Abstract
Introduction
Post-COVID-19 complications require simultaneous characterisation and management to plan policy and health system responses. We describe the 12-month experience of the first UK dedicated post-COVID-19 clinical service to include hospitalised and non-hospitalised patients.
Methods
In a single-centre, observational analysis, we report the demographics, symptoms, comorbidities, investigations, treatments, functional recovery, specialist referral and rehabilitation of 1325 individuals assessed at the University College London Hospitals post-COVID-19 service between April 2020 and April 2021, comparing by referral route: posthospitalised (PH), non-hospitalised (NH) and post emergency department (PED). Symptoms associated with poor recovery or inability to return to work full time were assessed using multivariable logistic regression.
Results
1325 individuals were assessed (PH: 547, 41.3%; PED: 212, 16%; NH: 566, 42.7%). Compared with the PH and PED groups, the NH group were younger (median 44.6 (35.6–52.8) years vs 58.3 (47.0–67.7) years and 48.5 (39.4–55.7) years), more likely to be female (68.2%, 43.0% and 59.9%), less likely to be of ethnic minority (30.9%, 52.7% and 41.0%) or seen later after symptom onset (median (IQR): 194 (118–298) days, 69 (51–111) days and 76 (55–128) days; all p<0.0001). All groups had similar rates of onward specialist referral (NH 18.7%, PH 16.1% and PED 18.9%, p=0.452) and were more likely to require support for breathlessness (23.7%, 5.5% and 15.1%, p<0.001) and fatigue (17.8%, 4.8% and 8.0%, p<0.001). Hospitalised patients had higher rates of pulmonary emboli, persistent lung interstitial abnormalities and other organ impairment. 716 (54.0%) individuals reported <75% optimal health (median 70%, IQR 55%–85%). Less than half of employed individuals could return to work full time at first assessment.
Conclusion
Post-COVID-19 symptoms were significant in PH and NH patients, with significant ongoing healthcare needs and utilisation. Trials of interventions and patient-centred pathways for diagnostic and treatment approaches are urgently required.
Keywords: COVID-19, clinical epidemiology
Key messages.
There is high symptom burden for non-hospitalised patients post-COVID-19, even compared with post-hospitalised patients post-COVID-19.
The significant, long-lasting health and social consequences of SARS-CoV-2 infection are not confined to those who required hospitalisation.
This is the first study to report the baseline characteristics, investigations and outcomes of initial assessment of all eligible patients in a dedicated multiprofessional post-COVID-19 service, including 547 posthospitalisation, 566 non-hospitalised and 212 patients discharged from the emergency department.
Introduction
Chronic post-viral sequelae are well known,1 but the SARS-CoV-2 pandemic’s scale poses an unprecedented threat to long-term health.2 3 Initially, funding, research, clinical practice and policy emphasised acute, hospitalised patients in areas with a high burden of COVID-19 cases and deaths. However, more recently, there has been an increasing focus on the longer-term effects of acute infection (‘Long COVID’ or ‘post-COVID syndrome’), including non-hospitalised patients4 5 (figure 1).
Figure 1.
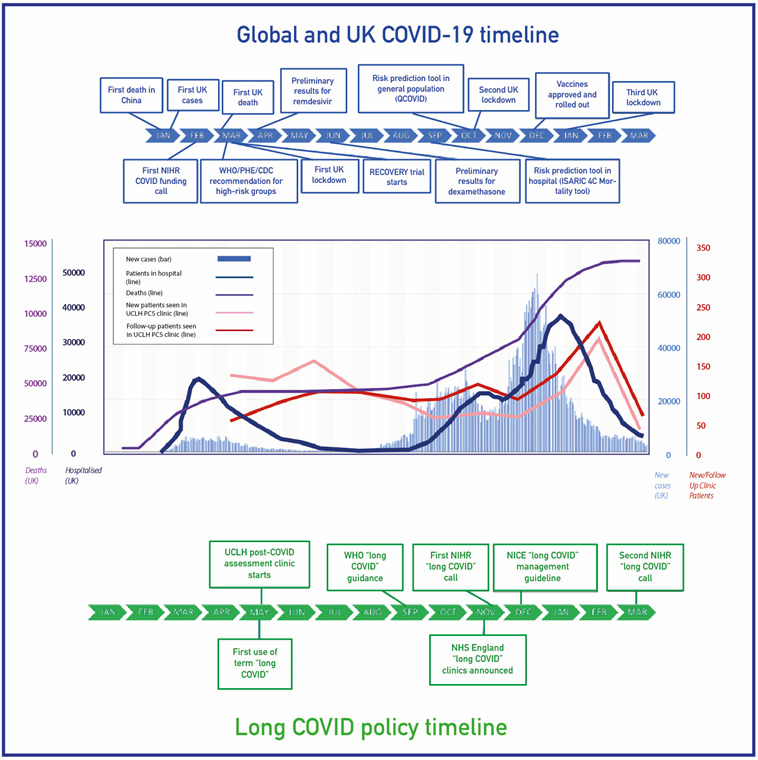
Post-COVID-19 assessment in the context of the pandemic. CDC, Centers for Disease Control and Prevention; ISARIC 4C, International Severe Acute Respiratory and emerging Infection Consortium (Coronavirus Clinical Characterisation Consortium); NHS, National Health Service; NIHR, National Institute of Health Research; PCS, Post COVID Syndrome; PHE, Public Health England; RECOVERY, Randomised Evaluation of COVID-19 Therapy; UCLH, University College London Hospitals NHS Foundation Trust.
Clinically, long COVID remains a poorly defined disease.5 6 To address the healthcare needs of an estimated 1.1 million individuals with long COVID in the UK7 and the millions around the world,8 there is a need to deliver clinical care while simultaneously reviewing clinical data in ‘learning health system’ approaches.9 Research on clinical characterisation and management has been identified by both recent expert consensus groups and patients as a priority area.10
COVID-19 hospitalisation is associated with a significant risk of end-organ impact, functional impairment, readmission and mortality.11–13 Recent analysis of 6-month post-COVID-19 outcomes in China highlighted persistent physical, mental and functional impact during follow-up, but excluded non-hospitalised patients.14 Recent electronic health record (EHR) analyses in the USA and Denmark, respectively, suggested major healthcare resource implications of long COVID in non-hospitalised individuals,15 16 but no studies to date have reported on the models of care for long COVID.
More than 80 dedicated post-COVID-19 assessment clinics have been announced in England,17 but many centres only started accepting referrals 1 year after the first wave of the pandemic. Various care models have been proposed through expert and patient consensus,18 19 but real-world data are lacking, particularly in community settings, where majority of patients with COVID-19 and long COVID are managed.
In April 2020, we established a dedicated service for assessment of post-COVID-19 complications at University College London Hospitals NHS Foundation Trust (UCLH) for both hospitalised and non-hospitalised individuals. We report the baseline characteristics, clinical presentation, management and outcomes of all individuals referred to this specialist clinic following suspected or confirmed SARS-CoV-2 infection over a 12-month period.
