Abstract
Background: Heart failure with preserved ejection fraction (HFpEF) is a complex disease which accounts for more than half of all HF hospital admissions with high prevalence and lack of effective evidence-based management. Sodium-glucose cotransporter 2 (SGLT2) inhibitor is a new antidiabetic drug that recently gained a new role in the management of heart failure with reduced ejection fraction but its role in HFpEF had yet to be studied.
Study and results: EMPEROR-Preserved trial set out to evaluate the effects of SGLT2 inhibition with empagliflozin on major heart failure outcomes in patients with HFpEF. The patients were randomized in a 1:1 fashion into two groups; to receive either empagliflozin 10 mg per day (n = 2, 997) or placebo (n = 2, 991) in addition to usual therapy. Empagliflozin led to a 21% risk reduction of the composite of cardiovascular death or hospitalization for heart failure, which was mainly related to a 29% lower risk of hospitalization for heart failure rather than effect on cardiovascular death empagliflozin. The effects SGLT2 inhibitors were consistent in all patients.
What we have learnt: The EMPEROR-Preserved trial is the first randomized controlled trial testing the efficacy and safety of SGLT2 inhibitor (empagliflozin) in patients with HFpEF. The trial proves that SGLT2 inhibitors (empagliflozin) can significantly reduce HF hospitalization with neutral effect on cardiovascular (CV) death.
Introduction
Heart failure (HF) is not a single pathological diagnosis, but rather represents a wide variety of pathologies. Structural and/or functional abnormalities in the heart result in elevated intracardiac pressures and/or inadequate cardiac output at rest and/or during exercise1. Three distinct phenotypes of HF have been identified; HF with reduced ejection fraction (HFrEF), HF with mildly reduced EF, and HF with preserved EF (HFpEF)1.
HFpEF is defined as: symptoms and signs of HF, with evidence of structural and/or functional cardiac abnormalities and/or raised natriuretic peptides (NPs), and with a left ventricular (LV) EF > 50%. It accounts for more than half of all HF hospital admissions with rising prevalence and a lack of effective evidence-based management.
Multiple randomized controlled trials (RCT) have been conducted with HFpEF patients, but unfortunately failed to show any significant reduction in morbidity and mortality, although some improvements have been observed in some phenotypes under the umbrella of HFpEF. These trials include; PEP-CHF (perindopril)2, CHARM-Preserved (candesartan)3, I-PRESERVE (irbesartan)4, TOPCAT (spironolactone)5, and PARAGON-HF (sacubitril/valsartan)6.
Sodium-glucose cotransporter 2 (SGLT2) inhibitor is a new anti-diabetic drug that reduces blood glucose levels through increased glucose excretion in the proximal renal tubule. Additionally, it increases sodium and water excretion, leading to natriuresis and diuresis. SGLT2 inhibitors have recently gained a new role in delaying and preventing heart failure in patients with type-2 diabetes mellitus (DM)7,8.
Moreover, the DAPA-HF and EMPEROR-Reduced trials have shown a significant reduction in morbidity and mortality with SGLT2 inhibitors (Dapagliflozin and empagliflozin) in patients with HFrEF, regardless of DM8–10. The role of SGLT2 inhibitors in patients with HFpEF has not yet been studied in a large RCT.
The EMPEROR-Preserved study
The Empagliflozin Outcome Trial in Patients with Chronic Heart Failure with Preserved Ejection Fraction (EMPEROR-Preserved) trial was a randomized, multicenter, double-blinded, placebo-controlled trial that set out to evaluate the effects of SGLT2 inhibition with empagliflozin on major heart failure outcomes in patients with HFpEF11.
After screening 11,583 patients, the study enrolled 5,988 patients with symptomatic heart failure and preserved ejection fraction (EF > 40%). The patients were randomized in a 1:1 fashion into two groups; to receive either empagliflozin 10 mg per day (n = 2,997) or placebo (n = 2,991) in addition to usual therapy.
The patients were followed up periodically for median duration of 26.2 months for symptoms, health status (assessed with the Kansas City Cardiomyopathy Questionnaire), and adverse events. It is noteworthy that about half of patients were diabetic.
