Abstract
Ginseng has long been considered as an herbal medicine. Recent data suggest that ginseng has anti-inflammatory properties and can improve learning- and memory-related function in the central nervous system (CNS) following the development of CNS neuroinflammatory diseases such as Alzheimer’s disease, cerebral ischemia, and other neurological disorders. In this review, we discuss the role of ginseng in the neurovascular unit, which is composed of endothelial cells surrounded by astrocytes, pericytes, microglia, neural stem cells, oligodendrocytes, and neurons, especially their blood-brain barrier maintenance, anti-inflammatory effects and regenerative functions. In addition, cell-cell communication enhanced by ginseng may be attributed to regeneration via induction of neurogenesis and angiogenesis in CNS diseases. Thus, ginseng may have therapeutic potential to exert cognitive improvement in neuroinflammatory diseases such as stroke, traumatic brain injury, multiple sclerosis, Parkinson’s disease, and Alzheimer’s disease.
Keywords: central nervous system, ginseng, neuroinflammation, neurovascular unit, regeneration
Graphical abstract
1. Introduction
Ginseng is a perennial plant, belonging to the genus Panax and family Aralliaceae. Three species are more usually applied among 11 different species of ginseng: (1) Panax ginseng, recognized as ginseng or Korean ginseng, (2) Panax notoginseng, known as Chinese notoginseng, and (3) Panax quinquefolius, identified as American ginseng [1]. Currently, approximately over 150 saponin monomers, ginsenosides, have been extracted and isolated from ginseng; pharmacological features of ginsenosides, which are active compounds, have also been tested [2]. Ginsenosides are categorized into three groups based on their structural features: the panaxadiol group, which contains Rb1, Rb2, Rb3, Rc, Rd, Rg3, Rh2, Rs1, Rs3 and compound K; the panaxatriol group, which includes Re, Rf, Rg1, Rg2, and Rh1; and the oleanolic acid group, which comprises Ro (Reviewed in [3]).
The effects of ginseng on pathophysiological conditions have been reported to be beneficial but sometimes some adverse effects may possibly stem based on the dose of ginseng, type of compound within ginseng, its bioavailability, and natural compound-drug interactions [4]. Therefore, ginseng or its isolated components should be used carefully for the treatment of central nervous system (CNS) diseases even though ginseng has been shown to have beneficial effects on neurodegenerative diseases [5].
In this review, we focused on the positive effects of ginseng and ginsenosides on various CNS injuries. Ginseng and ginsenosides have been widely used in traditional oriental medicine because of several medicinal effects such as anti-inflammatory, anti-aging, and anti-tumorigenic [[5], [6], [7], [8]]. In the CNS, learning and memory functions are enhanced by several ginsenosides and red ginseng [9,10]; however, the exact mechanism by which ginseng improves cognitive functions has not been demonstrated. Here, we have attempted to explain the underlying mechanism of cognitive improvement by ginseng through focusing on cellular components of neurovascular unit. Neurovascular unit in the CNS is a coordinated system constituted by vascular (pericytes and endothelial cells), neuronal (neurons and neural stem cells (NSCs)), glia (oligodendrocytes, astrocytes, and microglia) cells, and the extracellular matrix [11]. To better understand the pharmacological effects of ginseng, the role of ginseng in various neurovascular cells must be dissected. Thus, we have demonstrated the possible effects of ginseng on cell-cell communication in the CNS, which is inhibited in relation to regeneration after neuroinflammatory diseases.
2. Role of ginseng in the CNS repair processing
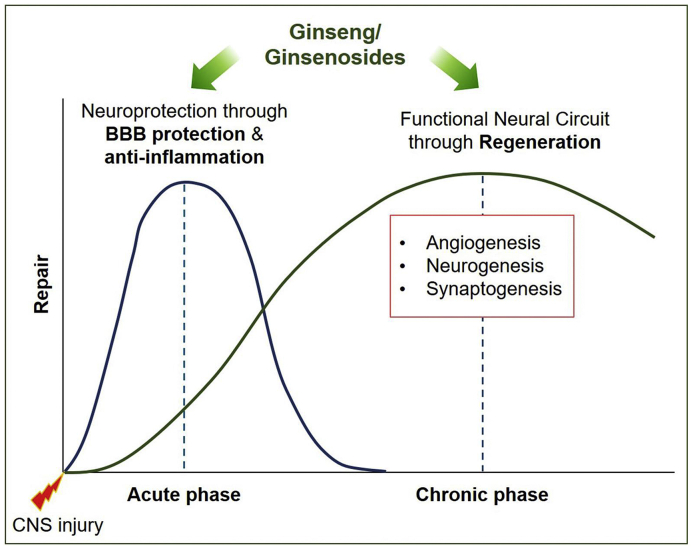
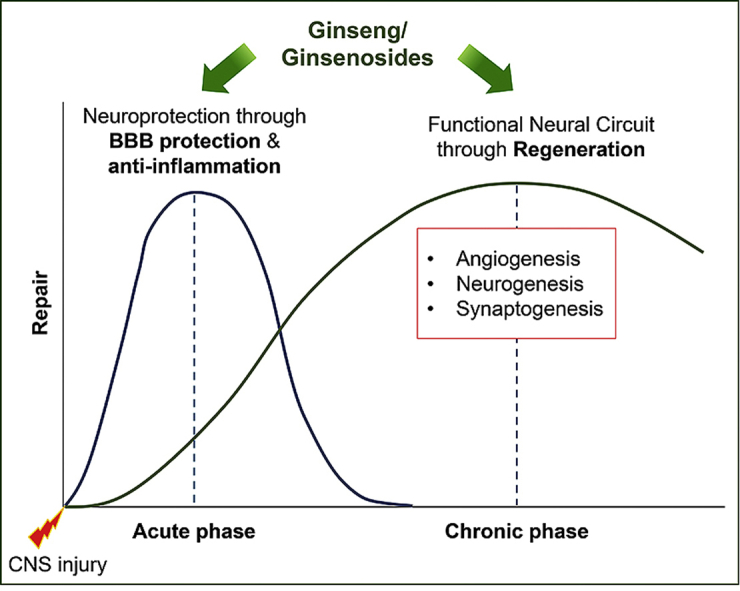
Neuroinflammation is a popular pathological feature that is associated with many neurodegenerative diseases such as stroke, traumatic brain injury (TBI), multiple sclerosis, Parkinson’s disease (PD), and Alzheimer’s disease (AD) [12,13]. Such inflammatory diseases are related to several damage-associated inflammatory disorders but without microbial pathogens [14]. In the acute phase of such neuroinflammatory diseases, local injury to the brain tissue often leads to vascular damage, breakdown blood-brain barrier (BBB), hypoxia/ischemia, death of pericytes, reactive glia formation, axonal loss, and demyelination [15,16]. Then, the modest recovery phase contributes to reorganization of function using remaining intact circuits via the intrinsic pathway and regeneration through synthesis of new endothelial precursor cells and NSCs, resulting in the induction of angiogenesis and neurogenesis [17,18]. Hence, potential therapeutic drugs may require the ability to reduce inflammation at the acute phase, and to increase regeneration at the chronic phase of neuroinflammatory diseases (Fig. 1). In this review, we suggest that ginseng and ginsenosides can act as therapeutic agents for CNS injuries by stimulating both steps (Fig. 1). Extensive research has supported the beneficial effects of ginseng and ginsenosides on the reduction of neuroinflammation and the induction of regeneration.
Fig. 1.
Schematic figure demonstrates the possible therapeutic mechanisms of ginseng and ginsenosides. At the early phase of central nervous system (CNS) injury, ginseng/ginsenosides may facilitate neuroprotection through anti-inflammatory effects. Then, at the late phase of CNS injury, ginseng/ginsenosides-mediated recovery of cellular communication may enhance the formation of functional neural circuit through regeneration, such as angiogenesis, neurogenesis, and synaptogenesis.
2.1. Blood-brain barrier by ginseng
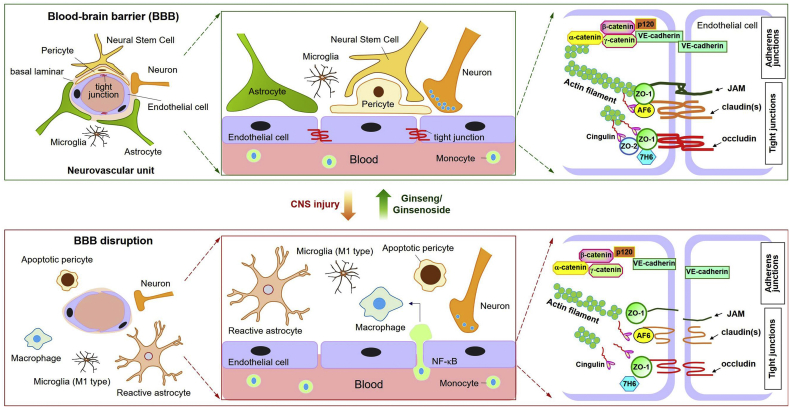
The cerebral microvascular barrier (or blood-brain barrier (BBB)) segregates the CNS from the systemic circulation. Endothelial tight junctions and adherence junctions play critical roles in BBB function. These junctions are regulated by surrounding cells (i.e. pericytes and astrocytes) and extracellular signaling pathways [[19], [20], [21]]. The association of brain microvessels with surrounding cells such as pericytes, astrocytes, microglia, NSCs, and neurons results in formation of functional ‘neurovascular units’; these intercellular networks enhance BBB functional characteristics [22,23] (Fig. 2). Tight and adherence junctions form a circumferential zipper-like seal between adjacent endothelia (Fig. 2), blocking entry of circulating hydrophilic substances with a molecular weight exceeding 180 Da [24]. Tight junction maintenance is regulated by three major transmembrane proteins: claudins, occludins, and junction adhesion molecules. These proteins are associated with cytoplasmic scaffold proteins such as zonula occludens (ZO-1, -2, and -3), which in turn are anchored to the actin cytoskeleton [20] (Fig. 2). Several tight junction accessory proteins such as cingulin, 7H6, and afadin have been identified; these also regulate vascular permeability [20].
Fig. 2.
Blood-brain barrier (BBB) integrity is maintained by ginseng/ginsenosides following central nervous system (CNS) injury. The neurovascular unit refers to functional coupling of brain endothelia with other local cells (e.g. pericytes, astrocytes, microglia, neural stem cells, and neurons) and the extracellular matrix. Endothelial tight junctions and integral proteins are involved in regulation of paracellular permeability. Following CNS injury, expression patterns - including site and level - of tight junction and integral proteins become altered. Damaged endothelia exhibit markedly increased NF-κB activation, leading to infiltration of monocytes into the brain parenchyma, where they become activated macrophages. Additionally, concomitant disruption of functional cellular associations is accompanied by breakdown of BBB integrity. Damaged brain parenchyma exhibits activated astrocytes and M1-polarized microglia, apoptotic pericytes, and neurotransmitter-degraded neurons. Abbreviations: JAM, junction adhesion molecule; ZO-1, zonula occluden-1; AF6, afadin 6; VE-cadherin, vascular endothelial cadherin; NF-κB, nuclear factor κ-light-chain-enhancer of activated B cells.