Methods
Context of the UCLH post-COVID-19 service
The UCLH post-COVID-19 service is a one-stop model of assessment (by physician and physiotherapist), diagnostics and exercise test to triage need for further specialist input, treatment or rehabilitation. It accepts referrals from three sources: (1) posthospitalised (PH): postadmission to UCLH with COVID-19; (2) non-hospitalised (NH): individuals referred from primary care with suspected long COVID ≥6 weeks post-SARS-CoV-2 infection; and (3) post emergency department (PED): referral for individuals with persistent symptoms at 4–6 weeks after attendance. As per the British Thoracic Society guidance,20 postdischarge review in PH patients was at 6 weeks for those who received respiratory support via continuous positive airway pressure or invasive ventilation, or with chest imaging abnormality, and at 12 weeks in all other patients. PH patients under the care of other specialist services or without chest X-ray abnormalities were not routinely booked into the post-COVID-19 service.
Clinical population
Our analysis included all patients assessed at the UCLH post-COVID-19 service between 20 April 2020 and 25 April 2021 (figure 2), excluding follow-up assessments and individuals who did not attend. Due to restricted access to testing during the first wave of the pandemic, SARS-CoV-2 infection was defined by either laboratory confirmation (viral positive oropharyngeal/nasopharyngeal swab when tested by reverse-transcriptase PCR or anti-N antigen IgG detected on convalescent serum) or strong clinical suspicion (assessed by both the referring and consulting clinicians).21 We followed the Strengthening the Reporting of Observational Studies in Epidemiology criteria (checklist).22
Figure 2.
Population undergoing assessment at the post-COVID-19 assessment clinic. CXR, Chest X-Ray; GP, General Practice; ICS, Integrated Care System; UCLH, University College London Hospitals NHS Foundation Trust.
Post-COVID-19 assessment
Clinical assessment was developed by secondary care clinicians and therapists and consists of a consultation and multiprofessional assessment, delivered primarily face to face, or where necessary virtually. An EHR (Epic; Epic Systems Corporation, Wisconsin) structured assessment tool (accessible via the patient portal before the appointment and during assessment) was used to record the sociodemographics, medical history, current symptoms and functional status. Where appropriate, the following outcome measures were recorded: percentage of best health (as used in other tools; eg, EuroQoL-5 domain-5 level), symptom severity for breathlessness, fatigue, cough, sleep disturbance and palpitations, MRC (Medical Research Council) Dyspnoea Scale, Post-Traumatic Stress Disorder Scale (PTSD), Fatigue Assessment Scale, two-item Generalised Anxiety Disorder (GAD-2), and two-item Patient Health Questionnaire (PHQ-2)5 11 13 21 (online supplemental methods).
bmjresp-2021-001041supp001.pdf (460.1KB, pdf)
Selected patients underwent further investigation at the discretion of the clinician or following multidisciplinary team meetings with respiratory, cardiology and neurology input according to clinical need. These tests included full blood count, liver and renal function, D-dimer, troponin and NT pro-brain natriuretic peptide (NT-proBNP), as well as sit-to-stand test, chest X-ray, Computed Tomography Pulmonary Angiography (CTPA) with HRCT (High-Resolution Computed Tomography) precontrast, ECG, cardiac MRI (cMRI), brain MRI, echocardiography and Holter monitoring. Lung function testing was unavailable due to local infection control requirements.
Clinical management
After assessment, patients were either discharged to the community, booked for further clinical follow-up, and/or referred for specialist opinion, physical rehabilitation, respiratory physiotherapy, fatigue management, vocational support and psychological support. Patients with elevated GAD-2 or PHQ-2 scores (≥3) were advised on self-referral to ‘improving access to psychological therapies’ services.
Data extraction and statistical analysis
Demographic and clinical data at first assessment were extracted from the EHR. Age, time since symptom onset in days and self-reported percentage of best health were recorded as continuous variables, and the presence or absence of individual symptoms as binary variables. Self-reported ability to return to work, for employed individuals, was recorded on an ordinal scale (‘Not at all’ to ‘Full time’). The Index of Multiple Deprivation (IMD) decile was derived from each patient’s postcode. We used descriptive statistics to summarise the baseline characteristics. Continuous variables are reported as median with IQR, while categorical variables are reported as frequency (%). For group-wise comparison, we used the Kruskal-Wallis test for continuous variables and the χ2 test or Fisher’s exact test for categorical variables. We used multivariable logistic regression models, adjusted for age (modelled as a continuous variable) and gender (male vs female as the reference group), to investigate symptoms associated with (1) optimal (≥75%) patient-reported functional recovery at first presentation and (2) patient-reported ability to return to full-time employment at first presentation. The model for returning to employment excluded those not employed or retired before COVID-19. Age and gender were included in the models and all recorded symptoms (represented as presence vs absence) were available for selection in a backwards stepwise selection process with a threshold of p<0.05. A sensitivity analysis also considered time since onset for selection in the models. Referral rates of local General Practices (GPs) (per 1000 practice population) were determined using locally available EHR data for practice size and referring practice. All analyses were performed using Python V.3.7.6.
Results
Sociodemographic profile
The number of referrals to the UCLH post-COVID-19 clinic mirrored successive pandemic waves (figure 1). Excluding patients who did not attend and who cancelled appointments, 1325 patients were reviewed: PH, n=547; NH, n=566; and PED, n=212 (figure 2). Of the patients, 614 (46.3%) were tested for SARS-CoV-2 infection using RT-PCR(reverse transcription polymerase chain reaction), of whom 378 (61.6%) were positive, while among those who underwent serological testing (n=241, 18.2%) 114 (47.3%) were positive. The remaining 470 (35.5%) patients had strong clinical suspicion of a prior SARS-CoV-2 infection.
The median age was 49.9 (IQR 40.1–60.1) years, and 748 (56.5%) were women and 550 (41.5%) were of non-white ethnicity. Compared with PH and PED individuals, NH individuals were younger (44.6 (IQR 35.6–52.8) years vs 58.3 (IQR 47.0–67.7) years and 48.5 (IQR 39.4–55.7) years, p<0.001), more likely to be female (68.2% vs 43.0% and 59.9%, p<0.001), less likely to be non-white (30.9% vs 52.7% and 41.0%, p<0.001) and less likely to live in an area of social deprivation (IMD category, median (IQR): 5 (3–7) vs 4 (2–6) and 4 (3–6), p<0.001) (table 1 and online supplemental figure 1). General practice referral rates in the catchment population ranged from 0.09 to 3.64 patients per 1000 practice population, with 49 of 201 (24.4%) practices referring no patients (online supplemental figure 2).
Table 1.
