Editor's note.
The Editors of the 5 journals of the American College of Rheumatology and European Alliance of Associations for Rheumatology have been reminded by this editorial that ACR and EULAR have jointly agreed on various classification criteria, definitions, recommendations, or points to consider, which do not always find reflection in manuscripts submitted to the journals. Consequently, in the future, the Editors will enforce the use of the products obtained in the course of joint ACR/EULAR or EULAR/ACR activities in all respective papers. For rheumatoid arthritis this would mean use of the ACR/EULAR or EULAR/ACR classification criteria, remission definitions, recommendations on what to report in clinical trials, and others, as pertinent. The same applies to other diseases. There are valid and important reasons that these activities have been undertaken by ACR and EULAR, and therefore, the conclusions of the various task forces, which have been endorsed by ACR and EULAR, should be respected by investigators and study administrators. This does not mean other methods could not be used in a study, but at the least, the reports should address the methods agreed upon by the 2 organizations. Maintaining uniformity across major publications regarding rheumatoid arthritis remission or other definitions not only allows for more appropriate comparison across analyses, but also enhances readers' ability to interpret results. Author instructions across the 5 journals will more strongly reflect this requirement.
Over the last 30 years, treatment for RA has improved dramatically. By the early 2000s, disease remission had become a realistic goal, although definitions of remission varied widely, making it difficult to compare treatment strategies and gauge how often remission occurred. In 2009, the ACR and the EULAR created a joint committee whose charge was to recommend a definition of remission. Members of the committee suggested a large number of candidate definitions and using a data-driven consensus process, statisticians and programmers tested these candidates in a bank of RA trial data to see which definitions performed best in predicting long-term good function and lack of radiographic progression. The committee endorsed a stringent definition using measures from the validated core set of outcome measures.
After reviewing analysis results, the committee selected two definitions of remission that were approved by the ACR and EULAR.1 2 The first was a Boolean version in which, to be classified as having attained remission, a patient had to have tender and swollen joint counts (SJCs) of ≤1, a C reactive protein (CRP) level of ≤1 mg/dL and a patient global assessment of arthritis activity of ≤1 (on a 0–10 scale). The second recommended definition was a score of ≤3 on the Simplified Disease Activity Index (SDAI),3 a scoring system that is based on the same core set outcome measures. While designed and validated in trials, these definitions could help assess treatment ‘success’ in clinical practice as well as in trials and, in practice, could serve as a ‘treat-to-target’ goal for some patients.
Like all developed criteria, the ACR/EULAR 2011 RA remission criteria were labelled as provisionally approved and awaited validation in an independent sample for final approval. A revised validated version of the remission criteria is pending for full approval by ACR/EULAR. Many concerns have arisen since the publication of the provisional remission criteria. Among them is the continuing use in trials of 28-joint Disease Activity Score (DAS28) thresholds4 to define remission, questions about the use of CRP as an element of remission definitions and questions about the appropriateness of patient global assessment in defining RA remission. This editorial will address each of these issues.
Using the DAS28: when ‘remission’ is often not remission
The DAS28 is a widely used measure of disease activity. An ACR committee that critically evaluated RA disease activity measures for use in clinical settings found that the DAS28 met predefined criteria, including providing a score that stratified patients into at least three disease activity states, being measurable in the clinical setting and having adequate psychometric properties. The DAS28 was one of the four recommended RA disease activity measures.5
The committee on RA remission considered several DAS28 thresholds as candidate definitions of remission, including the popular threshold of a DAS28 using the CRP level (DAS28-CRP) of <2.6 and an even lower threshold of <2.0. The DAS28 formula weights SJC half as much as tender joint count (TJC) and also underweights it relative to CRP (or erythrocyte sedimentation rate (ESR)). Therefore, a patient can achieve a low DAS28 Score but still have a substantial number of swollen joints. The committee’s analyses showed that 10% of patients with a DAS28 of <2.6 had ≥4 swollen joints and one patient had >20 swollen joints. When a lower DAS28 threshold of <2.0 was used, SJCs of 2 or 3 were common and scores of up to 6 were possible. In fact, if the TJC is 0, values for the other components of the DAS28 become irrelevant (figure 1). Values of up to 60 (of 100) for patient global assessment are consistent with remission according to the DAS28. Even if the TJC is 1, the DAS28 Score can be in the remission range when other core set measures show active disease. DAS28-CRP thresholds differ substantially from those obtained with the DAS28 using the ESR (DAS28-ESR),6 and with the DAS28-ESR, RA would be even more likely to be classified as being in remission when disease is in fact active.
Figure 1.