Ischemia/reperfusion injury-mediated release of pro-inflammatory cytokines (e.g. tumor necrosis factor α (TNFα), interleukin-1β (IL-1β)) and other soluble mediators (e.g. vascular endothelial growth factor (VEGF)) trigger paracellular permeability and tight junction disruption [25,26]. It is in this manner that BBB tight junctions are disrupted during neuroinflammatory diseases such as ischemic stroke, TBI, PD, and AD, which result in infiltration of monocytes into the brain parenchyma, where they become activated macrophages [20,27]. Endothelial damage, pericyte apoptosis, glial activation, and inflammatory cytokines exacerbate central nervous system (CNS) neurodegeneration via uncoupling normal cell-cell communication [13] (Fig. 2). Ginseng-mediated preservation of BBB structure and function during neuroinflammatory disease is due in part to anti-inflammatory effects, thereby preventing detrimental (neurodegenerative) signaling cascades.
2.2. Anti-inflammation by ginseng
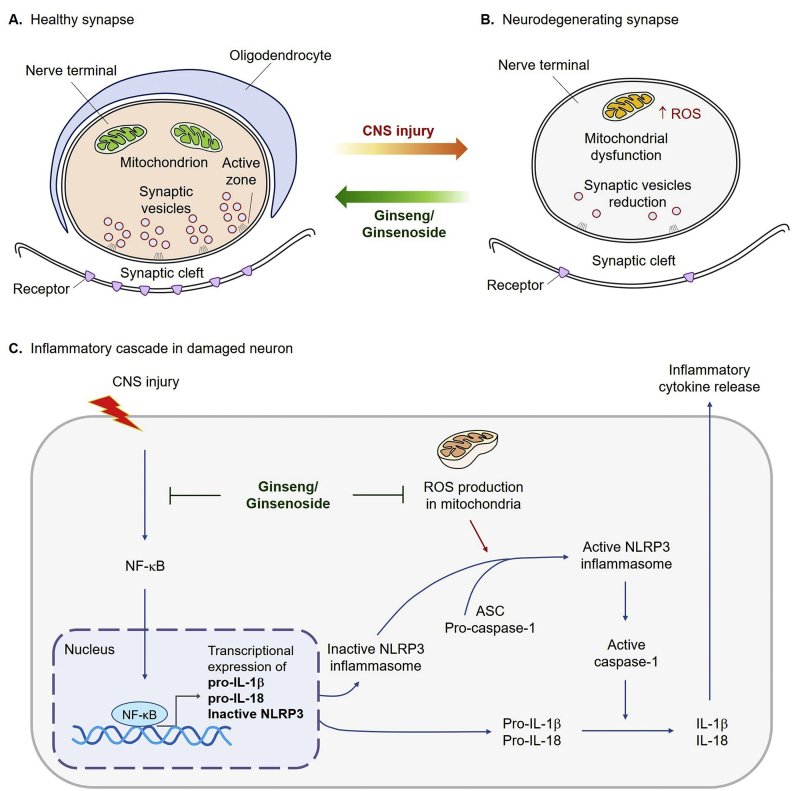
Since 2010, much increased studies showing the anti-inflammatory effects of ginseng and ginsenosides on various cell and animal models have been published (Reviewed in [6]). Excessive mitochondrial reactive oxygen species (ROS) triggers inflammatory cascades [28], which are abolished by ginseng and ginsenosides in the peripheral nervous system and CNS [29]. In CNS, synapses are packed with the mitochondria at presynaptic terminal, and the damaged synaptic mitochondria produces excessive ROS compared with that of the healthy mitochondria (Fig. 3A–B), leading to the activation of inflammatory responses (Fig. 3C). The nucleotide-binding leucine-rich repeat (LRR)-containing (NLR; also known as NOD-like receptors) proteins have emerged as a key family of sensors and regulators responding to damage-associated molecular patterns that are produced under neuroinflammatory conditions [30]. IL-1β and IL-18 are important mediators of neuroinflammation, and are produced by the inflammasomes [31]. Inflammasomes are multi-protein cytoplasmic complexes that mediate the maturation of IL-1β and IL-18 by activating caspase-1, and are associated with tauopathies in neurodegenerative diseases [30,32].
Fig. 3.
Mitochondrial reactive oxygen species (ROS) triggers neuroinflammation, and this effect is suppressed by ginseng/ginsenosides. (A) At the healthy synapses, mitochondria are packed in presynaptic terminal, synaptic vesicles are clustered at presynaptic release sites (active zone), and transmitter receptors are clustered in the postsynaptic membrane. Nerve terminals are coated with processes of oligodendrocytes. (B) At the neurodegenerating synapses, damaged mitochondria produce ROS and synaptic vesicles are reduced in presynaptic terminal, leading to less transmitter receptors in the postsynaptic membrane. (C) Mitochondrial ROS and neurotoxic materials can exacerbate neuronal functions by inducing inflammation cascades. Ginseng/ginsenosides can block both the NF-κB signaling and excessive mitochondrial ROS generation, which can reduce the production of pro-IL-1β, pro-IL18, and inactive NLRP3. Then, NLRP3-ASC-pro-caspase-1 complex is further processed, and active caspase-1 cleaves pro-IL-1β and pro-IL-18 into mature form of IL-1β and IL-18, respectively. Then, those inflammatory cytokines are released. Abbreviations: IL, interleukin; NLRP3, NOD-, LRR- and pyrin domain-containing 3; ASC, apoptosis-associated speck-like protein containing a CARD; CARD, caspase recruitment domain-containing protein.
Caspase-1 catalyzes more than 70 substrates, including pro-IL-1β and IL-18 into mature IL-1β and IL-18 [32]. In addition, apoptosis-associated speck-like protein containing a caspase recruitment domain-containing protein (CARD) (ASC) dimers mediating caspase-1 activation can also enhance a pro-inflammatory form of cell death called pyroptosis [33]. Several inflammasomes have been identified, including NLRP1 (NOD-, LRR- and pyrin domain-containing 1), NLRP3 and NLR family and CARD domain-containing 4 (NLRC4) inflammasome, which recognizes various danger signals [30]. NLRP3 is activated when it senses proteins such as misfolded or aggregated prion protein, amyloid-β (Aβ), and α-synuclein [30]. Neuroinflammatory diseases have been involved in nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB)-NLRP3-IL-1β axis-mediated inflammation [30]. Absent in melanoma 2 (AIM2) inflammasome distinguishes double stranded DNA, and NLRC4 inflammasome recognizes flagellin [34]. NLRC4 directly associates with pro-caspase-1 through CARD-CARD interactions, leading to autocatalytic processing of pro-caspase-1 to caspase-1 [35]. NLRC4 is involved in non-microbial inflammation, for example, expression of NLRC4 is enhanced in astrocyte-rich regions of the human brain during neuroinflammation [36]. In primary macrophage bone marrow-derived macrophage (BMDM) cells, IL-1β response to LPS and flagellin is ablated in Nlrc4−/− [36].
Macrophages and microglia are among the most activated cell types in CNS inflammatory diseases [37]. Macrophages and microglia are classified into M1, observed in interferon-γ (INF-γ)-mediated activation, or M2 type, induced by the T helper 2 cytokines interleukin-4 (IL-4) and IL-13, according to their phenotypes [38]. Activation of macrophages and microglia can increase the release of neurotoxic substances such as TNFα and nitric oxide (NO), leading to the induction of inflammatory processes in the injured brain region [39]. In the inflammatory phase, ginseng and ginsenosides downregulate the pro-inflammatory cytokines (i.e., TNF-α, IL-1β, and IL-6)) and NF-κB downstream enzymes (i.e., cyclooxygenase-2 (COX-2) and inducible NO synthase (iNOS)), which are related to the activation of M1-polarized macrophages and microglia (reviewed in [6]). Recently, the role of ginseng and ginsenosides (i.e., Rb1, Rg1, Rg3, Rh2, and compound K) in the upregulation of M2-polarized macrophages and microglia has been investigated during the resolution phase of CNS injuries (reviewed in [6]).
In addition, astrocytes are an important cell type for NLRP3- and NLRC4-mediated formation of inflammasomes [36]. Inflammatory astrocytes can be classified as an A1 type, similar to M1 type in macrophages and microglia. On the contrary, A2 reactive astrocytes are beneficial as they encapsulate the injury or seal the damaged BBB by forming a glial scar [40]. Under neuroinflammatory conditions, the A1 type astrocytes demonstrate upregulation of genes that are detrimental to synapses, whereas, in ischemic conditions, the A2 type astrocytes facilitate the expression of the genes beneficial to neuronal survival and growth [40].
Ginsenosides - including Rb1, Rg1, and Rg3 - decrease IL-1β secretion by suppressing NLRP3 inflammasome activation in an endotoxin-injected inflammatory mouse model [8,41]. In addition, anti-inflammatory responses to systemically-administered ginseng have been reported in in vivo CNS injury models [41] as well as in peripheral nervous system injury [42]. More precise effects of ginsenosides on anti-inflammation will be introduced in various CNS cell type.
2.3. Regeneration by ginseng
Regeneration after CNS injury requires angiogenesis, neurogenesis, and synaptic plasticity [43]. VEGF is an angiogenic and neurogenic factor, and a permeability factor [44,45]. Ginsenoside Rg1 promotes cerebral angiogenesis and formation of new vessels from existing ones through the hypoxia-inducible factor-1α (HIF-1α)-VEGF axis in ischemic mice [46]. The upstream signaling pathway of HIF-1α is phosphatidylinositol-3-kinase (PI3K)-protein kinase B (Akt)-mammalian target of rapamycin axis [46]. However, ginsenoside Rh2 inhibits excessive neovascularization and protect vessels from VEGF or toxic molecules [47]. It is thus tempting to speculate that ginseng may possess a dual ability to inhibit excessive neovascularization even as it stimulates angiogenesis in ischemic conditions, via modulation of the HIF-1α-VEGF pathway. However, additional studies on the angiogenic capacity of ginseng are required to confirm or disprove this hypothesis.