Baseline characteristics of 1325 individuals referred to the post-COVID-19 assessment clinic
| Overall (N=1325) | Hospitalised (n=547) | Non-hospitalised (n=566) | ED (n=212) | P value* | |
| n (%) | n (%) | n (%) | n (%) | ||
| Age, years | |||||
| Median (IQR) | 49.9 (40.1–60.1) | 58.3 (47.0–67.7) | 44.6 (35.6–52.8) | 48.5 (39.4–55.7) | <0.001 |
| Range | 18–93 | 18–93 | 19–85 | 22–85 | |
| Female gender | 748 (56.5) | 235 (43.0) | 386 (68.2) | 127 (59.9) | <0.001 |
| Ethnicity | <0.001 | ||||
| Ethnic minority | 550 (41.5) | 288 (52.7) | 175 (30.9) | 87 (41.0) | |
| White | 655 (49.4) | 213 (38.9) | 332 (58.7) | 110 (51.9) | |
| Unknown/not stated | 120 (9.1) | 46 (8.4) | 59 (10.4) | 15 (7.1) | |
| SARS-CoV-2 laboratory testing | |||||
| RT-PCR | 614 (46.3) | 448 (81.9) | 75 (13.3) | 91 (42.9) | <0.001 |
| Positive where tested | 378 (61.6) | 322 (71.9) | 12 (16.0) | 44 (48.4) | <0.001 |
| Serology | 241 (18.2) | 28 (5.1) | 162 (28.6) | 51 (24.1) | <0.001 |
| Positive where tested | 114 (47.3) | 17 (60.7) | 70 (43.2) | 27 (52.9) | 0.153 |
| No testing performed | 470 (35.5) | 71 (13.0) | 329 (58.1) | 70 (33.0) | <0.001 |
| IMD decile | |||||
| Median (IQR) | 4 (3–6) | 4 (2–6) | 5 (3–7) | 4 (3–6) | <0.001 |
| Unknown | 4 (0.3) | 2 (0.4) | 2 (0.4) | 0 (0.0) | ~1.000 |
| Chronic diseases and risk factors | |||||
| Cardiovascular disease | 165 (12.5) | 138 (25.2) | 16 (2.8) | 11 (5.2) | <0.001 |
| Hypertension | 232 (17.5) | 177 (32.4) | 32 (5.7) | 23 (10.8) | <0.001 |
| Asthma | 178 (13.4) | 66 (12.1) | 79 (14.0) | 33 (15.6) | 0.398 |
| Diabetes mellitus | 156 (11.8) | 129 (23.6) | 13 (2.3) | 14 (6.6) | <0.001 |
| Thyroid disease | 87 (6.6) | 42 (7.7) | 31 (5.5) | 14 (6.6) | 0.333 |
| Anxiety | 44 (3.3) | 19 (3.5) | 15 (2.7) | 10 (4.7) | 0.346 |
| Depression | 45 (3.4) | 16 (2.9) | 17 (3.0) | 12 (5.7) | 0.139 |
| Malignancy | 55 (4.2) | 36 (6.6) | 15 (2.7) | 4 (1.9) | 0.001 |
| Chronic obstructive pulmonary disease | 23 (1.7) | 17 (3.1) | 4 (0.7) | 2 (0.9) | 0.006 |
| Chronic kidney disease | 19 (1.4) | 19 (3.5) | 0 (0.0) | 0 (0.0) | <0.001 |
| Chronic fatigue syndrome | 10 (0.8) | 0 (0.0) | 8 (1.4) | 2 (0.9) | 0.009 |
| Current smoker | 17 (1.3) | 12 (2.2) | 3 (0.5) | 2 (0.9) | 0.043 |
| Time since symptom onset | |||||
| Median, days (IQR) | 108 (61–197) | 69 (51–111) | 194 (118–298) | 76 (55–128) | <0.001 |
| 0–3 months | 559 (42.2) | 356 (65.1) | 77 (13.6) | 126 (59.4) | |
| 3–6 months | 387 (29.2) | 151 (27.6) | 183 (32.3) | 53 (25.0) | |
| 6–9 months | 164 (12.4) | 19 (3.5) | 128 (22.6) | 17 (8.0) | |
| 9–12 months | 168 (12.7) | 14 (2.6) | 143 (25.3) | 11 (5.2) | |
| 12+ months | 47 (3.5) | 7 (1.3) | 35 (6.2) | 5 (2.4) | |
| Symptoms† | |||||
| Median (IQR) | 2 (1–4) | 1 (0–2) | 3 (2–5) | 2 (1–4) | <0.001 |
| Breathlessness | 651 (49.1) | 211 (38.6) | 342 (60.4) | 98 (46.2) | <0.001 |
| Fatigue | 644 (48.6) | 187 (34.2) | 359 (63.4) | 98 (46.2) | <0.001 |
| Cough | 312 (23.5) | 106 (19.4) | 150 (26.5) | 56 (26.4) | 0.011 |
| Chest pain | 305 (23.0) | 76 (13.9) | 176 (31.1) | 53 (25.0) | <0.001 |
| Myalgia | 251 (18.9) | 57 (10.4) | 168 (29.7) | 26 (12.3) | <0.001 |
| Headache | 233 (17.6) | 38 (6.9) | 166 (29.3) | 29 (13.7) | <0.001 |
| Brain fog‡ | 200 (15.1) | 35 (6.4) | 136 (24.0) | 29 (13.7) | <0.001 |
| Palpitations | 167 (12.6) | 31 (5.7) | 104 (18.4) | 32 (15.1) | <0.001 |
| Arthralgia | 170 (12.8) | 40 (7.3) | 110 (19.4) | 20 (9.4) | <0.001 |
| Disturbed sleep | 142 (10.7) | 25 (4.6) | 92 (16.3) | 25 (11.8) | <0.001 |
| Anosmia | 122 (9.2) | 29 (5.3) | 78 (13.8) | 15 (7.1) | <0.001 |
| Postural symptoms | 105 (7.9) | 14 (2.6) | 74 (13.1) | 17 (8.0) | <0.001 |
| Diarrhoea | 82 (6.2) | 20 (3.7) | 56 (9.9) | 6 (2.8) | <0.001 |
| Skin rash | 75 (5.7) | 12 (2.2) | 47 (8.3) | 16 (7.5) | <0.001 |
| Abdominal pain | 75 (5.7) | 13 (2.4) | 51 (9.0) | 11 (5.2%) | <0.001 |
| Functional status | |||||
| % best health, median (IQR) (n=1325) | 70 (55–85) | 80 (65–95) | 60 (50–75) | 75 (60–90) | <0.001 |
| Fatigue Assessment Scale score, median (IQR) (n=806) | 29 (21–37) | 24 (16–34) | 30 (24–38) | 28 (23–36) | <0.001 |
| MRC Dyspnoea Scale score assessed | 593 (44.8) | 192 (35.1) | 324 (57.2) | 77 (36.3) | <0.001 |
| MRC Dyspnoea Scale score ≥3 where assessed | 214 (36.1) | 69 (35.9) | 115 (35.5) | 30 (39.0) | 0.849 |
| PTSD score assessed | 399 (30.1) | 151 (27.6) | 200 (35.3) | 48 (22.6) | <0.001 |
| PTSD score≥6 where assessed | 95 (23.8) | 30 (19.9) | 53 (26.5) | 12 (25) | 0.345 |
| GAD-2 score assessed | 853 (64.4) | 295 (53.9) | 454 (80.2) | 104 (49.1) | <0.001 |
| GAD-2 score ≥3 where assessed | 255 (29.9) | 63 (21.4) | 159 (35.0) | 33 (31.7) | <0.001 |
| PHQ-2 score assessed | 841 (63.5) | 287 (52.5) | 451 (79.7) | 103 (48.6) | <0.001 |
| PHQ-2 score ≥3 where assessed | 204 (24.3) | 54 (18.8) | 119 (26.4) | 31 (30.1) | 0.022 |
*Kruskal-Wallis test used for continuous variables. χ2 test used for categorical variables. Fisher’s exact test used for categorical variables where one or more frequencies <5.
