Abstract
Marine macroalgae are well known to release a wide spectrum of volatile organic components, the release of which is affected by environmental factors. This paper aimed to identify the essential oil (EO) compounds of the brown algae Cystoseira compressa collected in the Adriatic Sea monthly, from May until August. EOs were isolated by hydrodistillation using a Clavenger-type apparatus and analyzed by gas chromatography coupled with mass spectrometry (GC–MS). One hundred four compounds were identified in the volatile fraction of C. compressa, accounting for 84.37–89.43% of the total oil. Samples from May, June, and July were characterized by a high share of fatty acids (56, 69, and 34% respectively) with palmitic acid being the dominant one, while in the August sample, a high content of alcohols (mainly phytol and oleyl alcohol) was found. Changes in the other minor components, which could be important for the overall aroma and biological activities of the algal samples, have also been noted during the vegetation periods. The results of this paper contribute to studies of algal EOs and present the first report on C. compressa EOs.
Keywords: Cystoseira compressa, brown algae, essential oil, harvest period, GC–MS
1. Introduction
More than 70% of the Earth’s surface is covered with oceans and seas, so it is not surprising that marine ecosystems are extremely complex with tremendous biodiversity. Recently, there is a growing trend in the investigation of new, inexpensive, and valuable sources of biologically active compounds, and marine origin products, like algae, are one of the most interesting sources, due to their production of a great variety of unique secondary metabolites [1]. Algae are vegetative organisms widely distributed throughout the world. Although many of them are of commercial importance in some parts of the world due to their nutritional, biological, and functional properties, only a small number of species are currently exploited for industrial food applications [2]. Studies on marine algae are usually focused on the isolation of structurally different bioactive compounds like polysaccharides (e.g., fucoidan, alginate, and laminarin), photosynthetic pigments (carotenoids, chlorophylls, and phycobilins), sterols, polyphenolics, etc. [3,4,5,6,7,8,9]. In comparison to the research on these non-volatile compounds, studies on volatiles of marine origin are still scarce.
Essential oils (EOs), as a special chemical group of algal metabolites, play an important role in communication in marine ecosystems, both interspecies and intraspecies, as well as in interactions with the surrounding environment. These compounds are involved in various algal ecological functions: they are defenses against predators and herbivores; they act as pheromones (allelochemicals; take part in the adaptation to abiotic stresses; and are important for the inhibition of bacterial and/or fungal fouling [1,10,11,12]. The essential oil metabolites present in marine algae species contain a mixture of different chemical classes such as hydrocarbons, fatty acids, esters, alcohols, carboxylic acids, aldehydes, ketones, terpenes, polyphenols, furans, pyrazines, pyridines, halogenated amines, and sulphur compounds [1,2]. The production of algal EOs is closely related to the physiology of the species [11,12]. Studies on EOs of green and red algae mainly report the presence of monoterpenoids, halogenated compounds, and sulphur compounds that have a low impact on their aroma perception. In contrast to those species, brown algae is responsible for strong and pleasant marine odors (the so-called “beach note”), which is usually related to the presence of C11-hydrocarbons. Among other aroma compounds, these species contain a wide range of monoterpenoids and sesquiterpenoids [13]. Although the functions of algal EOs are similar to those in terrestrial plants, studies dealing with algal EOs and their role are still in the primary stage, and there is a lack of reports on this subject [12]. EO profiles differ between species, but they are also influenced by various factors as age, geographical origin, growth and nutrition conditions, season, temperature, light, salinity, and processing/extraction parameters [2,12].
There are about 40 species of algae from the genera Cystoseira (Phaeophyta), which are widely distributed along the Eastern Atlantic and Mediterranean coasts [14], and C. compressa, is one of the most widespread brown algae in the Adriatic Sea. C. compressa is attached to the substratum by a small disc and its thallus shows morphological plasticity. Changes are most evident in the spring/summer period, when the winter rosette shape of the branches shifts to dense and ramified branches with aerocysts [15]. These changes might be related to the length of the photoperiod and sea temperature, and their effect on the EOs or other chemical components of the algae (phenolic profile, pigments, etc.) is unknown.
Compounds from C. compressa were characterized from extracts and associated with various biological activities, e.g., polysaccharides and phlorotannins with antioxidant activity [16,17], phlorotannins with antidiabetic activity [17], and phenolic compounds with antibacterial activity [5]. Furthermore, a connection between total phenolic content and the seawater temperature was observed, showing that the amount of phenolics is influenced by the temperature [18]. However, characterization of EO components has been done for C. sedoides [13], C. barbata [19,20], C. crinita [19], and C. tamariscifolia [21], but to our knowledge, there are no reports on compounds of C. compressa and their comparison over the spring/summer period, when the algae are under the influence of the thallus change, a rise in sea temperature, and an intensive photoperiod. For these reasons, this work aimed to study the EO profiles of C. compressa, collected in the Adriatic Sea monthly from May until August, to identify the molecules characterizing this species.
2. Results and Discussion
Seaweeds are widespread around the world, being of commercial importance in some parts, where they are consumed fresh, dry, or as an ingredient. Although in some regions they are widely used in the human diet, only a small number of species are currently exploited for food applications. One of the main limitations of the use of algal materials in the food industry is their flavor, which is the main parameter of quality directly related to consumers’ acceptance of food [2]. In comparison to the terrestrial odoriferous plants, only some algae possess an attractive, pleasant odor and characteristic marine flavor, and, therefore, great potential to be used in various food and cosmetic preparations [1,13].
Different extraction methods like hydrodistillation, solvent extraction, microwave-assisted extraction, supercritical fluid extraction, headspace extraction, etc., are commonly used for the isolation of volatile analytes from algal materials. In recent times, the conventional extraction procedures are usually being replaced by novel techniques that are less time-consuming, often (fully) automated, more environmentally friendly, require less solvent, and are more efficient [8]. However, despite all its disadvantages (duration, high temperatures, low efficiency, potential degradation of compounds, etc.), hydrodistillation is still the most used method. On the other hand, identification of the EO components is usually performed using capillary gas chromatography coupled with mass spectrometry (GC–MS), as this method of characterization covers a wide spectrum of compounds, from non-polar to polar ones [11,13].
