Abstract
Background and Objectives
The objective of the retrospective analysis was to test the hypothesis that changes in serum anti-myelin-associated glycoprotein (MAG) autoantibodies are associated with clinical response to immunotherapy in patients with anti-MAG neuropathy.
Methods
As of January 29, 2020, we used anti-myelin-associated glycoprotein-related search strings in the Medline database to identify studies that provided information on anti-MAG immunoglobulin M (IgM) autoantibodies and clinical outcomes during immunotherapies. The relative change in anti-MAG IgM titers, paraprotein levels, or total IgM was determined before, during, or posttreatment, and the patients were assigned to “responder,” “nonresponder,”’ or “acute deteriorating” category depending on their clinical response to treatment. The studies were qualified as “supportive” or “not supportive” depending on the percentage of patients exhibiting an association between relative change of anti-MAG antibody titers or levels and change in clinical outcomes.
Results
Fifty studies with 410 patients with anti-MAG neuropathy were included in the analysis. Forty studies with 303 patients supported the hypothesis that a “responder” patient had a relative reduction of anti-MAG antibody titers or levels that is associated with clinical improvements and “nonresponder” patients exhibited no significant change in anti-MAG IgM antibodies. Six studies with 93 patients partly supported, and 4 studies with 26 patients did not support the hypothesis.
Discussion
The retrospective analysis confirmed the hypothesis that a relative reduction in serum anti-MAG IgM antibodies is associated with a clinical response to immunotherapies; a sustained reduction of at least 50% compared with pretreatment titers or levels could be a valuable indicator for therapeutic response.
Anti-myelin-associated glycoprotein (MAG) neuropathy is a rare form of acquired demyelinating polyneuropathy associated with a monoclonal gammopathy of undetermined significance (MGUS).1 The gammopathy leads to the production of monoclonal anti-MAG immunoglobulin M (IgM) antibodies that recognize the CD57/HNK-1 carbohydrate epitope, which is highly expressed on adhesion molecules such as MAG, myelin protein zero, or sulphated glucuronyl glycolipids in the peripheral nervous system.2-5 There is considerable evidence that the deposition of anti-MAG IgM autoantibodies on myelin sheaths is responsible for widening of the myelin lamellae and demyelination. The slowly progressing neuropathy causes sensorimotor deficits, sensory ataxia, paresthesia, muscle weakness, neuropathic pain, and tremor.6-10 Typically, the disease onset occurs after the age of 50 years and is 2.7 times more frequent in men than in women with a prevalence of approximately 1 in 100,000.10-12 Currently, there is no approved treatment for anti-MAG neuropathy. However, given the high unmet medical need, over the last 3 decades, many different immunotherapies have been used for the management of anti-MAG neuropathy including IV immunoglobulins (IVIg), therapeutic plasma exchange, chemotherapeutic drugs, and various biologic drugs such as rituximab and obinutuzumab.8,10,13,14
The significance of the anti-MAG antibody titers or levels as predictive of response to therapy is controversial. Although there is considerable evidence for the pathogenicity of anti-MAG IgM autoantibodies, the association of reduced serum levels of anti-MAG IgM autoantibodies and clinical improvement of neuropathic symptoms is less clear based on the available literature and reviews.8,15 Therefore, we performed a systematic literature search and a retrospective analysis to investigate a relationship of change in serum anti-MAG IgM titers or levels and clinical outcome during immunotherapies and to evaluate whether the change in anti-MAG IgM antibodies is a predictive biomarker of response to immunotherapies in patients with anti-MAG neuropathy.
