Abstract
It is now established that two species of Bartonella, namely, Bartonella henselae and B. quintana, cause bacillary angiomatosis in human immunodeficiency virus-infected patients. In addition, B. henselae causes cat scratch disease and B. quintana, B. henselae, and B. elizabethae can cause bacteremia and endocarditis in immunocompetent persons. We have developed a PCR-restriction fragment length polymorphism-based assay for direct detection and identification to species level of Bartonella in clinical specimens. This is accomplished by PCR amplification of Bartonella DNA using primers derived from conserved regions of the gene carrying the 16S ribosomal DNA, followed by restriction analysis using DdeI and MseI restriction endonucleases. We amplified a Bartonella genus-specific 296-bp fragment from 25 clinical samples obtained from 25 different individuals. Restriction analysis of amplicons showed that identical patterns were seen from digestion of B. henselae and B. quintana amplicons with DdeI, whereas a different unique pattern was seen by using the same enzyme with B. vinsonii and B. elizabethae. With MseI digestion, B. henselae and B. vinsonii gave nearly identical patterns while B. quintana and B. elizabethae gave a different pattern. By combining the restriction analysis data generated with MseI and DdeI, unique “signature” restriction patterns characteristic for each species were obtained. These patterns were useful in identifying the Bartonella species associated with each tissue specimen.
Twelve species of Bartonella have been identified. Four, Bartonella henselae, B. quintana, B. elizabethae, and B. bacilliformis, are recognized as human pathogens, and one, B. clarridgeiae, has been implicated as a human pathogen by serology (8, 13, 15). B. bacilliformis, the first organism in the genus to be identified, causes bartonellosis (3). Recently, B. henselae was detected by PCR (1) and isolated from the lymph nodes of patients with cat scratch disease (CSD) (6), as well as from the blood of pet cats (11, 12), providing strong evidence for B. henselae as the causative agent of CSD. Both B. henselae and B. quintana cause bacteremia and bacillary angiomatosis in both immunocompromised and immunocompetent persons (10, 11, 18). B. quintana is also the etiologic agent of trench fever (20, 21), and both B. quintana and B. elizabethae can cause endocarditis (4, 7, 16, 20).
The implication of these Bartonella species in a variety of human diseases and the difficulty in isolating them from clinical specimens underscore an urgent need for better detection and identification methods. Birtles identified Bartonella species by PCR-restriction fragment length polymorphism (RFLP) analysis (2). We describe the development of a PCR-RFLP assay for the identification of Bartonella to the species level and demonstrate the application of this method directly to clinical diagnostic specimens.
MATERIALS AND METHODS
Bartonella DNA fragments were amplified from 25 tissue specimens, including fresh or paraffin-embedded clinical specimens (lymph node or lymph node aspirates, skin, subcutaneous nodules, or other tissues), obtained from patients participating in epidemiologic studies of CSD and bacillary angiomatosis (19). Amplifications of control tissues were also run in parallel. Control isolates were B. henselae B91-002000, B. vinsonii B92-010225, B. elizabethae B92-002005, and B. quintana ATCC VR-358.
Bartonella species were grown on Trypticase soy agar with 5% sheep blood or on chocolate agar plates for 4 to 7 days at 37°C. A few colonies (8 to 10) were harvested in 1 ml of 0.1 M phosphate-buffered saline, pH 7.0. Template DNA was prepared from suspended cells or from fresh or paraffin-embedded clinical specimens by the method described by Heller et al. (9). Positive and negative controls were processed for DNA preparation and PCR amplification along with the clinical specimens. The negative control was a section of fresh or paraffin-embedded skin free of Bartonella infection. The positive control was either Bartonella organisms or paraffin slices that had been artificially contaminated with Bartonella organisms.