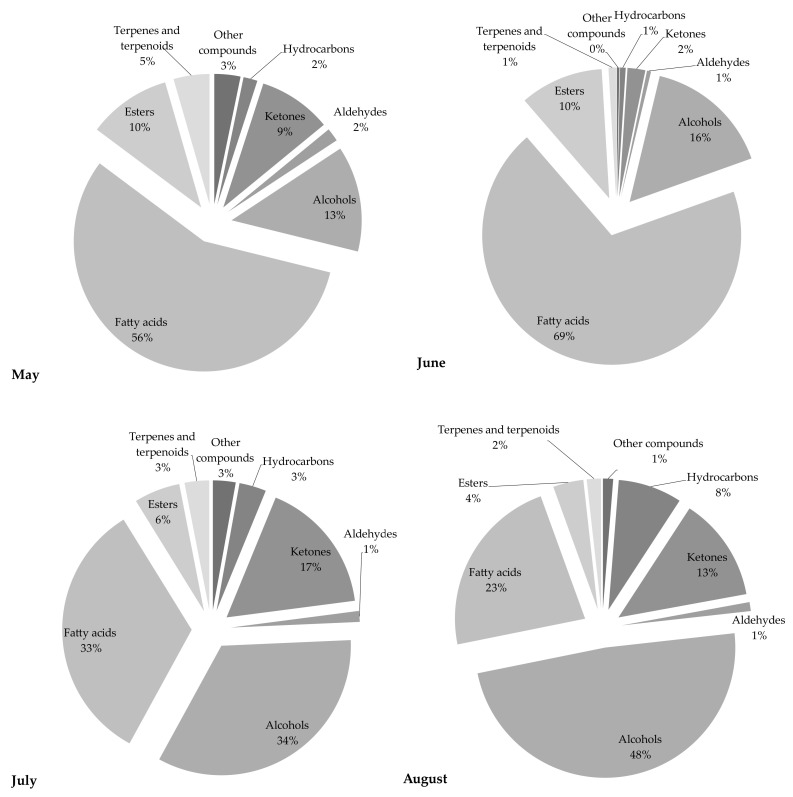
The chemical profile of volatile fractions and the relative content of detected components obtained by hydrodistillation of C. compressa are reported in Table 1. One hundred four compounds were identified, accounting for 84–89% of the total chemical composition. Figure 1 presents the relative share of the sum of compounds from the same chemical class to get better insight into the algal EOs profile. The GC–MS chromatograms of the essential oils obtained from C. compressa collected in different months are shown in Figure 2.
Table 1.
Differences in the chemical composition of essential oils of C. compressa harvested in different periods. The data are expressed as relative percentages of each single peak area with respect to the total peak area.
| Compound | C. compressa Samples | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | Rt (min) | Name | Similarity (%) | MW | Formula | CAS | May | June | July | August |
| 1 | 7.10 | Pent-1-en-3-one | 95 | 84.12 | C5H8O | 1629-58-9 | 0.07 | n.d. | n.d. | n.d. |
| 2 | 7.55 | Tetrahydro-2,5-dimethyl-furan | 97 | 100.15 | C6H12O | 1003-38-9 | 0.88 | 0.06 | 0.35 | 0.16 |
| 3 | 10.24 | Tetrahydro-2-furanmethanol | 92 | 102.13 | C5H10O2 | 97-99-4 | 0.06 | n.d. | 0.02 | 0.01 |
| 4 | 10.85 | Hex-5-enal | 93 | 98.14 | C6H10O | 764-59-0 | n.d. | 0.01 | 0.01 | 0.01 |
| 5 | 11.07 | Hexan-2-one | 93 | 100.16 | C6H12O | 591-78-6 | 0.05 | n.d. | 0.03 | 0.01 |
| 6 | 11.33 | Hexan-3-ol | 90 | 102.17 | C6H14O | 623-37-0 | 0.07 | n.d. | 0.03 | 0.03 |
| 7 | 11.48 | Hexanal | 95 | 100.16 | C6H12O | 66-25-1 | 0.10 | 0.04 | 0.07 | 0.07 |
| 8 | 14.16 | (E)-Hex-2-enal | 91 | 98.14 | C6H10O | 6728-26-3 | 0.04 | 0.01 | 0.04 | 0.04 |
| 9 | 16.75 | Heptanal | 92 | 114.19 | C7H14O | 111-71-7 | 0.04 | 0.01 | 0.01 | 0.01 |
| 10 | 19.81 | 1-methyl-pentyl hydroperoxide | 88 | 118.17 | C6H14O2 | 24254-55-5 | 0.11 | 0.01 | 0.05 | 0.02 |
| 11 | 20.97 | Oct-1-en-3-one | 90 | 126.19 | C8H14O | 4312-99-6 | 0.03 | 0.02 | 0.05 | 0.01 |
| 12 | 24.17 | Propylcyclohexane | 82 | 126.24 | C9H18 | 696-29-7 | 0.01 | n.d. | 0.02 | n.d. |
| 13 | 25.31 | (E)-Oct-2-enal | 90 | 126.20 | C8H14O | 2548-87-0 | n.d. | 0.01 | n.d. | n.d. |
| 14 | 25.79 | 1-Phenylethanone | 95 | 120.15 | C8H8O | 98-86-2 | n.d. | 0.01 | n.d. | n.d. |
| 15 | 26.05 | Octan-1-ol | 94 | 130.23 | C8H18O | 111-87-5 | n.d. | n.d. | n.d. | 0.03 |
| 16 | 27.30 | Non-1-en-4-ol | 85 | 142.24 | C9H18O | 35192-73-5 | n.d. | n.d. | 0.03 | 0.01 |
| 17 | 27.58 | Linalool | 90 | 154.25 | C10H18O | 78-70-6 | n.d. | n.d. | n.d. | 0.01 |
| 18 | 27.78 | Nonanal | 92 | 142.24 | C9H18O | 124-19-6 | 0.03 | n.d. | 0.02 | 0.02 |
| 19 | 29.85 | 2,6,6-trimethyl-2-cyclohexene-1,4-dione | 92 | 152.19 | C9H12O2 | 1125-21-9 | n.d. | n.d. | 0.03 | 0.02 |
| 20 | 32.95 | Decanal | 91 | 156.26 | C10H20O | 112-31-2 | n.d. | n.d. | 0.02 | 0.01 |
| 21 | 33.64 | 2,6,6-trimethyl-1-cyclohexene-1-carboxaldehyde | 83 | 152.23 | C10H16O | 4884-24-6 | 0.02 | n.d. | n.d. | n.d. |
| 22 | 35.67 | 2-(2,6,6-trimethylcyclohexen-1-yl) acetaldehyde | 89 | 166.26 | C11H18O | 472-66-2 | n.d. | n.d. | 0.02 | n.d. |
| 23 | 35.80 | Ethyl 5-methyl-3,4-dihydro-2H-pyran-4-carboxylate | 83 | 170.21 | C9H14O3 | 38858-64-9 | n.d. | n.d. | 0.05 | n.d. |
| 24 | 35.92 | 3,5,5-trimethyl-hex-1-ene | 86 | 126.24 | C9H18 | 4316-65-8 | n.d. | 0.02 | 0.29 | 0.03 |
| 25 | 36.18 | Decan-1-ol | 93 | 158.28 | C10H22O | 112-30-1 | n.d. | 0.01 | 0.09 | 0.36 |
| 26 | 36.58 | Dec-1-en-3-one | 92 | 154.22 | C10H18O | 56606-79-2 | 0.42 | 0.02 | 0.10 | 0.17 |
| 27 | 37.17 | Undecan-2-one | 95 | 170.29 | C11H22O | 112-12-9 | n.d. | n.d. | 0.14 | 0.02 |
| 28 | 37.80 | Undecanal | 96 | 170.29 | C11H22O | 112-44-7 | n.d. | n.d. | n.d. | 0.09 |
| 29 | 38.26 | (2E,4E)-deca-2,4-dienal | 94 | 152.23 | C10H16O | 25152-84-5 | n.d. | n.d. | 0.05 | 0.02 |
| 30 | 38.37 | Ethyl cyclohexanecarboxylate | 87 | 156.22 | C9H16O2 | 3289-28-9 | 0.25 | n.d. | 0.54 | 0.07 |