†Commonly reported symptoms as shown in the online supplemental material.
‡‘Brain fog’ encompasses problems with memory, cognition and concentration.
ED, emergency department; GAD-2, two-item Generalised Anxiety Disorder; IMD, Index of Multiple Deprivation; MRC, Medical Research Council; PHQ-2, two-item Patient Health Questionnaire; PTSD, Post-Traumatic Stress Disorder Scale; RT-PCR, Reverse Transcription Polymerase Chain Reaction.
Chronic diseases and risk factors
Hypertension (17.5%), asthma (13.4%), cardiovascular disease (12.5%), diabetes (11.8%) and thyroid disease (6.6%) were the most common comorbidities. Most premorbid chronic diseases were more common among PH patients, compared with NH and PED patients, except asthma (12.1% vs 14.0% and 15.6%), anxiety (3.5% vs 2.7% and 4.7%), depression (2.9% vs 3.0% and 5.7%) and chronic fatigue syndrome (0.0% vs 1.4% and 0.9%). Current smoking was uncommon (1.3% overall) (table 1). Overall, 86 PH patients died before a post-COVID-19 clinical assessment, with a further two PH patients dying after clinical review.
Symptoms and functional status
The median time from symptom onset (of acute SARS-CoV-2 infection) to first clinical assessment was 108 days (IQR 61–197), and was delayed in NH patients (194 (118–298) days) compared with PH patients (69 (51–111) days) and PED patients (76 (55–128) days) (p<0.001).
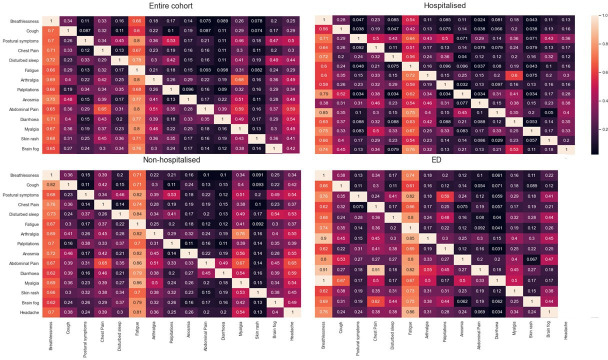
At first visit, the median (IQR) number of reported symptoms was 2 (1–4) overall (PH, NH and PED groups: 1 (0–2), 3 (2–5) and 2 (1–4), p<0.001). The most commonly reported symptoms were breathlessness (49.1%), fatigue (48.6%), cough (23.5%), myalgia (18.9%), chest pain (23.0%), headache (17.6%), ‘brain fog’ (15.1%) and palpitations (12.6%). All symptoms were more frequently reported by NH patients than PH and PED patients (table 1). Overall, 36.1% had MRC Dyspnoea Scale score ≥3 (n=593 assessed), 23.8% PTSD score >6 (n=399 assessed), 29.9% GAD-2 ≥3 (n=853 assessed) and 24.3% PHQ-2 ≥3 (n=841 assessed), with significant differences (p<0.001) between referral sources (table 1). Breathlessness, fatigue, palpitations, sleep quality and chest pain were more common, and where reported were rated as more severe (online supplemental table 3) in the NH group compared with the PH group. Symptom co-occurrence varied among the groups (figure 3). At first assessment, 716 (54.0%) individuals reported <75% optimal health, more frequently in NH (71.8%) compared with PED (37.8%) and PH (48.6%) individuals (online supplemental table 1).
Figure 3.
Co-occurrence of symptoms at first assessment of 1325 individuals referred to the post-COVID-19 assessment clinic. ED, emergency department.
Investigations
Investigations requested according to clinician judgement included chest X-ray (n=694, 52.4%), echocardiography (n=330, 24.9%), Holter monitor (n=222, 16.8%), CTPA (n=204, 15.4%), 6 min walk test (n=142, 10.7%), cMRI (for chest pain or troponin elevation, n=76, 5.7%) and brain MRI (n=40, 3.0%) (table 2). Of 204 CTPAs requested, 5.9% (0.9% of cohort) showed pulmonary embolism (PE) and 30.9% (4.8% of cohort) showed persistent lung interstitial changes. Outside the post-COVID-19 service, 8 patients had PE identified via acute medical services and 49 (9.0%) PH patients had PE on CTPA during inpatient stay. Echocardiography showed left ventricular systolic dysfunction in seven patients (2.2% of those examined). cMRI showed mild myocarditis in 24 (31.6% of those scanned) and evidence of ischaemic heart disease in 11 PH patients (35.5% of those scanned). On brain MRI, no changes attributed to COVID-19 were identified. Where tested, NT-proBNP was raised in 8.0% of individuals, absolute eosinophil count in 5.8%, troponin T in 13.4%, creatine kinase in 16.9%, alanine transaminase in 18.0% and D-dimer in 18.9%. Abnormal blood investigations were more common in PH than in NH individuals (D-dimer: 28.3% vs 10.3%; troponin T: 27.0% vs 4.7%; NT-proBNP: 17.0% vs 0.0%; and eosinophil count: 6.8% vs 2.5%) (online supplemental table 2).
Table 2.
