Abstract
Purpose:
Accurate preoperative assessment of tumor invasion/adhesion is crucial for planning appropriate operative procedures. Recent advances in digital radiography allow a motion analysis of lung tumors with dynamic chest radiography (DCR) with total exposure dose comparable to that of conventional chest radiography. The aim of this study was to investigate the feasibility of preoperative evaluation of pleural invasion/adhesion of lung tumors with DCR through a virtual clinical imaging study, using a four-dimensional (4D) extended cardiac-torso (XCAT) computational phantom.
Methods:
An XCAT phantom of an adult man (50th percentile in height and weight) with simulated respiratory and cardiac motions was generated to use as a virtual patient. To simulate lung tumors with and without pleural invasion, a 30-mm diameter tumor sphere was inserted into each lobe of the phantom. The virtual patient during respiration was virtually projected using an x-ray simulator in posteroanterior (PA) and oblique directions, and sequential bone suppression (BS) images were created. The measurement points (tumor, rib, and diaphragm) were automatically tracked on simulated images by a template matching technique. We calculated five quantitative metrics related to the movement distance and directions of the targeted tumor and evaluated whether DCR could distinguish between tumors with and without pleural invasion/adhesion.
Results:
Precise tracking of the targeted tumor was achieved on the simulated BS images without undue influence of rib shadows. There was a significant difference in all five quantitative metrics between the lung tumors with and without pleural invasion both on the oblique and PA projection views (P < 0.05). Quantitative metrics related to the movement distance were effective for tumors in the middle and lower lobes, while, those related to the movement directions were effective for tumors close to the frontal chest wall on the oblique projection view. The oblique views were useful for the evaluation of the space between the chest wall and a moving tumor.
Conclusion:
DCR could help distinguish between tumors with and without pleural invasion/adhesion based on the two-dimensional movement distance and direction using oblique and PA projection views. With anticipated improved image: processing to evaluate the respiratory displacement of lung tumors in the upper lobe or behind the heart, DCR holds promise for clinical assessment of tumor invasion/adhesion in the parietal pleura.
Keywords: dynamic chest radiography, flat-panel detector, tumor invasion/adhesion, virtual clinical trial, XCAT phantom
1. INTRODUCTION
Accurate preoperative assessment of tumor invasion/adhesion is crucial for planning appropriate operative procedures. Recent advances in imaging technologies may help predict pleural invasion/adhesion of lung cancers before surgery. For example, image findings of a moving lung tumor independent from the movement of the parietal or mediastinal pleura mean that the tumor does not invade or strongly adhere to the pleura and therefore is usually resectable.1 Several researchers have reported visual assessment of tumor invasion/adhesion using cine-magnetic resonance imaging (MRI) or combined inspiratory and expiratory chest computed tomography (CT) by experienced chest radiologists or physicians.2–6 These diagnostic methods are promising for preoperatively predicting pleural invasion/adhesion of tumors but are still considered to be experimental because of special imaging requirements, that is, MRI with low availability and high cost or CT with high radiation dose for inspiratory- and expiratory-gated imaging.
Recently, the four-dimensional (4D) dynamic-ventilation CT approach has become available for clinical use as a quick and powerful tool for chest examinations under limited radiation exposure.7,8 A previous study indicated that 4D dynamic-ventilation CT combined with quantitative analysis of the tumor movement could distinguish patients with and without pleural invasion/adhesion of lung cancers.9 Based on the fact that lung tumors with pleural invasion/adhesion exhibit similar movements as the adjacent structure, the movement of lung tumors was successfully quantified using the five quantitative metrics (Fig. 1):
FIG. 1.

Illustrations of quantitative parameters to distinguish lung tumors with invasion from that without invasion. (a) Tracking points of the lung tumor and rib as the reference on a projection image to measure (b) time–distance curves, (c) time–total moved distance curves, and (d)(e) two-dimensional (2D) loci of the lung tumor and rib as the reference, and two types of cosine similarity. (d) Cosine similarity between inspiratory and expiratory vectors (the arrows from the tumor to the reference structure) and (e) cosine similarity between the vectors of the lung tumor and the reference structure (the arrows from the maximum inspiratory frame to the maximum expiratory frame). Black and gray polygonal lines represent three-dimensional (3D) movements of the tumor and the rib, respectively. Ins: Inspiration, Exp: Expiration.