| 31 | 38.76 | Bicyclo(3.3.1)nonane-2,6-dione | 83 | 152.19 | C9H12O2 | 16473-11-3 | 0.17 | n.d. | 0.35 | 0.08 |
| 32 | 39.00 | (R)-5,7-dimethyl-1,6-octadiene (Isocitronellene) | 87 | 138.25 | C10H18 | 85006-04-8 | 0.88 | n.d. | 0.04 | 0.03 |
| 33 | 39.42 | Hexacosan-1-ol | 81 | 382.7 | C26H54O | 506-52-5 | n.d. | n.d. | 0.30 | n.d. |
| 34 | 40.20 | Eugenol | 95 | 164.20 | C10H12O2 | 97-53-0 | 0.19 | n.d. | n.d. | n.d. |
| 35 | 41.98 | Nona-3,5-dien-2-one | 84 | 138.21 | C9H14O | 80387-31-1 | 0.11 | n.d. | 0.45 | 0.16 |
| 36 | 42.18 | 6,10-dimethylundecan-2-one | 95 | 198.34 | C12H26O | 1604-34-8 | 0.05 | n.d. | 0.32 | 0.09 |
| 37 | 44.29 | Geranylacetone | 84 | 194.31 | C13H22O | 689-67-8 | 0.14 | n.d. | 0.70 | n.d. |
| 38 | 42.37 | Dodecanal | 92 | 184.32 | C12H24O | 112-54-9 | n.d. | n.d. | n.d. | 0.05 |
| 39 | 43.40 | (E)-4-[(1R,5S)-2,5,6,6-tetramethylcyclohex-2-en-1-yl]but-3-en-2-one | 85 | 206.32 | C14H22O | 79-69-6 | n.d. | n.d. | n.d. | 0.15 |
| 40 | 44.08 | 3,7,11-trimethyldodecan-1-ol (hexahydrofarnesol) | 92 | 228.41 | C15H32O | 6750-34-1 | n.d. | 0.14 | n.d. | 0.24 |
| 41 | 44.29 | (Z)-6,10-dimethyl-5,9-undecadien-2-one | 93 | 194.31 | C13H22O | 3879-26-3 | n.d. | n.d. | n.d. | 0.17 |
| 42 | 44.73 | 3,7,11-trimethyl-dodecan-1-ol | 83 | 228.31 | C15H32O | 6750-34-1 | 0.32 | 0.07 | 0.18 | 0.29 |
| 43 | 45.08 | (Z)-dec-3-enyl acetate | 89 | 198.30 | C12H22O2 | 81634-99-3 | n.d. | n.d. | 0.18 | n.d. |
| 44 | 45.17 | Dodecan-1-ol | 94 | 186.33 | C12H26O | 112-53-8 | 1.06 | 1.02 | 1.03 | 3.65 |
| 45 | 45.91 | (E)-4-(2,6,6-trimethyl-1-cyclohexen-1-yl)-3-buten-2-one | 85 | 192.30 | C13H20O | 14901-07-6 | 2.76 | 0.53 | 5.41 | 4.34 |
| 46 | 46.07 | Tridecan-2-one | 88 | 198.34 | C13H26O | 593-08-8 | 0.12 | 0.36 | 0.67 | 0.31 |
| 47 | 46.22 | Hexadecane | 96 | 226.44 | C16H34 | 544-76-3 | 0.58 | 0.08 | 0.84 | 1.30 |
| 48 | 46.34 | Dimethyl 1,4-benzenedicarboxylate | 88 | 194.18 | C10H10O4 | 120-61-6 | n.d. | 0.06 | 0.19 | 1.17 |
| 49 | 46.89 | n-pentadecan-1-ol | 94 | 228.41 | C15H32O | 629-76-5 | n.d. | n.d. | 0.02 | 0.23 |
| 50 | 47.33 | (E)-Octadec-5-ene | 91 | 252.47 | C18H36 | 7206-21-5 | n.d. | 0.09 | n.d. | n.d. |
| 51 | 47.86 | 5,6,7,7a-Tetrahydro-4,4,7a-trimethyl-2(4H)-benzofuranone | 90 | 180.24 | C11H16O2 | 15356-74-8 | 0.27 | n.d. | 0.45 | 0.28 |
| 52 | 48.31 | (Z)-dodec-5-enoic acid | 95 | 198.30 | C11H22O2 | 2430-94-6 | n.d. | 2.79 | n.d. | n.d. |
| 53 | 48.92 | (E)-3,7,11-Trimethyl-1,6,10-dodecatrien-3-ol | 89 | 222.36 | C15H26O | 40716-66-3 | n.d. | n.d. | 0.08 | n.d. |
| 54 | 49.12 | Dodecanoic acid (lauric acid) | 97 | 200.31 | C12H24O2 | 143-07-7 | n.d. | 3.78 | 0.22 | 0.08 |
| 55 | 49.31 | Tetradecan-1-ol | 84 | 214.38 | C14H30O | 112-72-1 | n.d. | 0.26 | 0.37 | 0.31 |
| 56 | 49.90 | 2-O-(4-methylpentyl) 1-O-octadecyl oxalate | 84 | 426.70 | C26H50O4 | 29590-28-1 | 0.04 | n.d. | 0.11 | 0.05 |
| 57 | 50.16 | Heptadecane | 92 | 240.46 | C17H36 | 629-78-7 | 0.08 | n.d. | 0.05 | 0.05 |
| 58 | 50.62 | Tetradecanal | 94 | 212.37 | C14H28O | 124-25-4 | 0.14 | n.d. | 0.08 | 0.20 |
| 59 | 51.26 | 2-methylhexadecan-1-ol | 90 | 256.46 | C17H36O | 2490-48-4 | n.d. | 0.35 | 0.18 | 0.39 |
| 60 | 51.42 | Diphenylmethanone | 95 | 182.21 | C13H10O | 119-61-9 | 0.34 | 0.29 | 0.39 | n.d. |
| 61 | 52.16 | 2-ethyldodecan-1-ol | 88 | 214.38 | C14H30O | 19780-33-7 | 0.24 | n.d. | 0.23 | 0.18 |
| 62 | 52.30 | alpha-Cadinol | 87 | 222.36 | C15H26O | 481-34-5 | 1.24 | 0.12 | n.d. | n.d. |
| 63 | 52.65 | Hexadecan-1-ol | 92 | 242.44 | C16H34O | 36653-82-4 | 1.56 | 2.60 | 7.20 | 7.99 |
| 64 | 52.91 | (E)-heptadec-8-ene | 77 | 238.45 | C17H34 | 54290-12-9 | n.d. | n.d. | 0.11 | 0.09 |
| 65 | 53.03 | Pentadec-1-ene | 92 | 210.40 | C15H30 | 13360-61-7 | n.d. | n.d. | 0.14 | 2.62 |
| 66 | 53.22 | 11-pentan-3-ylhenicosane | 89 | 366.70 | C26H54 | 55282-11-6 | 0.65 | 0.43 | 1.11 | 1.41 |
| 67 | 53.38 | 2-tetradecoxyethanol | 81 | 258.44 | C16H34O2 | 2136-70-1 | 0.24 | 0.32 | 0.44 | 0.37 |