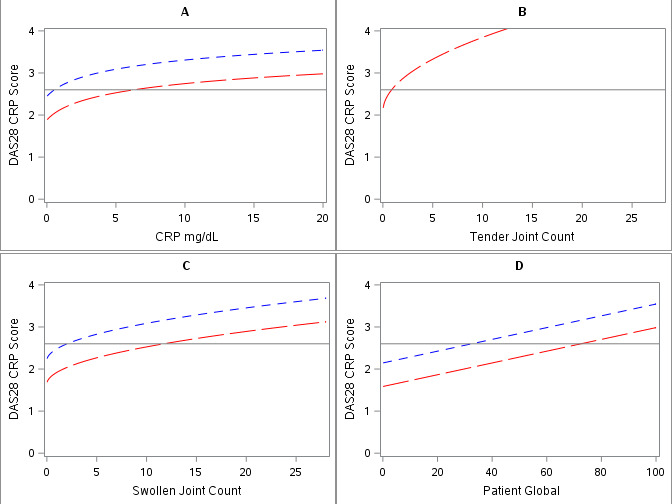
The contribution of each component of the 28-joint Disease Activity Score using the C reactive protein level (DAS28-CRP) to remission (score <2.6 (solid horizontal line)) when other components are in the range of remission. The DAS28-CRP is composed of four components: CRP level (A), tender joint count (TJC) (B), swollen joint count (SJC) (C) and patient global assessment of arthritis activity (D). In each graph, it is assumed that the three components other than the one depicted met the threshold for remission (CRP 0.5, TJC 0 [red dashed lines] or 1 [blue dashed lines], SJC 0, patient global assessment 1). Note that when the TJC is 0, most values of CRP and patient global assessment yield a DAS28 of <2.6 (‘remission’), and SJC values of <10 yield DAS28 ‘remission’.
One other major criterion was that patients whose disease was in remission at 6 months or 12 months in a 2-year trial should be likely to have both good and stable functional and radiographic outcomes later in the same trial. Patients in whom DAS28 remission was achieved had worse radiographic outcomes than those achieving remission according to other definitions (no change in the Sharp Score7 or the Sharp/van der Heijde Score8). Ultimately, the committee rejected DAS28 candidates as definitions of remission because SJCs were too high to be consistent with clinical remission and because DAS28 ‘remission,’ even with the use of stricter thresholds, did not predict good combined functional and radiographic outcomes as well as the predictive ability that was observed using the remission definitions selected by the committee.
Other studies carried out since the publication of ACR/EULAR remission criteria provided additional evidence that the DAS28 should not be used to define remission. Saleem et al9 demonstrated that among patients whose RA was in remission according to the DAS28, power Doppler ultrasound showed considerable disease activity unless disease was also in remission according to the SDAI. Lee et al10 reported that joint pain was present and persisted in patients whose disease was in remission according to the DAS28 but was absent if remission was classified according to the Boolean definition. Analyses from the AGREE trial of abatacept versus placebo11 confirmed that patients in whom remission was achieved according to the DAS28 subsequently had worse mean scores on the Health Assessment Questionnaire (HAQ)12 than those in whom remission was attained according to the SDAI. Schoels et al reported, from an analysis of three large multicenter RA trials, that among patients with a DAS28 of <1.9, those whose disease was not in remission according to the ACR/EULAR criteria still had an average of two to three swollen joints.13
Given the problems with the use of the DAS28 to define remission, why is it so widely used? First, the DAS28 is a commonly used disease activity measure and it is easy to apply a threshold in data already being acquired, although the requisite elements of the ACR/EULAR definitions of remission are also acquired. Another potential reason relates to industry-sponsored RA trials. A definition based on a DAS28 of <2.6 yields remission rates far higher than definitions endorsed by the ACR/EULAR and treatments therefore appear more efficacious with the use of the DAS28. Further, the use of a definition that yields a higher remission rate improves statistical power. The same absolute difference in remission rates between two drugs is more likely to reach statistical significance when remission rates are higher. Finally, DAS28 use is mandated by some regulatory agencies. Many reports do not even include data on other measures of remission.
When remission definitions favor some treatments over others
Reliance on the CRP level to define RA remission is an emerging concern.14 CRP is the second most heavily weighted variable in the DAS28 formula. The armamentarium for treatment of RA includes effective biologic agents that have different effects on CRP; interleukin-6 and JAK inhibitors both directly reduce CRP, whereas abatacept and rituximab do not. If the DAS28-CRP is used in a trial comparing the efficacy of abatacept and JAK inhibitors, even if effects on joint counts and patient-reported outcomes are the same, JAK inhibitors would score better, as seen in one recent trial.15 In another trial comparing biologic agents, the authors acknowledged avoiding the use of the DAS28-CRP because of this bias.16 The ACR/EULAR provisional criteria allow for remission definitions that exclude acute-phase reactants, using a three-variable version of the Boolean definition and the Clinical Disease Activity Index17 instead of the SDAI. Further, while the full ACR/EULAR remission definitions include acute-phase reactants, they are not weighted as heavily as in the DAS28-CRP (or the DAS28-ESR).
