.57.03.19.6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meng Y., Xue Y., Yu B., Gao C., Ma Y. Efficient production of l-lactic acid with high optical purity by alkaliphilic Bacillus sp. WL-S20. Bioresour. Technol. 2012;116:334–339. doi: 10.1016/j.biortech.2012.03.103. [DOI] [PubMed] [Google Scholar]
- 55.Abdel-Rahman M., Hassan S., Azab M., Mahin A., Gaber M. High improvement in lactic acid productivity by new alkaliphilic bacterium using repeated batch fermentation integrated with increased substrate concentration. BioMed Res. Int. 2019;2019:7212870. doi: 10.1155/2019/7212870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mamo G., Mattiasson B. Alkaliphiles: The versatile tools in biotechnology. Adv. Biochem. Eng. Biotechnol. 2020;172:1–51. doi: 10.1007/10_2020_126. [DOI] [PubMed] [Google Scholar]
- 57.Pal P., Sikder J., Roy S., Giorno L. Process intensification in lactic acid production: A review of membrane based processes. Chem. Eng. Process. Process. Intensif. 2009;48:1549–1559. doi: 10.1016/j.cep.2009.09.003. [DOI] [Google Scholar]
- 58.Bailly M., Balmann H.R., Aimar P., Lutin F., Cheryan M. Production process of fermented organic acids targeted around membrane operations: Design of the concentration step by conventional electrodialysis. J. Membr. Sci. 2001;191:129–142. doi: 10.1016/S0376-7388(01)00459-8. [DOI] [Google Scholar]
- 59.Choi S.Y., Cho I.J., Lee Y., Park S., Lee S.Y. Biocatalytic synthesis of polylactate and its copolymers by engineered microorganisms. Methods Enzymol. 2019;627:125–162. doi: 10.1016/bs.mie.2019.04.032. [DOI] [PubMed] [Google Scholar]
- 60.Selmer T., Willanzheimer A., Hetzel M. Propionate CoA-transferase from Clostridium propionicum: Cloning of gene and identification of glutamate 324 at the active site. Eur. J. Biochem. 2002;269:372–380. doi: 10.1046/j.0014-2956.2001.02659.x. [DOI] [PubMed] [Google Scholar]
- 61.Yokimiko D., Joo J.C., Yang J.E., Oh Y.H., Lee S.Y., Park S.J. Biosynthesis of 2-hydroxyacid-containing polyhydroxyal-kanoates by employing butyryl-CoA transferases in metabolically engineered Escherichia coli. Biotechnol. J. 2017;12:1700116. doi: 10.1002/biot.201700116. [DOI] [PubMed] [Google Scholar]
- 62.Mizuno S., Enda Y., Saika A., Hiroe A., Tsuge T. Biosynthesis of polyhydroxyalkanoates containing 2-hydroxy-4-methylvalerate and 2-hydroxy-3-phenylpropionate units from a related or unrelated carbon source. J. Biosci. Bioeng. 2018;125:295–300. doi: 10.1016/j.jbiosc.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 63.Yang J.E., Park S.J., Kim W.J., Kim H.J., Kim B.J., Lee H., Shin J., Lee S.Y. One-step fermentative production of aromatic polyesters from glucose by metabolically engineered Escherichia coli strains. Nat. Commun. 2018;9:1–10. doi: 10.1038/s41467-017-02498-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang T.H., Jung Y.K., Kang H.O., Kim T.W., Park S.J., Lee S.Y. Tailor-made type II Pseudomonas PHA synthases and their use for the biosynthesis of polylactic acid and its copolymer in recombinant Escherichia coli. Appl. Microbiol. Biotechnol. 2011;90:603–614. doi: 10.1007/s00253-010-3077-2. [DOI] [PubMed] [Google Scholar]
- 65.Choi S.Y., Park S.J., Kim W.J., Yang J.E., Lee H., Shin J., Lee S.Y. One-step fermentative production of poly(lactate-co-glycolate) from carbohydrates in Escherichia coli. Nat. Biotechnol. 2016;34:435–440. doi: 10.1038/nbt.3485. [DOI] [PubMed] [Google Scholar]