Ratio of the maximum displacement from the first frame of the lung tumor (D1) to that of the adjacent structure (D2) (D1/D2),
Cross-correlation coefficient (r) between two time–distance curves of the lung tumor and adjacent structure,
Ratio of the total moved distance of the lung tumor (T1) to that of the adjacent structure (T2) (T1/T2),
Cosine similarity between the inspiratory and expiratory vectors, both directed from the tumor to the adjacent structure, and
Cosine similarity between vectors of the lung tumor and the adjacent structure, both directed from the inspiratory to the expiratory frame.Here, D1, D2, T1, and T2 refer to the distances and the adjacent structure refers to the reference point (rib) indicated in Fig. 1.9
While 4D ventilation-dynamic CT is useful to predict tumor invasion/adhesion preoperatively, if such information could be more readily obtained, that could significantly facilitate and simplify the preoperative evaluation of tumors. Dynamic chest radiography (DCR) can be considered as a potential alternative. The technique can be performed as a supplement to conventional chest radiography using a flat-panel detector (FPD)-based x-ray imaging in a general x-ray room.10 In a standing posteroanterior (PA) position, sequential chest radiographs are obtained, at a high temporal resolution up to 30 frames/s, over the whole respiratory cycle in about 10 s. The total radiation exposure is adjustable based on changes in the imaging time, imaging rate, and source-to-image distance (SID). Given the high detective quantum efficiency (DQE) of FPDs, the total entrance surface dose can be less than 1.9 mGy, which is the guidance level for two projections (posteroanterior + lateral) recommended by the International Atomic Energy Agency.11,12 A recent preliminary study has demonstrated that DCR has the potential for preoperatively assessing tumor invasion to the parietal pleura.13 However, the lack of 3D visualization and overlying rib shadows can influence the quantitative performance of the technique. The 3D limitations of DCR can potentially be mitigated using an oblique view and/or the bone suppression (BS) image-processing techniques.14,15* However, these potentials have not been quantitatively assessed.
This study investigated the feasibility of preoperative evaluation of tumor invasion/adhesion to the parietal pleura based on a motion analysis with DCR. The study was designed to be based on known ground truth to ensure reliable quantitation. To that end, the study was performed via simulation using a 4D extended cardiac-torso (XCAT) phantom as a virtual patient.16–18 The XCAT phantom library provides virtual patients including thousands of defined structures and parameterized models for cardiac and respiratory motions. In the XCAT phantom, lung tumors with or without pleural invasion can be simulated anywhere in the lungs. The two-dimensional (2D) projection images of the XCAT phantom allow the objective and quantitative evaluations of tumor motion during respiration based on DCR without any patient irradiation. The simulations were used to identify effective quantitative metrics based on DCR to distinguish tumors with and without pleural invasion/adhesion, and to examine whether the oblique view and/or the BS image-processing technique could be useful for evaluating tumor invasion/adhesion.
2. MATERIALS AND METHODS
2.A. Phantom preparation
An XCAT phantom of an adult man (50th percentile in height and weight: 175 cm and 73 kg, respectively) with a normal heart rate (60 beats/min), slow breathing (6 breaths/min), and diaphragmatic motion (4 cm) was generated to obtain 4DCT images of a virtual patient comprising 150 phases in 10 s. The pulmonary airways, venous, and arterial vasculature (to approximately the 5th generation) are included in the XCAT phantom.18 Lung density changes according to lung volume during respiration, and the inflated lung density was set to be 0.26 g/cm3, which is the average density for a healthy adult.19 To simulate a solid tumor with and without pleural invasion showing CT values approximately 100 HU, a tumor sphere of 30-mm diameter made of 100% adipose materials was generated19,20 and were inserted into each lobe of a virtual patient in the right upper, middle, and lower lobes-the left upper and lower lobes and beside the descending aorta (six locations in total). We simulated three conditions of a tumor location in each lobe: involving the chest wall or descending aorta, partially attached with the chest wall or descending aorta, and away from the chest wall or descending aorta (Fig. 2). The total of 18 tumors (= 6 × 3; six tumors with invasion, and 12 tumors without invasion) were prepared for this simulation study. The tumor motion is known, even for the case of the tumor set to move with the underlying tissue. The program outputs the vectors for the tumor, where the tumor goes in the phantom images. For the tumor sphere without pleural invasion, the design incorporated simultaneous movement with the underlying tissue, depending on the locations in the lung. That is, the movement pattern of an inserted tumor follows that of the inserted part in the XCAT phantom, and if any portion of tumor was involved in the chest wall or descending aorta, the tumor would show a restricted movement. For a tumor partially attached with the chest wall or descending aorta, the tumor was inserted close to the chest wall or descending aorta in the lung without any inclusion in it, showing a similar loci pattern of the tumor away from the chest wall or descending aorta.