Methods
Data Sources and Search Strategy
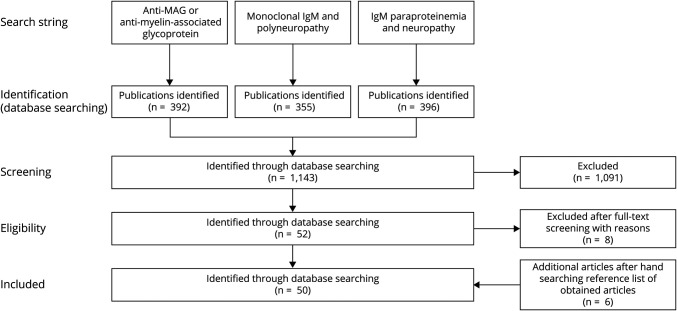
A systematic literature search in Medline Epub has been performed for all published work up to January 29, 2020 (as detailed in Figure 1), to investigate whether changes in clinical signs of neuropathy are associated with changes in anti-MAG IgM titers or levels of patients with anti-MAG neuropathy during treatment with immunotherapies. The search strings “anti-MAG neuropathy OR anti-myelin-associated glycoprotein,” “monoclonal IgM AND polyneuropathy,” and “IgM paraproteinemia AND neuropathy” were used to identify studies, providing information on anti-MAG autoantibody titers or levels and clinical outcomes at different time points, that is, particularly pretreatment and posttreatment.
Figure 1. Overview of the Systematic Literature Search in Medline Epub.
An All published work has been included until January 29, 2020, independent of the type of intervention or class of evidence given the limited number of Class I evidence studies. Data of 50 publications were included and analyzed.
This search yielded 1,143 hits, of which 1,091 were excluded after abstract screening for the following reasons: duplicates, not original publications (e.g., reviews), publications with nonclinical data (e.g., animal studies), or focus on a nonrelevant disease (e.g., neuropathy without anti-MAG antibodies). During full-text appraisal of the 52 remaining publications, 8 publications were excluded because they did not provide information on anti-MAG IgM titers, paraprotein levels, total IgM levels, and/or pretreatment and posttreatment clinical data (supplemental data, eTable 1, links.lww.com/NXI/A649).16-23 In addition, 6 publications were hand-selected and added to the list of 44 publications, for example, because they were presented as abstracts at conferences.24-29 Data were extracted from the 50 remaining publications and summarized (supplemental data, eTable 2, links.lww.com/NXI/A649).1,13,14,24-50,e1-e21 Of note, all publications that provided information on anti-MAG IgM, paraprotein, and total IgM levels as well as on clinical symptoms at different time points were included in this analysis, regardless of the results and the class of evidence (given the limited number of randomized controlled trials).
Data Extraction, Analysis, and Synthesis
Data from the 50 identified clinical publications were analyzed for the relative change in anti-MAG IgM autoantibody titer units, paraprotein levels (g/L), total IgM levels (g/L) from pretreatment (baseline) to posttreatment, and compared with changes in clinical outcomes (supplemental data, eTable 2, links.lww.com/NXI/A649). The methods used to assay the anti-MAG IgM antibodies are listed in the supplemental data Table e-3, links.lww.com/NXI/A649. In accordance with the recently suggested cutoff value of >7′000 BTU instead of >1′500 BTU in the Bühlmann test by Liberatore et al. 2020e22, in 48 of the analyzed studies, patients exhibited titers above this higher cutoff value. Only in 2 studies, patients were included with titers values below the 7′000 BTU cutoff value.33,43
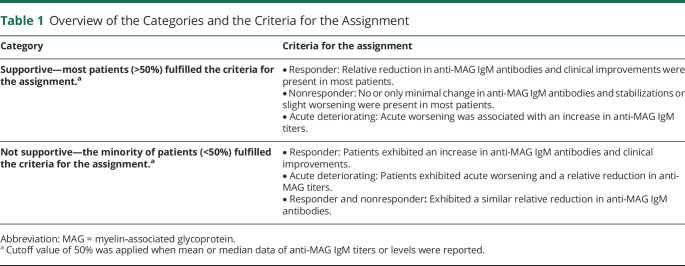
Individual patients were assigned to 1 of 3 categories (“responder,” “nonresponder,” or “acute deteriorating”) depending on their clinical response to treatment in primary and secondary outcome measures as defined by the authors of the original publications. Consistent with the original articles and to avoid a potential bias of the analysis, we separated the small subset of “acute deteriorating” patients from the “nonresponder” patients because they exhibited, mostly, a transient worsening of the clinical symptoms.17 However, the transient worsening was not necessarily a sign of long-term treatment failure.