| 68 | 53.64 | Tridecanal | 93 | 198.34 | C13H26O | 10486-19-8 | 0.60 | 0.37 | 0.81 | 0.43 |
| 69 | 53.72 | 2-hexadecoxyethanol | 87 | 286.49 | C18H38O2 | 2136-71-2 | 0.25 | n.d. | n.d. | n.d. |
| 70 | 54.12 | 2-octadecoxyethanol | 90 | 314.54 | C20H42O2 | 2136-72-3 | n.d. | 0.42 | 0.41 | 0.50 |
| 71 | 54.30 | n-Nonadecan-1-ol | 88 | 284.52 | C19H40O | 1454-84-8 | n.d. | 0.11 | n.d. | n.d. |
| 72 | 54.52 | (E)-icos-3-ene | 86 | 280.53 | C20H40 | 74685-33-9 | n.d. | 0.14 | n.d. | n.d. |
| 73 | 54.99 | Myristic acid | 97 | 228.37 | C14H28O2 | 544-63-8 | n.d. | 7.60 | 0.31 | 0.18 |
| 74 | 55.22 | n-Pentadecanol | 93 | 228.41 | C15H32O | 629-76-5 | 0.46 | 0.85 | 0.72 | 1.19 |
| 75 | 55.63 | Eicosanoic acid | 87 | 312.53 | C20H40O2 | 506-30-9 | 2.58 | 0.51 | 1.14 | n.d. |
| 76 | 55.69 | (Z)-Undec-4-enal | 87 | 168.27 | C11H20O | 68820-32-6 | 0.36 | n.d. | n.d. | n.d. |
| 77 | 56.17 | Isopropyl myristate | 93 | 270.45 | C17H34O2 | 110-27-0 | 0.30 | n.d. | n.d. | n.d. |
| 78 | 56.63 | 6,10,14-Trimethyl-2-pentadecanone | 96 | 268.5 | C18H36O | 502-69-2 | 2.99 | 0.75 | 5.98 | 5.72 |
| 79 | 56.89 | 2,3-diisopropyl-naphthalene | 77 | 212.33 | C16H20 | 94133-81-0 | 1.47 | 0.36 | 0.55 | 0.42 |
| 80 | 56.98 | Oleyl alcohol | 93 | 268.5 | C18H36O | 143-28-2 | n.d. | 0.68 | 4.41 | 5.96 |
| 81 | 57.18 | bis(2-methylpropyl) benzene-1,2-dicarboxylate | 97 | 278.34 | C16H22O4 | 84-69-5 | 2.11 | 0.51 | 1.11 | n.d. |
| 82 | 57.52 | Nonadec-1-ene | 93 | 266.50 | C19H38 | 18435-45-5 | n.d. | n.d. | 0.20 | 1.30 |
| 83 | 58.11 | Farnesyl acetone | 90 | 266.43 | C18H30O | 1117-52-8 | 0.93 | 0.57 | 1.28 | 1.05 |
| 84 | 58.62 | Palmitoleic acid | 95 | 254.40 | C16H30O2 | 373-49-9 | 2.79 | 11.94 | n.d. | n.d. |
| 85 | 58.90 | Palmitic acid | 93 | 256.42 | C16H32O2 | 57-10-3 | 40.15 | 31.92 | 26.81 | 18.62 |
| 86 | 60.14 | Arachidonic acid | 90 | 304.46 | C20H32O2 | 506-32-1 | 0.96 | 1.45 | 0.14 | 0.27 |
| 87 | 60.30 | Methyl Arachidonate | 83 | 318.49 | C21H34O2 | 2566-89-4 | 4.00 | 2.49 | 1.55 | 1.74 |
| 88 | 60.81 | n-Nonadecan-1-ol | 89 | 284.52 | C19H40O | 1454-84-8 | 1.67 | 3.13 | 4.13 | 4.34 |
| 89 | 60.84 | (Z,Z)-2,13-Octadecadien-1-ol | 85 | 266.46 | C18H34 | 123551-47-3 | 0.21 | n.d. | 1.67 | n.d. |
| 90 | 61.21 | 5-dodecyloxolan-2-one | 90 | 254.41 | C16H30O2 | 730-46-1 | 1.44 | 0.21 | 1.94 | 0.88 |
| 91 | 61.29 | Phytol | 96 | 296.53 | C20H40O | 150-86-7 | 3.97 | 2.90 | 5.76 | 14.20 |
| 92 | 61.69 | Oleic acid | 91 | 282.46 | C18H34O2 | 112-80-1 | 0.23 | 1.63 | 0.12 | 0.29 |
| 93 | 61.81 | Heptadecyl heptadecanoate | 81 | 508.92 | C34H68O2 | n.a. | 0.22 | n.d. | 0.12 | 0.40 |
| 94 | 61.99 | Ascorbyl palmitate | 70 | 414.53 | C22H38O7 | 137-66-6 | 0.32 | n.d. | n.d. | 0.12 |
| 95 | 62.39 | Octadecyl propan-2-yl sulphite | 82 | 376.60 | C21H44O3S | n.a. | 0.22 | n.d. | n.d. | 0.19 |
| 96 | 62.58 | Tetradecyl benzoate | 78 | 318.50 | C21H34O2 | 70682-72-3 | 1.10 | 4.37 | 0.53 | 0.09 |
| 97 | 63.04 | Pentadecyl benzoate | 80 | 332.50 | C22H36O2 | n.a. | 0.07 | 0.50 | 0.10 | 0.04 |
| 98 | 63.98 | Tridecyl benzoate | 88 | 304.50 | C20H32O2 | 29376-83-8 | 0.16 | 1.28 | 0.40 | 0.05 |
| 99 | 64.22 | 2-(Octadecyloxy)ethanol | 91 | 314.55 | C20H42O2 | 9005-00-9 | 0.28 | 1.04 | 0.76 | 1.46 |
| 100 | 64.39 | Stearic acid | 80 | 284.48 | C18H36O2 | 57-11-4 | 0.36 | 0.09 | n.d. | n.d. |
| 101 | 64.96 | 1-Decylsulfonyldecane | 79 | 346.61 | C20H42O2S | 111530-37-1 | 0.13 | n.d. | n.d. | 0.14 |
| 102 | 66.14 | Icosane | 94 | 282.55 | C20H42 | 112-95-8 | 0.16 | n.d. | 0.30 | 0.11 |
| 103 | 67.28 | Nonacosane | 93 | 408.79 | C29H60 | 630-03-5 | 0.34 | 0.05 | 0.09 | 0.14 |
| 104 | 70.37 | Squalene | 90 | 410.73 | C30H50 | 111-02-4 | 0.18 | 0.04 | 0.20 | 0.07 |
| TOTAL IDENTIFIED COMPOUNDS (from peak total area) | 84.37 | 89.43 | 85.44 | 87.41 | ||||||
Compounds detected in amounts higher than 0.5% are written in bold; n.d.—not detected; n.a.—not available.
