Abstract
Objective
The differential diagnosis and management of seronegative enteropathies is challenging due to the rarity of these conditions, the overlap of clinical and histopathological features and the current lack of an international consensus on their nomenclature.
Design
This is a narrative review providing pragmatic guide on the investigation and clinical management of seronegative enteropathies in adults based on the available literature and our clinical experience.
Conclusions
Seronegative coeliac disease is the most frequent cause among the heterogeneous group of seronegative enteropathies and its diagnosis is confirmed by the clinical and histological response to a gluten-free diet after the exclusion of other causes of villous atrophy. Correct identification and targeted management of seronegative enteropathies is mandatory because of the variation in terms of clinical outcomes and prognosis.
Keywords: celiac disease, gluten free diet, malabsorption, small intestinal biopsy
Introduction
Villous atrophy (VA) is the histopathological hallmark of many chronic enteropathies, which, despite being aetiologically heterogeneous, can manifest clinically with a malabsorption syndrome. Conventional coeliac disease (CD) and its complications are the major cause of VA, particularly in the Western World.1–4 Diagnosis of CD in adults is based on positive specific serology (IgA tissue transglutaminase-TTA, endomysial antibodies-EmA, deamidated gliadin peptides-DGP) and a certain degree of VA responding to a gluten-free diet (GFD).1 2 When VA occurs in patients without IgA coeliac specific serology (negative IgA EmA/TTA/DGP) the term seronegative enteropathy (SNE) is usually adopted.3 4 This term refers to a group of rare and aetiologically heterogeneous clinical entities, whose common hallmark is duodenal VA (table 1).3–11 Differential diagnosis and clinical management of SNEs is still challenging, because of their rarity, the overlapping clinical and histopathological features and the lack of a standard consensus on the nomenclature and the diagnostic criteria for many of these conditions.3 4 We specify that patients with total IgA deficiency and positive IgG-based coeliac serology (IgG EmA/tTG/DGP) should be diagnosed with CD associated to IgA deficiency, rather than being considered as affected by SNEs.3 4
Table 1.
Aetiological classification of non-coeliac seronegative enteropathies with villous atrophy
| Type of enteropathy | Clinical and laboratory features | Histological/molecular features on duodenal biopsy | Diagnostic tests | Treatment |
| Immuno-mediated | ||||
| Autoimmune enteropathy | Severe malabsorption with intractable diarrhoea, weight loss and electrolyte imbalance unresponsive to dietary restrictions | IELs can be reduced, decreased globet cells, lymphoplasmacytic infiltrate in lamina propria, neutrophilic cryptitis | Positive anti-enterocyte antibodies | Immunosuppressants (steroids, azathioprine, infliximab) and parenteral nutritional support |
| Common variable immunodeficiency | Malabsorption of different severity, arising after age 2 years, poor response to vaccines, recurrent infections of upper airways | Absence of plasma cells, polymorphonuclear infiltrate of the lamina propria, GVHD-like lesions, Crohn’s like lesions | IgG <5 g/L+ low IgA or IgM | Steroids, budesonide, immunoglobulin replacement therapy |
| Lymphoproliferative | ||||
| EATL (type 1 and type 2) | Severe malabsorption, abdominal pain, fever, bleeding, obstruction and/or perforation; type 1 usually associated to CD, unlike type 2 | Monoclonal population of T cells on IHC or flow cytometry | Inflammatory markers, abdomen CT/PET scan, capsule endoscopy | Consult haematologist+chemotherapy |
| CD4+indolent lymphomas | Severe malabsorption, abdominal pain, fever, bleeding, obstruction and/or perforation |
|
Inflammatory markers, abdomen CT/PET scan, capsule endoscopy | Consult haematologist+chemotherapy |
| IPSID | Malabsorption syndrome of different severity | TCR gamma/beta clonality on duodenal biopsy Full thickness intestinal biopsy |
Heavy chains of immunoglobulin | Consult haematologist+chemotherapy+antibiotics |
| Iatrogenic | Severe malabsorption and suggestive pharmacological history | VA undistinguishable form CD | Duodenal biopsy and drug withdrawal | Drug withdrawal |
| Angiotensin type 2 receptor blockers | Severe malabsorption and suggestive pharmacological history | VA undistinguishable form CD | Duodenal biopsy and drug withdrawal | Drug withdrawal |
| Chemotherapy | Severe malabsorption and suggestive pharmacological history | VA undistinguishable from CD, lamina propria fibrosis | Duodenal biopsy | Steroids, consult oncologist to evaluate alternative regimens |
| Radiotherapy | Severe malabsorption and history of radiotherapy | Lamina propria fibrosis | Duodenal biopsy | Steroids |
| GVHD | Severe malabsorption and history of bone marrow transplantation | Crypt cell necrosis, loss of epithelium | Duodenal biopsy | Steroids or budesonide |
| Infectious | ||||
| Giardiasis | Malabsorption syndrome of different severity. Consider immune-deficiencies as predisposing conditions | Identification trophozoites on duodenal biopsy | PCR from duodenal aspirate, positive stool specific immunoassay | Metronidazole |
| HIV enteropathy | Known history of AIDS, presence of opportunistic infections | Decrease CD4+ T lymphocytes, increase in CD8+ T lymphocytes | HIV test | Antiretroviral therapy, treatment of opportunistic infections |
| Tuberculosis | Cough, ascites, night sweats, fever | Granulomatous disease | Interferon-gamma release assay, CT, ascetic fluid/sputum analysis | Specific anti-TB regimens |
