Abstract
Severe hypertriglyceridemia is a major risk factor for acute pancreatitis. In exceptional cases, it is caused by plasma components inhibiting lipoprotein lipase activity. This phenomenon is predominantly associated with autoimmune diseases. Here, we report a case of severe hypertriglyceridemia due to a transient reduction in lipoprotein lipase activity following an episode of COVID-19 in an otherwise healthy 45-year-old woman. The lipoprotein lipase activity of the patient was markedly reduced compared with a healthy control and did recover to 20% of the healthy control’s lipoprotein lipase activity 5 months after the COVID-19 episode. Mixing tests substantiated reduced lipolytic capacity in the presence of the patient’s plasma at presentation compared with a homozygous lipoprotein lipase-deficient control, which was no longer present at follow-up. Western blotting confirmed that the quantity of lipoprotein lipase was not aberrant. Fibrate treatment and a strict hypolipidemic diet improved the patient’s symptoms and triglyceride levels.
Keywords: COVID-19, lipid disorders, pancreatitis
BACKGROUND
Severe hypertriglyceridemia (triglyceride concentration >10 mmol/L) is relatively rare and characterised by a markedly increased risk of (potentially lethal) pancreatitis.1 The pathogenesis of severe hypertriglyceridemia may reflect a lack of lipoprotein lipase (LPL) functionality due to pathogenic variants in the LPL gene and/or genes associated with LPL function or due to a combination of environmental factors such as alcohol consumption and medication use,2 co-morbidities leading to elevated triglycerides such as insulin resistance and hypothyroidism,3 4 and predisposing genetic variants associated with higher triglycerides.5 In addition, triglyceride concentrations of approximately 23 mmol/L have recently been observed in patients with COVID-19.6 7 These patients were treated with tocilizumab or propofol (lipid containing), which have both previously been reported to cause hypertriglyceridemia.8 9 In exceedingly rare cases, hypertriglyceridemia caused by an acquired LPL deficiency syndrome has been described associated with autoimmune diseases.10–13 Here, we report the first case of extreme hypertriglyceridemia due to a transient, severe reduction in LPL activity following an episode of COVID-19.
Case presentation
A 45-year-old woman, with stage I hypertension, was diagnosed with COVID-19 after developing mild symptoms that resolved within 2 weeks without hospital admission or newly prescribed medication. One month after the initial onset of these COVID-19 symptoms, the patient experienced nausea, and an aversion for fatty foods, and her symptoms worsened after consumption of small amounts of wine or after copious meals on various occasions. She was referred to our out-patient clinic 3 months after the COVID-19 episode, when her general practitioner had noted markedly elevated triglyceride levels of 44 mmol/L (reference value 0.6 to 2.2 mmol/L). There were no signs or symptoms of acute pancreatitis. Five years earlier, her non-fasting triglyceride concentration was 1.53 mmol/L at a routine check-up. The patient previously used on average one or two units of alcohol per week and did not use tobacco or oral contraceptives. Her blood pressure was well controlled with lisinopril 40 mg once a day and labetalol 100 mg two times per day. On physical examination, her blood pressure was 132/93 mm Hg. Her weight was 82 kg with a body mass index of 28.7 kg/m2. The patient appeared non-critically ill. Physical examination was unremarkable: there was no abdominal tenderness, peripheral oedema, eruptive xanthomas, xanthelasmas or hepatosplenomegaly. Blood and urinary tests (table 1) ruled out conditions causing acute severe hypertriglyceridemia such as hypothyroidism, diabetes mellitus, renal insufficiency, Addison’s disease or autoimmune diseases. Abdominal CT showed no signs of pancreatitis. The suspicion of acquired LPL-deficiency was raised.
Table 1.