Investigations, outcomes and onward referrals of 1325 individuals referred to the post-COVID-19 assessment clinic
| Overall (N=1325) | Hospitalised (n=547) | Non-hospitalised (n=566) | ED (n=212) | P value* | |
| n (%) | n (%) | n (%) | n (%) | ||
| Investigations | |||||
| Chest X-ray | 694 (52.4) | 355 (64.9) | 204 (36.0) | 135 (63.7) | <0.001 |
| CT pulmonary angiogram with HRCT precontrast | 204 (15.4) | 104 (19.0) | 64 (11.3) | 36 (17.0) | 0.001 |
| Pulmonary embolism detected on scans performed | 12 (5.9) | 9 (8.7) | 1 (1.6) | 2 (5.6) | 0.159 |
| Persistent interstitial abnormalities on scans performed | 63 (30.9) | 49 (47.1) | 8 (12.5) | 6 (16.7) | <0.001 |
| Echocardiogram | 330 (24.9) | 121 (22.1) | 160 (28.3) | 49 (23.1) | 0.048 |
| Ejection fraction <55% in investigations performed | 7 (2.1) | 3 (2.5) | 4 (2.5) | 0 (0.0) | 0.763 |
| Holter monitor | 222 (16.8) | 51 (9.3) | 143 (25.3) | 28 (13.2) | <0.001 |
| 6 min walk test | 142 (10.7) | 24 (4.4) | 98 (17.3) | 20 (9.4) | <0.001 |
| Cardiac MRI | 76 (5.7) | 31 (5.7) | 33 (5.8) | 12 (5.7) | 0.992 |
| Mild myocarditis on scans performed | 24 (31.6) | 9 (29.0) | 15 (45.5) | 0 (0) | 0.008 |
| Brain MR | 40 (3.0) | 27 (4.9) | 9 (1.6) | 4 (1.9) | 0.004 |
| Lung function | 17 (1.3) | 8 (1.5) | 9 (1.6) | 0 (0.0) | 0.171 |
| Sleep study | 8 (0.6) | 3 (0.5) | 4 (0.7) | 1 (0.5) | ~1.000 |
| Sit-to-stand test | 749 (56.5) | 238 (43.5) | 415 (73.3) | 96 (45.3) | <0.001 |
| Post-test oxygen saturation ≤92% in tests undertaken | 58 (7.7) | 35 (14.7) | 18 (4.3) | 5 (5.2) | <0.001 |
| Discharged at first appointment | 98 (58–170) | 63 (46–101) | 183 (118–329) | 72 (51–112) | <0.001 |
| At least one follow-up visit | 126 (70–221) | 80 (59–126) | 198 (124–274) | 89 (58–172) | <0.001 |
| First visit outcomes | |||||
| Discharged from clinic | 740 (55.8) | 338 (61.8) | 288 (50.9) | 114 (53.8) | <0.001 |
| Specialist referral | 234 (17.7) | 88 (16.1) | 106 (18.7) | 40 (18.9) | 0.452 |
| Seen by physiotherapist | 776 (58.6) | 236 (43.1) | 398 (70.3) | 142 (67.0) | <0.001 |
| Psychology referrals to iAPT | Advised if GAD-2 or PHQ-2 elevated (see table 1): patients can self refer to this service. | ||||
| Specialist referral | |||||
| Cardiology | 110 (8.3) | 41 (7.5) | 46 (8.1) | 23 (10.8) | 0.317 |
| Neurology | 125 (9.4) | 26 (4.8) | 87 (15.4) | 12 (5.7) | <0.001 |
| ENT | 31 (2.3) | 4 (0.7) | 20 (3.5) | 7 (3.3) | 0.002 |
| Other | 53 (4.0) | 19 (3.5) | 24 (4.2) | 10 (4.7) | 0.683 |
| Physiotherapist outcomes | 776 (58.6) | 236 (43.1) | 398 (70.3) | 142 (67.0) | |
| Discharge with self-management support† | 363 (46.8) | 133 (56.4) | 152 (38.2) | 78 (54.9) | <0.001 |
| Cognitive rehabilitation† | 17 (2.2) | 5 (2.1) | 9 (2.3) | 3 (2.1) | ~1.000 |
| Speech and language therapy† | 46 (5.9) | 31 (13.1) | 7 (1.8) | 8 (5.6) | <0.001 |
| Respiratory physiotherapy† (including ENO) | 196 (25.3) | 30 (12.7) | 134 (33.7) | 32 (22.5) | <0.001 |
| Fatigue management† | 144 (18.6) | 26 (11.0) | 101 (25.4) | 17 (12.0) | <0.001 |
*Kruskal-Wallis test for continuous variables. χ2 test for categorical variables. Fisher’s exact test used for categorical variables where one or more frequencies <5.
†Percentages reported as % of individuals seen by physiotherapist.
ED, emergency department; ENO, English National Opera 'Breathe' programme; ENT, ear, nose and throat; GAD-2, two-item Generalised Anxiety Disorder; HRCT, High-Resolution Computed Tomography; iAPT, improving access to psychological therapies; PHQ-2, two-item Patient Health Questionnaire.
Outcomes and onward referrals
Of the individuals, 740 (55.8%) were discharged after first assessment (61.8%, 50.9% and 53.8% in PH, NH and PED individuals). Individuals who required follow-up had longer symptom duration at presentation (median 126, IQR 70–221 days) than those discharged at first visit (median 98, IQR 58–170 days). PH, NH and PED individuals had similar specialist referral rates (16.1%, 18.7% and 18.9%, p=0.452), most commonly to cardiology (8.3%) and neurology (9.4%) (table 2). Of 776 (58.6% of the study population) individuals assessed by physiotherapists, 363 (46.8%) were discharged with self-management support at first visit. NH individuals were more likely than PH and PED individuals to require support for disordered breathing pattern (23.7%, 5.5% and 15.1%, p<0.001) and referral for fatigue management (25.4%, 11.0% and 12.0%, p<0.001) (table 2).
Optimal health and employment
In the PH, NH and PED groups, the median self-reported proportion with optimal health was 80% (65%–95%), 60% (50%–75%) and 75% (60%–90%), respectively, correlating negatively with symptom duration at the time of assessment (table 1 and online supplemental figure 3). Overall, less than half of employed individuals felt able to return to work full time at first assessment (online supplemental table 1).
In the multivariable logistic regression analysis (table 3), younger age was associated with return to work (PH and NH) and male gender (PH and PED). For PH individuals, fatigue, brain fog, chest pain and breathlessness were associated with inability to work full time. Arthralgia and headache were associated with full-time return to work. For NH individuals, fatigue, brain fog and headache were associated with inability to return to work. For PED individuals, breathlessness and myalgia were associated with non-return to work, whereas cough and arthralgia were associated with return to work. For some included symptoms, the 95% CIs were wide, suggesting uncertainty about their effect, perhaps due to the small number of patients with that symptom. Older age and male gender were associated with return to optimal health status (≥75%). Fatigue and postural symptoms were significantly associated with suboptimal (<75%) health status in the PH and NH groups, and brain fog in the NH and PED groups (table 3). Postural symptoms were rare in PH patients, and few with this symptom achieved optimal health. The extreme OR probably reflected the small number recovering in this group, rather than the importance of this symptom. Sensitivity analyses including time since onset in the backwards selection process only changed the models for recovery in NH patients, where longer times since onset and disturbed sleep were associated with lower odds of recovery, replacing postural symptoms in the model.
Table 3.