| Whipple’s disease | History of seronegative migratory arthritis preceding onset of severe malabsorption and fever, enlarged lymphnodes, neurological symptoms | PAS+ macrophagic infiltration of the lamina propria |
|
Ceftriaxone/meropenem followed by TMP-SMX/hydroxychloroquine and doxycycline |
| Tropical sprue | History of travel to/residency in endemic areas, anaemia with vitamin B12 and folate deficiency | Increased plasma cells and eosinophils in lamina propria, changes in duodenum, jejunum and ileum | Duodenal biopsy, VCE, exclusion of other causes of VA | Tetracycline or doxycycline+folic acid |
| Inflammatory | ||||
| Eosinophilic gastro-enteritis | History of atopy and allergies | Massive eosinophilic infiltration on duodenal biopsy | Duodenal biopsy and peripheral hyper eosinophilia | Steroids and dietary therapy |
| Collagenous sprue | Severe malabsorption | Villous atrophy and subepithelial collagen deposition | Duodenal biopsy | GFD and immunosuppression (budesonide, prednisone, azathioprine) |
| Crohn’s disease | Chronic diarrhoea with blood, abdominal pain, fever, weight loss | Villous atrophy, granulomas | Duodenal biopsy, colonoscopy + biopsies, abdomen CT, entero-MRI | Steroids, antibiotics, azathioprine, biological therapy |
| Idiopathic | ||||
| IVA 1—transient VA likely post-infective | Diarrhoea, weight loss, dyspepsia | Histology usually undistinguishable from CD | Abdominal CT, VCE | Spontaneous resolution within 6 months |
| IVA 2—persistent non-lymphoproliferative VA | Severe malabsorption | Histology usually undistinguishable from CD | Abdominal CT, VCE | Immunosuppressants |
| IVA 3—persistent VA with lymphoproliferative features | Severe malabsorption, history of lymphoproliferative disorders | Histology usually undistinguishable from CD, monoclonal rearrangement for gamma-TCR | Abdominal CT, VCE | Immunosuppressants, consider haematological consultation |
CD, coeliac disease; CT, computed tomography; EATL, enteropathy associated T-cell lymphoma; GFD, gluten-free diet; GVHD, graft versus host disease; IELs, intraepithelial lymphocytes; IHC, immunohistochemistry; IPSID, immune-proliferative small intestinal disease; IVA, idiopathic villous atrophy; MEITL, monomorphic epitheliotropic T-cell lymphoma; MRI, magnetic resonance imaging; PAS, periodic acid Shiff staining; PET, positron emission tomography; PET, positron emission tomography; TMP-SMX, trimethoprim sulfamethoxazole; VA, villous atrophy; VCE, video-capsule endoscopy.
In this narrative review, we summarise the current knowledge on SNEs and provide a practical guide on the differential diagnosis and clinical management of these enteropathies in adult patients based on the available literature and our clinical experience.
Epidemiology of SNEs
Non-coeliac enteropathies (NCEs) are rare conditions, although data on the epidemiology are scarce and figures vary according to the population under investigation.
When the denominator consists of all the patients affected by VA, up to 5.1% of patients with VA can have a form of NCE. An Italian referral centre study over 15 years revealed that the remaining 94.9% of patients with VA was due to conventional seropositive CD.8 Conversely, when the general population is considered, the true prevalence of NCEs is still unknown.
There is a limited data on the prevalence of the different aetiologies of NCEs. When the denominator consists of all the patients affected by a form of NCEs, it emerges that, in adults, seronegative coeliac disease (SNCD) is the most common cause of SNEs followed by iatrogenic and infectious causes. Remarkably, in up to 14% of adult patients, a definitive aetiology was not found and a final diagnosis of idiopathic VA/undefined sprue was made.6–10 While conversely, in children, SNCD and iatrogenic causes are virtually absent and the most common aetiologies are inflammatory, infectious and immune-mediated.12 13
When the setting is the general population, there are no data on the prevalence of these enteropathies and it is only possible to make an estimation of how common these conditions may be. For example, prevalence of olmesartan-associated enteropathy, which is certainly the most common form of drug-induced SNEs is estimated to vary in between 1/4000 and 1/5000 case of olmesartan users.3 14 Autoimmune enteropathy has an estimated incidence of 1/million person years.15–18 Gastrointestinal involvement in common variable immunodeficiency (CVID) is frequent, and persistent diarrhoea has been described in up to 20%–60% of cases.19–23 However, the true prevalence of VA in patients affected by CVID is still unknown.3 The prevalence of Whipple’s disease can be estimated around three cases per million people, according to a paper from Northern Italy.24
Finally, as far as SNCD is concerned, although it is the most common aetiology for SNEs,3–10 25 the true prevalence of SNCD among coeliac patients and in the general population still needs to be elucidated. While the first papers on SNCD reported a prevalence of SNCD of 10%–20% of all coeliac patients,26–30 more recent studies show a lower prevalence between 2%–6.5%.8 9 31 These last figures on the prevalence of SNCD among all the coeliac patients are in line with the sensitivity of coeliac specific antibody testing.32 It is still unclear, however, if the difference between the first and the latest figures may be due to a true change in the prevalence of SNCD over the years, the higher sensitivity of Ema and TTA, or to the different diagnostic criteria adopted for this condition.25 There are currently no data on the prevalence of SNCD in the general population.