Studies were assigned to 1 of 2 categories (“supportive” or “not supportive”) depending on whether most patients exhibited an association between change in anti-MAG antibody titers or levels and change in clinical outcome or not (Table 1).
Table 1.
Overview of the Categories and the Criteria for the Assignment
Patient Cohort and Treatment Interventions
All participants with anti-MAG IgM antibody-associated demyelinating peripheral neuropathy with MGUS were included, independent of age, pretreatment and treatment status, severity, and duration of the neuropathy.
The evaluated studies included the following interventions: (1) plasma exchange, plasmapheresis, or selective apheresis (protein A column); (2) IVIg; (3) rituximab or obinutuzumab; (4) interferon alpha-2a; (5) purine analogs: fludarabine or cladribine; (6) alkylating agents: cyclophosphamide, chlorambucil, or bendamustine; (7) corticosteroids: dexamethasone, prednisone, and other immunosuppressants such as cyclosporine and lenalidomide; and (8) placebo or no treatment. The studies included either single treatment interventions, combination treatment protocols, or comparisons vs placebo. Overall, most patients (n = 162) received either rituximab alone (39.5%) or in combination (9.5%) with plasma exchange, fludarabine, cyclophosphamide, dexamethasone, or bendamustine. In a few studies, this regimen was shortened or prolonged based on clinical response observed in patients. Almost a fifth (18.5%) of the participants received placebo (15.6%) or no treatment (2.9%). For our analysis, these patients are considered an important indicator for treatment-unrelated changes and fluctuation in anti-MAG titers. Symptom severity, clinical improvement, or acute deterioration were assessed with different methods, such as grip strength, the Inflammatory Neuropathy Cause and Treatment disability score, the Medical Research Council sum score, the Neuropathy Disability Score, the Overall Neuropathy Limitations Scale, or the Total Neuropathy Score, Neuropathy Impairment Scale, the modified Rankin Scale (mRS), the Rasch-built Overall Disability Scale, 10-meter walk time, and electrophysiologic parameters. Change in subjective clinical scores and scales were assessed at various time points in the course of treatment course (supplemental data, eTable 2, links.lww.com/NXI/A649). Patients with Waldenström macroglobulinemia (WM), multiple myeloma (MM), lymphoma, or monoclonal gammopathy of non-IgM type (e.g., IgG, IgA, and IgD) were excluded. In many studies performed in WM or MM, different clinical assessments were used (primarily oncological outcome measures), making an evaluation of the neurologic outcome measures difficult.
Data Availability
All data and the statistical analysis are available in the manuscript; the supplemental data are reported in the original articles cited in the manuscript.
Results
To obtain a more homogenous patient population, only patients with anti-MAG neuropathy and MGUS associated with elevated anti-MAG IgM were included in the analysis. Other pathologies associated with monoclonal anti-MAG IgM including WM were excluded from the analysis, unless indicated (supplemental data, eTable 2, links.lww.com/NXI/A649).
The systematic literature analysis showed that of the 50 studies (n = 410 participants), 40 studies (n = 303 participants) support the hypothesis that (1) clinical improvements are associated with a relative reduction in anti-MAG IgM antibodies, (2) nonresponders exhibit no, or only minimal change in anti-MAG IgM antibodies, and (3) acute deteriorating was associated with an increase in anti-MAG titers.
Of note, of the 10 studies that were not supportive (n = 119 participants), only 4 studies did not support the hypothesis at all (n = 26 participants). However, in 6 of these studies (n = 93 participants), at least some patients with anti-MAG IgM neuropathy (<50%) showed a relationship between change in anti-MAG antibodies and clinical outcome (supplemental data, eTable 2, links.lww.com/NXI/A649).