Figure 1.
The relative content of each chemical class of compounds detected in C. compressa samples.
Figure 2.
GC–MS chromatograms of the essential oils obtained from Cystoseira compressa collected from May to August.
Samples from May and June were characterized by a high share of fatty acids, while in the July and August samples the dominant chemical class of compounds were alcohols (34 and 48%, respectively). EOs from May and June were characterized by an extremely high content of fatty acids, 56 and 69%, respectively, while almost two-fold lower results were obtained for the July extract. The major acid in all samples was palmitic acid (C16:0), with the highest amount found in the May extract (40.15%), and shares of 31.92%, 26.81%, and 18.62%, in the June, July, and August samples, respectively. It is interesting to note that this saturated fatty acid was present in high amounts in all samples and followed a regular trend characterized by a continued decrease in content during the collecting months. In comparison to the May samples, there was a more than two-fold lower amount detected in the August samples. This compound was also previously reported as an abundant fatty acid in different Cystoseira species [3,4,8,14,21,22]. The May extract also contained the highest share of eicosanoic acid (2.58%). Significant amounts of this acid were also found in June (0.51%) and July (1.14%), while it was not detected in the August sample. The content of all other fatty acids was the highest in the June fraction: palmitoleic acid (11.94%) > myristic acid (7.60%) > lauric acid (3.78%) > (Z)-dodec-5-enoic acid (2.79%) > oleic acid (1.64%) > arachidonic acid (1.45%) > stearic acid (0.36%). It is well known that fatty acids with >12 carbon atoms are odorless, so although present in high amounts they do not affect significantly the flavor of the samples [2].
Among monounsaturated fatty acids, the presence of (Z)-5-dodecenoic acid was confirmed only in the June sample, where the content of oleic acid was also the highest in comparison with the others. Arachidonic acid was the only detected polyunsaturated acid, with the highest amount again found in the June sample, but significant amounts were also detected in May (0.96%). Cvitković et al. [8] reported the domination of total unsaturated fatty acids in the lipid fraction of different Adriatic brown algae species and two Cystoseira species, C. barbata and C. compressa. These authors also reported the domination of oleic acid among unsaturated fatty acids, as well as the presence of arachidonic acid in high amounts in brown algae samples. Similar results were also reported by Oucif et al. [4]. Kord et al. [22] also identified fatty acids (14 to 20 carbon atoms) of which palmitic acid was the major compound in C. sauvageauana lipid fractions, while among polyunsaturated fatty acids, arachidonic acid was the found in highest concentration.
Compounds from the chemical class of hydrocarbons, alkanes, and alkenes are common compounds in the majority of marine macroalgae EOs [1]. Although unsaturated hydrocarbons from C8 to C19 with the presence of 1 to 4 degrees of unsaturation are common, our study mainly reported the presence of compounds with one double bond. From the class of hydrocarbons, the straight chain saturated hydrocarbon 11-pentan-3-ylhenicosane was found in high amounts (from 0.43% to 1.41%), as well as hexadecane (from 0.08% to 1.30%). Both of these compounds followed similar trends, with the lowest concentrations found in the July sample, while their content significantly increased in next two collecting months, with the highest concentration in August. Also, pentadec-1-en was found in July (0.14%) and in even higher amounts in August (2.62%), while in the first two collection months this compound was not detected. The presence of squalene, which is the biosynthetic precursor of triterpenes and steroids, was confirmed in all samples, with the highest amounts detected in July.
Previous studies on volatile components from Cystoseira species confirmed the domination of hydrocarbons in C. barbata, while this class of compounds was found only in traces in C. crinite, where the majority of compounds were monoterpenoids [19]. The domination of hydrocarbons in the volatile oil of C. barbata was also reported by Ozdemir et al. [20], while Bouzidi et al. [13] reported that the most important class of VOCs obtained by hydrodistillation in C. sedoides were fatty acids and derivatives, with a content of 53.1%. Gressler et al. [11] reported the identification of hexadecane in different algae, among which were two Cystoseira species: C. barbata and C. mediterranea. Furthermore, heneicosan was also detected in C. barbata [20]. In their study, Bouzidi et al. [13] confirmed the presence of hexadecane and pentadec-1-en in samples of the Algerian endemic algae C. sedoides. It is interesting to note that these compounds were found in samples obtained by hydrodistillation, while they were not present in fractions obtained by focused microwave hydrodistillation and supercritical fluid extraction, which could be confirmation that aggressive isolation conditions (e.g. high temperature, long extraction duration, oxidation, and contact with water) cause the degradation of volatiles.