Long-term outcomes and mortality
Only two papers7 8 have evaluated long-term outcomes in SNEs showing that these enteropathies are characterised by poor prognosis. In the UK paper by Aziz et al7 patients affected by non-coeliac SNEs and those affected by SNCD had a higher mortality than conventional seropositive CD. In the Italian paper by Schiepatti et al, data about development of complications and mortality were provided. 4/260 patients with CD developed complications (type 1 refractory CD, abdominal B-cell lymphoma, small bowel carcinoma and enteropathy-associated T-cell lymphoma), compared with 4/14 SNEs patients, with a complication rate of 0.2 (95% CI 0.1 to 0.6) and 6.3 per 100 person years (95% CI 2.4 to 17.0), respectively.8
4/260 patients died in the CD group (three of unrelated causes and one of enteropathy-associated T-cell lymphoma) and 4/14 in the SNEs group, therefore corresponding to a mortality rate of 0.2 (95% CI 0.1 to 0.6) and 6.0 deaths per 100 person years (95% CI 2.2 to 16), respectively (HR=25.37, 95% CI 6.15 to 104.63, p<0.001).8
However, both the Italian and UK study did not compare risk of complications and mortality in SNCD and in each subtype of NCE to CD.7 8
This is a relevant point, as in our clinical experience it is likely that long-term outcomes are slightly different within the heterogeneous group of NCEs. In fact, while prognosis of SNEs due to an identifiable cause, such as drugs and infections, is usually excellent on removal of the trigger agent, patients affected by CVID, AE, idiopathic VA can develop severe complications worsening their prognosis.3 4 7 8 33–41
Patients affected by CVID enteropathy are burdened by a high mortality,33 39 mainly due to development of infectious and malignant complications. Also patients with AE can develop lymphoproliferative complications,35 40 and an American study showed an increased mortality in patients with AE, comparable to those affected by refractory CD.41
A recent dual-centre Italian-UK study, on forms VA of undetermined origin, also known as idiopathic villous atrophy (IVA), showed that these enteropathies can be subclassified into three main groups, with distinct clinical phenotypes and prognosis.37 IVA group 1 is characterised by forms of transient self-limiting partial VA, likely due to an infectious agent and good prognosis (5-year survival 96%). IVA group 2 is characterised by persistent non-clonal IVA with peculiar association with HLA DQB1*0301 and DQB1*06 and long-term survival (5-year survival was 100%). Finally, IVA group 3 is characterised by a cluster of enteropathies with lymphoproliferative features and poor outcome (5-year survival 27%). Hypoalbuminaemia and age at diagnosis were major predictors of mortality in IVA.
Finally, although a recent UK paper suggested that a more extensive small-bowel disease on capsule endoscopy correlated with a higher mortality (p=0.019) in SNEs,42 a thorough study of clinical predictors of poor long-term outcomes and mortality may be helpful to address the clinical follow-up of these patients.
Seronegative coeliac disease
A minority of coeliac patients present with VA and negative serology at diagnosis. These patients are affected by SNCD. Diagnosis of SNCD is based on the clinical and histological response to a GFD, after the exclusion of other NCEs.3 4 25 In the last years, our understanding of SNCD has been growing and it is likely that different forms of SNCD may exist. According to the literature, forms of true SNCD may be found in an early phase of the disease with a lesser degree of VA, as well as in the late stage of disease (with possible refractory CD or lymphoma) and in dermatitis herpetiformis.25 Remarkably, up to 30% of patients with biopsy proven dermatitis herpetiformis can have a negative serology at diagnosis.43 Forms of SNCD may also rarely occur in the first degree relatives of coeliac patients.44 45 Patients who had already been started on a GFD or steroids/immunosuppressive therapies prior to serological testing may also present with IgA negative TTA/EmA at diagnosis.25 However, when immunosuppressants are withdrawn or a GFD re-started a positive coeliac serology is found in these patients. Therefore, they should be considered as affected by conventional seropositive CD and not by SNCD.25
Debates still exist on whether patients affected by total IgA deficiency who show positive class IgG TTA or IgG EmA and VA should be considered as affected by SNCD. Taking into consideration that these patients generate an IgG-based serological response, this point may be in favour of a diagnosis of conventional seropositive CD instead of that of true SNCD.25 However, comparative data on the clinical phenotypes and long-term outcomes of patients with CD associated with IgA deficiency and SNCD are still lacking.
Finally, some authors suggest that SNCD can be found in association with CVID.46–49 Although this point is highly debated, the histological recovery of VA after gluten withdrawal is the mainstay for confirming CD in this specific setting. Moreover, a negative HLA-DQ2 and DQ8 typing is very useful to rule out CD.48 49 On the contrary, coeliac antibodies have no role in this diagnostic work-up. In fact, class IgG EmA has been found in CVID patients who had previously received intravenous immunoglobulin replacement therapy and in whom CD was excluded, due to the absence of HLA-DQ2 and DQ8 molecules.22 48 49 Finally, certain histopathological features such as absence of plasma cells and the presence of a polymorphonuclear infiltrate and graft-versus-host disease like lesions48–50 were described in patients affected by CVID and duodenal VA but not in untreated CD. However, their role is only supportive for diagnosis of CVID.
Although the main literature findings suggest that patients with SNCD are older at diagnosis and can more frequently present with severe malabsorption,7–9 25 29 there is a lack of clinical data about the long-term follow-up of SNCD.
Clinical features of patients with seronegative enteropathies
In the vast majority of cases, the clinical picture of SNEs is dominated by a severe malabsorption syndrome requiring upper GI endoscopy despite the negative coeliac serology.3 4 According to the literature, adult patients with SNEs (both SNCD and non-CD enteropathies) are older than patients with conventional seropositive CD.3 4 7–9 Therefore, the overlap in presenting symptoms between NCEs, SNCD and complications of CD is certainly a major challenge in the differential diagnosis of these conditions.
Some clinical clues can be useful to guide the differential diagnosis between seronegative CD and non-coeliac SNEs. For example, a thorough pharmacological and travel history can guide towards a diagnosis of iatrogenic forms of SNEs or tropical sprue, respectively. A personal history of primary (such as CVID or IgA deficiency) or secondary immunodeficiency can represent a predisposing factor to giardiasis.3–11 A history of recurrent upper and lower airways infections in early childhood can support the diagnosis of CVID. Finally, for other conditions such as Whipple’s disease, tuberculosis and HIV, VA and a malabsorption syndrome are usually not the typical primary manifestations prompting the diagnosis, especially if other more relevant findings unrelated to malabsorption are not present. For example, Whipple’s disease should be suspected predominantly in middle-aged Caucasians men with a long-lasting history of seronegative arthritis, lymphadenopathy and fever, which anticipates the onset of a severe malabsorption syndrome.24 51 A potentially life-threatening neurological involvement can complete the clinical picture.