Concerns about inclusion of the patient global assessment
Yet another concern about the provisional definitions of remission has been championed by Ferreira et al.18 They point out that a patient’s global assessment of their arthritis activity often is based on considerations unrelated to current disease activity, such as pain from joint damage, and that this measure should not be included in definitions of remission. The factors that most influence the patient global activity measure are pain and fatigue. Ferreira et al’s analyses suggest that removing the patient global assessment would not compromise the ability to predict later radiographic outcomes in RA, although they acknowledge that patient global assessment is a powerful predictor of function (as measured by the HAQ). High patient global assessment scores not only correlate with poor concurrent physical function but they also identify patients whose physical function is worsening.19 20 If patient global assessment is removed, remission criteria no longer predict future patient function well.
In addition to it being the only patient-reported outcome measure included in remission definitions and the importance of including the patient perspective, there are other critical reasons to include patient global assessment as a component of remission. First, the patient global assessment reflects components of disease activity that are otherwise not captured, including fatigue and pain, as well as inflammation in joints not included in a 28-joint count, such as the feet and ankles. This may be why high patient global assessment scores, even when 28-joint counts are low, identify patients at high risk of later functional loss. Second, the patient global assessment is among the most sensitive, if not the most sensitive, outcome measure in RA.20 It improves much more with active RA treatment than with placebo, suggesting that it provides a window into disease activity related to systemic inflammation not detected by tender and SJCs. Therefore, eliminating patient global assessments from RA trial outcomes would compromise the ability to distinguish the comparative efficacy of different treatments. This would occur at a time when, given the large armamentarium of treatments available, there is a particular need to maximise the ability to differentiate their efficacy. In addition, inclusion of patient global assessment markedly increases the likelihood that patients in whom remission is attained will have both good radiographic outcomes and good functional outcomes later (table 1) and it ensures that the definition of remission captures non-radiographic outcomes that are important to patients.
Table 1.
Proportion of patients with good outcomes (both radiographic and functional) in three multicenter rheumatoid arthritis trials*
| Patients with good outcomes† | Candidate remission definition | |
| TJC, SJC and CRP level, all ≤1 | TJC, SJC, CRP level and patient global assessment, all ≤1 | |
| In remission, % | 46 | 66 |
| Not in remission, % | 17 | 17 |
| Positive likelihood ratio (95% CI) | 3.1 (1.9 to 5.3) | 7.2 (3.5 to 14.8) |
*Excluding patient global assessment compromises the ability to predict good outcomes.1
†Based on remission status at 6 months after baseline. Good radiographic outcome was defined as a change of 0 in the Sharp/van der Heijde Score between 12 months and 24 months after baseline. Good functional outcome was defined as a change of 0 in the. Health Assessment Questionnaire between 12 months and 24 months after baseline and a score of ≤0.5 at both the 12-month and 24-month time points.
CRP, C reactive protein; SJC, swollen joint count; TJC, tender joint count.
Conclusions
With remission achievable in RA, making the definition of remission stringent will ensure that patients benefit from comprehensive control of their disease. The DAS28 should not be used to define remission because, even with the use of low thresholds, many patients whose disease is in ‘remission’ will still have a number of swollen joints and active disease. Also, given its dependence on the CRP value, the use of the DAS28 makes it difficult to differentiate efficacious treatments with dissimilar effects on acute-phase reactant levels. Defining remission without asking patients to provide any information about their disease activity—not to mention failing to collect data on any patient-reported outcomes—risks losing valuable information on treatment efficacy.