Ten microliters of bacterial or tissue lysates was used to amplify the 16S ribosomal DNA (rDNA) fragment by the method of Relman et al. (14). PCR amplification was carried out in 100-μl reaction mixtures consisting of 10 μl of DNA and 90 μl of the amplification mix, which contained the following components: 20 pmol each of p12B and p24E primers, 0.5 mM MgCl2, 200 μM each deoxynucleoside triphosphate, 10 μl of Gene Amp PCR buffer (Perkin-Elmer, Norwalk, Conn.), and 2.5 U of Taq DNA polymerase (Perkin-Elmer).
The PCR amplification was performed in a PC-100 Thermal controller (MJ Research, Watertown, Mass.) for 35 cycles. Each cycle consisted of 120 s at 94°C, 60 s at 60°C, and 90 s at 72°C, and a final extension of 10 min at 72°C was done. The amplified products were detected by electrophoresis on a 1% agarose gel (14 by 14 cm) in 1× Tris-borate-EDTA buffer at 100 V for 60 min. Gels were stained with ethidium bromide and photographed. PCR controls included a known positive DNA extract and a reagent blank.
For digestion of PCR-amplified DNA from cultures and clinical specimens, 10 to 15 μl of PCR-amplified DNA was restricted with 10 U of DdeI and MseI in a total volume of 15 to 20 μl, respectively, according to the manufacturer's specifications (New England Biolabs, Inc., Beverly, Mass.). The restriction fragments were separated by electrophoresis on agarose gels (4% NuSieve agarose [3:1] [FMC Bioproducts, Rockland, Maine] at 60 V for 2 h. Gels were photographed and fragment sizes were determined with interpolation by using the BioImage system whole-band software analysis (Millipore Corp., Ann Arbor, Mich.).
For clinical specimens with PCR-amplified DNA revealing more than one band, the 296-bp DNA in the agarose gel was detected with ethidium bromide, excised, and placed in a 1.5-ml microcentrifuge tube. The agarose slice was washed once with 500 μl of Tris-EDTA buffer and twice with enzyme buffer. The buffer was discarded, and the agarose plug was cut into small pieces. Ten units of the restriction enzymes (DdeI and MseI) was added in a 100-μl solution (10× buffer [10 μl], bovine serum albumin [2.5 μl], and deionized water to a volume of 100 μl). DNA was precipitated from the supernatant with 3 M sodium acetate and 2.5 volumes of 95% cold ethanol. The pellet was washed once in cold 70% ethanol, dried in a vacuum dessicator, and dissolved in 20 μl of water. Fragments were detected by electrophoresis on 4% NuSieve agarose gels and were analyzed as described previously.
RESULTS
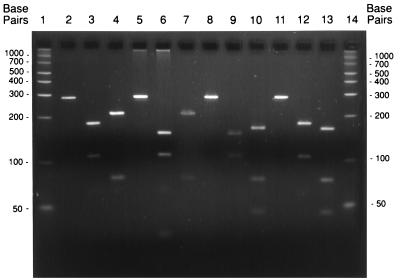
Figure 1 shows the 296-bp fragment as well as the various RFLP patterns derived from digestion of amplicons of the four species with DdeI and MseI, and Table 1 lists the observed and predicted sizes of the fragments from the digested amplicons. B. henselae and B. quintana gave nearly identical patterns with DdeI, whereas B. vinsonii and B. elizabethae gave a different and unique pattern. With MseI, B. henselae and B. vinsonii had nearly identical patterns, and B. quintana and B. elizabethae had patterns different from those of B. henselae and B. vinsonii but nearly identical to each other. Composite types generated from restriction analysis using DdeI and MseI were unique for each species (Table 1).
FIG. 1.
PCR-RFLP of B. henselae, B. vinsonii, B. elizabethae, and B. quintana. Lane 1, 50-bp ladder; lanes 2 to 4, B. henselae B91-002000; lanes 5 to 7, B. vinsonii B92-010225; lanes 8 to 10, B. elizabethae B92-002005; lanes 11 to 13, B. quintana ATCC VR-358; lane 14, 50-bp ladder. PCR products were uncut (lanes 2, 5, 8, and 11) or cut with DdeI (lanes 3, 6, 9, and 12) or MseI (lanes 4, 7, 10, and 13).