Histological features of seronegative enteropathies
Although VA is the key histological feature for NCEs and it is mandatory for the diagnosis, there are currently no specific histological markers allowing the differential diagnosis between SNCD and other non-coeliac SNEs. Some of these histological findings may be supportive for a specific aetiology (see table 1), but need always to be considered in the whole clinical and biochemical scenario. The only two conditions that can be directly diagnosed through histology are giardiasis and Whipple’s disease. However, these two conditions usually do not pose a problem of differential diagnosis with SNCD and other seronegative enteropathies, as VA is not always present and the diagnostic tests are very specific. PCR on duodenal biopsies aspirate or direct identification of Giardia lamblia by the pathologist on formalin-fixed paraffin embedded H&E stained small-bowel specimens are reliable diagnostic methods for making the diagnosis of giardiasis.3 4 Duodenal biopsy showing a periodic acid Shiff staining+diastase resistant macrophagic infiltration of the lamina propria is key to the diagnosis of Whipple’s disease. PCR for Tropheryma whipplei is highly specific but should be reserved for certain sterile sites, such as the central nervous system.51
Traditional histology, immunohistochemistry, flow cytometry and molecular diagnostics (PCR on duodenal specimens for detecting monoclonal rearrangements of gamma/beta-TCR genes) remain the key diagnostic tests for some kind of rare primary lymphoproliferative disorders of the small bowel that can manifest with VA in the uninvolved non-neoplastic mucosa.52 These conditions can pose a problem of differential diagnosis with SNCD and include enteropathy associated T-cell lymphoma- type 1 and type 2, indolent T-cell lymphoproliferative disease of gastrointestinal tract, and immune-proliferative small intestinal disease. Abdomen CT, positron emission tomography (PET)-CT and bone marrow aspiration can complete the diagnostic work-up for these disorders.3 4
Some interest was initially dedicated to intestinal deposits of IgA tTG2 antibodies that were found in the small bowel mucosa of seronegative coeliac patients, but not in other NCEs.29 However, their specificity has recently been questioned,53 so their relevance for differentiating SNCD from other NCEs in everyday clinical practice is still limited.
More recently, it has been suggested that analysis of intraepithelial lymphocytes by means of flow cytometry allows the distinction of SNCD from non-coeliac SNEs on the basis of the so called ‘coeliac lymphogram’.10 54 These methods, however, need to be validated on larger sample sizes.
Serological markers and faecal tests
Enterocyte antibodies (AEA) and dosage of serum immunoglobulins are the most relevant blood test in the differential diagnosis of SNEs.3 4 AEA detected by means of indirect immunofluorescence on monkey jejunum slides are the mainstay for serological diagnosis of AE in adults.16 18 55 In children, also cases of AE with negative AEA have been described, particularly in association with rare and complex immunodeficiency syndromes.56 Although standard diagnostic criteria for the detection of AEA in indirect immunofluorescence are still lacking, on the basis of our clinical experience, we and others suggest that in adult patients with VA unresponsive to a GFD, positive AEA confirm the diagnosis of AE.3 4 16 26 34 35 55 Anti-goblet cell antibodies were also proposed as a further marker for AE.18 However, they are totally non-specific and their use should definitely be discouraged for the diagnosis of AE.3 4 57
A marked decrease of IgG (at least 2 SD below the mean for age) and at least one of either IgM or IgA is required to make a diagnosis of CVID. The following diagnostic criteria must also be fulfilled: onset of immunodeficiency after the age of 2 years; absent isohaemagglutinins and/or poor response to vaccines; exclusion of secondary causes of hypogammaglobulinaemia such as malignancies, drugs, infections and genetic disorders.19
Finally, for giardiasis, identification of cysts or trophozoites in the stool, stool specific Giardia antigens, PCR on duodenal biopsies aspirate or direct identification of the parasite by the pathologist on formalin-fixed paraffin embedded H&E stained small-bowel specimens are reliable diagnostic methods.3 4
Conditions that should not be considered in the differential diagnosis
It has been reported that also small intestinal bacteria overgrowth (SIBO) and Helicobacter pylori may be associated with non-coeliac VA.6–8 However, the strength of evidence is poor for these two conditions. In patients affected by SIBO a wide variety of histological lesions have been reported,58–60 but VA is not a key diagnostic element. Whereas mild VA seems to occur only in the most severe cases of SIBO,6 7 9 in most patients a normal villous architecture has been described.58 59 Moreover, in patients with VA and absence of any predisposing conditions to SIBO a positive glucose H2 breath test is quite likely to be a consequence of the histological lesions themselves rather than their cause.60 Few reports suggest peptic duodenitis with or without H. pylori infection5 7 61 as the cause of SNVA, but there is scarce evidence in favour of its causative role in SNVA.
Methodological approach to differential diagnosis of seronegative enteropathies
Currently, there is no standardised international consensus about the nomenclature and the clinical management of SNEs. However, based on our experience and the recommendations by the American Gastroenterology Association, and other centres with international expertise on SNEs,3–11 we have developed an investigational work-up for the differential diagnosis of SNEs (figure 1).
Figure 1.
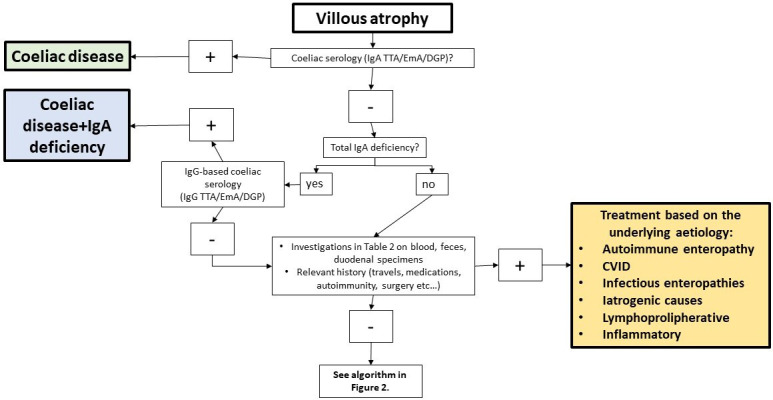
Algorithm for the differential diagnosis of duodenal villous atrophy. CVID, common variable immunodeficiency; DGP, deamidated gliadin antibodies; EmA, endomysial antibodies; TTA, tissue transglutaminase antibodies.