Korean Red Ginseng treated (100 mg/kg orally) mice group showed enhanced adult hippocampal neurogenesis, as detected by doublecortin (DCX, migratory mature NSC marker)-5-Bromo-2′-deoxyuridine (BrdU, de novo DNA synthesis marker) double positive cells compared with that of the vehicle-treated mice group [48], suggesting that ginseng may promote normal adult neurogenesis. Adult neurogenesis after neuroinflammatory diseases such as ischemia/reperfusion can be also enhanced by ginseng [49]. During regeneration after CNS injuries, adult neurogenesis occurred in the subventricular and subgranular zones of the postnatal rodent brain [12,15]. In humans, new neurons could be generated in the dentate gyrus of the hippocampus [50]. Saponins induced the BrdU-nestin (immature NSC marker) double positive cells when subjected to oxygen glucose deprivation (OGD) [49]. In addition, Panax notoginseng saponin also promoted in vitro mature neuronal differentiation, as assessed by neuron specific class III β-tubulin detection in OGD-conditioned rat hippocampal postnatal day 1 NSCs [49].
Adult neurogenesis activator combined with brain-derived neurotrophic factor (BDNF) significantly enhanced the cognitive functions, which could mimic the effects of exercise on cognition in an 5xFAD Alzheimer’s mouse model [51]. Similarly, treatment of human NSCs with BDNF and ginsenosides (Rg1 and Rb1) promoted cell survival and neurite outgrowth, and the expression of synaptic plasticity-related proteins [52]. Rg1 is also involved in BDNF-mediated neurogenesis and exhibits antidepressant activity [53,54]. Furthermore, Rg5 was able to ameliorate streptozotocin-induced loss of BDNF-positive cells in the cerebral cortex and hippocampus [55]. Besides angiogenesis and neurogenesis, ginseng and ginsenosides also contribute to neuroprotection during stroke, TBI, multiple sclerosis, PD, and AD [3,56].
3. Functions of ginseng in various cell types in neurovascular unit
In pathophysiological condition, ginseng plays key roles in anti-inflammation and in establishing an environment enriched in neurotrophic factors. In addition, ginseng induces angiogenic and neurogenic growth factors, cytokines, and molecules. In this section, we have discussed the effects of ginseng on each neurovascular cell type in pathophysiological condition. Each cell type also shows heterogeneity, and their functions and characteristics can be altered by cellular communications in glia-neuron interface and vessel-neuron interface.
3.1. Endothelial cells
Anti-angiogenic ginsenoside has been uncovered; however, most research data demonstrate angiogenic capacities of ginsenosides. Rb1 (10 nM) inhibited angiogenesis, which involved the activation of peroxisome proliferator-activated receptor γ and downregulation of microRNA-33, and thereby stimulating pigment epithelial-derived factor in human umbilical vein endothelial cells (HUVECs) [57]. Various ginsenosides (i.e., Rb1, Rb2, Rc, Rd, RF, Rg1, Rg2, Rg3, Rg5, Rh1, or Rh2) were tested to examine their effect on proliferation by [3H]thymidine incorporation in HUVECs [58]. Proliferative ability of Rg1, Rg3, or Rg5 (10 μM or 20 μM) was demonstrated, and Rg5 showed the most prominent effect [58]. Rg5 may activate insulin-like growth factor-1 receptor (IGF-1R) and its downstream signaling cascade such as the PI3K-Akt-endothelial NOS (eNOS) pathway, which is similar to the VEGF-mediated angiogenesis pathway [58]. However, unlike VEGF, Rg5 does not induce vascular inflammation and permeability [58]. Another study demonstrates that a low dose of Rg1 (150 nM) induces the production of VEGF in HUVECs, which is mediated by the PI3K-Akt pathway and β-catenin activation [59].
20(S)-Rg3 (15 μM) facilitates in vitro angiogenesis through the activation of the PI3K-Akt-eNOS pathway in HUVECs; in contrast, the stereoisomer 20(R)-Rg3 does not have the ability to activate that signaling axis [60]. 20(S)-Rg3 (10 μg/ml) can inhibit the mitochondrial caspase pathway, leading to enhanced survival of EC under serum starvation in HUVECs [61]. Ginsenoside F1 (GF1, 20, and 40 μM) promotes in vitro, ex vivo, and in vivo angiogenesis by activating the IGF-1/IGF-1R pathway [62]. The pretreatment with GF1 (16 μM) diminishes oxidized low-density-lipoprotein-induced HUVEC cell death, and ameliorates the inflammatory responses by suppressing the NF-κB translocation from cytoplasm to nucleus [63]. GF1 (10 μM) or Rh1 (10 μM) inhibited the VEGF-mediated change in cell permeability in HUVECs and in vivo vascular leakage through NR4A1 (also known as NAK-1, NGFI-B, Nur77, TIS1, and TR3), a member of the nuclear receptor superfamily 4 group A [64]. GF1 and Rh1 may suppress VEGF-mediated TNFα signaling via NF-κB [64]. Taken together, some ginsenosides may protect endothelial cells against vascular permeability and induce moderate angiogenesis.
3.2. Astrocytes
Astrocytic endfeet processes communicate with the blood vessels, regulating the BBB formation and angiogenesis in the CNS [65]. Amounts of Ca2+ in the astrocytic endfeet processes can modulate vascular tone through big conductance potassium channels in the mouse brain slices [66,67], and consequently supporting O2 supply to injured ischemic tissues. Activation of acute heme oxygenase-1 (HO-1) can be observed in injured ischemic tissue, contributing to endogenous anti-inflammation and bioenergetic regeneration in CNS repair [67,68].
In rat primary astrocytes, Rh1 (300 μM) abolishes H2O2-induced ROS production and increases the expression of phase II antioxidant enzymes such as HO-1, catalase, superoxide dismutase 2 (SOD2), and nicotinamide adenine dinucleotide phosphate (NAD(P)H):quinone oxidoreductase 1 (NQO1) to regulate the nuclear factor erythroid 2-related factor 2 (Nrf2)/antioxidant response element (ARE) signaling pathway [69]. Similarly, protective effects of ginseng root extract (0.1, 0.5, or 1 mg/ml) on the antioxidant enzymatic activities of astrocytes including glutathione content (reduced GSH and oxidized GSSG forms) can be observed during exposure to H2O2 [70]. Ginseng root extract influences the activity of antioxidant defense enzymes such as catalase, SOD, glutathione peroxidases and glutathione reductase on glutathione content (GSH and GSSG and red/ox index) and intracellular ROS formation [70].
Similar to the in vivo AD model, treatment of in vitro rat primary brain astrocytes with Aβ plus Rh2 (1 μM) reversed the Aβ-mediated growth suppression of astrocytes [71]. Since astrocytes act as a bridge between blood vessels and neurons [72], ginseng- and ginsenosides-induced increase in healthy astrocytes may facilitate the crosstalk among neurovascular cells, and thus promote angiogenesis, neurogenesis, and synaptogenesis.
3.3. Pericytes
Pericytes located in the brain and retina play a key role in the BBB protection, angiogenesis, and neurogenesis, which promotes neurological recovery [13,15]. GF1, isolated from Panax notoginseng saponins, increases the proliferation of HUVECs and human brain microvascular endothelial cells at 20 μM and 40 μM concentrations [62]. The angiogenic effect of GF1 is confirmed in the in vivo MCAO model [62]. GF1 also increases pericyte recruitment to the endothelial cells, as assessed by the in vitro Matrigel-based co-culture assay [62].
Cellular network formation between pericytes and endothelial cells is facilitated by multiple ligands and their receptors such as stromal-derived factor-1α (SDF-1α)/C-X-C chemokine receptor type 4 (CXCR4), platelet-derived growth factor B (PDGF-B)/PDGF receptor β (PDGFRβ), angiopoietin 1 (Ang1)/tyrosine-protein kinase receptor 2 (Tie-2), and heparin-binding epidermal growth factor-like growth factor (HB-EGF)/Erb-B2 receptor tyrosine kinase 2 (ErbB) (Reviewed in [73]). The crosstalk between pericytes and endothelial cells through the SDF-1α/CXCR4, PDGF-B/PDGFRβ, Ang1/Tie-2 and HB-EGF/ErbB pathway may be assisted by ginseng and ginsenosides; however, few studies have examined the effects of cellular crosstalk in CNS vessels on neuroinflammatory diseases. Moreover, comparing other cell types, the protective role of ginseng in healthy pericytes has not been well investigated. Loss of pericytes reveals AD-like neurodegeneration pathogenic steps in APP overexpressing mice [74]. Thus, further studies are necessary to elucidate the mechanisms by which ginseng and ginsenosides influence the functions of pericytes, will represent a novel therapeutic target to for the treatment of neuroinflammatory diseases.
3.4. Neural stem cells and neuron
Protection of BBB exerts neuroprotection and boosts cellular crosstalk [22]. NSCs followed by CNS injury may exhibit crosstalk with pericytes by localizing in close proximity of the brain pericytes, and this effect can be accelerated by HO-1 byproducts, consequently leading to neurogenesis [15]. Since ginseng and ginsenosides can induce HO-1 [6], induced HO-1 may further induces neurogenesis. Rg1 increases dendritic spine number and hippocampal neurogenesis after chronic mild stress, which may be due to activation of the CREB (cAMP response element-binding protein)/BDNF signaling pathway [53]. Rg1 and Rb1 increases proliferation and differentiation of neural progenitor cells in dentate gyrus of hippocampus of normal adult mice and global ischemia model in gerbils [75]. Rg1 increases BDNF expression and antioxidant enzyme expression [76], possibly affecting both regeneration and anti-inflammation. Treatment of human NSCs with Rg1 and Rb1 promotes cell survival and neurite outgrowth and the expression of synaptic marker proteins microtubule associated protein-2 (MAP-2) [52].
In embryonic (E14.5) rat primary neural progenitor cells, 300 μM tert-butylhydroperoxide (t-BHP)-induced oxidative injury can be diminished by Rb1 (10 μM), but not by Rd, Rg1, and Re [77]. Rb1 may protect neural progenitor cells against oxidative stress by enhancing the anti-oxidant molecules such as HO-1 through Nrf2-mediated transcription [77]. The mRNA expression of other phase II enzymes such as SOD2, NQO1 and CAT is unchanged by Rb1 [78]. Neuroprotective role of Rb1 is not associated with proliferation and differentiation of neural progenitor cells, because direct treatment of neural progenitor cells with Rb1 cannot enhance neuronal and glial differentiation as well as proliferation [78]. Interestingly, combinatory treatment of 10 ng/ml BDNF with ginsenosides (5 μM Rg1 and 5 μM Rb1) synergistically induces MAP-2 and synapsin proteins in human NSCs [52], suggesting that ginsenosides promote BDNF-mediated NSCs differentiation into mature neurons strengthened by synapse connections.