- 66.Tran T.T., Charles T.C. Genome-engineered Sinorhizobium meliloti for the production of poly(lactic-co-3-hydroxybutyric) acid copolymer. Can. J. Microbiol. 2016;62:130–138. doi: 10.1139/cjm-2015-0255. [DOI] [PubMed] [Google Scholar]
- 67.Park S.J., Jang Y.A., Lee H., Park A.R., Yang J.E., Shin J., Oh Y.H., Song B.K., Jegal J., Lee S.H., et al. Metabolic engineering of Ralstonia eutropha for the biosynthesis of 2-hydroxyacid-containing polyhydroxyalkanoates. Metab. Eng. 2013;20:20–28. doi: 10.1016/j.ymben.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 68.Song Y., Matsumoto K., Yamada M., Gohda A., Brigham C.J., Sinskey A.J., Taguchi S. Engineered Corynebacterium glutamicum as an endotoxin-free platform strain for lactate-based polyester production. Appl. Microbiol. Biotechnol. 2012;93:1917–1925. doi: 10.1007/s00253-011-3718-0. [DOI] [PubMed] [Google Scholar]
- 69.Kim Y.J., Chae C.G., Kang K.H., Oh Y.H., Joo J.C., Song B.K., Lee S.Y., Park S.J. Biosynthesis of lactate-containing polyhydroxyalkanoates in recombinant Escherichia coli by employing new CoA transferases. KSBB J. 2016;31:27–32. doi: 10.7841/ksbbj.2016.31.1.27. [DOI] [Google Scholar]
- 70.Lindenkamp N., Schürmann M., Steinbüchel A. A propionate CoA-transferase of Ralstonia eutropha H16 with broad sub-strate specificity catalyzing the CoA thioester formation of various carboxylic acids. Appl. Microbiol. Biotechnol. 2013;97:7699–7709. doi: 10.1007/s00253-012-4624-9. [DOI] [PubMed] [Google Scholar]
- 71.Volodina E., Schürmann M., Lindenkamp N., Steinbüchel A. Characterization of propionate CoA-transferase from Ralstonia eutropha H16. Appl. Microbiol. Biotechnol. 2014;98:3579–3589. doi: 10.1007/s00253-013-5222-1. [DOI] [PubMed] [Google Scholar]
- 72.Lee S.Y. Bacterial polyhydroxyalkanoates. Biotechnol. Bioeng. 1996;49:1–14. doi: 10.1002/(SICI)1097-0290(19960105)49:1<1::AID-BIT1>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 73.Park S.J., Kim T.W., Kim M.K., Lee S.Y., Lim S.-C. Advanced bacterial polyhydroxyalkanoates: Towards a versatile and sustainable platform for unnatural tailor-made polyesters. Biotechnol. Adv. 2012;30:1196–1206. doi: 10.1016/j.biotechadv.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 74.Schubert P., Steinbüchel A., Schlegel H.G. Cloning of the Alcaligenes eutrophus genes for synthesis of poly-beta-hydroxybutyric acid (PHB) and synthesis of PHB in Escherichia coli. J. Bacteriol. 1988;170:5837–5847. doi: 10.1128/jb.170.12.5837-5847.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huisman G.W., Wonink E., Meima R., Kazemier B., Terpstra P., Witholt B. Metabolism of poly(3-hydroxyalkanoates) (PHAs) by Pseudomonas oleovorans. Identification and sequences of genes and function of the encoded proteins in the synthesis and degradation of PHA. J. Biol. Chem. 1991;266:2191–2198. doi: 10.1016/S0021-9258(18)52227-4. [DOI] [PubMed] [Google Scholar]
- 76.Matsusaki H., Manji S., Taguchi K., Kato M., Fukui T., Doi Y. Cloning and molecular analysis of the poly(3-hydroxybutyrate) and poly(3-hydroxybutyrate- co -3-hydroxyalkanoate) biosynthesis genes in Pseudomonas sp. strain 61-3. J. Bacteriol. 1998;180:6459–6467. doi: 10.1128/JB.180.24.6459-6467.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liebergesell M., Steinbuchel A. Cloning and nucleotide sequences of genes relevant for biosynthesis of poly(3-hydroxybutyric acid) in Chromatium vinosum strain D. JBIC J. Biol. Inorg. Chem. 1992;209:135–150. doi: 10.1111/j.1432-1033.1992.tb17270.x. [DOI] [PubMed] [Google Scholar]