Samples from July and August contained high percentages of alcohols, 34% and 48%, respectively. Phytol, an acyclic diterpene alcohol, also known as a precursor of vitamin E and a degradation product of chlorophyll, was found in all samples at the highest percentage, especially in the August sample, where its content was 14.20% of all detected compounds [1]. This compound was detected in the lowest concentration in the June sample (2.9%), but in the next two months its content was almost 2 and 5-fold greater. El Amrani Zerrifi et al. [21] confirmed the domination of phytol in C. tamariscifolia from their study, as well as Bouzidi et al. [13] in C. sedoides. Other dominant components from the chemical class of alcohols were oleyl alcohol and n-nonadecan-1-ol, for which the regular amount increase during the collecting months was recorded. The presence of oleyl alcohol in the May sample was not confirmed, while its content in June was 0.68%, in July 5.76%, and in August almost 6%. On the other hand, the share of n-nonadecan-1-ol was 1.67% in May, 3.13% in June, 4.13% in July, and 4.34% in August, and an increase in its concentration during the collection periods could be noted. The great impact of unsaturated alcohols on the overall aroma and sensory perception of food has been previously reported [2].
The share of ketones was 9% in the May sample, 13% in the August sample and 17% in the July sample, while the lowest amount was found in the June sample (only 2%). Among detected compounds, (E)-4-(2,6,6-trimethyl-1-cyclohexen-1-yl)-3-buten-2-one (ranging from 0.53% to 5.41%) and 6,10,14-trimethyl-pentadecan-2-one (ranging from 0.75% to 5.98%), were found in the highest amounts. It is interesting to note that the amounts and the variations in their content among samples for both compounds followed the same trend: July > August (5.72% and 5.41%, respectively) > May (2.76% and 2.58%, respectively) > June. Bouzidi et al. [13] also reported the identification of 6,10,14-trimethyl-pentadecan-2-one in C. sedoides. Among other detected ketones, significant amounts of tridecan-2-one and dec-1-en-3-one were found. The first component was detected in the highest amount in the July sample (0.67%), while the other one was found in the May sample (0.42%). The July sample was also rich in monoterpene ketone geranyl acetone (0.70%).
Among all detected compounds, aldehydes, which are important odor compounds, were detected in the lowest percentages in all samples (1–2%), with only a few compounds present at a percentage above 0.10%. Aldehydes with low molecular weight are associated with unpleasant aroma, while those with higher molecular weight are responsible for sweet and fruity notes [2]. Tridecanal was dominant in all samples ranging from 0.37% in June to 0.81% in July. Tetradecanal was found in the highest amount in August (0.20%), while its presence in June was not confirmed. On the other hand, (Z)-undec-4-enal was found in the May sample at a percentage of 0.36%, while in other samples it was not detected.
The share of esters in the first two collecting months was equal (10%), while in July and August it was significantly lower, at 6% and 4%, respectively. The dominant ester was methyl arachidonate, with the highest amount found in the May sample (4%). Its content was significantly lower in June (2.49%), July (1.55%), and August (1.74%). Other benzoic acid esters were also found in high amounts in all samples, especially tetradecyl ester in the June sample (4.37%). The highest content of other esters, namely pentadecyl and tridecyl benzoate, were detected in samples harvested in June. Finally, it is interesting to note that all these compounds—tri, tetra, and penta-decyl esters—showed a similar trend across the collecting months: June > July > May > August.
Terpenes are a class of compounds that play an important role as chemical defense agents, but are also involved in some metabolic processes and functions, like the stability of cell membranes and photosynthesis [1]. It has been reported that terpenes are responsible for the distinctive ocean smell of algae, particularly acyclic and cyclic non-isoprenoid C11-hydrocarbons, while the disagreeable odor is related to amines and halogenated, sulphurous, and other specific compounds [1]. However, for the detection of polycyclic aromatic hydrocarbons, substituted phenols, and sulphur compounds, liquid chromatography is required, as they are semi-volatile [11]. From the group of terpenes, a terpene ketone farnesyl acetone (6,10,14-trimethylpentadeca-5,9,13-trien-2-one) was found in the highest amount in all samples (from 0.57% in June to 1.28% in July). The joint FAO/WHO Expert Committee on Food Additives put this compound on its list of flavoring agents, as it is characterized by an intensely sweet and floral odor, which makes it interesting for further applications [23]. Among others, alpha-cadinol was dominant in the May sample at 1.24%; its content was significantly lower in June, while in samples from other to collecting months it was not detected. Bouzidi et al. [13] also reported the presence of this compound in their study, though again, only in samples prepared by hydrodistillation.
Previous studies on Cystoseira species confirmed the potential health benefits of algae extracts and present individual compounds. Bruno de Sousa et al. (2017) in their review paper reported various biological activities of the Cystoseira algae samples, among which properties like antioxidant, antimicrobial (antibacterial, antifungal, antiviral), cytotoxic, antiproliferative, anticancer, antifouling, anti-inflammatory, antileishmanial, cholinesterase inhibitory, anti-diabetic, anti-obesity, hepatoprotective, etc. were confirmed by different studies.
Among recent studies, Hentati et al. [16] detected good antioxidant activity of water-soluble polysaccharides (fucoidan and a sodium alginate), while antidiabetic and antioxidant activity of phlorotannins extracted from C. compressa were reported by Gheda et al. [17]. Abu-Khudir et al. [24] investigated and confirmed the good free radical scavenging activity of the C. crinita extracts, antimicrobial activity against various pathogenic microorganisms, and strong cytotoxic effects against a panel of cancer cells. The authors, using GC–MS analysis, also confirmed the presence of a vast array of medicinally valuable phytochemical compounds belonging to various classes. Ahmed et al. [25] investigated the antimicrobial and cytotoxic activity of the extract, fractions, and pure compounds from C. trinodis, and their results pointed out the good activity of the samples.
Although the yield of EOs obtained from algal samples is low, C. compressa could be an interesting subject of further analysis on algae biological activities, due to the results of previous studies and the interesting chemical profiles of isolates (EOs and extracts from our other study).
3. Materials and Methods
3.1. Algal Material
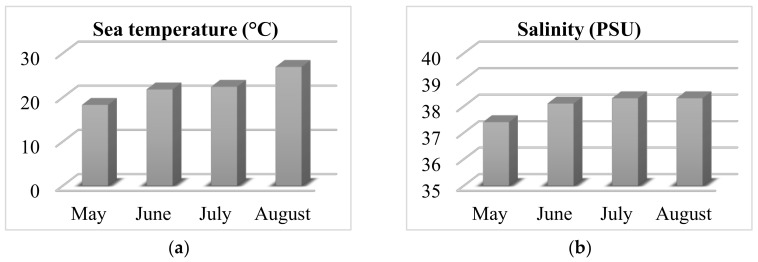
The wild-growing populations of C. compressa (Phaeophyceae) were collected monthly from May to August 2020 on the coast of Čiovo Island, Central Dalmatia, Croatia (43.493389° N, 16.272505° E). Samples were collected throughout a lagoon at 25 points in depth, ranging from 20 to 120 cm. During every sampling, the sea parameters (temperature in °C and salinity in Practical Salinity Unit, PSU) were measured using an YSI Pro2030 probe (YSI Inc., Yellow Springs, OH, USA) and the obtained results are shown in Figure 3. The sea temperature rose during the months of sampling, while the salinity changed under the influence of water springs (typical only in periods with sufficient rainfall, while in periods of drought the springs cease to flow). Pre-treatment of the algal material involved removal of sand, epiphytes, and other organisms from the surface by washing it with tap water. The algal materials were air-dried (for 7 days at room temperature in a shaded and aerated place) and dried algal materials were used for the isolation of the volatile organic compounds.