The first step is the assessment of VA on correctly duodenal specimens taken from the second duodenal portion. It is mandatory to ascertain that investigations leading to the diagnosis of VA were conducted while on a gluten-containing diet, and if not, additional or repeat testing should be performed after gluten-challenge (box 1). For patients with a history of VA, a careful review of duodenal biopsy slides by an expert gastrointestinal pathologist informed about the pharmacological history and clinical features of the patient is mandatory. This means that patients presenting with increased intraepithelial lymphocyte count only, without VA, cannot be considered in this algorithm. Unfortunately, the poor orientation of duodenal specimens still account for a substantial proportion of the diagnostic errors in SNEs, leading to overestimation of seronegative CD and unnecessary treatment.62
Box 1. Diagnostic panel for differential diagnosis of seronegative enteropathies.
Laboratory tests
HLA typing.
Serum IgA, IgG, IgM.
IgA and IgG Ema, TTA and DGP.
Anti-enterocyte antibodies.
HIV testing.
Quantiferon.
Stool tests
Giardia lamblia and other parasites.
Viruses.
Helicobacter pylori antigens.
Faecal calprotectin.
Duodenal biopsies
H&E and PAS staining.
PCR for Giardia, tuberculosis and Whipple’s disease.
Small bowel aspirate.
PCR for beta and gamma-TCR clonality assessment.
Flow cytometry for aberrant IELs.
Other examinations
Capsule endoscopy.
Abdomen CT/PET.
Colonoscopy+biopsies.
The second key requirement is the synchronous presence of negative IgA EmA/ttG/DGP.4
Once VA and negative coeliac antibodies are confirmed, all the possible aetiologies must be excluded before considering the possibility of SNCD or IVA.3 4 25 37 In this regard, a pertinent pharmacological and clinical history, together with specific laboratory and molecular tests can guide clinicians to the appropriate diagnosis in a substantial number of cases (table 1). HLA typing for DQ2.5 (DQA1*05, DQB1*0201), DQ2.2 (DQA1*02, DQB1*0202), DQ8 (DQA1*03, DQB1*0302) and DQ7.5 (DQA1*05, DQB1*0301) should be carefully considered, particularly when negative in order to exclude SNCD.3 4 25
Clinical management and follow-up of seronegative enteropathies
The optimal management of SNEs is still unknown. Data from major referral centres have tried to address the issue of the differential diagnosis of SNVA, but there is still a complete lack of data on the best treatment options and timing for clinical and endoscopic follow-up of these patients.
Based on our clinical experience, we propose the following options for the treatment and follow-up of these patients.
Treatment
While for patients affected by SNEs due to a known cause, the treatment depends on the underlying aetiology (see table 1), it is still unclear what the best approach for patients with SNEs and no identifiable cause should be. Although histological response to a GFD is the mainstay for diagnosing SNCD, uncertainties still exist about the correct timing for a patient affected by SNVA, positive HLA-DQ2/DQ8/DQ7.5 and negative investigations to be started on a GFD. We think that it can be reasonable to take into account factors such as ethnicity, HLA typing and severity of the clinical picture and length of involved small-bowel on capsule endoscopy3 4 7 37 42 to guide the decision on whether or not to start a GFD. We suggest that in Caucasian patients carrying HLA-DQ2/DQ8 and in whom no alternative aetiology has been found, a GFD could be started. In patients with these characteristics and presenting with severe features of malabsorption and age >50 years corticosteroids followed by oral budesonide may be considered to promote mucosal recovery. Parenteral nutrition should be considered for patients with severe malabsorption and malnutrition. In patients with no cause found for VA, mild symptoms and absence of laboratory abnormalities at diagnosis (IVA 1), it may be reasonable to adopt a ‘watch and wait’ approach and a histological follow-up, as this resulted in histological recovery of VA within 12 months from diagnosis.3 7 37 Figure 2 shows a flow chart for the diagnosis and management of SNCD and idiopathic forms of VA.
Figure 2.
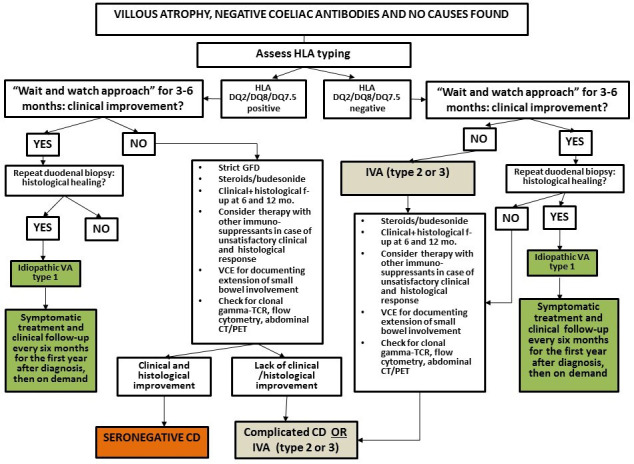
Flow-chart for the diagnosis and management of seronegative coeliac disease and IVA. CD, coeliac disease; GFD, gluten-free diet; IVA, idiopathic villous atrophy; PET, positron emission tomography; VCE, video capsule endoscopy; TCR, T-cell receptor.
Follow-up
Given the risk of poor long-term outcomes in SNEs,3 4 7 8 we strongly recommend a strict clinical and endoscopic follow-up in these patients, regardless of the underlying cause. While it could be debatable whether to perform a follow-up duodenal biopsy in patients with SNEs due to an identifiable and reversible cause (such as iatrogenic) and in whom a complete and satisfactory clinical response to treatment has occurred, we believe it is mandatory to document both histological recovery and extension of the involved small-bowel by means of VCE in patients with SNCD, CVID, autoimmune enteropathy, IVA, iatrogenic causes and lymphomas.
Conclusions
Differential diagnosis and clinical management of SNEs in adults are still challenging, and in the absence of specific international guidelines, pitfalls are still common. In our review, we have suggested investigation and management strategies based on expert opinion, which we hope can nevertheless be helpful and largely pragmatic, despite the lack of a true evidence base.