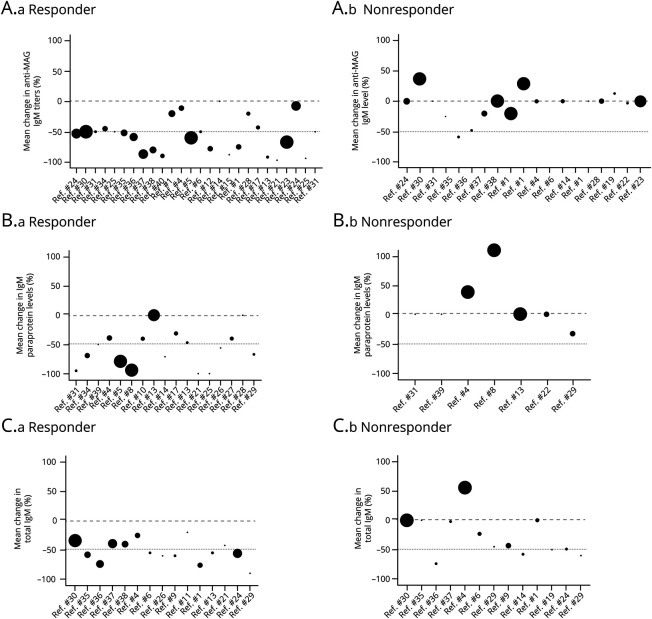
To further test the hypothesis, the studies and the extracted data of each group (responder, nonresponder, and acute deteriorating) were analyzed for the relative change in anti-MAG IgM titers, paraprotein levels, or total IgM levels (Figure 2). Of importance, all studies and participants were included in the analysis regardless of whether the study was categorized as supportive or not supportive. In most studies the anti-MAG IgM titers were assessed but only a minority of studies measured paraprotein (commonly referred to as M-protein or monoclonal protein) or total IgM levels. Based on the systematic literature search, a strong association was observed between clinical improvements in the responder group (n = 208 participants) and a significant reduction in anti-MAG titers, paraprotein, and/or total IgM levels (p > 0.001) compared with the nonresponder group (n = 191 participants) or the acute deteriorating group (n = 11 participants). If the 2 follow-up studies are excluded, therefore reducing the bias of including the same patient twice,36,38 the total number of participants is 394 patients with anti-MAG, of which 197 patients (50.0%) are considered as responders and 185 (47.0%) as nonresponders to the treatment.
Figure 2. Relative Change in Serum Anti-MAG IgM Titers or Levels and Response to Immunotherapies in Patients With Anti-MAG Neuropathy.
An overview of the studies that assessed the relative change in anti-MAG IgM autoantibodies (pretreatment and posttreatment) and clinical response to immunotherapies. (A) Relative change in anti-MAG IgM titers in the responder (a) and the nonresponder group (b); (B) Relative change in paraprotein levels in the responder and the nonresponder group; and (C) Relative change in total IgM levels in the responder and the nonresponder group. Data are indicated as mean values and the circle size represents comparative size of the study (number of participants). MAG = myelin‐associated glycoprotein.
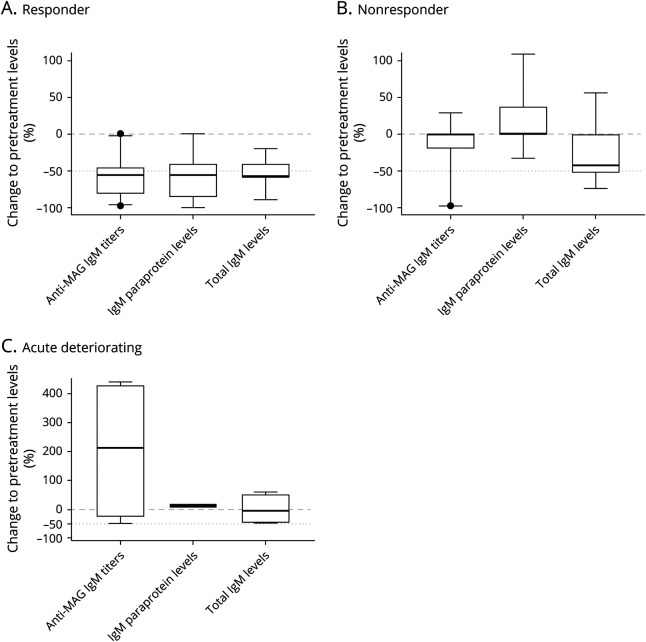
Regardless of whether the anti-MAG IgM antibodies were assessed in titer units (e.g., Bühlmann Titer Units or Western blotting), paraprotein levels (g/L), or total IgM levels (g/L), a significant reduction was observed in the responder group compared with the nonresponder or acute deteriorating group (Figure 3). In the responder group, the mean anti-MAG IgM titers were reduced by 57.5% ± 28.1% SD, the mean paraprotein levels by 57.5% ± 31.3% SD, and the mean total IgM levels by 52.3% ± 19.3% SD compared with pretreatment levels. The nonresponder group exhibited a reduction of 11.3% ± 30.9% SD in anti-MAG IgM titers, an increase in paraprotein levels of 16.3% ± 45.8% SD, and in total IgM levels of 26.8% ± 36.0% SD compared with the pretreatment levels. The acute deteriorating group exhibited an increase in anti-MAG titers of 204.3% ± 253.4% SD, an increase in paraprotein levels of 11.50% ± 3.5 SD, and a reduction of −0.5% ± 54.64% SD in total IgM levels. However, the small number of patients and the large SD makes it difficult to conclude that the transient acute worsening is associated with an increase in anti-MAG titers.