Main blood and urinary tests at presentation
| Test | Value | Normal range |
| ESR (mm/U) | 25 | 0–20 |
| Creatinine (µmol/L) | 87 | 65–95 |
| EGFR CKD-EPI (mL/min/1.73 m2) | 69 | >60 |
| Glucose (mmol/L) | 5.5 | 3.5–6.0 |
| Lipase (U/L) | 33 | 0–60 |
| TSH (mU/L) | 2.94 | 0.27–4.20 |
| Cortisol (nmol/L) | 322 | 250–650 |
| CRP (mg/L) | 1 | 0–5 |
| hCG in urine (E/L) | <2 | <2 |
CKD-EPI, chronic kidney disease epidemiology collaboration; CRP, C reactive protein; EGFR, estimated glomerular filtration rate; ESR, erythrocyte sedimentation rate; hCG, human chorionic gonadotropin; TSH, thyroid stimulating hormone.;
Investigations
The patient’s plasma lipid concentrations at presentation and 2-month follow-up are listed in table 2. For LPL activity, a mixture of [9,10-3H(N)]-trioleylglycerol, unlabeled trioleylglycerol and lecithin was emulsified in glycerol. Non-fasting plasma samples of the patient were incubated with this mixture, BSA-heparin buffer (18% BSA, 0.31 mg/mL heparin and 120 mg NaCl in 0.3 M Tris buffer, pH 8.5) and human serum at 37°C in a shaking waterbath for 60 min. The reaction was stopped by adding a mixture of chloroform–methanol–heptane (33:40:27) and a buffer containing 0.1 M K2CO3 and 0.1 M H3BO3, pH 10.5. The samples were centrifuged at 3000g for 5 min after which the radioactivity in the upper layer was determined. To correct for hepatic lipase activity, the same procedure was performed but with plasma samples incubated in 2 M NaCl. The resultant LPL activity assay (figure 1) showed decreased LPL activity in the patient’s plasma compared with plasma of a healthy volunteer.
Table 2.
Plasma lipid concentrations at presentation and follow-up
| Test | Presentation | Two-week follow-up | One-month follow-up | Five-month follow-up | Normal range |
| Total cholesterol (mmol/L) | 15.1 | 5.0 | 4.4 | 4.2 | <5.0 |
| HDL cholesterol (mmol/L) | 0.8 | 1.6 | 1.8 | 1.7 | >1.0 |
| Non-HDL cholesterol (mmol/L) | 14.3 | 3.4 | 2.7 | 2.5 | <3.8 |
| LDL cholesterol (mmol/L) | 1.5 | 1.3 | 1.3 | 1.8 | <3.0 |
| Triglycerides (mmol/L) | 44.0 | 4.7 | 3.1 | 1.4 | <2.0 |
HDL, high density lipoprotein; LDL, low density lipoprotein;
Figure 1.
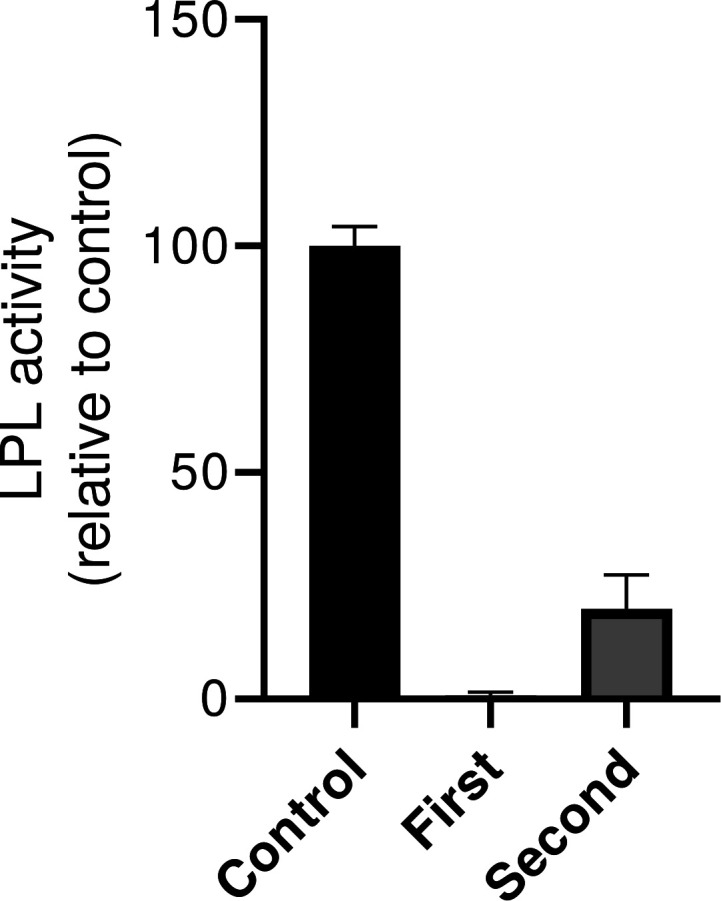
LPL activity of the patient at presentation (first) and 2-month follow-up (second) relative to a healthy volunteer (control). LPL, lipoprotein lipase.