A systematic and algorithmic approach may be useful to appropriately categorise these patients and treat them accordingly. Assessment of histological response to treatment is the mainstay to make the diagnosis of SNCD, but also to guide clinical management and follow-up of the other forms of SNEs.
Future research perspectives may include the development of standard consensus guidelines for the diagnosis and a better definition of natural history of different forms of SNEs. Identification of molecular mechanisms beyond SNVA will also be precious to develop biomarkers and target therapies, particularly for patients at higher risk of poor outcomes.
Footnotes
Contributors: AS and DSS conceived the study. AS drafted the manuscript with MC, FB and DSS. DSS revised the study for important intellectual content. All the authors approved the final version of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Commissioned; internally peer reviewed.
Data availability statement
No data are available. Not applicable.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Ludvigsson JF, Bai JC, Biagi F, et al. Diagnosis and management of adult coeliac disease: guidelines from the British Society of gastroenterology. Gut 2014;63:1210–28. 10.1136/gutjnl-2013-306578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Toma A, Volta U, Auricchio R, et al. European Society for the study of coeliac disease (ESsCD) guideline for coeliac disease and other gluten-related disorders. United European Gastroenterol J 2019;7:583–613. 10.1177/2050640619844125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schiepatti A, Sanders DS, Zuffada M, et al. Overview in the clinical management of patients with seronegative villous atrophy. Eur J Gastroenterol Hepatol 2019;31:409–17. 10.1097/MEG.0000000000001340 [DOI] [PubMed] [Google Scholar]
- 4.Leonard MM, Lebwohl B, Rubio-Tapia A, et al. AGA clinical practice update on the evaluation and management of seronegative Enteropathies: expert review. Gastroenterology 2021;160:437–44. 10.1053/j.gastro.2020.08.061 [DOI] [PubMed] [Google Scholar]
- 5.Pallav K, Leffler DA, Tariq S, et al. Noncoeliac enteropathy: the differential diagnosis of villous atrophy in contemporary clinical practice. Aliment Pharmacol Ther 2012;35:380–90. 10.1111/j.1365-2036.2011.04938.x [DOI] [PubMed] [Google Scholar]
- 6.DeGaetani M, Tennyson CA, Lebwohl B, et al. Villous atrophy and negative celiac serology: a diagnostic and therapeutic dilemma. Am J Gastroenterol 2013;108:647–53. 10.1038/ajg.2013.45 [DOI] [PubMed] [Google Scholar]
- 7.Aziz I, Peerally MF, Barnes J-H, et al. The clinical and phenotypical assessment of seronegative villous atrophy; a prospective UK centre experience evaluating 200 adult cases over a 15-year period (2000-2015). Gut 2017;66:1563–72. 10.1136/gutjnl-2016-312271 [DOI] [PubMed] [Google Scholar]
- 8.Schiepatti A, Biagi F, Fraternale G, et al. Short article: mortality and differential diagnoses of villous atrophy without coeliac antibodies. Eur J Gastroenterol Hepatol 2017;29:572–6. 10.1097/MEG.0000000000000836 [DOI] [PubMed] [Google Scholar]
- 9.Volta U, Caio G, Boschetti E, et al. Seronegative celiac disease: shedding light on an obscure clinical entity. Dig Liver Dis 2016;48:1018–22. 10.1016/j.dld.2016.05.024 [DOI] [PubMed] [Google Scholar]
- 10.Fernández-Bañares F, Crespo L, Núñez C, et al. Gamma delta+ intraepithelial lymphocytes and coeliac lymphogram in a diagnostic approach to coeliac disease in patients with seronegative villous atrophy. Aliment Pharmacol Ther 2020;51:699–705. 10.1111/apt.15663 [DOI] [PubMed] [Google Scholar]
- 11.Jansson-Knodell CL, Murray JA, Rubio-Tapia A. Management of small bowel villous atrophy in patients seronegative for celiac disease. Am J Gastroenterol 2020;115:492–7. 10.14309/ajg.0000000000000575 [DOI] [PubMed] [Google Scholar]
- 12.Gustafsson I, Repo M, Popp A, et al. Prevalence and diagnostic outcomes of children with duodenal lesions and negative celiac serology. Dig Liver Dis 2020;52:289–95. 10.1016/j.dld.2019.11.011 [DOI] [PubMed] [Google Scholar]
- 13.Mandile R, Maglio M, Pellino N, et al. Seronegative villous atrophy in children: clinical and immunohistochemical features. J Pediatr Gastroenterol Nutr 2021;72:282–7. 10.1097/MPG.0000000000002917 [DOI] [PubMed] [Google Scholar]
- 14.Marietta EV, Cartee A, Rishi A, et al. Drug-Induced enteropathy. Dig Dis 2015;33:215–20. 10.1159/000370205 [DOI] [PubMed] [Google Scholar]
- 15.Unsworth DJ, Walker-Smith JA. Autoimmunity in diarrhoeal disease. J Pediatr Gastroenterol Nutr 1985;4:375–80. 10.1097/00005176-198506000-00009 [DOI] [PubMed] [Google Scholar]
- 16.Corazza GR, Biagi F, Volta U, et al. Autoimmune enteropathy and villous atrophy in adults. Lancet 1997;350:106–9. 10.1016/S0140-6736(97)01042-8 [DOI] [PubMed] [Google Scholar]
- 17.Masia R, Peyton S, Lauwers GY, et al. Gastrointestinal biopsy findings of autoimmune enteropathy: a review of 25 cases. Am J Surg Pathol 2014;38:1319–29. 10.1097/PAS.0000000000000317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akram S, Murray JA, Pardi DS, et al. Adult autoimmune enteropathy: Mayo clinic Rochester experience. Clin Gastroenterol Hepatol 2007;5:1282–90. 10.1016/j.cgh.2007.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park MA, Li JT, Hagan JB, et al. Common variable immunodeficiency: a new look at an old disease. Lancet 2008;372:489–502. 10.1016/S0140-6736(08)61199-X [DOI] [PubMed] [Google Scholar]