Ginseng and ginsenosides exert beneficial effects on neuronal survival, axonal outgrowth, and synaptic plasticity that follows neuroinflammatory diseases. Ginseng extracts partly inhibited the cyclosporine-induced tau phosphorylation in SY-5Y cells [79]. Rb1 reduced tau phosphorylation at Thr205, Ser396, and Ser404 induced by Aβ(25-35) and Aβ(1-42) [80]. When SK-N-SH cells (human neuroblastoma cell) were transfected with Swedish amyloid precursor protein (SweAPP), Rg3 reduced the levels of Aβ(1-40) and Aβ(1-42), probably because of an increase in the gene expression of neprilysin [81]. Neprilysin is the rate-limiting enzyme in Aβ degradation in the brain [82].
In adult primary hippocampal neuronal cells, 1 μM Rb1 was co-treated with high glucose (50 mM), and Rb1 was found to protect neurons against high glucose-mediated endoplasmic reticulum (ER) stress via downregulation of the glucose synthase kinase 3β or protein kinase RNA-like ER kinase-mediated C/EBP homologous protein pathway [83]. In cultured AD cortical neurons, Rb1 and its metabolic compounds M1 increased the axonal outgrowth, which appeared to be the maturation step in the reorganization process and is involved in synaptic plasticity [84]. Rg1 activated the PI3K/Akt pathway, leading to neuronal survival against 6-hydroxydopamine (6-OHDA)-induced cell death [85]. In addition, Rg1 modulated 6-OHDA-induced mitochondria dysfunction by enhancing the mitochondria membrane potential in human neuroblastoma SK-N-SH cells [86]. Hence, the observed effects of ginseng on axonal outgrowth, neurite extension, and synaptic plasticity may be involved in the underlying mechanism by which ginseng improves the regeneration of neural circuits after CNS diseases.
3.5. Microglia and macrophage
Immune reactions in the neuroinflammatory diseases that are mediated by microglia and macrophages involve the release of cytokines that can inhibit extensive regrowth of neurons and induce BBB protection [37]; therefore, inhibition of excessive neurotoxic cytokine release from microglia and macrophages may contribute to neurovascular repair processes.
Korean Red Ginseng extracts have the ability to inhibit the activation of both NLRP3 and AIM2 inflammasome in BMDM cells [87]. Rg6 (10, 20, or 50 μM) may augment M2 macrophages by reducing the expression of TNFα in LPS-induced inflammatory conditions in BMDMs, and by inducing the secretion of IL-10 in mice administered with 30 mg/kg LPS 2 h after administering 20 mg/kg Rg6 intraperitoneally [88]. Rg3 enhanced the microglial Aβ uptake, internalization, and digestion. The target for Rg3 appeared to be macrophage scavenger receptor type A [89]. In macrophage cells such as THP-1 (human cell) and RAW264.7 (mouse cell), Rg3 (10 μg/ml) significantly reduced the formation of NLRP3 inflammasome and consequent secretion of IL-1β in LPS/nigericin or LPS/ATP treated cells [41].
In murine N9 microglia cells, two protopanaxadiols (i.e., 0.1-100 μM Rd or Rb2) did not affect the lipopolysaccharide (LPS)-mediated NO production, whereas two protopanaxatriols (10 μM Rg1 or 10 μM Re) reduced the LPS-induced production of NO [90]. In the same cells, all four ginsenosides, Rg1, Re, Rb2, or Rd, could reduce the LPS-induced secretion of TNF-α and phosphorylation-based activation of extracellular signal-regulated kinase (ERK) [90]. Rf (50 μM) reduced the LPS-induced increase in the mRNA expression of IL-1β, IL-6, and iNOS [91]. Rg5:Rk1 in a 1:1 weight ratio mixture of ginsenosides that attenuated the LPS-induced production of NO via downregulation of NF-κB-p38 MAPK-Signal Transducer and Activator of Transcription 1 axis in RAW264.7 macrophages [92].
4. Role of ginseng/ginsenosides in neuroinflammatory diseases
Reactive glia cells secrete various inflammatory cytokines that are detrimental to the neighboring cells, such as neurons and NSCs. To restore the neural circuit function after CNS injuries, reestablishing cell-cell communication may help to reconstruct neural circuits through the stimulation of angiogenesis, neurogenesis, oligodendrogenesis, and synaptogenesis [12,13]. After neuroinflammatory diseases, extended and variable phases of adaptive and maladaptive reactions occur in damaged tissue to reorganize and recover [93]. In this section, we discuss the therapeutic effects of ginseng and ginsenosides on neuroinflammatory diseases such as stroke, TBI, multiple sclerosis, PD, and AD (Table 1). Potential therapies for CNS injuries should combine multifactorial effects on acute injury and inflammation along with prolonged multicellular recovery phenomenon [94].
Table 1.
Therapeutic Effects of Ginseng/Ginsenoside on Neuroinflammatory Diseases in the Brain
| Disease model | Ginseng/Ginsenoside | Location (Neurovascular Unit) | Species | Therapeutic effects | Reference |
|---|---|---|---|---|---|
| Stroke | Rb1, Rg3 | Brain | Rat | Neuroprotection Anti-inflammation |
Cheng Z et al, 2019 Shi YH et al, 2020 |
| Rb1 | Brain | Rat | Improved behavioral abilities BBB protection Neuroprotection |
Shi YH et al, 2020 | |
| Rg3 | Brain | Rat | Improved cerebral blood flow | Tian J et al, 2005 | |
| Rd | Brain | Rat | Anti-inflammation Improved behavioral ability |
Ye R et al, 2011 | |
| Traumatic brain injury (TBI) | Rb1 | Brain | Rat | Neuroprotection | Chen W et al, 2016 |
| Saponin | Brain (hippocampus) | Rat | Neuroprotection | Ji YC et al, 2005 | |
| Multiple Sclerosis | Rd | Brain | Mouse | Improved behavioral ability BBB protection Increased neurotrophic factors |
Zhu D et al, 2014 |
| Parkinson’s disease (PD) | Rg1, Rd | Brain (Neuronal cell) | Mouse | Neuroprotection | Chen XC et al, 2005 |
| Rg1 | Brain (nigrostriatum) | Rat | Neuroprotection | Xu L et al, 2009 | |
| Rg1 | Brain (Astrocyte, Microglia, Neuron) | Mouse | BBB protection Anti-inflammation Neuroprotection |
Heng Y et al, 2016 | |
| Rb1 | Human neuroblastoma cell | Human | Neuroprotection | Ardah MT et al, 2015 | |
| Alzheimer’s disease (AD) | Rg1 | Brain | Mouse | Anti-inflammation | Chen Y et al, 2020 |
| Re, Rg1, Rg3 | Brain | Mouse (human APP overexpression) | Aβ reduction | Shi YH et al, 2020 | |
| Rg1 | Brain (hippocampus) | Rat | Improved behavioral ability Neuroprotection |
Quan Q et al, 2013 | |
| Rg1 | Brain (hippocampus) | Mouse | Improved behavioral abilities Aβ reduction |
Shi YQ et al, 2010 | |
| Ginseng extract | Brain | Mouse | Enhanced memory Neuronal plasticity Anti-oxidation |
Zhao H et al, 2009 | |
| Rg5 | Brain (Cerebral cortex, hippocampus) | Rat | Improved behavioral abilities Aβ reduction |
Chu S et al, 2014 |
4.1. stroke
Protective effects of ginseng extracts or ginsenosides on hypoxia/ischemic stroke model have been reported [3]. In middle cerebral artery occlusion (MCAO) (1.5 h) rat model, 10 mg/kg of each ginsenoside (i.e., Rg1, Rb1, Rh2, Rg3, Rg5, or Re) was administered via intravenous injection 2 h after reperfusion [95]. Among these, Rb1 and Rg3 possess greater neuroprotective and anti-inflammatory effects [95]. In particular, many preclinical studies have demonstrated that Rb1 plays a beneficial role in ischemic stroke [96]. Varying concentrations of Rb1 (20 μg/kg to 200 mg/kg) can improve neurological and vascular functions in the MCAO rat model through the protection of BBB, permeability reducing effects, reduction in infarct size, increase in neuroprotective factors (i.e., BDNF, growth associated protein 43, SOD, and GSH), and improved memory functions, as detected via the Morris water maze test (Reviewed in [96]). Cerebral energy metabolism and regional cerebral blood flow were improved by 5 mg/kg 20(S)-Rg3, administered through sublingual vein injection 0.5 h after the onset of ischemia, when the rat model was subjected to MCAO [97]. Rd (0.1, 1, 10, 50, 200 mg/kg) was applied intraperitoneally 30 min before 2 h MCAO in rat model, and Rd treatment (10, 50 mg/kg) increased the expression of antioxidant proteins and improved behavioral ability tested by modified neurological severity scores [98].
4.2. Traumatic brain injury
After traumatic brain injury (TBI), acute mechanical trauma triggers multiple mechanisms including NLRP3-mediated inflammation and injury, which are then followed by extended and variable phases of adaptive reactions to injury as the damaged tissue attempts to adjust and reorganize [15,93]. Ginseng and ginsenosides may provide novel therapeutic approaches for TBI by preventing inflammation and facilitating prolonged multicellular recovery phenomenon [94].
Rg1 could increase cell survival following in vitro ischemia/reperfusion injury at 10-60 μM, and this effect was partly a result of the activation of Nrf2/HO-1 axis [99]. Evidence has indicated the critical role of HO-1 metabolites in diminishing inflammation, ischemia, oxidation, and more diverse biological functions [13] even in the TBI mouse model [15]. Rb1 (10, 20, or 40 mg/kg) suppressed the TBI-induced expression of connexin 40 protein via activation of the ERK1/2 signaling pathway [100]. Adult male Sprague-Dawley rats were subjected to a controlled cortical impact injury, and total saponins from ginseng (100 mg/kg or 200 mg/kg) were administered via intraperitoneal injection right after TBI injury [56]. The neuroprotective effect of ginseng saponins was observed at 24 h after injury in the hippocampus [56]. Hence, further research focused on the anti-inflammatory effects and regenerative capacity of ginseng and ginsenosides can lead to therapeutic drug development.
4.3. Multiple sclerosis
Multiple sclerosis displays characteristic prototypical inflammatory demyelination in the CNS caused by autoimmune disease. To study the pathogenesis of multiple sclerosis, experimental autoimmune encephalomyelitis (EAE) model was established [101]. In this model, neurovascular disruption could be observed through the breakdown of BBB, migration of T lymphocytes into the CNS, demyelination, and axonal loss (reviewed in [102]). Administration of Rd (40 mg/kg/d) via intraperitoneal injection could improve neurological behavior, as shown by a decrease in Evans blue-stain based detection of BBB leakage and IFN-γ levels, and upregulation of BDNF and NGF protein levels in the EAE model brain [103].