FIG. 2.
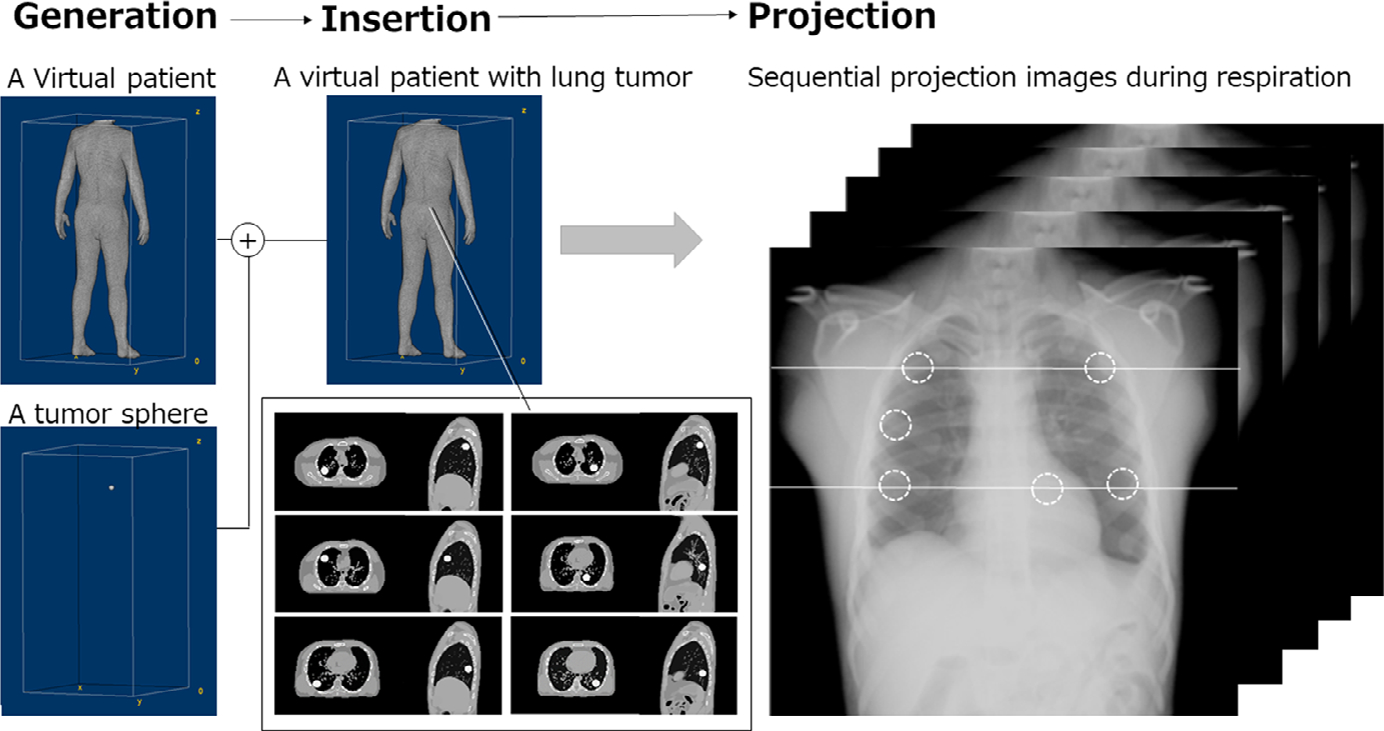
Procedures for preparing a virtual patient with a lung tumor in each lobe and beside the descending aorta (six locations in total). Each tumor was manually inserted into each lobe of the extended cardiac-torso (XCAT) phantom in three conditions of tumor location: involving, partially attached with, and away from the chest wall or the descending aorta.