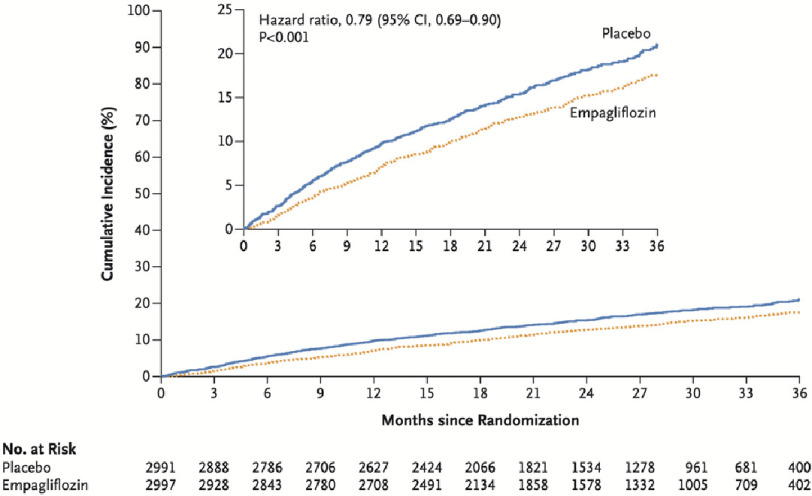
The primary composite outcome (cardiovascular death or heart failure hospitalization) was significantly lower in the empagliflozin group compared to the placebo group (415 patients; 13.8% versus 511 patients 17.1%, hazard ratio, 0.79; 95% CI [0.69–0.90]; P < 0.001). The number of patients treated with empagliflozin needed to prevent one primary outcome event was 31 (95% CI [20–69]).
Hospitalization for heart failure occurred in 8.6% of the empagliflozin group and 11.8% of the placebo group. However, cardiovascular death numbers were not significantly different (7.3% in empagliflozin group, versus 8.2% in placebo group) (Figure 1).
Figure 1. Estimated cumulative incidence of the primary outcome (composite of cardiovascular death or hospitalization for HF) in the empagliflozin group compared to the placebo group11.
Regarding secondary outcomes, the total number of hospitalizations for heart failure was significantly lower with empagliflozin than with placebo (hazard ratio, 0.73; 95% CI [0.61–0.88]; P < 0.001) and the rate of decline in the eGFR was slower in the empagliflozin group than in the placebo group (−1.25 vs. −2.62 ml per minute per 1.73 m2 per year; P < 0.001).
Discussion
In patients with HFpEF, empagliflozin led to a 21% risk reduction of the composite of cardiovascular death or hospitalization for heart failure, which was mainly related to a 29% lower risk of hospitalization for heart failure, rather than any substantial effect on cardiovascular death. The effects on the incidence of primary outcome events were seen consistently across all prespecified subgroups, including patients with or without diabetes.
Empagliflozin also led to a lower total number of hospitalizations for heart failure and a longer time to first hospitalization for heart failure. It is noteworthy that the percentage of patients who discontinued treatment for reasons other than death was 23% and was similar in the two treatment groups.
What have we learned?
The EMPEROR-Preserved trial is the first randomized controlled trial testing the efficacy and safety of SGLT2 inhibitors (empagliflozin) in patients with HFpEF. The EMPEROR-Preserved trial has proven that SGLT2 inhibitors can significantly reduce HF hospitalization with neutral effect on cardiovascular (CV) death, and now the door is open for further exploration by other members of the SGLT2 family in also reducing CV mortality in HFpEF patients.
References
- 1.McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkiene J, Chioncel O, Clel JGF, Coats AJS, Crespo-Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A, ESC Scientific Document Group 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. European Heart Journal. 2021;42(36):3599–3726. doi: 10.1093/eurheartj/ehab368. doi: 10.1093/eurheartj/ehab368. doi: [DOI] [PubMed] [Google Scholar]
- 2.Cleland JGF, Tendera M, Adamus J, Freemantle N, Polonski L, Taylor J, PEP-CHF Investigators The perindopril in elderly people with chronic heart failure (PEP-CHF) study. European Heart Journal. 2006;27(19):2338–2345. doi: 10.1093/eurheartj/ehl250. doi: 10.1093/eurheartj/ehl250. doi: [DOI] [PubMed] [Google Scholar]
- 3.Granger CB, McMurray JJV, Yusuf S, Held P, Michelson EL, Olofsson B, Ostergren J, Pfeffer MA, Swedberg K, CHARM Investigators and Committees Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: the CHARM-Alternative trial. Lancet. 2003;362(9386):772–776. doi: 10.1016/S0140-6736(03)14284-5. doi: 10.1016/S0140-6736(03)14284-5. doi: [DOI] [PubMed] [Google Scholar]