4.4. Parkinson’s disease (PD)
Damage to the dopaminergic neurons in the substantia nigra pars compacta is the key characteristic of PD. Ginseng and ginsenosides (i.e., Rg1 and Rd) exert a protective effect against the neuronal cell death caused by 1-methyl-4-phenylpyridinium ion (MPP+)-mediated toxicity [5]. Pretreatment of Rg1 or N-acetylcysteine (ROS scavenger) protects neurons of substantia nigra against MPP+-induced neuronal loss [104]. Rats were infused unilaterally with 6-hydroxydopamine (6-OHDA) into the medial forebrain bundle to cause lesions in the nigrostriatal dopamine signaling and were treated with 10 mg/kg Rg1 via intraperitoneal injection (1.5 h after 6-OHDA injections) in the absence or presence of IGF-IR antagonist (1 h before Rg1 injections) [105]. Administration of Rg1 diminishes the neurotoxicity, as assessed by the expression of tyrosine hydroxylase, dopamine transporter, and anti-apoptotic Bcl-2 protein in the substantia nigra, which are abolished by IGF-1R antagonist [105]. In addition, oral Rg1 treatment attenuated 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced glial (astrocytic and microglial) activation and BBB degradation in a PD mouse model [106]. Rb1 demonstrates a stronger ability to disaggregate fibrils and inhibit the polymerization of α-synuclein, a neurotoxic protein, as compared the effects of Rg1 and Rg3 [107].
4.5. Alzheimer’s disease (AD)
AD is a neuroinflammatory disease that reveals Aβ accumulation and tau pathology in injured or aged brains [108]. Aβ accumulation may be responsible for BBB disruption by alteration of tight junction proteins in human AD pathogenesis [[109], [110], [111]]. Individuals diagnosed with AD show activation of NLRP3 and NLRP1 inflammasomes in Aβ stimulated peripheral monocytes, and such migratory peripheral monocytes cross the BBB and accelerate tau pathology [16]. Ginsenosides (10 mg/kg Rg1, intragastric injection) may protect senescence-accelerated mouse prone 8 strain (SAMP8) against the formation of NADPH oxidase 2 (NOX2)-ROS-mediated inflammasome complex such as NLRP1, ASC, and procaspase-1 [112]. NOX2 is expressed in neurovascular cells (i.e., endothelial cells and neuron) in the brain, a key source of ROS over-production in age-related neurodegenerative diseases, including AD [113].
Acute effects of each ginsenoside (25 mg/kg of Re, R1, or Rg3) on Aβ reduction can be detected in the brains of 3-4 month-old female Tg2576 mice overexpressing the human APP gene containing the Swedish mutation that causes familial AD [114]. A single, orally administered Re, Rg1, or Rg3 results in a significant reduction of Aβ(1-42) detected in Tg2576 brains at 18 h post administration of each ginsenoside [114]. In this study, in vitro cell culture experiments also showed reduction of Aβ(1-42) in conditioned medium of CHO 2B7 cells (Chinese hamster ovary cell line stably transfected with human βAPP 695wt) after 3 h of ginsenoside treatment [114]. Chronic effects of 100 mg/kg/day or 200 mg/kg/day ginseng on 4-month-old senescence-accelerating SAMP8 have been investigated by administration of ginseng through drinking water for 7 months [10]. Ginseng exerts protective and regenerative effects that can be attributed to its antioxidant activity, and effect on the neuronal plasticity-related proteins including phosphorylation-based activation of N-methyl-D-aspartate receptor 1, postsynaptic density 95, PKA, Ca2+-calmodulin dependent kinase II, and CREB [10,115]. Improvement of memory function in response to ginsenosides (e.g. Rg1 [116,117] and Rg5 [55]) has also been reported, as detected via the Morris water maze test. This suggests that ginseng and ginsenosides may repair cognitive functions in chronic neurodegenerative diseases as well as in acute neurovascular injuries.
5. Clinical trials in human study
Ginseng is one of the best-selling natural products in the world, which has recently gained popularity in USA, Canada, and Europe, and Eastern countries [7]. The World Health Organization established International Clinical Trials Registry Platform (ICTRP) demonstrating clinical trial registries and information on clinical trials conducted around the world (http://apps.who.int/trialsearch). Since 2005 254 trials have been conducted using “ginseng or ginsenoside”. More than 10 ginseng trials since 2012 have been reported every year [9]. Phase 0 to 2 and Phase 3 to 4 trials have been reported at approximately 36% and 29%, respectively [9]. Table 2 summarizes recent clinical trials (https://www.clinicaltrials.gov) focused on the therapeutic effects of ginseng and ginsenosides in CNS injury and on cognitive function.
Table 2.
Clinical Trial of the Ginseng/Ginsenosides on CNS Pathogenesis or Cognitive Function
| Ginseng/Ginsenoside | Conditions | Clinical trial number |
|
Phase |
|---|---|---|---|---|
| Ginsenoside-Rd | Ischemic Stroke | NCT00591084 |
|
Phase 2 |
| Ginsenoside-Rd | Ischemic Stroke | NCT00815763 |
|
Phase 3 |
| Ginseng Ginkgo Biloba |
Blood pressure Cognition |
NCT02386852 |
|
Early Phase 1 |
| American Ginseng Root Extract | Cognitive change Cognitive function |
NCT03579005 |
|
- |
| Korean Red Ginseng Extract | Brain waves | NCT04167449 |
|
Phase 4 |
| Hydrolysed Red Ginseng Extract | Cognitive function | NCT04184388 |
|
- |
6. Perspectives
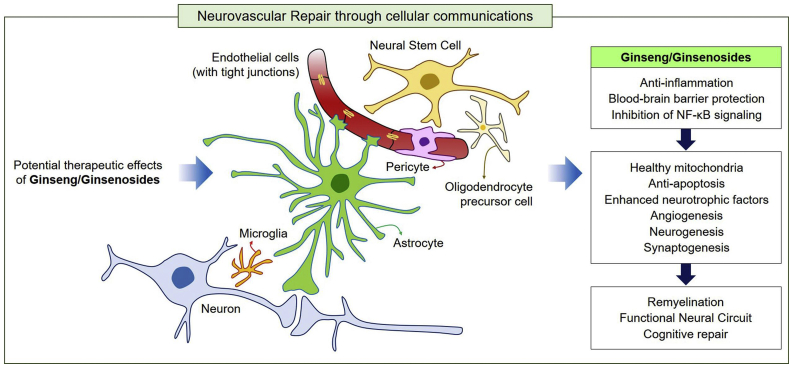
Most previous studies have focused on anti-inflammatory functions of ginseng and ginsenosides in neurovascular unit. Regeneration following CNS injury in higher mammals is very limited. Thus, investigators should attempt to elucidate the precise mechanisms that can augment the limited ability of CNS neurons to recover to normal functions [17,18]. Ginseng and ginsenosides may play key roles in neurovascular repair by inhibiting vascular leakage and inflammatory responses and stimulating regeneration after CNS injuries (Fig. 4). In addition to the intrinsic regenerative pathway, boosting regeneration through interaction with the surrounding environment and cellular communications may enhance the regenerative capacity such as angiogenesis, neurogenesis, synaptogenesis of ginseng. To restore the proper neural circuit after CNS injuries, further studies on the recovery of glia-neuron communication in neurovascular systems by ginseng are necessary.
Fig. 4.
Possible therapeutic mechanism of ginseng/ginsenosides is the induction of cellular network in neurovascular unit. Ginseng/ginsenosides possess multifaceted repair mechanisms including anti-inflammation through BBB protection and inhibition of NF-κB signaling and oxidative stress, and induction of healthy mitochondria. In addition, ginseng/ginsenosides play important roles in the protection of blood-brain barrier, inhibition of apoptosis, and enhancement of cellular interactions. Consequently, functional neural circuit may be partly restored by ginseng-mediated glia-neuron and vessel-neuron communications, leading to angiogenesis, neurogenesis, synaptogenesis, and remyelination. In conclusion, ginseng and ginsenosides can contribute to cognitive repair in cases of neurovascular inflammatory diseases.
Declaration of competing interest
The authors declare no conflicts of interest.
Acknowledgments
This paper was supported by the Konkuk University Premier Research Fund received in 2019.