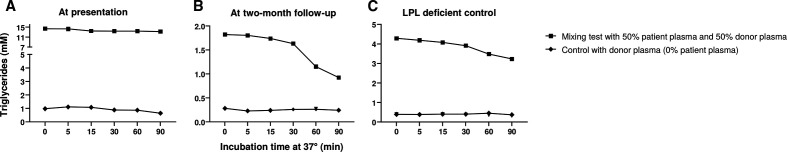
For LPL mixing tests, non-fasting plasma of the patient and plasma from a control with a homozygous LPL deficiency were mixed in equal proportions with post-heparin plasma of a healthy volunteer and incubated at 37°C in a waterbath. Small samples were collected at various time-points for triglyceride (Sopachem) and free glycerol (Sopachem) measurements using a SELECTRA auto-analyser (Sopachem). The free glycerol levels were subtracted from the total triglyceride levels in order to retrieve the net triglyceride concentration. Figure 2 illustrates the absolute triglyceride concentration on addition of 50% of the patient’s plasma to plasma from a healthy volunteer. As a control, the triglyceride concentration of the donor plasma (0% patient’s plasma) is shown. Triglyceride lipolysis is inhibited at baseline (figure 2A), but not inhibited at 2-month follow-up (figure 2B). As an extra control, the LPL mixing test was repeated in a patient with congenital LPL deficiency (ie, without an LPL inhibitor). Mixing this patient’s plasma with the same healthy donor’s plasma resulted in normal triglyceride lipolysis (figure 2C). These LPL mixing tests substantiated that the patient’s plasma at presentation contained an LPL-inhibiting component that impairs the lipolysis activity in the healthy volunteer’s plasma.
Figure 2.
Free glycerol-corrected triglycerides during in vitro plasma mix incubation of plasma from a healthy control and 0% and 50% plasma from our case (A) at presentation and (B) at 2-month follow-up compared with (C) plasma from a healthy control mixed with 0% and 50% plasma from a patient with congenital homozygous LPL deficiency. LPL, lipoprotein lipase.
LPL immunoblotting was performed with a post-heparin plasma sample diluted in reducing sample buffer and denatured at 90°C for 10 min. The sample was electrophoresed on a 4%–12% Bis-Tris gel (Invitrogen) and blotted onto a polyvinylidene difluoride membrane (Bio-Rad). Membranes were blocked in 5% skim milk powder (Sigma-Aldrich) in tris-buffered saline (TBS) with 0.1% Tween-20 for 1 hour at RT and incubated overnight at 4°C with an LPL antibody (5D2; 1:5000) in TBS containing 0.1% Tween-20% and 5% skim milk powder. Next, the membranes were washed and incubated for 1 hour at RT with a horseradish peroxidase-conjugated polyclonal rabbit anti-goat antibody (Dako; 1:3000) in TBS containing 0.1% Tween-20% and 5% milk powder. Quantification was performed with the ChemiDoc MP system (Bio-Rad) and SuperSignal West Femto Maximum Sensitivity Substrate (Thermo Scientific). The Western blot (figure 3) showed that the quantity of LPL in the patient’s plasma was not aberrant and is thus not causing the reduced LPL activity.