Figure 3. Comparison of the Relative Change in Serum anti-MAG IgM Titers or Levels Between Responder, Nonresponder, and Acute Deteriorating Groups.
Comparison of clinical improvement and relative change in serum anti-MAG IgM titers, paraprotein levels, and total IgM levels in the (A) responder group, (B) nonresponder group, (C) and the acute deteriorating group. Data are shown as median and 95% confidence intervals. MAG = myelin‐associated glycoprotein.
Remarkably, 77.7% of all responders exhibited a relative reduction of more than 50.0% in anti-MAG IgM titers compared with pretreatment titers. In responders, 62.1% experienced more than a 50.0% reduction in IgM paraprotein and 49.2% experienced more than a 50% reduction in total IgM levels. Conversely, more than 90.0% of nonresponders showed a reduction of less than 20.0% in anti-MAG IgM titers (94.1%) or IgM paraprotein (93.3%), and 70.9% of nonresponders showed a reduction of less than 20.0% in total IgM levels compared with pretreatment levels.
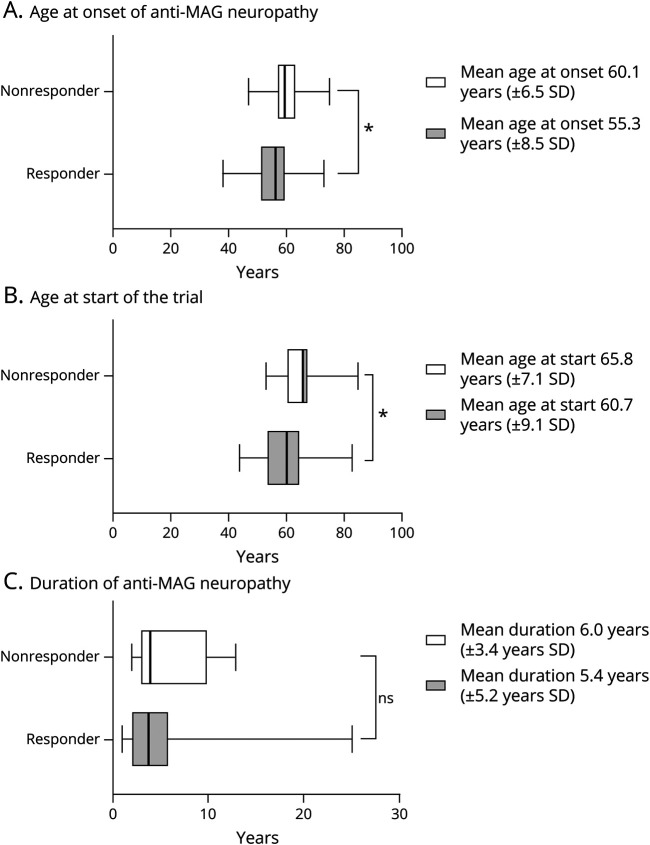
Besides the comparison of the relative change in serum anti-MAG IgM titers or levels and clinical outcome measures, we analyzed the responder and nonresponder groups in age at neuropathy onset, age when patients participated in the clinical studies, and the duration of the neuropathy until the patients participated in the clinical trial and received the specific treatment (Figure 4).
