Nicolau syndrome (synonym: embolia cutis medicamentosa) is a rare complication of subcutaneous and IM drug injections, typically independent of the drug itself.1 Patients develop painful purpuric and necrotizing lesions at the injection site, often requiring surgical intervention. The pathogenesis of the ischemic skin necrosis is poorly understood. We report 2 cases of skin necrosis associated with injection of glatiramer acetate (GA) in patients with multiple sclerosis (MS), which occurred in close temporal relation to the second dose of a severe acute respiratory syndrome coronavirus 2 (SARS-CoV2) mRNA vaccine.
Case 1
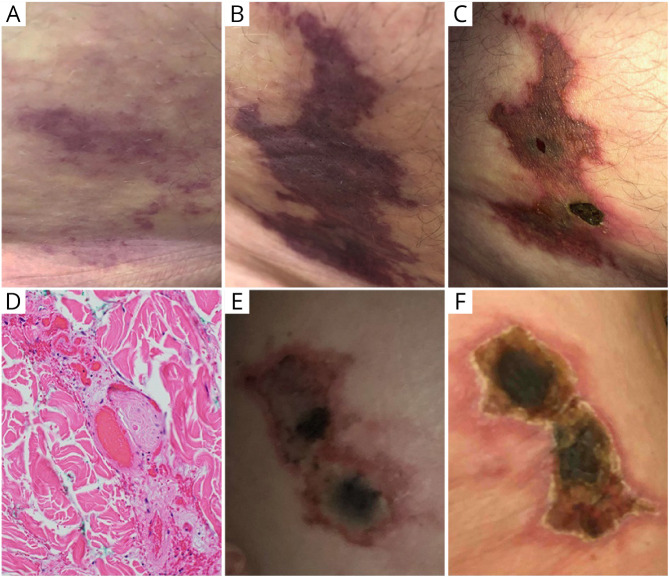
A 62-year-old man with a history of hyperlipidemia, obesity (body mass index of 30.1 kg/m2), and MS received the first dose of the BNT162b2 vaccine in late March 2021.2 The patient had been stable on GA 40 mg three times a week (Glatopa) for the past 5 years without new MRI lesions or relapses and no serious injection reactions. An injection of GA given in the lateral abdomen 17 days after the first vaccine dose resulted in retiform purpura surrounding the injection site (Figure, A). The purpura appeared 1 day before the second dose of the BNT162b2 vaccine and subsequently continued to spread (Figure, B). The injection site developed a necrotic black eschar (Figure, C). A punch biopsy obtained 14 days after vaccine dose 2 contained fibrin thrombi in the superficial and deep dermis, along with tissue necrosis indicative of thrombotic vasculopathy (Figure, D). Continued administration of GA after appearance of purpura did not lead to any further injection site reactions.
Figure. Development of Retiform Purpura With Central Necrosis After Injection of Glatiramer Acetate (GA) in Close Association With Administration of BNT162b2 (A–D) and mRNA-1273 Vaccine (E and F).
(A–C) Days 2 (A), 5 (B), and 14 (C) after injection of GA in close association with the BNT162b2 vaccine. (D) Representative micrograph of punch biopsy of cutaneous lesion revealing intravascular fibrin thrombi at day 14 (×200). (E and F) Four weeks (E) and 5 weeks (F) after GA injection in close association with the mRNA-1273 vaccine.
Case 2
A 59-year-old woman with a history of hypertension and MS received the first dose of the mRNA-1273 vaccine in early March 2021.3 The patient had been stable on GA 40 mg three times a week (Copaxone) for more than 10 years without new MRI lesions or relapses. One week after the first vaccine dose, a large welt developed at the site of a GA injection near her right hip. Over the next few weeks, retiform purpura developed around the injection site. A central necrotic eschar developed when she received the second dose of the mRNA-1273 vaccine 4 weeks later, which enlarged over the next 7 days (Figure, E and F). She developed severe burning pain at the site of the wound, and the wound was debrided. Her local dermatologist diagnosed her with Nicolau syndrome but did not perform a biopsy. GA injections continued at other sites throughout the development of this purpuric skin lesion without further reactions. Roughly 10 years ago, she had experienced 1 skin reaction after GA injection that did not develop into the large ischemic lesion observed in the current case.
Discussion
The COVID-19 pandemic has placed enormous health and economic burden on the worldwide community. The mRNA vaccines from Pfizer (BNT162b2) and Moderna (mRNA-1273) are notable achievements, highly effective in preventing severe COVID-19 syndromes.2,3 Our second patient reported typical side effects including fever, fatigue, headache, chills, and muscle pain, whereas the first patient had no side effects. Complications related to drug interactions in specific populations have not been reported.
Nicolau syndrome is an exceedingly rare, adverse event in GA-treated patients with MS.1,4 Occurrence of Nicolau syndrome in 2 patients with MS at our MS center in close proximity to SARS-CoV2 vaccination with mRNA vaccines raises the possibility of an association between the 2. No other reports of similar skin lesions were observed in our vaccine program at UC Irvine. Our MS center currently has 224 patients on GA, with an estimated 70%–80% having received a SARS-CoV2 vaccine. Of interest, the joint targets of both interventions are professional antigen-presenting dendritic cells (DCs): mRNA vaccines work via mRNA delivery to DCs at the injection site, which then produce and present mRNA-encoded viral antigen to initiate an immune response.5 Although the mechanisms of GA on the immune system are broad, DCs act as the initial target for drug uptake and presentation.6 Both patients have been stable on GA, and both tested positive for antibodies against SARS-CoV2 4 months after vaccination.
We report these cases to make MS centers aware of this potential complication. Although our 2 observations are by no means a proof of causation, they suggest that providers and patients should carefully monitor GA injections in close proximity to SARS-CoV2 vaccinations with mRNA vaccine. A timely response coordinated with dermatology has been reported to prevent necrosis and scar formation, but it is unclear whether this approach may also be successful under these new circumstances.7 Of note, our observation may also be of relevance for other injectables, as Nicolau syndrome has also been observed with other substances, and may be attributed to mechanical affection of the tissue rather than to drug-related effects.
Appendix. Authors
Study Funding
No targeted funding reported.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/NN for full disclosures.
References
- 1.Kimbrough DJ, Newsome SD. Case report: two cases of Nicolau syndrome associated with glatiramer acetate. Int J MS Care. 2017;19(3):148-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(3):2603-2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baden LR, Sahly HME, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(3):403-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bosca I, Bosca M, Belengeur A, et al. Necrotising cutaneous lesions as a side effect of glatiramer acetate. J Neurol. 2006;253(3):1370-1371. [DOI] [PubMed] [Google Scholar]
- 5.Pardi N, Hogan MJ, Porter FW, Weissman D.. mRNA vaccines—a new era in vaccinology. Nat Rev Drug Discov. 2018;17(3):261-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prod'homme T, Zamvil SS.. The evolving mechanisms of action of glatiramer acetate. Cold Spring Harb Perspect Med. 2019;9(3):a029249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geukens J, Rabe E, Bieber T.. Embolias cutis medicamentosa of the foot after sclerotherapy. Eur J Dermatol. 1999;9(3):132-133. [PubMed] [Google Scholar]