Multivariable logistic regression analysis showing symptoms associated with ability to return to work full time and ≥75% functional recovery at first assessment of 1325 individuals referred to the post-COVID-19 assessment clinic
| Return to work full time (n=1028) | ≥75% functional recovery (n=1325) | |||||
| Covariate | Multivariable OR (95% CI) | P value | Covariate | Multivariable OR (95% CI) | P value | |
| Hospitalised | ||||||
| Age | 0.97 (0.95 to 0.99) | 0.008 | Age | 1.01 (1.00 to 1.03) | 0.019 | |
| Male gender | 1.88 (1.33 to 2.67) | <0.001 | Male gender | 2.58 (1.94 to 3.42) | <0.001 | |
| Brain fog | 0.13 (0.03 to 0.58) | 0.008 | Postural symptoms | 0.05 (0.01 to 0.46) | 0.007 | |
| Chest pain | 0.28 (0.13 to 0.60) | 0.001 | Chest pain | 0.43 (0.25 to 0.74) | 0.002 | |
| Fatigue | 0.29 (0.17 to 0.52) | <0.001 | Fatigue | 0.47 (0.33 to 0.68) | <0.001 | |
| Breathlessness | 0.54 (0.33 to 0.90) | 0.019 | Arthralgia | 2.69 (1.22 to 5.92) | 0.014 | |
| Arthralgia | 2.55 (1.01 to 6.42) | 0.048 | ||||
| Headache | 2.75 (1.04 to 7.25) | 0.041 | ||||
| Non-hospitalised | ||||||
| Age | 0.98 (0.96 to 0.99) | 0.008 | Age | 1.02 (1.00 to 1.04) | 0.018 | |
| Male gender | 1.20 (0.84 to 1.74) | 0.319 | Male gender | 1.44 (0.99 to 2.09) | 0.058 | |
| Brain fog | 0.54 (0.35 to 0.86) | 0.008 | Postural symptoms | 0.08 (0.02 to 0.32) | <0.001 | |
| Headache | 0.64 (0.42 to 0.97) | 0.034 | Fatigue | 0.49 (0.35 to 0.68) | <0.001 | |
| Fatigue | 0.67 (0.5 to 0.92) | 0.012 | Myalgia | 0.49 (0.30 to 0.81) | 0.005 | |
| Brain fog | 0.53 (0.31 to 0.89) | 0.017 | ||||
| Emergency department | ||||||
| Age | 0.98 (0.95 to 1.01) | 0.155 | Age | 1.01 (0.99 to 1.04) | 0.266 | |
| Male gender | 1.79 (1.04 to 3.10) | 0.037 | Male gender | 2.98 (1.78 to 4.98) | <0.001 | |
| Breathlessness | 0.25 (0.13 to 0.48) | <0.001 | Brain fog | 0.29 (0.1 to 0.85) | 0.025 | |
| Myalgia | 0.26 (0.09 to 0.75) | 0.013 | Fatigue | 0.40 (0.24 to 0.67) | 0.001 | |
| Cough | 2.71 (1.28 to 5.74) | 0.009 | ||||
| Arthralgia | 3.92 (1.12 to 13.77) | 0.033 | ||||
Discussion
Our 12-month experience with the earliest post-COVID-19 clinical service in the UK to include NH, PH and PED patients highlights five findings. First, we document significant functional impairment across all patient groups, particularly NH individuals. Second, we identify the need for multidisciplinary, structured assessment following SARS-CoV-2 infection in all patient groups. Third, we show variations in symptoms and diagnostic features across the patient groups. Fourth, we describe a high burden of specialist input, onward therapy and psychology support in different patient groups in a real-world post-COVID clinical service. Fifth, we identify factors which could contribute to inequitable access to post-COVID-19 care.
Our results underscore the patient and system need for comprehensive measurement and reporting of effects of SARS-CoV-2 infection in all individuals (PH and NH) on health, function and healthcare utilisation, and the profound ramifications for the individuals, their families and communities beyond measures of functional impairment and organ dysfunction. Patients with long COVID have called for ‘recognition, research and rehabilitation’,23 but COVID-19 research and care have focused primarily on acute physiology, management and mortality among hospitalised individuals. It has been previously established that the burden of specific diseases can only be compared across population groups when morbidity in addition to mortality is accurately recorded.24 The burden of long COVID must be documented in this way.
The extent of multimorbidity and functional impairment in long COVID requires cross-specialty, multiprofessional, integrated working, and development of broader clinical expertise, with transferable benefits to other conditions beyond the pandemic,21 rather than siloed, organ-based approaches.19 25 Such integrated care pathways have been effective in other diseases prepandemic26 and could support rapid upskilling and skills transference needed for the wider workforce in post-COVID-19 clinical care. To enable a consistent, holistic approach, we developed structured EHR tools to capture functional and psychological impact. We implemented multispecialty, multidisciplinary meetings to enhance efficiency and share learning, seeking patient feedback from all encounters to improve services. Further analysis of the correlation between symptoms, functional consequences and investigations via data-driven approaches are essential to guide mechanistic studies, treatment approaches and risk stratification. Hospital-based and clinic-based analyses are important given the slow uptake of new long COVID coding in primary care.27
As in other studies, we document severe functional impairment11–13 and significant mortality risk post hospital discharge. We also document severe functional impairment in NH and PED individuals despite low predicted2 and observed mortality risk. UK guidance for posthospital follow-up prioritised those requiring greater levels of organ support or with more severe chest imaging abnormality.20 This focus risks lack of recognition of morbidity in patients with less severe acute respiratory presentations of SARS-CoV-2 infection. The multivariable models suggested that fatigue, brain fog, chest pain and breathlessness were the common factors for continued suboptimal health and not returning to work. Other factors were less consistently included in the models and some were associated in an unexpected direction, which may be due to a real effect or confounding with other symptoms. Underlying mechanisms and management require further research.
Like other studies, we show prolonged variation in symptoms and extent of diagnostic abnormality between different cohorts, suggesting different phenotypes of post-COVID-19 syndrome/long COVID.28 Absence of a specific diagnostic test or biomarker29 makes the balance between adequate investigation of significant symptoms and ‘over-medicalising’ challenging. For example, elevated D-dimer levels triggered frequent CTPA requests, but with low diagnostic yield in NH patients. Exercise testing showed oxygen desaturation most frequently in PH patients, but also in the NH group without clear evidence of lung or heart abnormalities. Echocardiography rarely showed abnormal findings despite frequent use to investigate chest pain and breathlessness. The clinical significance of detectable abnormalities on cMRI in patients with chest pain requires further evaluation.30 Future research must determine whether and how diagnostic abnormalities relate to end-organ effects or mechanisms underlying long COVID.