Figure 4. Analysis of the Clinical Study Patient Population.
(A) Mean age at onset of the neuropathy and (B) the mean age at the start of the clinical study was significantly lower in the responder group (n = 208 participants) compared with the nonresponder group (n = 191 participants); (C) The difference in disease duration until the patients participated in the clinical study was not significant. Data are shown as median and 95% confidence intervals, an independent t-test and Tukey Kramer test were performed (p < 0.05).
The mean age at onset of the neuropathy in the nonresponder group (60.1 ± 6.5 years SD) was significantly (p ≤ 0.05) higher compared with the responder group (55.3 ± 8.5 years SD). Likewise, the mean age when the patients with anti-MAG neuropathy were included in the clinical study was significantly higher in the nonresponder group with 65.8 years (±7.1 years SD) compared with 60.7 years (±9.1 years SD) in the responder group (p ≤ 0.05). Surprisingly, there was no significant difference in the duration of the neuropathy at the time point when the participants were included in the clinical study and receiving immunotherapies in the nonresponder group (6.0 ± 3.4 years SD) compared with the responder group (5.4 ± 5.2 years SD, p > 0.05).
Discussion
To date, an association between anti-MAG IgM titers or paraprotein levels and either the severity spectrum or the progression of anti-MAG neuropathy has not been convincingly shown.17,47 However, this retrospective analysis of 50 clinical trials in anti-MAG neuropathy demonstrates that most analyzed studies are supportive of the hypothesis that (1) a relative reduction in anti-MAG IgM antibodies was associated with clinical improvement in the responder group, (2) the nonresponder group exhibited no or only minimal change in anti-MAG IgM titers and levels, and (3) acute worsening was associated with an increase in transient anti-MAG titers.
The variety of the clinical outcome measures, including disability scores, strength, and ataxia scores or patient-reported outcomes, makes a direct comparison of the clinical outcomes among studies difficult, especially as many of the measures are nominal or ordinal and, therefore, are descriptive values. They are often misinterpreted as numerical values including the assumption of linearity, which would be required for statistical calculations.e23 Hence, a correlation coefficient was not calculated for reduction of autoantibodies and clinical improvements. Nonetheless, a strong association between relative reduction in anti-MAG antibodies and clinical improvements is supported by this retrospective analysis. Independent of whether the assessment was performed in anti-MAG IgM titer units, or paraprotein levels (g/L), most responders (77.7%) exhibited a mean reduction of more than 50% compared with their pretreatment values. In addition, only 6% of nonresponders exhibited a reduction of more than 20% in anti-MAG IgM titers or paraprotein during the clinical studies. These findings suggest that a relative reduction of more than 50% in anti-MAG IgM titer units or paraprotein levels during the course of treatment is a useful biomarker for sustained clinical improvement; although a relative reduction of less than 20% indicates an insufficient response to the immunotherapy, regardless of whether it is assessed in anti-MAG titer units or paraprotein levels.
In most of the studies not supporting the hypothesis, the authors commented on possible reasons for the contradictory observations. For example, in several studies, patients exhibited anti-MAG IgM titers above the upper cutoff value of the ELISA, making it difficult to detect a reduction of anti-MAG IgM titers.27,28,e6,e24 In contrast to anti-MAG IgM titers, monoclonal IgM paraprotein levels are assessed as absolute amounts (g/L) with no specific upper cutoff value and may be considered as more reliable indicators of the hematologic response in patients with high anti-MAG IgM titers.e25 Nonetheless, measuring changes in paraprotein levels in patients with low baseline levels is challenging because of the lower limit of detection.e16,e17,e26 In addition, paraprotein measurements neither assess the reactivity nor affinity of the monoclonal component. Total IgM measurement is the least sensitive method as shown in different studies.e9,e14 In light of these findings, it might be beneficial for future clinical studies to measure both anti-MAG IgM titers and paraprotein levels in order to cover the entire range of anti-MAG IgM autoantibodies during immunotherapy.