2.B. Acquisition of projection images
An x-ray simulator, set to model a dynamic FPD system (PaxScan, 4343CB, Varian Medical Systems, Inc.), was used to acquire sequential projection images of the XCAT virtual patient during respiration under the same imaging conditions as the actual DCR (100 kV; 0.2 mAs/frame; 15 frame/s; SID = 2.0 m). The matrix size was 1024 × 1024 pixels, the pixel size was 417 × 417 μm2, and the gray-scale image range was 16 bits. The projection images included Poison noise which determined based on the mAs value, with image contrast based on the x-ray energy spectrum.21 The sharpness of the image is determined by the pixel size. In this study, the original sharpness was not changed by any image processing. The XCAT phantom was sequentially projected both in PA and oblique directions to obtain dynamic chest radiographs comprising of 150 respiratory phases in 10 s (matrix size: 512 × 512; pixel size: 0.834 mm; 32-bit real grayscale) (Fig. 3) (Movies S-1–6). Assuming that the perfect BS technique was applied to the resulting projection images, bone structures, such as the ribs, clavicles, and scapula, were removed from the XCAT phantom before projection (Movie S-7). A space for removed objects was filled with generic soft tissue. The projection images were rescaled into 16-bit grayscale images, and the image histogram was then matched with that of actual dynamic chest radiographs.
FIG. 3.

Projection images of the extended cardiac-torso (XCAT) phantom with and without pleural invasion of lung tumor in the left lower lobe. The solid (pink) and broken (light blue) arrows indicate the x-ray paths for posteroanterior (PA) and oblique (OBL) projection views, respectively (Movies 5).
2.C. Motion tracking
In all projection images, three measurement points were set on the first frame (maximum inspiration) (Fig. 4): one at the center of the target lung tumor and the remaining two at the top of the diaphragm and the rib edge close to the lung tumor, as the reference. The respiratory phase was determined based on the diaphragm movement. The measurement points (tumor, rib, and diaphragm) were automatically tracked on the sequential projection images by template matching with the cross-correlation algorithm where the region of interest in the previous frame was considered as the template. Motion tracking was implemented using custom-written programs (C++BuilderXE8; Embarcadero Technologies, Austin, TX, USA).
FIG. 4.

Measurement points for motion tracking of the tumor in the (a) oblique and (b) posteroanterior projection views and (c)(d) the corresponding bone suppression images in the left lower lung. The red circle, blue triangle, and green rectangle indicate the center of the target tumor and the reference points at the rib and diaphragm, respectively.
2.D. Quantitative analysis
Based on the 2D loci of a given lung tumor and reference points in the PA and oblique directions, five quantitative metrics described in the second paragraph of “INTRODUCTION” were calculated. The cosine similarity was derived from the equation in Fig. 1. The metrics were the analogue of those shown by 4D ventilation-dynamic CT approach to be reflective of the difference between the patients with and without pleural invasion/adhesion.9 The reference points were set on the rib edge close to the lung tumor or thoracic vertebra. In all the targeted tumors, motion tracking was completed on the BS images but interrupted by the rib shadows on conventional images. Therefore, quantitative parameters were obtained only from BS images. The loci of tumors were manually corrected in several frames when automatic tracking was stacked due to influence of overlapping structures. Quantitative analysis was conducted to identify effective quantitative metrics based on DCR to distinguish tumors with and without invasion. Therefore, the results were compared in each group of tumor: “with invasion” (n = 6) and “without invasion” (n = 12).
For statistical analysis, a simple t-test was used to determine the performance of DCR-based parameters to distinguish between lung tumors with and without pleural invasion/adhesion. For all tests, a P = 0.05 was deemed statistically significant.
2.E. Visual evaluation
For the lung tumors inserted into each lobe away from the chest wall (n = 5), we examined the usefulness of the oblique projection view for the visual evaluation of the space between the lung tumors and the chest wall.
3. RESULTS
Figures 5 and 6 show a representative 2D loci of the tumor in the right lower lobe and the reference point (rib, diaphragm) measured over the 150 phases on sequential BS images in both the oblique and PA projection views of the XCAT virtual patient, respectively. As shown in Fig. 7, the analysis of time–distance curves successfully helped to quantify the differences in movements between the lung tumors with and without pleural invasion in both the oblique and PA projection views.
FIG. 5.

Two-dimensional loci of the tumor in the left lower lung (a) involving the chest wall (b), partially attached with the chest wall, and (c) away from the chest wall, and the reference point at the rib tracking over the 150 phases in oblique projection views, respectively.
FIG. 6.