Patients often needed further specialist opinion and onward referral to community rehabilitation and psychological support, with significant resource implication given such pathways are currently poorly defined. In addition to the ongoing waves of COVID-19 infection, threats of new variants and effects on non-COVID-19 services,2 the ‘long tail’ of the pandemic in terms of long COVID is a concern for policy and health service planning in all countries. Rehabilitation needs are complex and likely to require novel multimodal therapy approaches which integrate psychological support and workforce capacity and capability, which are not currently in place. Expertise in fatigue management, treatment for disordered breathing patterns and programmes to support return to employment are particular priorities. Novel digital self-management solutions could be a useful adjunct but need further evaluation. Although different post-COVID-19 care models are evolving,31 32 the evidence base is minimal, with few, large-scale, pragmatic trials of treatment and rehabilitation. Developing integrated, research-oriented pathways in EHR-enabled health systems could provide a platform for rapid evaluation of investigation, treatment and rehabilitation approaches alongside delivering care, which has been effective in acute COVID-19.33
There are signs of inequitable access to care in our cohort. Although the observed ethnicity of NH patients reflected the catchment population, it contrasted with the disproportionate burden of acute SARS-CoV-2 in ethnic minorities. NH patients were also less likely to live in areas of deprivation and may have had easier access to referral. Varying referral rates by primary care provider were seen, requiring further investigation to understand contributing factors. Underdiagnosis of long COVID has been described in primary care in England.32 NH patients had delayed referral. Consideration should be given to proactive follow-up of patients managed in the community, particularly given the severity of illness in patients referred. Follow-up strategies should also account for the needs of frail, elderly, comorbid and hard-to-reach patients, who were under-represented in our cohort.
Our findings have several limitations. We report outcomes from a single centre, unlikely to be representative of the whole UK population. As post-COVID-19 clinics are established around the UK and in other countries, prospective data collection and comparison will enable variations to be investigated. We used subjective symptom and functional status data, which may have self-reporting bias. We report real-world, clinical findings and therefore data regarding premorbid status, baseline characteristics, investigations and follow-up (particularly for patients with persistent disease for 12 months or more) are more limited than in dedicated research cohorts. NH patients were self-selecting while PH patients were included as part of routine follow-up. This selection bias precludes both making precise estimates of the burden of long COVID among NH patients and also making comparisons between NH patients and other patient groups. In our basic logistic regression models, we modelled age as a linear variable, which may have obscured true non-linear effects of age in these models. Our models were based on cross-sectional data at presentation to the clinic. We did not include a control population. Although it is possible that a proportion of patients would present with ‘medically unexplained symptoms’,34 we only included patients referred to the post-COVID-19 assessment service with clinical or serological confirmation of SARS-CoV-2 infection. Moreover, there is evidence that long-term symptoms are significantly higher in individuals who are PCR-positive for SARS-CoV-2, compared with PCR-negative individuals,35 and higher in individuals with long COVID compared with postinfluenza.36
Our findings have several implications for research and policy. First, we identified differences in the longer-term effects of SARS-CoV-2 infection in PH versus NH individuals, supporting the existence of a number of different phenotypes of long COVID. Further correlation of symptom clusters, functional impact and diagnostic information is required to better define phenotypes and to inform studies of underlying mechanisms and potential treatments. Second, a wide range of patient-reported outcome measures were required to capture the impact of long COVID on individuals. Development of a validated clinical assessment tool will further enable investigation of the natural history of the condition across larger data sets and differing EHR systems. Third, we identified the need for a novel model of rehabilitation, incorporating psychological support and urgent solutions to address the current workforce shortfalls. Fourth, the multisystem nature of the condition will require broadening of clinical expertise both within primary and secondary care and effective integrated pathways. Finally, given the severity of illness documented in all patient groups, our analysis supports the need for swift, equitable access to assessment for all patients suffering from ongoing illness after SARS-CoV-2 infection, as well as effective infection suppression policy for all.
Post-COVID-19 morbidity can be severe, regardless of severity of acute illness, and the scale of healthcare utilisation and inability to return to employment represent a major burden to individuals, healthcare and welfare systems, and economies. Definition of long COVID phenotypes and development and evaluation of diagnostic, treatment and rehabilitation approaches are urgently required. Dissemination of clinical expertise in the management of post-COVID-19 complications needs to occur across integrated care systems and policy change is required to improve equity of access by individuals to appropriate levels of care and support.
Patient and public involvement
Patient views were incorporated in the development of the clinical pathways since inception, and two patients have been involved in the writing and review of the final manuscript (EA and LH). The views expressed by patient groups in meetings attended by MH and TEH (eg, NHS England’s Long COVID Taskforce, Department of Health and Social Care’s Long COVID Round Table) informed the study objectives and design, and through EA and LH the views of wider patient groups (ie, LongCOVIDSOS and UKDoctors#Longcovid) were also incorporated.
Footnotes
Contributors: MH has led the UCLH post-COVID-19 service since inception, with TEH. MM, RL, HAR, RB, MZ, PMcN, EW, RE, AC, EB, PM and ED provided clinical support to the service. MH, TEH and AB wrote the first and final drafts of the manuscript. JP conducted the analyses, with input from MH, SC and AB, and statistical input from HM-D and GP. All authors provided critical feedback on earlier and final drafts of the manuscript. AB is guarantor for the study.
Funding: The UCL/UCLH Biomedical Research Centre part-funded two research fellows who were involved in the clinical service delivery. The clinic was based at UCLH. AB, TEH, MZ, GP, EA, LH, EW and MH have received funding from the NIHR (LT2-0043) for the STIMULATE-ICP study.
Competing interests: AB has received research grants from AstraZeneca, unrelated to this work. All other authors report no relevant conflicts of interest.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
This study was confirmed to be a service evaluation, and ethical approval was waived, by UCLH’s Research and Development and Information Governance Directorates as part of the UCLH Data Access Committee set up in response to the COVID-19 pandemic (https://www.uclhospitals.brc.nihr.ac.uk/clinical-research-informatics-unit/data-explorer), following UK COVID-19 guidelines for use of patient data (https://www.hra.nhs.uk/covid-19-research/guidance-using-patient-data/).