References
- 1.Angelova N., Kong H.W., van der Heijden R., Yang S.Y., Choi Y.H., Kim H.K., et al. Recent methodology in the phytochemical analysis of ginseng. Phytochem Anal. 2008;19(1):2–16. doi: 10.1002/pca.1049. [DOI] [PubMed] [Google Scholar]
- 2.Leung K.W., Wong A.S. Pharmacology of ginsenosides: a literature review. Chin Med. 2010;5:20. doi: 10.1186/1749-8546-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim H.J., Kim P., Shin C.Y. A comprehensive review of the therapeutic and pharmacological effects of ginseng and ginsenosides in central nervous system. J Ginseng Res. 2013;37(1):8–29. doi: 10.5142/jgr.2013.37.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu J.M., Yao Q., Chen C. Ginseng compounds: an update on their molecular mechanisms and medical applications. Curr Vasc Pharmacol. 2009;7(3):293–302. doi: 10.2174/157016109788340767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim K.H., Lee D., Lee H.L., Kim C.E., Jung K., Kang K.S. Beneficial effects of Panax ginseng for the treatment and prevention of neurodegenerative diseases: past findings and future directions. J Ginseng Res. 2018;42(3):239–247. doi: 10.1016/j.jgr.2017.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Im D.S. Pro-resolving effect of ginsenosides as an anti-inflammatory mechanism of Panax ginseng. Biomolecules. 2020;10(3) doi: 10.3390/biom10030444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu S.E., Mwesige B., Yi Y.S., Yoo B.C. Ginsenosides: the need to move forward from bench to clinical trials. J Ginseng Res. 2019;43(3):361–367. doi: 10.1016/j.jgr.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohanan P., Subramaniyam S., Mathiyalagan R., Yang D.C. Molecular signaling of ginsenosides Rb1, Rg1, and Rg3 and their mode of actions. J Ginseng Res. 2018;42(2):123–132. doi: 10.1016/j.jgr.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He Y., Yang J., Lv Y., Chen J., Yin F., Huang J., et al. A review of ginseng clinical trials registered in the WHO international clinical trials Registry platform. Biomed Res Int. 2018;2018:1843142. doi: 10.1155/2018/1843142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao H., Li Q., Zhang Z., Pei X., Wang J., Li Y. Long-term ginsenoside consumption prevents memory loss in aged SAMP8 mice by decreasing oxidative stress and up-regulating the plasticity-related proteins in hippocampus. Brain Res. 2009;1256:111–122. doi: 10.1016/j.brainres.2008.12.031. [DOI] [PubMed] [Google Scholar]
- 11.Lo E.H., Rosenberg G.A. The neurovascular unit in health and disease: introduction. Stroke; a Journal of Cerebral Circulation. 2009;40(3 Suppl):S2–S3. doi: 10.1161/STROKEAHA.108.534404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jung E., Koh S.H., Yoo M., Choi Y.K. Regenerative potential of carbon monoxide in adult neural circuits of the central nervous system. Int J Mol Sci. 2020;21(7) doi: 10.3390/ijms21072273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee H., Choi Y.K. Regenerative effects of heme oxygenase metabolites on neuroinflammatory diseases. Int J Mol Sci. 2018;20(1) doi: 10.3390/ijms20010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rock K.L., Latz E., Ontiveros F., Kono H. The sterile inflammatory response. Annu Rev Immunol. 2010;28:321–342. doi: 10.1146/annurev-immunol-030409-101311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi Y.K., Maki T., Mandeville E.T., Koh S.H., Hayakawa K., Arai K., et al. Dual effects of carbon monoxide on pericytes and neurogenesis in traumatic brain injury. Nat Med. 2016;22(11):1335–1341. doi: 10.1038/nm.4188. [DOI] [PubMed] [Google Scholar]
- 16.Saresella M., La Rosa F., Piancone F., Zoppis M., Marventano I., Calabrese E., et al. The NLRP3 and NLRP1 inflammasomes are activated in Alzheimer’s disease. Mol Neurodegener. 2016;11:23. doi: 10.1186/s13024-016-0088-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahar M., Cavalli V. Intrinsic mechanisms of neuronal axon regeneration. Nature Reviews Neuroscience. 2018;19(6):323–337. doi: 10.1038/s41583-018-0001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tedeschi A., Bradke F. Spatial and temporal arrangement of neuronal intrinsic and extrinsic mechanisms controlling axon regeneration. Curr Opin Neurobiol. 2017;42:118–127. doi: 10.1016/j.conb.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 19.Abbott N.J., Ronnback L., Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nature Reviews Neuroscience. 2006;7(1):41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 20.Sandoval K.E., Witt K.A. Blood-brain barrier tight junction permeability and ischemic stroke. Neurobiol Dis. 2008;32(2):200–219. doi: 10.1016/j.nbd.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 21.Bell R.D., Winkler E.A., Sagare A.P., Singh I., LaRue B., Deane R., et al. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron. 2010;68(3):409–427. doi: 10.1016/j.neuron.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi Y.K., Kim K.W. Blood-neural barrier: its diversity and coordinated cell-to-cell communication. BMB Rep. 2008;41(5):345–352. doi: 10.5483/bmbrep.2008.41.5.345. [DOI] [PubMed] [Google Scholar]
- 23.Choi Y.K. Role of carbon monoxide in neurovascular repair processing. Biomol Ther (Seoul) 2018;26(2):93–100. doi: 10.4062/biomolther.2017.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mitic L.L., Anderson J.M. Molecular architecture of tight junctions. Annual Review of Physiology. 1998;60:121–142. doi: 10.1146/annurev.physiol.60.1.121. [DOI] [PubMed] [Google Scholar]
- 25.Bates D.O., Harper S.J. Regulation of vascular permeability by vascular endothelial growth factors. Vascul Pharmacol. 2002;39(4–5):225–237. doi: 10.1016/s1537-1891(03)00011-9. [DOI] [PubMed] [Google Scholar]
- 26.Min J.K., Cho Y.L., Choi J.H., Kim Y., Kim J.H., Yu Y.S., et al. Receptor activator of nuclear factor (NF)-kappaB ligand (RANKL) increases vascular permeability: impaired permeability and angiogenesis in eNOS-deficient mice. Blood. 2007;109(4):1495–1502. doi: 10.1182/blood-2006-06-029298. [DOI] [PubMed] [Google Scholar]
- 27.Desai B.S., Monahan A.J., Carvey P.M., Hendey B. Blood-brain barrier pathology in Alzheimer’s and Parkinson’s disease: implications for drug therapy. Cell Transplant. 2007;16(3):285–299. doi: 10.3727/000000007783464731. [DOI] [PubMed] [Google Scholar]
- 28.Chen L., Na R., Boldt E., Ran Q. NLRP3 inflammasome activation by mitochondrial reactive oxygen species plays a key role in long-term cognitive impairment induced by paraquat exposure. Neurobiol Aging. 2015;36(9):2533–2543. doi: 10.1016/j.neurobiolaging.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 29.Xu M., Ma Q., Fan C., Chen X., Zhang H., Tang M. Ginsenosides Rb1 and Rg1 protect primary cultured astrocytes against oxygen-glucose deprivation/reoxygenation-induced injury via improving mitochondrial function. Int J Mol Sci. 2019;20(23) doi: 10.3390/ijms20236086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heneka M.T., McManus R.M., Latz E. Inflammasome signalling in brain function and neurodegenerative disease. Nature Reviews Neuroscience. 2018;19(10):610–621. doi: 10.1038/s41583-018-0055-7. [DOI] [PubMed] [Google Scholar]
- 31.Martinon F., Burns K., Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10(2):417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 32.Beers D.R., Appel S.H. Immune dysregulation in amyotrophic lateral sclerosis: mechanisms and emerging therapies. Lancet Neurol. 2019;18(2):211–220. doi: 10.1016/S1474-4422(18)30394-6. [DOI] [PubMed] [Google Scholar]
- 33.Fernandes-Alnemri T., Wu J., Yu J.W., Datta P., Miller B., Jankowski W., et al. The pyroptosome: a supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ. 2007;14(9):1590–1604. doi: 10.1038/sj.cdd.4402194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carvalho F.A., Nalbantoglu I., Aitken J.D., Uchiyama R., Su Y., Doho G.H., et al. Cytosolic flagellin receptor NLRC4 protects mice against mucosal and systemic challenges. Mucosal Immunol. 2012;5(3):288–298. doi: 10.1038/mi.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Poyet J.L., Srinivasula S.M., Tnani M., Razmara M., Fernandes-Alnemri T., Alnemri E.S. Identification of Ipaf, a human caspase-1-activating protein related to Apaf-1. J Biol Chem. 2001;276(30):28309–28313. doi: 10.1074/jbc.C100250200. [DOI] [PubMed] [Google Scholar]
- 36.Freeman L., Guo H., David C.N., Brickey W.J., Jha S., Ting J.P. NLR members NLRC4 and NLRP3 mediate sterile inflammasome activation in microglia and astrocytes. J Exp Med. 2017;214(5):1351–1370. doi: 10.1084/jem.20150237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sevenich L. Brain-resident microglia and blood-borne macrophages orchestrate central nervous system inflammation in neurodegenerative disorders and brain cancer. Front Immunol. 2018;9:697. doi: 10.3389/fimmu.2018.00697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gordon S., Martinez F.O. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32(5):593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 39.Combs C.K., Karlo J.C., Kao S.C., Landreth G.E. beta-Amyloid stimulation of microglia and monocytes results in TNFalpha-dependent expression of inducible nitric oxide synthase and neuronal apoptosis. J Neurosci. 2001;21(4):1179–1188. doi: 10.1523/JNEUROSCI.21-04-01179.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liddelow S.A., Guttenplan K.A., Clarke L.E., Bennett F.C., Bohlen C.J., Schirmer L., et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541(7638):481–487. doi: 10.1038/nature21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shi Y., Wang H., Zheng M., Xu W., Yang Y., Shi F. Ginsenoside Rg3 suppresses the NLRP3 inflammasome activation through inhibition of its assembly. FASEB J. 2020;34(1):208–221. doi: 10.1096/fj.201901537R. [DOI] [PubMed] [Google Scholar]
- 42.Gao Y., Li J., Wang J., Li X., Li J., Chu S., et al. Ginsenoside Rg1 prevent and treat inflammatory diseases: a review. Int Immunopharmacol. 2020;87:106805. doi: 10.1016/j.intimp.2020.106805. [DOI] [PubMed] [Google Scholar]