TABLE 1.
Identification of Bartonella species by PCR-RFLP analysis of a 16S rDNA fragment
| Bartonella sp. |
DdeI
|
MseI
|
PCR-RFLP composite pattern | ||||
|---|---|---|---|---|---|---|---|
| Lengths of fragmentsa (bp)
|
No. of restriction sites | Length of fragments (bp)
|
No. of restriction sites | ||||
| O | P | O | P | ||||
| B. henselae | 190, 115 | 187, 109 | 1 | 214, 81 | 217, 79 | 1 | I |
| B. vinsonii | 158, 114 | 159, 109, 28 | 2 | 218, 82 | 217, 79 | 2 | II |
| B. elizabethae | 154, 110 | 159, 109, 28 | 2 | 166, 79, 52 | 173, 79, 44 | 2 | III |
| B. quintana | 180, 106 | 187, 109 | 1 | 168, 75, 47 | 173, 79, 44 | 2 | IV |
O, observed; P, predicted (GenBank sequences).
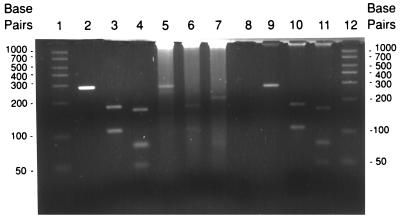
Table 2 shows the distribution of Bartonella species among 25 clinical specimens. Twenty-one of 26 (81%) of the isolates gave the restriction pattern for B. henselae, 4 (15.38%) gave that for B. quintana, and 1 (4%) gave that for B. elizabethae. The RFLP patterns of B. henselae and B. quintana obtained from clinical specimens are shown in Fig. 2. All 21 B. henselae amplified PCR products have the same composite restriction pattern with DdeI and MseI. This pattern is identical to that found for the B. henselae type strain (B91-002000). All four B. quintana amplified PCR products have the same composite restriction pattern, which is identical to that of the B. quintana type strain (ATCC VR-358). It should be noted that minor differences in fragment lengths among isolates within the same species may be encountered (5). The sizes of digested DNA fragments are shown in Table 1.
TABLE 2.
Distribution of Bartonella species among clinical diagnostic specimens evaluated
| Species | No. of specimens | Tissue | Clinical diagnosis |
|---|---|---|---|
| B. henselae | 21 | Lymph node | CSD |
| Skin | BAa | ||
| Blood | Bacteremia | ||
| B. quintana | 4 | Skin | BA |
| Bone | BA |
BA, bacillary angiomatosis.
FIG. 2.
PCR-RFLP of B. quintana and B. henselae from clinical specimens. Lane 1, 50-bp ladder, lanes 2 to 4, B. quintana H93-176; lanes 5 to 7, B. henselae B92-007003; lane 8, blank; lanes 9 to 11, B. quintana B93-007356; lane 12, 50-bp ladder. PCR products were left uncut (lanes 2, 5, and 9) or were cut with DdeI (lanes 3, 6, and 10) or MseI (lanes 4, 7, and 11).
DISCUSSION
We were able to differentiate among the different Bartonella species implicated in human disease by extending the 16S rDNA-based detection method for Bartonella species by using restriction analysis of the 296-bp amplicons. This was achieved by generating species-specific composite patterns based on digestion of 16S rDNA amplicons with DdeI and MseI restriction enzymes. The RFLP method was applied to amplicons from clinical specimens, allowing direct species identification for these specimens. The predicted and observed fragment lengths for all four species matched each other. Predicted fragments 28 for B. vinsonii and B. elizabethae cut with DdeI were not observed because they were small and were not visualized on gels.
Our method was shown to be more advantageous than a previously described method (1) because only one set of primers was used in this study, followed by restriction analysis, allowing for direct identification in a variety of clinical specimens of the Bartonella species implicated in human disease (Table 2). Although the method described by Anderson et al. (1) allows for the detection of B. quintana and B. henselae in lymph nodes and lymph node aspirates, it requires multiple steps and the use of radioactive probes.