Two-dimensional loci of the tumor in the left lower lung (a) involving the chest wall, (b) partially attached with the chest wall, and (c) away from the chest wall, and the reference point at the rib tracking over the 150 phases in posteroanterior projection views, respectively.
FIG. 7.

Time–distance curves measured on projection images of a virtual patient in the oblique (upper) and posteroanterior (lower) directions; (a)(c) time–distance curves and (b)(d) time–total moved distance curves of the lung tumor in the right lower lung and reference point at the rib.
Table I summarizes the metrics obtained through the simulations with and without pleural invasion. For both the oblique and PA projection views, the cross-correlation coefficients (r) between the two time-distance curves (tumor and rib) were significantly greater in with (oblique: r = 0.92 ± 0.07, PA: r = 0.91 ± 0.03) than without (oblique: r = 0.82 ± 0.12, PA: r = 0.75 ± 0.10) pleural invasion (P < 0.01). In addition, there was a significant difference in both the ratio of the maximum displacement (D1/D2) and the ratio of the total movement distances (T1/T2) between the tumors with vs without pleural invasion (P < 0.01). However, tumors with and without pleural invasion in the right middle lobes showed similar results in these parameters related to the movement distance on the oblique projection views (with invasion: r = 0.99, D1/D2 = 1.13, T1/T2 = 1.13, without invasion: r = 0.99, D1/D2 = 1.06, T1/T2 = 1.06).
TABLE I.
Quantitative metrics obtained from projection images of a virtual patient with and without pleural invasion.
| Oblique view |
PA view |
|||||
|---|---|---|---|---|---|---|
| Invasion (+) (n = 6) |
Invasion (−) (n = 12) |
P-value | Invasion (+) (n = 6) |
Invasion (−) (n = 12) |
P-value | |
| (i) Ratio of the maximum displacement (D1/D2) | 1.16 ± 0.25 | 3.48 ± 3.46 | <0.01 | 1.19 ± 0.29 | 4.73 ± 4.10 | <0.01 |
| (ii) Cross-correlation coefficient (r) | 0.92 ± 0.07 | 0.82 ± 0.12 | <0.01 | 0.91 ± 0.03 | 0.75 ± 0.10 | <0.01 |
| (iii) Ratio of the total moved distances (T1/T2) | 1.16 ± 0.25 | 3.51 ± 3.44 | <0.01 | 1.19 ± 0.29 | 4.85 ± 4.26 | <0.01 |
| (iv) Cosine similarity between inspiratory andexpiratory vectors | 0.99 ± 0.02 | 0.96 ± 0.05 | <0.01 | 1.00 ± 0.00 | 0.97 ± 0.04 | <0.05 |
| (v) Cosine similarity between vectors of the cancer and the reference | 0.98 ± 0.03 | 0.87 ± 0.15 | <0.05 | 0.98 ± 0.03 | 0.88 ± 0.14 | <0.05 |
Abbreviation: PA, posteroanterior.
With regard to the other metrics related to the movement direction, both metrics of cosine similarities were significantly different for the tumor with vs without pleural invasion. In particular, in the right middle lobe on the oblique view, decreased cosine similarity between vectors of the tumor and the reference structure was observed for tumors without invasion, showing a different movement direction from that of the reference point (with invasion: 0.95, without invasion: 0.64). Although quantitative analysis showed similar results among the PA and oblique projection views, the oblique views successfully captured the space between the chest wall and the moving tumor in all the tumors inserted away from the chest wall (n = 5). Quantitative metrics related to the movement distance effectively worked for tumors in the middle and lower lobes, while those related to the movement direction worked for tumors close to the frontal chest wall on oblique projection views. Tumors in the upper lobe, both with and without pleural invasion, indicated similar respiratory displacement to less than 5 mm.
4. DISCUSSION
In this simulation study, we assessed the pleural invasion of the tumors on dynamic chest radiographs using five quantitative metrics, indicated by 4D-CT to be reflective of pleural invasion/adhesion. Generally, lung tumors of patients without pleural invasion/adhesion move a greater distance during respiration than the distance seen for tumors with parietal pleural invasion/adhesion, which shows restricted movement. DCR could successfully quantify the difference between the tumors with and without pleural invasion based on all five metrics; a higher correlation in the time-distance curves of tumors with invasion and the reference point (rib), higher similarities in 2D loci between the lung tumors with invasion and the reference point (rib), and greater maximum and total moved distances from the first frame in tumors without invasion. These findings are supported by a previous study, reporting that the movements of the loci of a lung tumor and a reference point were similar in patients with parietal pleural invasion/adhesion, while they were independent in patients without invasion/adhesion.9 The use of DCR significantly simplifies the process. However, this simulation study suggested that these quantitative parameters should be properly utilized in the case of the DCR-based approach because of a lack of 3D information, according to the tumor locations and imaging directions. For example, the tumor with and without invasion to the frontal chest wall can be distinguished based on the quantitative metrics related to the movement direction on oblique projection view, where the movement direction of tumors and the reference point is different from each other, but having a similar movement distance.