Figure 3.
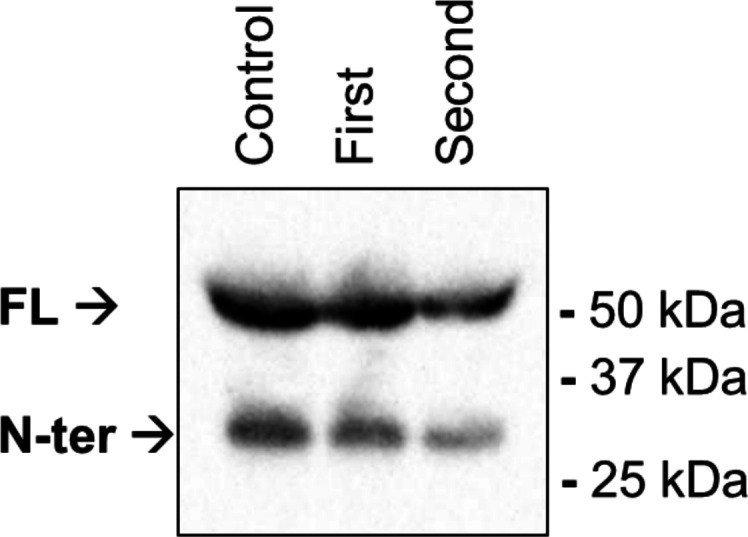
Western blot of lipoprotein lipase quantity of the patient at presentation (first) and 2-month follow-up (second), compared with a healthy volunteer. FL, full-length; N-ter, N-terminal.
Treatment
The patient was counselled by a dietician to follow a very-low-fat diet (≤15 g/day), combined with fibrate treatment (ciprofibrate, 100 mg/day).
Outcome and follow-up
After 2 weeks, triglyceride levels were 4.7 mmol/L and nausea improved significantly. One month after the initial diagnosis, the strict low-fat diet was loosened. The gastrointestinal symptoms did not recur and non-fasting triglycerides were 3.1 mmol/L. Plasma mix studies revealed normalised lipolysis at 2-month follow-up (figure 2), indicating marked lowering of the LPL-inhibiting capacity of patient’s plasma. The LPL activity assay (figure 1) confirmed an improved LPL activity, although that is still only 20% of the healthy volunteer’s plasma LPL activity.
Discussion
To our knowledge, this is the first case of severe hypertriglyceridemia following a recent COVID-19 episode. A striking feature of this case were the mild COVID-19 symptoms, which were managed in an outpatient setting. LPL activity assays, LPL mixing studies and LPL western blotting were essential for elucidating the rare cause of the hypertriglyceridemia. Treatment with fibrates and a very-low-fat diet proved to be effective in reducing both the patient’s symptoms and triglyceride levels.
Hypertriglyceridemia has previously been reported in patients with COVID-19 but was caused by different factors such as concomitant culprit medication or acute liver failure.6 7 COVID-19 has been shown to be accompanied by excessive production of proinflammatory cytokines.14 Previous studies have demonstrated the transient presence of lupus anticoagulant in patients with COVID-19 infection,15 which may be associated to the hypercoagulable state seen in COVID-19-infected patients.16 Analogous, we show a case of acquired LPL inhibition presumably due to excess production of autoantibodies from which the patient recovered 1 month after presentation at our outpatient clinic.
Knowledge of the described rare complication of COVID-19 is crucial for treating physicians in order to recognise the symptoms of acquired LPL deficiency preferably before the patient experiences pancreatitis due to the severe hypertriglyceridemia. The incidence of pancreatitis in patients with LPL deficiency is increased 360-fold,17 with poor prognosis once pancreatitis is present.18 If a patient develops severe hypertriglyceridemia following COVID-19, the diagnosis of acquired LPL deficiency should be considered. Mix assays using post-heparin plasma of patients and healthy volunteers are readily available and can be used to identify the presence of an LPL-inhibiting component in the patient’s plasma. Vice versa, in patients with acquired severe hypertriglyceridemia, anti-LPL components should be considered when other causes of genetic and secondary hypertriglyceridemia have been excluded, particularly if the patient recently had COVID-19.