Figure 3.
Sea parameters during the algal material sampling; (a) Temperature and (b) Salinity.
3.2. Extraction of Essential Oils
C. compressa essential oils were obtained by hydrodistillation of dried algal material (100 g) that was immersed in a flask with distilled water (1000 mL). The extraction process was performed in a Clavenger apparatus (Deotto Lab, Zagreb, Croatia) for 3 h. Pentane and diethyl ether (1:1, v/v) in the inner tube of the apparatus were used for trapping the volatile compounds carried through the system by vapor. Finally, after hydrodistillation, the distillate was dried over anhydrous sodium sulphate while nitrogen was used to evaporate the organic solvent. The samples of essential oils were stored at +4 °C in the dark until analysis [21,26,27].
3.3. GC–MS Analysis of Volatiles
The seaweed EOs were analyzed by GC–MS (Shimadzu QP2010, Shimadzu, Kyoto, Japan) using an autosampler and a DB-5 60 m × 0.25 mm × 0.25 μm column (Agilent Technologies Italia Spa, Milano, Italy). The EOs were resuspended in hexane and 1 µL was injected in the following gas chromatographic conditions: injection temperature 260 °C, interface temperature 280 °C, ion source 220 °C, carrier gas (He) flow rate 30 cm/s, splitting ratio 1:10. The oven temperature was programmed as follows: 40 °C for 4 min, from 40 °C to 175 °C with a 3 °C/min rate of increase, from 175 °C to 300 °C with a 7 °C/min increase, then holding for 10 min. EO constituents were identified by comparing their mass spectra with those reported in literature and the NIST Mass Spectral Database (NIST 08, National Institute of Standards and Technology, Gaithersburg, MD, USA). For each sample, the volatile profile composition was expressed as the relative percentage of each single peak area with respect to the total peak area.
4. Conclusions
This paper is the first report that provides information about the influence of the harvest period on essential oil aromatic compounds in C. compressa, and to obtain insight into the impact of individual components on the general sensory perception of the algae. According to the results obtained, C. compressa could be considered as a source of novel chemical entities with great potential to be used as an ingredient in different industrial applications such as functional foods, pharmaceuticals, and/or cosmeceuticals. The increase in the content of some of the key aroma compounds during the vegetation periods has been noted, while some detected compounds are probably products of degradation or modifications caused by aggressive isolation conditions. As new extraction methods have greatly developed in the last few years and have been widely used in the field of natural compounds due to their numerous benefits in comparison to conventional ones, this scientific research is still ongoing and opens a wide spectrum of possibilities for future research.
Author Contributions
Conceptualization: V.Š. and G.T.; Methodology: M.Č., G.T., F.B., and C.M.; Formal analyses: M.Č., C.M., and D.S.; Data curation: F.B., I.G.M., and G.T., Writing—original draft preparation: I.G.M.; writing—review and editing: V.Š. and G.T.; project administration, G.T. and V.Š.; funding acquisition: V.Š. and G.T.; supervision: V.Š. All authors have read and agreed to the published version of the manuscript.
Funding
This research is supported by the PRIMA program under the project BioProMedFood (Project ID 1467). The PRIMA program is supported by the European Union.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analysis, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Sample Availability
Samples of the EOs are not available from the authors.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.El Hattab M. Essential Oils—Bioactive Compounds, New Perspectives and Applications. IntechOpen Limited; London, UK: 2020. Algae Essential Oils: Chemistry, Ecology, and Biological Activities; pp. 1–25. [DOI] [Google Scholar]
- 2.Garicano Vilar E., O’Sullivan M.G., Kerry J.P., Kilcawley K.N. Volatile Compounds of Six Species of Edible Seaweed: A Review. Algal Res. 2020;45:101740. doi: 10.1016/j.algal.2019.101740. [DOI] [Google Scholar]
- 3.Vizetto-Duarte C., Pereira H., De Sousa C.B., Rauter A.P., Albericio F., Custódio L., Barreira L., Varela J. Fatty Acid Profile of Different Species of Algae of the Cystoseira Genus: A Nutraceutical Perspective. Nat. Prod. Res. 2015;29:1264–1270. doi: 10.1080/14786419.2014.992343. [DOI] [PubMed] [Google Scholar]
- 4.Oucif H., Benaissa M., Ali Mehidi S., Prego R., Aubourg S.P., Abi-Ayad S.M.E.A. Chemical Composition and Nutritional Value of Different Seaweeds from the West Algerian Coast. J. Aquat. Food Prod. Technol. 2020;29:90–104. doi: 10.1080/10498850.2019.1695305. [DOI] [Google Scholar]
- 5.Maggio A., Alduina R., Oddo E., Piccionello A.P., Mannino A.M. Antibacterial Activity and HPLC Analysis of Extracts from Mediterranean Brown Algae. Plant Biosyst. 2020:1–17. doi: 10.1080/11263504.2020.1829737. [DOI] [Google Scholar]
- 6.Generalić Mekinić I., Skroza D., Šimat V., Hamed I., Čagalj M., Perković Z.P. Phenolic Content of Brown Algae (Pheophyceae) Species: Extraction, Identification, and Quantification. Biomolecules. 2019;9:244. doi: 10.3390/biom9060244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mekinić I.G., Šimat V., Botić V., Crnjac A., Smoljo M., Soldo B., Ljubenkov I., Čagalj M., Skroza D. Bioactive Phenolic Metabolites from Adriatic Brown Algae Dictyota Dichotoma and Padina Pavonica (Dictyotaceae) Foods. 2021;10:1187. doi: 10.3390/foods10061187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cvitković D., Dragović-Uzelac V., Dobrinčić A., Čož-Rakovac R., Balbino S. The Effect of Solvent and Extraction Method on the Recovery of Lipid Fraction from Adriatic Sea Macroalgae. Algal Res. 2021;56:102291. doi: 10.1016/j.algal.2021.102291. [DOI] [Google Scholar]