rmdopen-2021-002034supp001.pdf (173.1KB, pdf)
Footnotes
Contributors: All authors drafted the article, revised it critically for important intellectual content and approved the final version to be published.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; internally peer reviewed.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Felson DT, Smolen JS, Wells G, et al. American College of rheumatology/European league against rheumatism provisional definition of remission in rheumatoid arthritis for clinical trials. Arthritis Rheum 2011;63:573–86. 10.1002/art.30129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Felson DT, Smolen JS, Wells G, et al. American College of rheumatology/European league against rheumatism provisional definition of remission in rheumatoid arthritis for clinical trials. Ann Rheum Dis 2011;70:404–13. 10.1136/ard.2011.149765 [DOI] [PubMed] [Google Scholar]
- 3.Smolen JS, Breedveld FC, Schiff MH, et al. A simplified disease activity index for rheumatoid arthritis for use in clinical practice. Rheumatology 2003;42:244–57. 10.1093/rheumatology/keg072 [DOI] [PubMed] [Google Scholar]
- 4.Prevoo ML, van 't Hof MA, Kuper HH, et al. Modified disease activity scores that include twenty-eight-joint counts. development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 1995;38:44–8. 10.1002/art.1780380107 [DOI] [PubMed] [Google Scholar]
- 5.England BR, Tiong BK, Bergman MJ. Update of the American college of rheumatology recommended rheumatoid arthritis disease activity measures. Arthritis Care Res 2019;2019:1540–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greenmyer JR, Stacy JM, Sahmoun AE, et al. DAS28-CRP cutoffs for high disease activity and remission are lower than DAS28-ESR in rheumatoid arthritis. ACR Open Rheumatol 2020;2:507–11. 10.1002/acr2.11171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharp JT, Young DY, Bluhm GB, et al. How many joints in the hands and wrists should be included in a score of radiologic abnormalities used to assess rheumatoid arthritis? Arthritis Rheum 1985;28:1326–35. 10.1002/art.1780281203 [DOI] [PubMed] [Google Scholar]
- 8.van der Heijde D. How to read radiographs according to the sharp/van der heijde method. J Rheumatol 1999;26:743–5. [PubMed] [Google Scholar]
- 9.Saleem B, Brown AK, Keen H, et al. Should imaging be a component of rheumatoid arthritis remission criteria? A comparison between traditional and modified composite remission scores and imaging assessments. Ann Rheum Dis 2011;70:792–8. 10.1136/ard.2010.134445 [DOI] [PubMed] [Google Scholar]
- 10.Lee YC, Cui J, Lu B, et al. Pain persists in DAS28 rheumatoid arthritis remission but not in ACR/EULAR remission: a longitudinal observational study. Arthritis Res Ther 2011;13:R83. 10.1186/ar3353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smolen JS, Wollenhaupt J, Gomez-Reino JJ, et al. Attainment and characteristics of clinical remission according to the new ACR-EULAR criteria in abatacept-treated patients with early rheumatoid arthritis: new analyses from the Abatacept study to Gauge Remission and joint damage progression in methotrexate (MTX)-naive patients with Early Erosive rheumatoid arthritis (AGREE). Arthritis Res Ther 2015;17:157. 10.1186/s13075-015-0671-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fries JF, Spitz P, Kraines RG, et al. Measurement of patient outcome in arthritis. Arthritis Rheum 1980;23:137–45. 10.1002/art.1780230202 [DOI] [PubMed] [Google Scholar]
- 13.Schoels M, Alasti F, Smolen JS, et al. Evaluation of newly proposed remission cut-points for disease activity score in 28 joints (DAS28) in rheumatoid arthritis patients upon IL-6 pathway inhibition. Arthritis Res Ther 2017;19:155. 10.1186/s13075-017-1346-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aletaha D, Smolen JS. Remission in rheumatoid arthritis: missing objectives by using inadequate DAS28 targets. Nat Rev Rheumatol 2019;15:633–4. 10.1038/s41584-019-0279-6 [DOI] [PubMed] [Google Scholar]
- 15.Rubbert-Roth A, Enejosa J, Pangan AL, et al. Trial of upadacitinib or abatacept in rheumatoid arthritis. N Engl J Med 2020;383:1511–21. 10.1056/NEJMoa2008250 [DOI] [PubMed] [Google Scholar]
- 16.Hetland ML, Haavardsholm EA, Rudin A, et al. Active conventional treatment and three different biological treatments in early rheumatoid arthritis: phase IV investigator initiated, randomised, observer blinded clinical trial. BMJ 2020;371:m4328. 10.1136/bmj.m4328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aletaha D, Nell VPK, Stamm T, et al. Acute phase reactants add little to composite disease activity indices for rheumatoid arthritis: validation of a clinical activity score. Arthritis Res Ther 2005;7:R796–806. 10.1186/ar1740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferreira RJO, Welsing PMJ, Jacobs JWG, et al. Revisiting the use of remission criteria for rheumatoid arthritis by excluding patient global assessment: an individual meta-analysis of 5792 patients. Ann Rheum Dis 2020. 10.1136/annrheumdis-2020-217171. [Epub ahead of print: 06 Oct 2020]. [DOI] [PubMed] [Google Scholar]
- 19.Studenic P, Felson D, de Wit M, et al. Testing different thresholds for patient global assessment in defining remission for rheumatoid arthritis: are the current ACR/EULAR Boolean criteria optimal? Ann Rheum Dis 2020;79:445–52. 10.1136/annrheumdis-2019-216529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Felson DT, Anderson JJ, Boers M, et al. The American College of rheumatology preliminary core set of disease activity measures for rheumatoid arthritis clinical trials. Arthritis & Rheumatism 1993;36:729–40. 10.1002/art.1780360601 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2021-002034supp001.pdf (173.1KB, pdf)