In our case, conservative treatment using fibrate and extreme dietary fat restriction led to an uncomplicated recovery after a few weeks. In case of severe complications such as pancreatitis, plasmapheresis may be considered,19 potentially in combination with B-cell reducing interventions.10–13
Patient’s perspective.
I was presumably infected with COVID-19 by a colleague. The diagnosis was made 5 days later. I had a mild disease course with just flu-like symptoms and a maximum temperature of 38.3°C. About a week later I recovered.
After another few weeks, nausea and excessive saliva production appeared, which was remarkably viscous: almost custard! Also an increasing aversion to fatty foods (especially meat products) with nocturnal vomiting arose, which almost always started between 3 and 5 AM. After I ate a lavish meal on my daughter’s birthday, the nausea was so strong that I decided to go to the doctor. A blood test was arranged. Because I had such an aversion to fatty food, I also asked the doctor for a lipid profile. This was determined and the most striking result was triglyceride levels of 44 mmol/L. I was referred to the university hospital where an acquired LPL deficiency was detected with the use of an LPL mixing test. An abdominal CT scan showed that there was no pancreatitis (yet). My complaints decreased quickly after starting a fat-free/very low-fat diet and a ciprofibrate. The triglyceride values also decreased slowly to an acceptable level. After 4 months all complaints had disappeared and the blood results had also normalised after another month.
Learning points.
Severe hypertriglyceridemia due to a transient inhibitor of lipoprotein lipase activity can occur following an episode of COVID-19.
Lipoprotein lipase mixing tests are helpful in the diagnostic process of severe hypertriglyceridemia to distinguish between absolute lipoprotein lipase deficiency or an inhibitor of lipoprotein lipase activity.
Fibrate treatment and extreme dietary fat restriction can quickly improve both clinical symptoms and triglyceride levels.
Acknowledgments
We thank Hans Jansen for his contribution to the laboratory analyses, Linda Landman-Booter and Shirin Ibrahim for the collection of plasma samples, and Erik Stroes and Nordin Hanssen for proofreading.
Footnotes
Twitter: @LaureFijen
Contributors: LMF, AG, JHML and DMC contributed to the planning, conduct and reporting of the work described in the article. DMC identified the case and had the idea for the article. LMF, AG and JHML collected and analysed the data presented. LMF, AG, JHML and DMC interpreted the test results. LMF wrote the first version of the article, and LMF, AG, JHML and DMC revised the article and gave their final approval of the version to be published. LMF is the guarantor.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s)
References
- 1.Brunzell JD, Schrott HG. The interaction of familial and secondary causes of hypertriglyceridemia: role in pancreatitis. J Clin Lipidol 2012;6:409–12. 10.1016/j.jacl.2012.06.005 [DOI] [PubMed] [Google Scholar]
- 2.Yuan G, Al-Shali KZ, Hegele RA. Hypertriglyceridemia: its etiology, effects and treatment. CMAJ 2007;176:1113–20. 10.1503/cmaj.060963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pavlic M, Valéro R, Duez H, et al. Triglyceride-Rich lipoprotein-associated apolipoprotein C-III production is stimulated by plasma free fatty acids in humans. Arterioscler Thromb Vasc Biol 2008;28:1660–5. 10.1161/ATVBAHA.108.169383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waring AC, Rodondi N, Harrison S, et al. Thyroid function and prevalent and incident metabolic syndrome in older adults: the health, ageing and body composition study. Clin Endocrinol 2012;76:911–8. 10.1111/j.1365-2265.2011.04328.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moulin P, Dufour R, Averna M, et al. Identification and diagnosis of patients with familial chylomicronaemia syndrome (FCS): Expert panel recommendations and proposal of an "FCS score". Atherosclerosis 2018;275:265–72. 10.1016/j.atherosclerosis.2018.06.814 [DOI] [PubMed] [Google Scholar]