The bone-suppression image-processing technique effectively worked for a precise motion tracking on projection images. In this simulation study, perfect BS projection images were created by removing the bone structures from the XCAT phantom before image projection. We confirmed that motion tracking was able to be completed on the BS images but negatively affected by the rib shadows in conventional images, suggesting the BS image-processing technique is essential for quantitative motion analysis of pleural invasion/adhesion of lung tumors based on DCR. However, there might be some differences in appearance from real bone-suppressed images. Therefore, further studies are required for the use of bone-suppression image-processing in a clinical study. For the comparison of projection views, the oblique views were useful for evaluation of the space between the chest wall and the moving tumor, while, the PA projection views provided effective quantitative parameters related to the movement distance. These results indicated that the motion analysis with combined DCR and BS techniques has the potential for preoperative prediction of pleural invasion/adhesion of lung tumors, either on oblique or on PA views.
Although we successfully demonstrated the differences between tumors with and without pleural invasion with DCR-based motion analysis, we need to address several methodological issues of DCR-based approach prior to clinical consideration. Among them is pleural invasion in the upper lobe: DCR-based motion analysis indicated similar results for the lung tumors with and without pleural invasion in the upper lobe, where it is difficult to distinguish the differences even by using dynamic-ventilation CT. The upper lobes, especially in the lung apex, have been reported to not clearly show respiratory movements, even in patients without parietal pleural invasion/adhesion.9 Smaller respiratory movements of lung cancers located in the upper lobe make imaging assessment of cancer invasion challenging. This limitation can be minimized by the body movements available in DCR, such as forearm pronation and supination, vertical or rotational movement of the arm, etc., which might induce a motion displacement of the upper lobe. Another challenge is the assessment of tumors in proximity of the descending aorta. DCR-based approach cannot be useful if the tumor is invisible on projection images due to overlapping organs and lung structures. Further studies are warranted to enhance the visibility of such tumors. Finally, this study was conducted under limited conditions of tumor and patient. Although the feasibility and limitations of the DCR-based approach were ascertained, further studies are required to confirm the diagnostic performance of this technique in multiple virtual patients of varying physiques, and various tumor shapes, sizes, tissue composition, and locations, under different respiratory patterns and vital capacities. In particular, tumor appearance shape should be more realistic. Such studies pave the way toward the clinical implementation of DCR for the preoperative evaluation of pleural invasion/adhesion of lung tumors.
5. CONCLUSIONS
DCR is capable of distinguishing between lung tumors with and without pleural invasion/adhesion through a quantitative analysis of tumor motion during respiration. The technique is aided by the use of both oblique and PA projection views, and by the use of bone-subtraction image-processing methods. Notwithstanding these findings, the technique has limitation for lung tumors in the upper lobe and behind the heart, where improved image-processing and imaging techniques will be necessary prior to clinical considerations for such tumor locations.
Supplementary Material
Video S7. Video that demonstrates simulated dynamic chest radiographs of a virtual patient with a lung tumor away from the chest wall (invasion (−)) in the right lower lobe (Left) and the corresponding bone suppressed images (Right).avi
Video S6. Video that demonstrates simulated dynamic chest radiographs of a virtual patient with lung tumor beside the descending aorta in both the posteroanterior (PA) and oblique projection views.avi
Video S5. Video that demonstrates simulated dynamic chest radiographs of a virtual patient with lung tumor in the left lower lobe in both the posteroanterior (PA) and oblique projection views.avi
Video S4. Video that demonstrates simulated dynamic chest radiographs of a virtual patient with lung tumor in the left upper lobe in both the posteroanterior (PA) and oblique projection views.avi
Video S3. Video that demonstrates simulated dynamic chest radiographs of a virtual patient with lung tumor in the right lower lobe in both the posteroanterior (PA) and oblique projection views.avi
Video S2. Video that demonstrates simulated dynamic chest radiographs of a virtual patient with lung tumor in the right middle lobe in both the posteroanterior (PA) and oblique projection views.avi
Video S1. Video that demonstrates simulated dynamic chest radiographs of a virtual patient with lung tumor in the right upper lobe in both the posteroanterior (PA) and oblique projection views.avi
ACKNOWLEDGMENTS
This work was supported in part by Grant-in-aid for MEXT KAKENHI (19K12841), Tateishi Science and Technology Foundation, and Shimadzu Science and Technology.