- 43.Kim S., Lee M., Choi Y.K. The role of a neurovascular signaling pathway involving hypoxia-inducible factor and notch in the function of the central nervous system. Biomol Ther (Seoul) 2020;28(1):45–57. doi: 10.4062/biomolther.2019.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abhinand C.S., Raju R., Soumya S.J., Arya P.S., Sudhakaran P.R. VEGF-A/VEGFR2 signaling network in endothelial cells relevant to angiogenesis. J Cell Commun Signal. 2016;10(4):347–354. doi: 10.1007/s12079-016-0352-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Argaw A.T., Asp L., Zhang J., Navrazhina K., Pham T., Mariani J.N., et al. Astrocyte-derived VEGF-A drives blood-brain barrier disruption in CNS inflammatory disease. J Clin Invest. 2012;122(7):2454–2468. doi: 10.1172/JCI60842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen J., Zhang X., Liu X., Zhang C., Shang W., Xue J., et al. Ginsenoside Rg1 promotes cerebral angiogenesis via the PI3K/Akt/mTOR signaling pathway in ischemic mice. Eur J Pharmacol. 2019;856:172418. doi: 10.1016/j.ejphar.2019.172418. [DOI] [PubMed] [Google Scholar]
- 47.Zhang X.P., Li K.R., Yu Q., Yao M.D., Ge H.M., Li X.M., et al. Ginsenoside Rh2 inhibits vascular endothelial growth factor-induced corneal neovascularization. FASEB J. 2018;32(7):3782–3791. doi: 10.1096/fj.201701074RR. [DOI] [PubMed] [Google Scholar]
- 48.Ryu S., Jeon H., Kim H.Y., Koo S., Kim S. Korean red ginseng promotes hippocampal neurogenesis in mice. Neural Regen Res. 2020;15(5):887–893. doi: 10.4103/1673-5374.268905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Si Y.C., Zhang J.P., Xie C.E., Zhang L.J., Jiang X.N. Effects of Panax notoginseng saponins on proliferation and differentiation of rat hippocampal neural stem cells. Am J Chin Med. 2011;39(5):999–1013. doi: 10.1142/S0192415X11009366. [DOI] [PubMed] [Google Scholar]
- 50.Eriksson P.S., Perfilieva E., Bjork-Eriksson T., Alborn A.M., Nordborg C., Peterson D.A., et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4(11):1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 51.Choi S.H., Bylykbashi E., Chatila Z.K., Lee S.W., Pulli B., Clemenson G.D., et al. Combined adult neurogenesis and BDNF mimic exercise effects on cognition in an Alzheimer’s mouse model. Science. 2018;361(6406) doi: 10.1126/science.aan8821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang L., Kisaalita W.S. Administration of BDNF/ginsenosides combination enhanced synaptic development in human neural stem cells. Journal of Neuroscience Methods. 2011;194(2):274–282. doi: 10.1016/j.jneumeth.2010.10.025. [DOI] [PubMed] [Google Scholar]
- 53.Jiang B., Xiong Z., Yang J., Wang W., Wang Y., Hu Z.L., et al. Antidepressant-like effects of ginsenoside Rg1 are due to activation of the BDNF signalling pathway and neurogenesis in the hippocampus. Br J Pharmacol. 2012;166(6):1872–1887. doi: 10.1111/j.1476-5381.2012.01902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jakaria M., Kim J., Karthivashan G., Park S.Y., Ganesan P., Choi D.K. Emerging signals modulating potential of ginseng and its active compounds focusing on neurodegenerative diseases. J Ginseng Res. 2019;43(2):163–171. doi: 10.1016/j.jgr.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chu S., Gu J., Feng L., Liu J., Zhang M., Jia X., et al. Ginsenoside Rg5 improves cognitive dysfunction and beta-amyloid deposition in STZ-induced memory impaired rats via attenuating neuroinflammatory responses. Int Immunopharmacol. 2014;19(2):317–326. doi: 10.1016/j.intimp.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 56.Ji Y.C., Kim Y.B., Park S.W., Hwang S.N., Min B.K., Hong H.J., et al. Neuroprotective effect of ginseng total saponins in experimental traumatic brain injury. J Korean Med Sci. 2005;20(2):291–296. doi: 10.3346/jkms.2005.20.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lu H., Zhou X., Kwok H.H., Dong M., Liu Z., Poon P.Y., et al. Ginsenoside-Rb1-Mediated anti-angiogenesis via regulating PEDF and miR-33a through the activation of PPAR-gamma pathway. Front Pharmacol. 2017;8:783. doi: 10.3389/fphar.2017.00783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cho Y.L., Hur S.M., Kim J.Y., Kim J.H., Lee D.K., Choe J., et al. Specific activation of insulin-like growth factor-1 receptor by ginsenoside Rg5 promotes angiogenesis and vasorelaxation. J Biol Chem. 2015;290(1):467–477. doi: 10.1074/jbc.M114.603142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leung K.W., Pon Y.L., Wong R.N., Wong A.S. Ginsenoside-Rg1 induces vascular endothelial growth factor expression through the glucocorticoid receptor-related phosphatidylinositol 3-kinase/Akt and beta-catenin/T-cell factor-dependent pathway in human endothelial cells. J Biol Chem. 2006;281(47):36280–36288. doi: 10.1074/jbc.M606698200. [DOI] [PubMed] [Google Scholar]
- 60.Kwok H.H., Guo G.L., Lau J.K., Cheng Y.K., Wang J.R., Jiang Z.H., et al. Stereoisomers ginsenosides-20(S)-Rg(3) and -20(R)-Rg(3) differentially induce angiogenesis through peroxisome proliferator-activated receptor-gamma. Biochemical Pharmacology. 2012;83(7):893–902. doi: 10.1016/j.bcp.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 61.Min J.K., Kim J.H., Cho Y.L., Maeng Y.S., Lee S.J., Pyun B.J., et al. 20(S)-Ginsenoside Rg3 prevents endothelial cell apoptosis via inhibition of a mitochondrial caspase pathway. Biochem Biophys Res Commun. 2006;349(3):987–994. doi: 10.1016/j.bbrc.2006.08.129. [DOI] [PubMed] [Google Scholar]
- 62.Zhang J., Liu M., Huang M., Chen M., Zhang D., Luo L., et al. Ginsenoside F1 promotes angiogenesis by activating the IGF-1/IGF1R pathway. Pharmacol Res. 2019;144:292–305. doi: 10.1016/j.phrs.2019.04.021. [DOI] [PubMed] [Google Scholar]
- 63.Qin M., Luo Y., Lu S., Sun J., Yang K., Sun G., et al. Ginsenoside F1 ameliorates endothelial cell inflammatory injury and prevents atherosclerosis in mice through A20-mediated suppression of NF-kB signaling. Front Pharmacol. 2017;8:953. doi: 10.3389/fphar.2017.00953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kang J.I., Choi Y., Cui C.H., Lee D., Kim S.C., Kim H.M. Pro-angiogenic ginsenosides F1 and Rh1 inhibit vascular leakage by modulating NR4A1. Sci Rep. 2019;9(1):4502. doi: 10.1038/s41598-019-41115-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Choi Y.K., Kim J.H., Kim W.J., Lee H.Y., Park J.A., Lee S.W., et al. AKAP12 regulates human blood-retinal barrier formation by downregulation of hypoxia-inducible factor-1alpha. J Neurosci. 2007;27(16):4472–4481. doi: 10.1523/JNEUROSCI.5368-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Girouard H., Bonev A.D., Hannah R.M., Meredith A., Aldrich R.W., Nelson M.T. Astrocytic endfoot Ca2+ and BK channels determine both arteriolar dilation and constriction. Proc Natl Acad Sci U S A. 2010;107(8):3811–3816. doi: 10.1073/pnas.0914722107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim Y., Park J., Choi Y.K. The role of astrocytes in the central nervous system focused on BK channel and heme oxygenase metabolites: a review. Antioxidants (Basel) 2019;8(5) doi: 10.3390/antiox8050121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nitti M., Piras S., Brondolo L., Marinari U.M., Pronzato M.A., Furfaro A.L. Heme oxygenase 1 in the nervous system: does it favor neuronal cell survival or induce neurodegeneration? Int J Mol Sci. 2018;19(8) doi: 10.3390/ijms19082260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jung J.S., Lee S.Y., Kim D.H., Kim H.S. Protopanaxatriol ginsenoside Rh1 upregulates phase II antioxidant enzyme gene expression in rat primary astrocytes: involvement of MAP kinases and Nrf2/ARE signaling. Biomol Ther (Seoul) 2016;24(1):33–39. doi: 10.4062/biomolther.2015.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Naval M.V., Gomez-Serranillos M.P., Carretero M.E., Villar A.M. Neuroprotective effect of a ginseng (Panax ginseng) root extract on astrocytes primary culture. J Ethnopharmacol. 2007;112(2):262–270. doi: 10.1016/j.jep.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 71.Shieh P.C., Tsao C.W., Li J.S., Wu H.T., Wen Y.J., Kou D.H., et al. Role of pituitary adenylate cyclase-activating polypeptide (PACAP) in the action of ginsenoside Rh2 against beta-amyloid-induced inhibition of rat brain astrocytes. Neurosci Lett. 2008;434(1):1–5. doi: 10.1016/j.neulet.2007.12.032. [DOI] [PubMed] [Google Scholar]
- 72.Eroglu C., Barres B.A. Regulation of synaptic connectivity by glia. Nature. 2010;468(7321):223–231. doi: 10.1038/nature09612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Harrell C.R., Simovic Markovic B., Fellabaum C., Arsenijevic A., Djonov V., Volarevic V. Molecular mechanisms underlying therapeutic potential of pericytes. J Biomed Sci. 2018;25(1):21. doi: 10.1186/s12929-018-0423-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sagare A.P., Bell R.D., Zhao Z., Ma Q., Winkler E.A., Ramanathan A., et al. Pericyte loss influences Alzheimer-like neurodegeneration in mice. Nat Commun. 2013;4:2932. doi: 10.1038/ncomms3932. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 75.Shen L.H., Zhang J.T. Ginsenoside Rg1 promotes proliferation of hippocampal progenitor cells. Neurological Research. 2004;26(4):422–428. doi: 10.1179/016164104225016047. [DOI] [PubMed] [Google Scholar]
- 76.Cheng Y., Shen L.H., Zhang J.T. Anti-amnestic and anti-aging effects of ginsenoside Rg1 and Rb1 and its mechanism of action. Acta Pharmacol Sin. 2005;26(2):143–149. doi: 10.1111/j.1745-7254.2005.00034.x. [DOI] [PubMed] [Google Scholar]
- 77.Ye J., Yao J.P., Wang X., Zheng M., Li P., He C., et al. Neuroprotective effects of ginsenosides on neural progenitor cells against oxidative injury. Mol Med Rep. 2016;13(4):3083–3091. doi: 10.3892/mmr.2016.4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ni N., Liu Q., Ren H., Wu D., Luo C., Li P., et al. Ginsenoside Rb1 protects rat neural progenitor cells against oxidative injury. Molecules. 2014;19(3):3012–3024. doi: 10.3390/molecules19033012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tu L.H., Ma J., Liu H.P., Wang R.R., Luo J. The neuroprotective effects of ginsenosides on calcineurin activity and tau phosphorylation in SY5Y cells. Cell Mol Neurobiol. 2009;29(8):1257–1264. doi: 10.1007/s10571-009-9421-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xie Y.H., Chen X.C., Zhang J., Huang T.W., Song J.Q., Fang Y.X., et al. [Ginsenoside Rb1 attenuates beta-amyloid peptide(25-35) -induced hyperphosphorylation of tau protein through CDK5 signal pathway] Yao Xue Xue Bao. 2007;42(8):828–832. [PubMed] [Google Scholar]