- 6.Morrison AR, Johnson JM, Ramesh M, et al. Acute hypertriglyceridemia in patients with COVID-19 receiving tocilizumab. J Med Virol 2020;92:1791–2. 10.1002/jmv.25907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomas CM, Vicent M, Moore S, et al. Treatment of severe hypertriglyceridemia with insulin infusions in severe COVID-19: a case series. J Pharm Pract 2021;8971900211010473:8971900211010473. 10.1177/08971900211010473 [DOI] [PubMed] [Google Scholar]
- 8.Giles JT, Sattar N, Gabriel S, et al. Cardiovascular safety of tocilizumab versus etanercept in rheumatoid arthritis: a randomized controlled trial. Arthritis Rheumatol 2020;72:31–40. 10.1002/art.41095 [DOI] [PubMed] [Google Scholar]
- 9.Devlin JW, Lau AK, Tanios MA. Propofol-associated hypertriglyceridemia and pancreatitis in the intensive care unit: an analysis of frequency and risk factors. Pharmacotherapy 2005;25:1348–52. 10.1592/phco.2005.25.10.1348 [DOI] [PubMed] [Google Scholar]
- 10.Pruneta V, Moulin P, Labrousse F, et al. Characterization of a new case of autoimmune type I hyperlipidemia: long-term remission under immunosuppressive therapy. J Clin Endocrinol Metab 1997;82:791–6. 10.1210/jc.82.3.791 [DOI] [PubMed] [Google Scholar]
- 11.Lilley JS, Linton MF, Kelley JC, et al. A case of severe acquired hypertriglyceridemia in a 7-year-old girl. J Clin Lipidol 2017;11:1480–4. 10.1016/j.jacl.2017.08.003 [DOI] [PubMed] [Google Scholar]
- 12.Ashraf AP, Beukelman T, Pruneta-Deloche V, et al. Type 1 hyperlipoproteinemia and recurrent acute pancreatitis due to lipoprotein lipase antibody in a young girl with Sjogren's syndrome. J Clin Endocrinol Metab 2011;96:3302–7. 10.1210/jc.2011-1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blom DJ, Marais AD. Severe hypertriglyceridemia in a patient with lupus. Am J Med 2005;118:443–4. 10.1016/j.amjmed.2004.10.023 [DOI] [PubMed] [Google Scholar]
- 14.Ragab D, Salah Eldin H, Taeimah M, et al. The COVID-19 cytokine storm; what we know so far. Front Immunol 2020;11:1446. 10.3389/fimmu.2020.01446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harzallah I, Debliquis A, Drénou B. Lupus anticoagulant is frequent in patients with Covid-19. J Thromb Haemost 2020;18:2064–5. 10.1111/jth.14867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bowles L, Platton S, Yartey N, et al. Lupus anticoagulant and abnormal coagulation tests in patients with Covid-19. N Engl J Med 2020;383:288–90. 10.1056/NEJMc2013656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaudet D, de Wal J, Tremblay K, et al. Review of the clinical development of alipogene tiparvovec gene therapy for lipoprotein lipase deficiency. Atheroscler Suppl 2010;11:55–60. 10.1016/j.atherosclerosissup.2010.03.004 [DOI] [PubMed] [Google Scholar]
- 18.Ewald N, Hardt PD, Kloer H-U. Severe hypertriglyceridemia and pancreatitis: presentation and management. Curr Opin Lipidol 2009;20:497–504. 10.1097/MOL.0b013e3283319a1d [DOI] [PubMed] [Google Scholar]
- 19.Joglekar K, Brannick B, Kadaria D, et al. Therapeutic plasmapheresis for hypertriglyceridemia-associated acute pancreatitis: case series and review of the literature. Ther Adv Endocrinol Metab 2017;8:59–65. 10.1177/2042018817695449 [DOI] [PMC free article] [PubMed] [Google Scholar]