Footnotes
CONFLICT OF INTEREST
The authors have no relevant conflict of interest to disclose.
ETHICAL ADHERENCE
N/A
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
Bone suppression (BS) image-processing technique: One of the image-processing methods to suppress the conspicuity of bones which was developed to improve the detection performance of lung nodules on a chest radiograph.
Contributor Information
Rie Tanaka, College of Medical, Pharmaceutical & Health Sciences, Kanazawa University, 5-11-80 Kodatsuno, Kanazawa, Ishikawa 920-0942, Japan.
Ehsan Samei, Carl E Ravin Advanced Imaging Labs, Department of Radiology, Duke University, Durham, NC 27705, United States.
William Paul Segars, Carl E Ravin Advanced Imaging Labs, Department of Radiology, Duke University, Durham, NC 27705, United States.
Ehsan Abadi, Carl E Ravin Advanced Imaging Labs, Department of Radiology, Duke University, Durham, NC 27705, United States.
Isao Matsumoto, Department of Thoracic Surgery, Kanazawa University, 13-1 Takara-machi, Kanazawa, Ishikawa 920-8641, Japan.
Masaya Tamura, Department of Thoracic Surgery, Kanazawa University, 13-1 Takara-machi, Kanazawa, Ishikawa 920-8641, Japan.
Munehisa Takata, Department of Thoracic Surgery, Kanazawa University, 13-1 Takara-machi, Kanazawa, Ishikawa 920-8641, Japan.
Tsuneo Yamashiro, Department of Diagnostic Radiology, Yokohama City University, 3-9 Fukuura, Kanazawa-ku, Yokohama, Kanagawa 236-0004, Japan.
REFERENCES
- 1.Troupis JM, Pasricha SS, Narayanan H, Rybicki FJ, Pick AW. 4D CT and lung cancer surgical resectability: a technical innovation. J Med Imaging Radiat Oncol. 2014;58:469–471. [DOI] [PubMed] [Google Scholar]
- 2.Murata K, Takahashi M, Mori M, et al. Chest wall and mediastinal invasion by lung cancer: evaluation with multi section expiratory dynamic CT. Radiology. 1994;191:251–255. [DOI] [PubMed] [Google Scholar]
- 3.Shirakawa T, Fukuda K, Miyamoto Y, Tanabe H, Tada S. Parietal pleural invasion of lung masses: evaluation with CT performed during deep inspiration and expiration. Radiology. 1994;192:809–811. [DOI] [PubMed] [Google Scholar]
- 4.Sakai S, Murayama S, Murakami J, Hashiguchi N, Masuda K. Bronchogenic carcinoma invasion of the chest wall: evaluation with dynamic cine MRI during breathing. J Comput Assist Tomogr. 1997;21:595–600. [DOI] [PubMed] [Google Scholar]
- 5.Shiotani S, Sugimura K, Sugihara M, et al. Diagnosis of chest wall invasion by lung cancer: useful criteria forexclusion of the possibility of chest wall invasion with MR imaging. Radiat Med. 2000;18:283–290. [PubMed] [Google Scholar]
- 6.Akata S, Kajiwara N, Park J, et al. Evaluation of chest wall invasion by lung cancer using respiratory dynamic MRI. J Med Imaging Radiat Oncol. 2008;52:36–39. [DOI] [PubMed] [Google Scholar]
- 7.Yamashiro T, Moriya H, Tsubakimoto M, Matsuoka S, Murayama S. Continuous quantitative measurement of the proximal airway dimensions and lung density on four-dimensional dynamic-ventilation CT in smokers. Int J Chron Obstruct Pulmon Dis. 2016;11:755–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamashiro T, Miyara T, Honda O, et al. Iterative reconstruction for quantitative computed tomography analysis of emphysema: consistent results using different tube currents. Int J Chron Obstruct Pulmon Dis. 2015;11:321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sakuma K, Yamashiro T, Moriya H, Murayama S, Ito H. Parietal pleural invasion/adhesion of subpleural lung cancer: quantitative 4-dimensional CT analysis using dynamic-ventilatory scanning. Eur J Radiol. 2017;87:36–44. [DOI] [PubMed] [Google Scholar]
- 10.Tanaka R Dynamic chest radiography: flat-panel detector (FPD) based functional X-ray imaging. Radiol Phys Technol. 2016;9:139–153. [DOI] [PubMed] [Google Scholar]