- 20.Kalha I, Sellin JH. Common variable immunodeficiency and the gastrointestinal tract. Curr Gastroenterol Rep 2004;6:377–83. 10.1007/s11894-004-0053-y [DOI] [PubMed] [Google Scholar]
- 21.Khodadad A, Aghamohammadi A, Parvaneh N, et al. Gastrointestinal manifestations in patients with common variable immunodeficiency. Dig Dis Sci 2007;52:2977–83. 10.1007/s10620-006-9736-6 [DOI] [PubMed] [Google Scholar]
- 22.Jørgensen SF, Reims HM, Frydenlund D, et al. A cross-sectional study of the prevalence of gastrointestinal symptoms and pathology in patients with common variable immunodeficiency. Am J Gastroenterol 2016;111:1467–75. 10.1038/ajg.2016.329 [DOI] [PubMed] [Google Scholar]
- 23.Hermaszewski RA, Webster AD. Primary hypogammaglobulinaemia: a survey of clinical manifestations and complications. Q J Med 1993;86:31–42. [PubMed] [Google Scholar]
- 24.Biagi F, Balduzzi D, Delvino P, et al. Prevalence of Whipple's disease in north-western Italy. Eur J Clin Microbiol Infect Dis 2015;34:1347–8. 10.1007/s10096-015-2357-2 [DOI] [PubMed] [Google Scholar]
- 25.Schiepatti A, Sanders DS, Biagi F. Seronegative coeliac disease: clearing the diagnostic dilemma. Curr Opin Gastroenterol 2018;34:154–8. 10.1097/MOG.0000000000000436 [DOI] [PubMed] [Google Scholar]
- 26.Abrams JA, Diamond B, Rotterdam H, et al. Seronegative celiac disease: increased prevalence with lesser degrees of villous atrophy. Dig Dis Sci 2004;49:546–50. 10.1023/B:DDAS.0000026296.02308.00 [DOI] [PubMed] [Google Scholar]
- 27.Rostami K, Kerckhaert J, Tiemessen R, et al. Sensitivity of antiendomysium and antigliadin antibodies in untreated celiac disease: disappointing in clinical practice. Am J Gastroenterol 1999;94:888–94. 10.1111/j.1572-0241.1999.983_f.x [DOI] [PubMed] [Google Scholar]
- 28.Rostami K, Kerckhaert J, von Blomberg BM, et al. Sat and serology in adult coeliacs, seronegative coeliac disease seems a reality. Neth J Med 1998;53:15–19. 10.1016/S0300-2977(98)00050-3 [DOI] [PubMed] [Google Scholar]
- 29.Salmi TT, Collin P, Korponay-Szabó IR, et al. Endomysial antibody-negative coeliac disease: clinical characteristics and intestinal autoantibody deposits. Gut 2006;55:1746–53. 10.1136/gut.2005.071514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dickey W, Hughes DF, McMillan SA. Reliance on serum endomysial antibody testing underestimates the true prevalence of coeliac disease by one fifth. Scand J Gastroenterol 2000;35:181–3. 10.1080/003655200750024362 [DOI] [PubMed] [Google Scholar]
- 31.Collin P, Kaukinen K, Vogelsang H, et al. Antiendomysial and antihuman recombinant tissue transglutaminase antibodies in the diagnosis of coeliac disease: a biopsy-proven European multicentre study. Eur J Gastroenterol Hepatol 2005;17:85–91. 10.1097/00042737-200501000-00017 [DOI] [PubMed] [Google Scholar]
- 32.Leffler DA, Schuppan D. Update on serologic testing in celiac disease. Am J Gastroenterol 2010;105:2520–4. 10.1038/ajg.2010.276 [DOI] [PubMed] [Google Scholar]
- 33.Pensieri MV, Pulvirenti F, Schiepatti A, et al. The high mortality of patients with common variable immunodeficiency and small bowel villous atrophy. Scand J Gastroenterol 2019;54:164–8. 10.1080/00365521.2019.1568543 [DOI] [PubMed] [Google Scholar]
- 34.Ciccocioppo R, Russo ML, Bernardo ME, et al. Mesenchymal stromal cell infusions as rescue therapy for corticosteroid-refractory adult autoimmune enteropathy. Mayo Clin Proc 2012;87:909–14. 10.1016/j.mayocp.2012.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ciccocioppo R, Croci GA, Biagi F, et al. Intestinal T-cell lymphoma with enteropathy-associated T-cell lymphoma-like features arising in the setting of adult autoimmune enteropathy. Hematol Oncol 2018;36:481–8. 10.1002/hon.2494 [DOI] [PubMed] [Google Scholar]
- 36.Schiepatti A, Biagi F, Cumetti D, et al. Olmesartan-associated enteropathy: new insights on the natural history? report of two cases. Scand J Gastroenterol 2016;51:152–6. 10.3109/00365521.2015.1074719 [DOI] [PubMed] [Google Scholar]
- 37.Schiepatti A, Sanders DS, Aziz I, et al. Clinical phenotype and mortality in patients with idiopathic small bowel villous atrophy: a dual-centre international study. Eur J Gastroenterol Hepatol 2020;32:938–49. 10.1097/MEG.0000000000001726 [DOI] [PubMed] [Google Scholar]
- 38.Basson M, Mezzarobba M, Weill A, et al. Severe intestinal malabsorption associated with olmesartan: a French nationwide observational cohort study. Gut 2016;65:1664–9. 10.1136/gutjnl-2015-309690 [DOI] [PubMed] [Google Scholar]
- 39.Resnick ES, Moshier EL, Godbold JH, et al. Morbidity and mortality in common variable immune deficiency over 4 decades. Blood 2012;119:1650–7. 10.1182/blood-2011-09-377945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Malamut G, Verkarre V, Callens C, et al. Enteropathy-Associated T-cell lymphoma complicating an autoimmune enteropathy. Gastroenterology 2012;142:726–9. 10.1053/j.gastro.2011.12.040 [DOI] [PubMed] [Google Scholar]