- 78.Liebergesell M., Rahalkar S., Steinbüchel A. Analysis of the Thiocapsa pfennigii polyhydroxyalkanoate synthase: Subcloning, molecular characterization and generation of hybrid synthases with the corresponding Chromatium vinosum enzyme. Appl. Microbiol. Biotechnol. 2000;54:186–194. doi: 10.1007/s002530000375. [DOI] [PubMed] [Google Scholar]
- 79.McCool G.J., Cannon M.C. PhaC and PhaR are required for polyhydroxyalkanoic acid synthase activity in Bacillus megaterium. J. Bacteriol. 2001;183:4235–4243. doi: 10.1128/JB.183.14.4235-4243.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Satoh Y., Minamoto N., Tajima K., Munekata M. Polyhydroxyalkanoate synthase from Bacillus sp. INT005 is composed of PhaC and PhaR. J. Biosci. Bioeng. 2002;94:343–350. doi: 10.1016/S1389-1723(02)80175-X. [DOI] [PubMed] [Google Scholar]
- 81.Ren Y., Meng D., Wu L., Chen J., Wu Q., Chen G.-Q. Microbial synthesis of a novel terpolyester P(LA-co-3HB-co-3HP) from low-cost substrates. Microb. Biotechnol. 2016;10:371–380. doi: 10.1111/1751-7915.12453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fukui T., Yokomizo S., Kobayashi G., Doi Y. Co-expression of polyhydroxyalkanoate synthase and (R)-enoyl-CoA hydra-tase genes of Aeromonas caviae establishes copolyester biosynthesis pathway in Escherichia coli. FEMS Microbiol. Lett. 1999;170:69–75. doi: 10.1111/j.1574-6968.1999.tb13356.x. [DOI] [PubMed] [Google Scholar]
- 83.Park S.J., Choi J.I., Lee S.Y. Engineering of Escherichia coli fatty acid metabolism for the production of polyhydroxyalka-noates. Enzyme Microb. Technol. 2005;36:579–588. doi: 10.1016/j.enzmictec.2004.12.005. [DOI] [Google Scholar]
- 84.Jung Y.K., Kim T.Y., Park S.J., Lee S.Y. Metabolic engineering of Escherichia coli for the production of polylactic acid and its copolymers. Biotechnol. Bioeng. 2010;105:161–171. doi: 10.1002/bit.22548. [DOI] [PubMed] [Google Scholar]
- 85.Shozui F., Matsumoto K., Motohashi R., Sun J., Satoh T., Kakuchi T., Taguchi S. Biosynthesis of a lactate (LA)-based polyester with a 96mol% LA fraction and its application to stereocomplex formation. Polym. Degrad. Stab. 2011;96:499–504. doi: 10.1016/j.polymdegradstab.2011.01.007. [DOI] [Google Scholar]
- 86.Choi S.Y., Kim W.J., Yu S.J., Park S.J., Im S.G., Lee S.Y. Engineering the xylose-catabolizing Dahms pathway for production of poly(d-lactate-co-glycolate) and poly(d-lactate-co-glycolate-co-d-2-hydroxybutyrate) in Escherichia coli. Microb. Biotechnol. 2017;10:1353–1364. doi: 10.1111/1751-7915.12721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yang J.E., Kim J.W., Oh Y.H., Choi S.Y., Lee H., Park A.-R., Shin J., Park S.J., Lee S.Y. Biosynthesis of poly(2-hydroxyisovalerate-co-lactate) by metabolically engineered Escherichia coli. Biotechnol. J. 2016;11:1572–1585. doi: 10.1002/biot.201600420. [DOI] [PubMed] [Google Scholar]
- 88.Sun L., Lee J.W., Yook S., Lane S., Sun Z., Kim S.R., Jin Y.S. Complete and efficient conversion of plant cell wall hemicellulose into high-value bioproducts by engineered yeast. Nat. Commun. 2021;12:4975. doi: 10.1038/s41467-021-25241-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sim S.J., Snell K.D., Hogan S.A., Stubbe J., Rha C., Sinskey A.J. PHA synthase activity controls the molecular weight and polydispersity of polyhydroxybutyrate in vivo. Nat. Biotechnol. 1997;15:63–67. doi: 10.1038/nbt0197-63. [DOI] [PubMed] [Google Scholar]