Our PCR-RFLP-based assay may offer the advantage of early diagnosis of suspected Bartonella species infections and can be used to differentiate among three species causing human disease in North America. This rapid and specific test is an alternative to culture that provides more-timely information to clinicians, who can then direct antibiotic therapy and suggest prevention strategies to their patients based on species-specific test results (11).
ACKNOWLEDGMENTS
This work was initiated in 1993 while G.M.M. was a Visiting Associate at the Centers for Disease Control and Prevention (CDC).
This study is supported in part by NIH R29 AI36075 and the California Universitywide AIDS Research Program (to J.E.K.).
REFERENCES
- 1.Anderson B, Sims K, Regnery R, Robinson L, Schmidt M J, Goral S, Hager C, Edwards K. Detection of Rochalimaea henselae DNA in specimens from cat scratch disease patients by PCR. J Clin Microbiol. 1994;32:942–948. doi: 10.1128/jcm.32.4.942-948.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Birtles J B. Differentiation of Bartonella species using restriction endonuclease analysis of PCR-amplified 16S rRNA genes. FEMS Microbiol Lett. 1995;129:261–266. doi: 10.1111/j.1574-6968.1995.tb07590.x. [DOI] [PubMed] [Google Scholar]
- 3.Brenner D J, O'Connor S P, Winkler H, Steigart A G. Proposals to unify the genera Bartonella and Rochalimaea, with descriptions of Bartonella quintana comb. nov., Bartonella vinsonii comb. nov., Bartonella henselae comb. nov., and Bartonella elizabethae comb. nov., and to remove the family Bartonellaceae from the order Rickettsialles. Int J Syst Bacteriol. 1993;43:777–786. doi: 10.1099/00207713-43-4-777. [DOI] [PubMed] [Google Scholar]
- 4.Daly J S, Worthington M G, Brenner D J, Moss C W, Hollis D G, Weyant R S, Steigerwalt A G, Weaver R E, Daneshvar M I, O'Connor S P. Rochalimaea elizabethae sp. nov. isolated from a patient with endocarditis. J Clin Microbiol. 1993;31:872–881. doi: 10.1128/jcm.31.4.872-881.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dauga C, Miras I, Grimont P A D. Identification of Bartonella henselae and B. quintana 16S rDNA sequences by branch-genus and species-specific amplification. J Med Microbiol. 1996;45:192–199. doi: 10.1099/00222615-45-3-192. [DOI] [PubMed] [Google Scholar]
- 6.Dolan M J, Wong M T, Regnery R L, Jorgensen J H, Garcia M, Peter J, Drehner D. Syndrome of Rochalimaea henselae adenitis suggesting cat scratch disease. Ann Intern Med. 1993;118:331–336. doi: 10.7326/0003-4819-118-5-199303010-00002. [DOI] [PubMed] [Google Scholar]
- 7.Drancourt M, Mainardi J L, Brouqui P, Vandenesch F, Carta A, Lehnert F, Etienne J, Goldstein F, Acar J, Raoult D. Bartonella (Rochalimaea) quintana endocarditis in three homeless men. N Engl J Med. 1995;332:419–423. doi: 10.1056/NEJM199502163320702. [DOI] [PubMed] [Google Scholar]
- 8.Ellis B A, Regenry R L, Beati L, Bacellar F, Rood M, Glass G G, Marston E, Ksiazek T G, Jones D, Childs J E. Rats of the genus Rattus are reservoir hosts for pathogenic Bartonella species: an Old World origin for a New World disease? J Infect Dis. 1999;180:220–224. doi: 10.1086/314824. [DOI] [PubMed] [Google Scholar]