- 81.Yang L., Hao J., Zhang J., Xia W., Dong X., Hu X., et al. Ginsenoside Rg3 promotes beta-amyloid peptide degradation by enhancing gene expression of neprilysin. J Pharm Pharmacol. 2009;61(3):375–380. doi: 10.1211/jpp/61.03.0013. [DOI] [PubMed] [Google Scholar]
- 82.Iwata N., Tsubuki S., Takaki Y., Shirotani K., Lu B., Gerard N.P., et al. Metabolic regulation of brain Abeta by neprilysin. Science. 2001;292(5521):1550–1552. doi: 10.1126/science.1059946. [DOI] [PubMed] [Google Scholar]
- 83.Liu D., Zhang H., Gu W., Liu Y., Zhang M. Ginsenoside Rb1 protects hippocampal neurons from high glucose-induced neurotoxicity by inhibiting GSK3beta-mediated CHOP induction. Mol Med Rep. 2014;9(4):1434–1438. doi: 10.3892/mmr.2014.1958. [DOI] [PubMed] [Google Scholar]
- 84.Tohda C., Matsumoto N., Zou K., Meselhy M.R., Komatsu K. Abeta(25-35)-induced memory impairment, axonal atrophy, and synaptic loss are ameliorated by M1, A metabolite of protopanaxadiol-type saponins. Neuropsychopharmacology. 2004;29(5):860–868. doi: 10.1038/sj.npp.1300388. [DOI] [PubMed] [Google Scholar]
- 85.Ge K.L., Chen W.F., Xie J.X., Wong M.S. Ginsenoside Rg1 protects against 6-OHDA-induced toxicity in MES23.5 cells via Akt and ERK signaling pathways. J Ethnopharmacol. 2010;127(1):118–123. doi: 10.1016/j.jep.2009.09.038. [DOI] [PubMed] [Google Scholar]
- 86.Gao Q.G., Chen W.F., Xie J.X., Wong M.S. Ginsenoside Rg1 protects against 6-OHDA-induced neurotoxicity in neuroblastoma SK-N-SH cells via IGF-I receptor and estrogen receptor pathways. Journal of Neurochemistry. 2009;109(5):1338–1347. doi: 10.1111/j.1471-4159.2009.06051.x. [DOI] [PubMed] [Google Scholar]
- 87.Kim J., Ahn H., Han B.C., Lee S.H., Cho Y.W., Kim C.H., et al. Korean red ginseng extracts inhibit NLRP3 and AIM2 inflammasome activation. Immunol Lett. 2014;158(1–2):143–150. doi: 10.1016/j.imlet.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 88.Paik S., Choe J.H., Choi G.E., Kim J.E., Kim J.M., Song G.Y., et al. Rg6, a rare ginsenoside, inhibits systemic inflammation through the induction of interleukin-10 and microRNA-146a. Sci Rep. 2019;9(1):4342. doi: 10.1038/s41598-019-40690-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Joo S.S., Lee D.I. Potential effects of microglial activation induced by ginsenoside Rg3 in rat primary culture: enhancement of type A Macrophage Scavenger Receptor expression. Arch Pharm Res. 2005;28(10):1164–1169. doi: 10.1007/BF02972981. [DOI] [PubMed] [Google Scholar]
- 90.Wu C.F., Bi X.L., Yang J.Y., Zhan J.Y., Dong Y.X., Wang J.H., et al. Differential effects of ginsenosides on NO and TNF-alpha production by LPS-activated N9 microglia. Int Immunopharmacol. 2007;7(3):313–320. doi: 10.1016/j.intimp.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 91.Ahn S., Siddiqi M.H., Aceituno V.C., Simu S.Y., Yang D.C. Suppression of MAPKs/NF-kappaB activation induces intestinal anti-inflammatory action of ginsenoside Rf in HT-29 and RAW264.7 cells. Immunol Invest. 2016;45(5):439–449. doi: 10.3109/08820139.2016.1168830. [DOI] [PubMed] [Google Scholar]
- 92.Ahn S., Siddiqi M.H., Aceituno V.C., Simu S.Y., Zhang J., Jimenez Perez Z.E., et al. Ginsenoside Rg5:Rk1 attenuates TNF-alpha/IFN-gamma-induced production of thymus- and activation-regulated chemokine (TARC/CCL17) and LPS-induced NO production via downregulation of NF-kappaB/p38 MAPK/STAT1 signaling in human keratinocytes and macrophages. Vitro Cell Dev Biol Anim. 2016;52(3):287–295. doi: 10.1007/s11626-015-9983-y. [DOI] [PubMed] [Google Scholar]
- 93.Xiong Y., Mahmood A., Chopp M. Emerging treatments for traumatic brain injury. Expert Opin Emerg Drugs. 2009;14(1):67–84. doi: 10.1517/14728210902769601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xiong Y., Mahmood A., Chopp M. Animal models of traumatic brain injury. Nature Reviews Neuroscience. 2013;14(2):128–142. doi: 10.1038/nrn3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cheng Z., Zhang M., Ling C., Zhu Y., Ren H., Hong C., et al. Neuroprotective effects of ginsenosides against cerebral ischemia. Molecules. 2019;24(6) doi: 10.3390/molecules24061102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shi Y.H., Li Y., Wang Y., Xu Z., Fu H., Zheng G.Q. Ginsenoside-Rb1 for ischemic stroke: a systematic review and meta-analysis of preclinical evidence and possible mechanisms. Front Pharmacol. 2020;11:285. doi: 10.3389/fphar.2020.00285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tian J., Fu F., Geng M., Jiang Y., Yang J., Jiang W., et al. Neuroprotective effect of 20(S)-ginsenoside Rg3 on cerebral ischemia in rats. Neurosci Lett. 2005;374(2):92–97. doi: 10.1016/j.neulet.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 98.Ye R., Yang Q., Kong X., Han J., Zhang X., Zhang Y., et al. Ginsenoside Rd attenuates early oxidative damage and sequential inflammatory response after transient focal ischemia in rats. Neurochem Int. 2011;58(3):391–398. doi: 10.1016/j.neuint.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 99.Li Q., Xiang Y., Chen Y., Tang Y., Zhang Y. Ginsenoside Rg1 protects cardiomyocytes against hypoxia/reoxygenation injury via activation of Nrf2/HO-1 signaling and inhibition of JNK. Cell Physiol Biochem. 2017;44(1):21–37. doi: 10.1159/000484578. [DOI] [PubMed] [Google Scholar]
- 100.Chen W., Guo Y., Yang W., Zheng P., Zeng J., Tong W. Involvement of Connexin40 in the protective effects of ginsenoside Rb1 against traumatic brain injury. Cell Mol Neurobiol. 2016;36(7):1057–1065. doi: 10.1007/s10571-015-0299-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gold R., Linington C., Lassmann H. Understanding pathogenesis and therapy of multiple sclerosis via animal models: 70 years of merits and culprits in experimental autoimmune encephalomyelitis research. Brain. 2006;129(Pt 8):1953–1971. doi: 10.1093/brain/awl075. [DOI] [PubMed] [Google Scholar]
- 102.Constantinescu C.S., Farooqi N., O’Brien K., Gran B. Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS) Br J Pharmacol. 2011;164(4):1079–1106. doi: 10.1111/j.1476-5381.2011.01302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhu D., Liu M., Yang Y., Ma L., Jiang Y., Zhou L., et al. Ginsenoside Rd ameliorates experimental autoimmune encephalomyelitis in C57BL/6 mice. J Neurosci Res. 2014;92(9):1217–1226. doi: 10.1002/jnr.23397. [DOI] [PubMed] [Google Scholar]
- 104.Chen X.C., Zhou Y.C., Chen Y., Zhu Y.G., Fang F., Chen L.M. Ginsenoside Rg1 reduces MPTP-induced substantia nigra neuron loss by suppressing oxidative stress. Acta Pharmacol Sin. 2005;26(1):56–62. doi: 10.1111/j.1745-7254.2005.00019.x. [DOI] [PubMed] [Google Scholar]
- 105.Xu L., Chen W.F., Wong M.S. Ginsenoside Rg1 protects dopaminergic neurons in a rat model of Parkinson’s disease through the IGF-I receptor signalling pathway. Br J Pharmacol. 2009;158(3):738–748. doi: 10.1111/j.1476-5381.2009.00361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Heng Y., Zhang Q.S., Mu Z., Hu J.F., Yuan Y.H., Chen N.H. Ginsenoside Rg1 attenuates motor impairment and neuroinflammation in the MPTP-probenecid-induced parkinsonism mouse model by targeting alpha-synuclein abnormalities in the substantia nigra. Toxicol Lett. 2016;243:7–21. doi: 10.1016/j.toxlet.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 107.Ardah M.T., Paleologou K.E., Lv G., Menon S.A., Abul Khair S.B., Lu J.H., et al. Ginsenoside Rb1 inhibits fibrillation and toxicity of alpha-synuclein and disaggregates preformed fibrils. Neurobiol Dis. 2015;74:89–101. doi: 10.1016/j.nbd.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fang E.F., Hou Y., Palikaras K., Adriaanse B.A., Kerr J.S., Yang B., et al. Mitophagy inhibits amyloid-beta and tau pathology and reverses cognitive deficits in models of Alzheimer’s disease. Nat Neurosci. 2019;22(3):401–412. doi: 10.1038/s41593-018-0332-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wan W., Cao L., Liu L., Zhang C., Kalionis B., Tai X., et al. Abeta(1-42) oligomer-induced leakage in an in vitro blood-brain barrier model is associated with up-regulation of RAGE and metalloproteinases, and down-regulation of tight junction scaffold proteins. Journal of Neurochemistry. 2015;134(2):382–393. doi: 10.1111/jnc.13122. [DOI] [PubMed] [Google Scholar]
- 110.Kook S.Y., Hong H.S., Moon M., Ha C.M., Chang S., Mook-Jung I. Abeta(1)(-)(4)(2)-RAGE interaction disrupts tight junctions of the blood-brain barrier via Ca(2)(+)-calcineurin signaling. J Neurosci. 2012;32(26):8845–8854. doi: 10.1523/JNEUROSCI.6102-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zipser B.D., Johanson C.E., Gonzalez L., Berzin T.M., Tavares R., Hulette C.M., et al. Microvascular injury and blood-brain barrier leakage in Alzheimer’s disease. Neurobiol Aging. 2007;28(7):977–986. doi: 10.1016/j.neurobiolaging.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 112.Chen Y., Dong S., Zhang H., Sun Z., Shen X., Sun L., et al. Protective effects of ginsenoside Rg1 on neuronal senescence due to inhibition of NOX2 and NLRP1 inflammasome activation in SAMP8 mice. J. Funct. Foods. 2020;65:103713. [Google Scholar]
- 113.Fan L.M., Geng L., Cahill-Smith S., Liu F., Douglas G., McKenzie C.A., et al. Nox2 contributes to age-related oxidative damage to neurons and the cerebral vasculature. J Clin Invest. 2019;129(8):3374–3386. doi: 10.1172/JCI125173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chen F., Eckman E.A., Eckman C.B. Reductions in levels of the Alzheimer’s amyloid beta peptide after oral administration of ginsenosides. FASEB J. 2006;20(8):1269–1271. doi: 10.1096/fj.05-5530fje. [DOI] [PubMed] [Google Scholar]
- 115.Razgonova M.P., Veselov V.V., Zakharenko A.M., Golokhvast K.S., Nosyrev A.E., Cravotto G., et al. Panax ginseng components and the pathogenesis of Alzheimer’s disease (Review) Mol Med Rep. 2019;19(4):2975–2998. doi: 10.3892/mmr.2019.9972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Quan Q., Wang J., Li X., Wang Y. Ginsenoside Rg1 decreases Abeta(1-42) level by upregulating PPARgamma and IDE expression in the hippocampus of a rat model of Alzheimer’s disease. PLoS One. 2013;8(3) doi: 10.1371/journal.pone.0059155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Shi Y.Q., Huang T.W., Chen L.M., Pan X.D., Zhang J., Zhu Y.G., et al. Ginsenoside Rg1 attenuates amyloid-beta content, regulates PKA/CREB activity, and improves cognitive performance in SAMP8 mice. J Alzheimers Dis. 2010;19(3):977–989. doi: 10.3233/JAD-2010-1296. [DOI] [PubMed] [Google Scholar]