- 41.Sharma A, Choung RS, Wang XJ, et al. Features of adult autoimmune enteropathy compared with refractory celiac disease. Clin Gastroenterol Hepatol 2018;16:877–83. 10.1016/j.cgh.2017.12.044 [DOI] [PubMed] [Google Scholar]
- 42.Chetcuti Zammit S, Schiepatti A, Aziz I, et al. Use of small-bowel capsule endoscopy in cases of equivocal celiac disease. Gastrointest Endosc 2020;91:1312–21. 10.1016/j.gie.2019.12.044 [DOI] [PubMed] [Google Scholar]
- 43.Reunala T, Hervonen K, Salmi T. Dermatitis herpetiformis: an update on diagnosis and management. Am J Clin Dermatol 2021;22:329–38. 10.1007/s40257-020-00584-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Esteve M, Rosinach M, Fernández-Bañares F, et al. Spectrum of gluten-sensitive enteropathy in first-degree relatives of patients with coeliac disease: clinical relevance of lymphocytic enteritis. Gut 2006;55:1739–45. 10.1136/gut.2006.095299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Henry DX, Sanders DS, Basu K, et al. Seronegative coeliac disease masquerading as irritable bowel syndrome type symptoms. J Gastrointestin Liver Dis 2020;29:111–3. 10.15403/jgld-718 [DOI] [PubMed] [Google Scholar]
- 46.Heneghan MA, Stevens FM, Cryan EM, et al. Celiac sprue and immunodeficiency states: a 25-year review. J Clin Gastroenterol 1997;25:421–5. 10.1097/00004836-199709000-00004 [DOI] [PubMed] [Google Scholar]
- 47.Webster AD, Slavin G, Shiner M, et al. Coeliac disease with severe hypogammaglobulinaemia. Gut 1981;22:153–7. 10.1136/gut.22.2.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Biagi F, Bianchi PI, Zilli A, et al. The significance of duodenal mucosal atrophy in patients with common variable immunodeficiency: a clinical and histopathologic study. Am J Clin Pathol 2012;138:185–9. 10.1309/AJCPEIILH2C0WFYE [DOI] [PubMed] [Google Scholar]
- 49.Malamut G, Verkarre V, Suarez F, et al. The enteropathy associated with common variable immunodeficiency: the delineated frontiers with celiac disease. Am J Gastroenterol 2010;105:2262–75. 10.1038/ajg.2010.214 [DOI] [PubMed] [Google Scholar]
- 50.Daniels JA, Lederman HM, Maitra A, et al. Gastrointestinal tract pathology in patients with common variable immunodeficiency (CVID): a clinicopathologic study and review. Am J Surg Pathol 2007;31:1800–12. 10.1097/PAS.0b013e3180cab60c [DOI] [PubMed] [Google Scholar]
- 51.Marth T, Moos V, Müller C, et al. Tropheryma whipplei infection and Whipple's disease. Lancet Infect Dis 2016;16:e13–22. 10.1016/S1473-3099(15)00537-X [DOI] [PubMed] [Google Scholar]
- 52.Foukas PG, de Leval L. Recent advances in intestinal lymphomas. Histopathology 2015;66:112–36. 10.1111/his.12596 [DOI] [PubMed] [Google Scholar]
- 53.Maglio M, Ziberna F, Aitoro R, et al. Intestinal production of anti-tissue transglutaminase 2 antibodies in patients with diagnosis other than celiac disease. Nutrients 2017;9. 10.3390/nu9101050. [Epub ahead of print: 21 Sep 2017]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fernández-Bañares F, Núñez C, Montoro M, et al. Management of small bowel villous atrophy in patients seronegative for celiac disease: high diagnostic accuracy of celiac Lymphogram. Am J Gastroenterol 2020;115:2110. 10.14309/ajg.0000000000000768 [DOI] [PubMed] [Google Scholar]
- 55.Volta U, Mumolo MG, Caio G, et al. Autoimmune enteropathy: not all flat mucosa mean coeliac disease. Gastroenterol Hepatol Bed Bench 2016;9:140–5. [PMC free article] [PubMed] [Google Scholar]
- 56.van Wanrooij RLJ, Bontkes HJ, Neefjes-Borst EA, et al. Immune-Mediated enteropathies: from bench to bedside. J Autoimmun 2021;118:102609. 10.1016/j.jaut.2021.102609 [DOI] [PubMed] [Google Scholar]
- 57.Biagi F, Bianchi PI, Trotta L, et al. Anti-goblet cell antibodies for the diagnosis of autoimmune enteropathy? Am J Gastroenterol 2009;104:3112. 10.1038/ajg.2009.511 [DOI] [PubMed] [Google Scholar]
- 58.Lappinga PJ, Abraham SC, Murray JA, et al. Small intestinal bacterial overgrowth: histopathologic features and clinical correlates in an underrecognized entity. Arch Pathol Lab Med 2010;134:264–70. 10.5858/134.2.264 [DOI] [PubMed] [Google Scholar]
- 59.Riordan SM, McIver CJ, Wakefield D, et al. Small intestinal mucosal immunity and morphometry in luminal overgrowth of Indigenous gut flora. Am J Gastroenterol 2001;96:494–500. 10.1111/j.1572-0241.2001.03533.x [DOI] [PubMed] [Google Scholar]
- 60.Strocchi A, Corazza G, Furne J, et al. Measurements of the jejunal unstirred layer in normal subjects and patients with celiac disease. Am J Physiol 1996;270:G487–91. 10.1152/ajpgi.1996.270.3.G487 [DOI] [PubMed] [Google Scholar]
- 61.Voutilainen M, Juhola M, Färkkilä M, et al. Gastric metaplasia and chronic inflammation at the duodenal bulb mucosa. Dig Liver Dis 2003;35:94–8. 10.1016/S1590-8658(03)00003-3 [DOI] [PubMed] [Google Scholar]
- 62.Schiepatti A, Savioli J, Vernero M, et al. Pitfalls in the diagnosis of coeliac disease and Gluten-Related disorders. Nutrients 2020;12:12. 10.3390/nu12061711 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data are available. Not applicable.