- 9.Heller M J, Burgart L J, TenEyck C J, Anderson M E, Greiner T C, Robinson R A. An efficient method for the extraction of DNA from formalin-fixed, paraffin-embedded tissue by sonication. BioTechniques. 1991;11:372–377. [PubMed] [Google Scholar]
- 10.Koehler J E, Quinn F D, Berger T G, Leboit P E, Tappero J W. Isolation of Rochalimaea species from cutaneous and osseous lesions of bacillary angiomatosis. N Engl J Med. 1992;327:1625–1631. doi: 10.1056/NEJM199212033272303. [DOI] [PubMed] [Google Scholar]
- 11.Koehler J E, Sanchez M A, Carrido C S, Whitfeld M J, Berger T G, Rodriguez-Barradas M C, LeBoit P E, Tappero J W. Molecular epidemiology of Bartonella infections in patients with bacillary angiomatosis-peliosis. N Engl J Med. 1997;337:1876–1883. doi: 10.1056/NEJM199712253372603. [DOI] [PubMed] [Google Scholar]
- 12.Koehler J E, Glasser C A, Tappero J W. Rochalimaea henselae infection: a new zoonosis with the domestic cat as reservoir. JAMA. 1994;271:531–535. doi: 10.1001/jama.271.7.531. [DOI] [PubMed] [Google Scholar]
- 13.Kordick D L, Hilyard E J, Hadfield T L, Wilson K H, Steigerwalt A G, Brenner D J, Breitschwerdt E B. Bartonella clarridgeiae, a newly recognized zoonotic pathogen causing inoculation papules, fever, and lymphadenopathy (cat scratch disease) J Clin Microbiol. 1997;35:1813–1818. doi: 10.1128/jcm.35.7.1813-1818.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Relman D A, Loutit J S, Schmidt T M, Falkow S, Tompkins L S. The agent of bacillary angiomatosis; an approach to the identification of uncultured pathogens. N Engl J Med. 1990;323:1573–1580. doi: 10.1056/NEJM199012063232301. [DOI] [PubMed] [Google Scholar]
- 15.Slater L, Welch D F, Hensel D, Coody D W. A newly recognized fastidious gram-negative pathogen as a cause of fever and bacteremia. N Engl J Med. 1990;323:1587–1593. doi: 10.1056/NEJM199012063232303. [DOI] [PubMed] [Google Scholar]
- 16.Spach D H, Kanter A S, Daniels N A, Nowowiejski D J, Larson A M, Schmidt R A, Swaminathan B, Brenner D J. Bartonella (Rochalimaea) species as a cause of apparent “culture negative” endocarditis. Clin Infect Dis. 1995;20:1044–1047. doi: 10.1093/clinids/20.4.1044. [DOI] [PubMed] [Google Scholar]
- 17.Spach D H, Kanter A S, Dougherty M J, Larson A M, Coyle M B, Brenner D J, Swaminathan B, Matar G M, Welch D F, Root R K, Stamm W E. Bartonella (Rochalimaea) quintana bacteremia in inner-city patients with chronic alcoholism. N Engl J Med. 1995;332:424–428. doi: 10.1056/NEJM199502163320703. [DOI] [PubMed] [Google Scholar]
- 18.Tappero J W, Koehler J E, Berger T G, et al. Bacillary angiomatosis and bacillary splenitis in immunocompetent adults. Ann Intern Med. 1993;118:363–365. doi: 10.7326/0003-4819-118-5-199303010-00007. [DOI] [PubMed] [Google Scholar]
- 19.Tappero J W, Mohle-Boetani J C, Koehler J E, et al. The epidemiology of bacillary angiomatosis and bacillary peliosis. JAMA. 1993;269:770–775. [PubMed] [Google Scholar]
- 20.Vinson J W, Fuller H S. Studies on trench fever. 1. Propagation of Rickettsia-like organisms from a patient's blood. Pathol Microbiol. 1961;24:S152–S166. [PubMed] [Google Scholar]
- 21.Vinson J W, Varela G, Molina-Pasquel C. Trench fever. 3. Introduction of clinical disease in volunteers inoculated with Rickettsia quintana propagated on blood agar. Am J Trop Med Hyg. 1969;18:713–722. [PubMed] [Google Scholar]