- 11.International basic safety standards for protection against ionizing radiation and for the safety of radiation sources. In: IAEA Safety Series No. 115. Vienna: International Atomic Energy Agency (IAEA); 1996;279. [Google Scholar]
- 12.Annex IV. Schedule III. Guidance levels of dose, dose rate and activity for medical exposure. In: Radiological Protection for Medical Exposure to Ionizing Radiation. Safety Guide. IAEA Safety Standards Series No. SSG-46. Vienna: International Atomic Energy Agency (IAEA). 2018. [Google Scholar]
- 13.Tamura M, Matsumoto I, Saito D, et al. Dynamic chest radiography: Novel and less-invasive imaging approach for preoperative assessments of pleural invasion and adhesion. Radiol Case Reports. 2020;15:702–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suzuki K, Abe H, MacMahon H, Doi K. Image-processing technique for suppressing ribs in chest radiographs by means of massive training artificial neural network (MTANN). IEEE Trans Med Imaging. 2006;25:406–416. [DOI] [PubMed] [Google Scholar]
- 15.Knapp J, Worrell S. Feature Based Neural Network Regression for Feature Suppression. U.S. Patent Number, 204,292 B2, June 12; 2012.
- 16.Segars WP, Mahesh M, Beck T, Frey E, Tsui B. Realistic CT simulation using the 4D XCAT phantom. Med Phys. 2008;35:3800–3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Segars WP, Bond J, Frush J, et al. Population of anatomically variable 4D XCAT adult phantoms for imaging research and optimization. Med Phys. 2013;40:043701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Segars WP, Sturgeon G, Mendonca S, Grimes J, Tsui BM. 4D XCAT phantom for multimodality imaging research. Med Phys. 2010;37:4902–4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.ICRU. International Commission on Radiation Units and Measurement, Photon, Electron, Proton and Neutron Interaction Data for Body Tissues, ICRU Report No 46, ICRU, Bethesda, MD; 1992. [Google Scholar]
- 20.Sone S, Hanaoka T, Ogata H, et al. Small peripheral lung carcinomas with five-year post-surgical follow-up: assessment by semi-automated volumetric measurement of tumour size, CT value and growth rate on TSCT. Eur Radiol. 2012;22:104–119. [DOI] [PubMed] [Google Scholar]
- 21.Spectrum processor SRS-78. http://linux.fjfi.cvut.cz/%7Emadlenka/medphys.htm Accessed Oct 16, 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S7. Video that demonstrates simulated dynamic chest radiographs of a virtual patient with a lung tumor away from the chest wall (invasion (−)) in the right lower lobe (Left) and the corresponding bone suppressed images (Right).avi
Video S6. Video that demonstrates simulated dynamic chest radiographs of a virtual patient with lung tumor beside the descending aorta in both the posteroanterior (PA) and oblique projection views.avi
Video S5. Video that demonstrates simulated dynamic chest radiographs of a virtual patient with lung tumor in the left lower lobe in both the posteroanterior (PA) and oblique projection views.avi
Video S4. Video that demonstrates simulated dynamic chest radiographs of a virtual patient with lung tumor in the left upper lobe in both the posteroanterior (PA) and oblique projection views.avi
Video S3. Video that demonstrates simulated dynamic chest radiographs of a virtual patient with lung tumor in the right lower lobe in both the posteroanterior (PA) and oblique projection views.avi
Video S2. Video that demonstrates simulated dynamic chest radiographs of a virtual patient with lung tumor in the right middle lobe in both the posteroanterior (PA) and oblique projection views.avi
Video S1. Video that demonstrates simulated dynamic chest radiographs of a virtual patient with lung tumor in the right upper lobe in both the posteroanterior (PA) and oblique projection views.avi