References
- 1.Chen J, Wu J, Hao S, et al. Long term outcomes in survivors of epidemic influenza A (H7N9) virus infection. Sci Rep 2017;7:17275. 10.1038/s41598-017-17497-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banerjee A, Pasea L, Harris S, et al. Estimating excess 1-year mortality associated with the COVID-19 pandemic according to underlying conditions and age: a population-based cohort study. Lancet 2020;395:1715–25. 10.1016/S0140-6736(20)30854-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Norton A, Olliaro P, Sigfrid L, et al. Long COVID: tackling a multifaceted condition requires a multidisciplinary approach. Lancet Infect Dis 2021. :;21:00043–8. Feb 3. 10.1016/S1473-3099(21)00043-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oronsky B, Larson C, Hammond TC. A review of persistent post-COVID syndrome (PPCS). Clin Rev Allergy Immunol 2021;20:1–9. 10.1007/s12016-021-08848-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Institute for Health and Care Excellence . COVID-19 rapid guideline: managing the long-term effects of COVID-19, 2020. Available: https://www.nice.org.uk/guidance/ng188 [PubMed]
- 6.Long COVID: let patients help define long-lasting COVID symptoms. Nature 2020;586:170. 10.1038/d41586-020-02796-2 [DOI] [PubMed] [Google Scholar]
- 7.Office for National Statistics . Prevalence of ongoing symptoms following coronavirus (COVID-19) infection in the UK, 2021. Available: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/prevalenceofongoingsymptomsfollowingcoronaviruscovid19infectionintheuk/1april2021
- 8.Altmann DM, Boyton RJ. Confronting the pathophysiology of long covid, 2020. BMJ opinion. Available: https://blogs.bmj.com/bmj/2020/12/09/confronting-the-pathophysiology-of-long-covid/
- 9.Madhavan S, Bastarache L, Brown JS, et al. Use of electronic health records to support a public health response to the COVID-19 pandemic in the United States: a perspective from 15 academic medical centers. J Am Med Inform Assoc 2021;28:393–401. 10.1093/jamia/ocaa287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carson G, Long Covid Forum Group . Research priorities for long Covid: refined through an international multi-stakeholder forum. BMC Med 2021;19:84. 10.1186/s12916-021-01947-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raman B, Cassar MP, Tunnicliffe EM, et al. Medium-term effects of SARS-CoV-2 infection on multiple vital organs, exercise capacity, cognition, quality of life and mental health, post-hospital discharge. EClinicalMedicine 2021;31:100683. 10.1016/j.eclinm.2020.100683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ayoubkhani D, Khunti K, Nafilyan V, et al. Post-covid syndrome in individuals admitted to hospital with covid-19: retrospective cohort study. BMJ 2021;372:n693. 10.1136/bmj.n693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evans RA, McAuley H, PHOSP-COVID Collaborative Group . Physical, cognitive and mental health impacts of COVID-19 following hospitalisation – a multi-centre prospective cohort study. Medrxiv. 10.1101/2021.03.22.21254057v1 [DOI] [Google Scholar]
- 14.Huang C, Huang L, Wang Y, et al. 6-Month consequences of COVID-19 in patients discharged from Hospital: a cohort study. Lancet 2021;397:220–32. 10.1016/S0140-6736(20)32656-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature 2021;594:259–64. 10.1038/s41586-021-03553-9 [DOI] [PubMed] [Google Scholar]
- 16.Lund LC, Hallas J, Nielsen H, et al. Post-Acute effects of SARS-CoV-2 infection in individuals not requiring hospital admission: a Danish population-based cohort study. Lancet Infect Dis 2021;21:1373–82. 10.1016/S1473-3099(21)00211-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.NHS . NHS to offer ‘long covid’ sufferers help at specialist centres, 2020. Available: https://www.england.nhs.uk/2020/10/nhs-to-offer-long-covid-help/
- 18.Park S, Elliott J, Berlin A, et al. Strengthening the UK primary care response to covid-19. BMJ 2020;370:m3691. 10.1136/bmj.m3691 [DOI] [PubMed] [Google Scholar]
- 19.Ladds E, Rushforth A, Wieringa S, et al. Developing services for long COVID: lessons from a study of wounded healers. Clin Med 2021;21:59–65. 10.7861/clinmed.2020-0962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.British Thoracic Society . British thoracic society guidance on respiratory follow up of patients with a clinico-radiological diagnosis of COVID-19 pneumonia, 2020. [Google Scholar]
- 21.Dennis A, Wamil M, Alberts J, et al. Multiorgan impairment in low-risk individuals with post-COVID-19 syndrome: a prospective, community-based study. BMJ Open 2021;11:e048391. 10.1136/bmjopen-2020-048391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008;61:344–9. 10.1016/j.jclinepi.2007.11.008 [DOI] [PubMed] [Google Scholar]
- 23.Wise J. Long covid: who calls on countries to offer patients more rehabilitation. BMJ 2021;372:n405. 10.1136/bmj.n405 [DOI] [PubMed] [Google Scholar]
- 24.Lopez AD, Murray CC. The global burden of disease, 1990-2020. Nat Med 1998;4:1241–3. 10.1038/3218 [DOI] [PubMed] [Google Scholar]
- 25.Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med 2021;27:601–15. 10.1038/s41591-021-01283-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Plishka CT, Rotter T, Penz ED, et al. Effects of clinical pathways for COPD on patient, professional, and systems outcomes: a systematic review. Chest 2019;156:864–77. 10.1016/j.chest.2019.04.131 [DOI] [PubMed] [Google Scholar]
- 27.Walker AJ, The OpenSAFELY collaborative . Clinical coding of long COVID in English primary care: a federated analysis of 58 million patient records in situ using OpenSAFELY. medrxiV 2021. 10.1101/2021.05.06.21256755v2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.NIHR . Living with covid-19. a dynamic review of the evidence around ongoing covid-19 symptoms (often called long covid), 2020. Available: https://evidence.nihr.ac.uk/themedreview/living-with-covid19
- 29.Sudre CH, Murray B, Varsavsky T, et al. Attributes and predictors of long COVID. Nat Med 2021;27:626–31. 10.1038/s41591-021-01292-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joy G, Artico J, Kurdi H. Prospective case-control study of cardiovascular abnormalitiesCase-Control Study of Cardiovascular Abnormalities 6 months following mild covid-19 in healthcare workersMonths Following Mild COVID-19 in Healthcare Workers. JACC Cardiovasc Imaging 2021;14. 10.1016/j.jcmg.2021.04.011. [Epub ahead of print: 05 May 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duncan E, Cooper K, Cowie J, et al. A national survey of community rehabilitation service provision for people with long Covid in Scotland. F1000Res 2020;9:1416. 10.12688/f1000research.27894.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parkin A, Davison J, Tarrant R, et al. A multidisciplinary NHS COVID-19 service to manage Post-COVID-19 syndrome in the community. J Prim Care Community Health 2021;12:215013272110109. 10.1177/21501327211010994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.RECOVERY Collaborative Group, Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med 2021;384:693–704. 10.1056/NEJMoa2021436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scott MJ, Crawford JS, Geraghty KJ, et al. The 'medically unexplained symptoms' syndrome concept and the cognitive-behavioural treatment model. J Health Psychol 2021:13591053211038042. 10.1177/13591053211038042 [DOI] [PubMed] [Google Scholar]
- 35.Sudre CH, Murray B, Varsavsky T, et al. Attributes and predictors of long COVID. Nat Med 2021;27:626–31. 10.1038/s41591-021-01292-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taquet M, Dercon Q, Luciano S, et al. Incidence, co-occurrence, and evolution of long-COVID features: a 6-month retrospective cohort study of 273,618 survivors of COVID-19. PLoS Med 2021;18:e1003773. 10.1371/journal.pmed.1003773 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjresp-2021-001041supp001.pdf (460.1KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.