- 9.Čagalj M., Skroza D., Tabanelli G., Özogul F., Šimat V. Maximizing the Antioxidant Capacity of Padina Pavonica by Choosing the Right Drying and Extraction Methods. Processes. 2021;9:587. doi: 10.3390/pr9040587. [DOI] [Google Scholar]
- 10.Akakabe Y., Kajiwara T. Bioactive Volatile Compounds from Marine Algae: Feeding Attractants. J. Appl. Phycol. 2008;20:661–664. doi: 10.1007/s10811-007-9309-x. [DOI] [Google Scholar]
- 11.Gressler V., Colepicolo P., Pinto E. Useful Strategies for Algal Volatile Analysis. Curr. Anal. Chem. 2010;5:271–292. doi: 10.2174/157341109788680255. [DOI] [Google Scholar]
- 12.Zuo Z. Why Algae Release Volatile Organic Compounds—The Emission and Roles. Front. Microbiol. 2019;10:491. doi: 10.3389/fmicb.2019.00491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bouzidi N., Seridi H., Daghbouche Y., Piovetti L., El Hattab M. Comparison of the Chemical Composition of “Cystoseira Sedoides (Desfontaines) C. Agardh” Volatile Compounds Obtained by Different Extraction Techniques. Rec. Nat. Prod. 2015;10:58–67. [Google Scholar]
- 14.Bruno de Sousa C., Gangadhar K.N., Macridachis J., Pavão M., Morais T.R., Campino L., Varela J., Lago J.H.G. Cystoseira Algae (Fucaceae): Update on Their Chemical Entities and Biological Activities. Tetrahedron Asymmetry. 2017;28:1486–1505. doi: 10.1016/j.tetasy.2017.10.014. [DOI] [Google Scholar]
- 15.Falace A., Zanelli E., Bressan G. Morphological and Reproductive Phenology of Cystoseira Compressa (Esper) Gerloff & Nizamuddin (Fucales, Fucophyceae) in the Gulf of Trieste (North Adriatic) Ann. Ser. Hist. Nat. 2005;5:5–12. [Google Scholar]
- 16.Hentati F., Delattre C., Ursu A.V., Desbrières J., Le Cerf D., Gardarin C., Abdelkafi S., Michaud P., Pierre G. Structural Characterization and Antioxidant Activity of Water-Soluble Polysaccharides from the Tunisian Brown Seaweed Cystoseira Compressa. Carbohydr. Polym. 2018;198:589–600. doi: 10.1016/j.carbpol.2018.06.098. [DOI] [PubMed] [Google Scholar]
- 17.Gheda S., Naby M.A., Mohamed T., Pereira L., Khamis A. Antidiabetic and Antioxidant Activity of Phlorotannins Extracted from the Brown Seaweed Cystoseira Compressa in Streptozotocin-Induced Diabetic Rats. Environ. Sci. Pollut. Res. 2021;28:22886–22901. doi: 10.1007/s11356-021-12347-5. [DOI] [PubMed] [Google Scholar]
- 18.Mancuso F.P., Messina C.M., Santulli A., Laudicella V.A., Giommi C., Sarà G., Airoldi L. Influence of Ambient Temperature on the Photosynthetic Activity and Phenolic Content of the Intertidal Cystoseira Compressa along the Italian Coastline. J. Appl. Phycol. 2019;31:3069–3076. doi: 10.1007/s10811-019-01802-z. [DOI] [Google Scholar]
- 19.Milkova T., Talev G., Christov R., Dimitrova-Konaklieva S., Popov S. Sterols and Volatiles in Cystoseira Barbata and Cystoseira Crinita from the Black Sea. Phytochemistry. 1997;45:93–95. doi: 10.1016/S0031-9422(96)00588-2. [DOI] [Google Scholar]
- 20.Ozdemir G., Horzum Z., Sukatar A., Karabay-Yavasoglu N. Antimicrobial Activities of Volatile Components and Various Extracts of Dictyopteris Membranaceae and Cystoseira Barbata from the Coast of Izmir, Turkey. Pharm. Biol. 2006;44:183–188. doi: 10.1080/13880200600685949. [DOI] [Google Scholar]
- 21.Amrani Zerrifi S.E., El Khalloufi F., Mugani R., El Mahdi R., Kasrati A., Soulaimani B., Barros L., Ferreira I.C.F.R., Amaral J.S., Finimundy T.C., et al. Seaweed Essential Oils as a New Source of Bioactive Compounds for Cyanobacteria Growth Control: Innovative Ecological Biocontrol Approach. Toxins. 2020;12:527. doi: 10.3390/toxins12080527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kord A., Cherif Y.F., Amiali M., Mustapha M.A., Benfares R., Soumia B., Belfadel O. Fatty Acids Composition of Cystoseira Sauvageauana and Laurencia Pinnatifida Collected from the Algerian Coast. Acta Period. Technol. 2019;50:113–122. doi: 10.2298/APT1950113K. [DOI] [Google Scholar]
- 23.FAO 2,6,10-Trimethyl-2,6,10-pentadecatrien-14-one. [(accessed on 27 September 2021)]. Available online: http://www.fao.org/food/food-safety-quality/scientific-advice/jecfa/jecfa-flav/details/en/c/1055/
- 24.Abu-Khudir R., Ismail G.A., Diab T. Antimicrobial, Antioxidant, and Anti-Tumor Activities of Sargassum Linearifolium and Cystoseira Crinita from Egyptian Mediterranean Coast. Nutr. Cancer. 2021;73:829–844. doi: 10.1080/01635581.2020.1764069. [DOI] [PubMed] [Google Scholar]
- 25.Ahmed S.A., Rahman A.A., Elsayed K.N.M., Abd El-Mageed H.R., Mohamed H.S., Ahmed S.A. Cytotoxic Activity, Molecular Docking, Pharmacokinetic Properties and Quantum Mechanics Calculations of the Brown Macroalga Cystoseira Trinodis Compounds. J. Biomol. Struct. Dyn. 2021;39:3855–3873. doi: 10.1080/07391102.2020.1774418. [DOI] [PubMed] [Google Scholar]
- 26.Mastelić J., Jerković I., Blažević I., Radonić A., Krstulović L. Hydrodistillation-Adsorption Method for the Isolation of Water-Soluble, Non-Soluble and High Volatile Compounds from Plant Materials. Talanta. 2008;76:885–891. doi: 10.1016/j.talanta.2008.04.051. [DOI] [PubMed] [Google Scholar]
- 27.Blažević I., Đulović A., Čikeš Čulić V., Burčul F., Ljubenkov I., Ruščić M., Generalić Mekinić I. Bunias Erucago L.: Glucosinolate Profile and in Vitro Biological Potential. Molecules. 2019;24:741. doi: 10.3390/molecules24040741. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is contained within the article.