- 4.Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, Anderson S, Donovan M, Iverson E, Staiger C, Ptaszynska A, I-PRESERVE Investigators Irbesartan in patients with heart failure and preserved ejection fraction. New England Journal of Medicine. 2008;359(23):2456–2467. doi: 10.1056/NEJMoa0805450. doi: 10.1056/NEJMoa0805450. doi: [DOI] [PubMed] [Google Scholar]
- 5.Pitt B, Pfeffer MA, Assmann SF, Boineau R, An IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Harty B, Heitner JF, Kenwood CT, Lewis EF, O’Meara E, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, Yang S, McKinlay SM, TOPCAT Investigators Spironolactone for heart failure with preserved ejection fraction. New England Journal of Medicine. 2014;370(15):1383–1392. doi: 10.1056/NEJMoa1313731. doi: 10.1056/NEJMoa1313731. doi: [DOI] [PubMed] [Google Scholar]
- 6.Solomon SD, McMurray JJV, An IS, Ge J, Lam CSP, Maggioni AP, Martinez F, Packer M, Pfeffer MA, Pieske B, Redfield MM, Rouleau JL, Veldhuisen DJvan, Zannad F, Zile MR, Desai AS, Claggett B, Jhund PS, Boytsov SA, Comin-Colet J, Clel J, Düngen H-D, Goncalvesova E, Katova T, Saraiva JFKerr, Lelonek M, Merkely B, Senni M, Shah SJ, Zhou J, Rizkala AR, Gong J, Shi VC, Lefkowitz MP, PARAGON-HF Investigators and Committees Angiotensin-neprilysin inhibition in heart failure with preserved ejection fraction. New England Journal of Medicine. 2019;381(17):1609–1620. doi: 10.1056/NEJMoa1908655. doi: 10.1056/NEJMoa1908655. doi: [DOI] [PubMed] [Google Scholar]
- 7.Sattar N, McLaren J, Kristensen SL, Preiss D, McMurray JJ. SGLT2 Inhibition and cardiovascular events: why did EMPA-REG Outcomes surprise and what were the likely mechanisms? Diabetologia. 2016;59(7):1333–1339. doi: 10.1007/s00125-016-3956-x. doi: 10.1007/s00125-016-3956-x. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Furtado RHM, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Sabatine MS. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. The Lancet. 2019;393(10166):31–39. doi: 10.1016/S0140-6736(18)32590-X. doi: 10.1016/S0140-6736(18)32590-X. doi: [DOI] [PubMed] [Google Scholar]
- 9.McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, An IS, Bělohlávek J, Böhm M, Chiang C-E, Chopra VK, De Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O’Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjöstr M, Langkilde A-M. Dapagliflozin in patients with heart failure and reduced ejection fraction. New England Journal of Medicine. 2019;381(21):1995–2008. doi: 10.1056/NEJMoa1911303. doi: 10.1056/NEJMoa1911303. doi: [DOI] [PubMed] [Google Scholar]
- 10.Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, Jamal W, Kimura K, Schnee J, Zeller C, Cotton D, Bocchi E, Böhm M, Choi D-J, Chopra V, Chuquiure E, Giannetti N, Janssens S, Zhang J, Gonzalez Juanatey JR, Kaul S, Brunner-La Rocca H-P, Merkely B, Nicholls SJ, Perrone S, Pina I, Ponikowski P, Sattar N, Senni M, Seronde M-F, Spinar J, Squire I, Taddei S, Wanner C, Zannad F, EMPEROR-Reduced Trial Investigators Cardiovascular and renal outcomes with empagliflozin in heart failure. New England Journal of Medicine. 2020;383(15):1413–1424. doi: 10.1056/NEJMoa2022190. doi: 10.1056/NEJMoa2022190. doi: [DOI] [PubMed] [Google Scholar]
- 11.Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, Brunner-La Rocca H-P, Choi D-J, Chopra V, Chuquiure-Valenzuela E, Giannetti N, Gomez-Mesa JE, Janssens S, Januzzi JL, Gonzalez-Juanatey JR, Merkely B, Nicholls SJ, Perrone SV, Piña IL, Ponikowski P, Senni M, Sim D, Spinar J, Squire I, Taddei S, Tsutsui H, Verma S, Vinereanu D, Zhang J, Carson P, Lam CSP, Marx N, Zeller C, Sattar N, Jamal W, Schnaidt S, Schnee JM, Brueckmann M, Pocock SJ, Zannad F, Packer M. Empagliflozin in heart failure with a preserved ejection fraction. New England Journal of Medicine. 2021 doi: 10.1056/NEJMoa2107038. doi: 10.1056/NEJMoa2107038. doi: [DOI] [Google Scholar]