Two factors that may affect the response to treatment are the advanced stage of disease and the severity of axonal damage as discussed by Rakocevic et al.14 They suggest advanced axonal damage as a reason for the observation that even an almost complete depletion of CD20+ B cells and circulating anti-MAG antibodies did not lead to clinical improvements. Biomarkers of axonal damage, such as neurofilament light chain, could prove to be a valuable indicator of poor response to treatment in anti-MAG neuropathy, as has been described in other peripheral neuropathies such as Guillain-Barré syndrome or chronic inflammatory demyelinating polyneuropathy.e27-e29 Furthermore, cases of CD20+ B cell depletion by rituximab without a reduction of anti-MAG IgM autoantibodies or clinical improvement suggests that anti-MAG IgM antibody producing cells were most likely late-stage CD20- B cells or plasma cells.e12
Another factor that should be taken in account is the time of the treatment relative to clinical assessment and, importantly, the follow-up phase after the course of treatment. Peripheral nerves have the potential for both remyelination and regeneration, which requires time.e30 As a consequence, efficient and sustained depletion of anti-MAG IgM autoantibodies from the circulation would not necessarily, immediately, remove the pathogenic antibodies from myelin, but could, at least, prevent the binding of new autoantibodies to myelin, leading to long-term stabilization or improvement of the disease. Current outcome measures are often limited in their ability to capture minimal but clinically important differences in disease status. Clinical assessment of patients with anti-MAG neuropathy may, thus, need to be adjusted to better capture early clinically meaningful signs of improvements.e31
Other parameters of the patient population were analyzed. Interestingly, the duration of the neuropathy until participation in the clinical studies had no significant impact on the response to treatment. However, responders had a significant lower age at onset of the neuropathy and were significantly younger at the time point when they participated in the clinical study. Based on the limited data, no firm conclusion can be made and a sufficiently large natural history study may be more appropriate to clarify the impact of the onset and duration of neuropathy on treatment outcome.
Taken together, most studies support the hypothesis that there is a strong association between relative changes in anti-MAG IgM autoantibodies and clinical outcomes in patients with anti-MAG neuropathy and, specifically, that a reduction in anti-MAG autoantibodies is associated with improvement of symptoms. The retrospective analysis indicates that a sustained relative reduction of more than 50% compared with the pretreatment anti-MAG IgM titers units or paraprotein levels is associated with clinical improvements. Thus, both of these parameters could be valuable biomarkers and predictors for long-term immunotherapy response in patients with anti-MAG neuropathy.
Glossary
- IgM
immunoglobulin M (IgM)
- IVIg
IV immunoglobulins
- MAG
anti-myelin-associated glycoprotein
- MGUS
monoclonal gammopathy of undetermined significance
- MM
multiple myeloma
- WM
Waldenström macroglobulinemia
Appendix. Authors
Contributor Information
Butrint Aliu, Email: b.aliu@unibas.ch.
Kea Martin, Email: k.martin@polyneuron.com.
Ruben Herrendorff, Email: r.herrendorff@polyneuron.com.
Andreas Johann Steck, Email: andreas.steck@unibas.ch.
Study Funding
The authors report no targeted funding.
Disclosure
P. Hänggi, R. Herrendorff, and A.J. Steck are cofounders of a University of Basel spin-off, Polyneuron Pharmaceuticals AG, whose activity is related to the subject matter of this article. R. Herrendorff is also a member of the board of directors, and P. Hänggi, K. Martin, and R. Herrendorff are employees of Polyneuron Pharmaceuticals AG. Go to Neurology.org/NN for full disclosures.
References
- 1.Talamo G, Mir MA, Pandey MK, Sivik JK, Raheja D. IgM MGUS associated with anti-MAG neuropathy: a single institution experience. Ann Hematol. 2015;94(6):1011-1016. [DOI] [PubMed] [Google Scholar]
- 2.Quarles RH. Myelin-associated glycoprotein (MAG): past, present and beyond. J Neurochem. 2007;100(6):1431-1448. [DOI] [PubMed] [Google Scholar]
- 3.Voshol H, van Zuylen CW, Orberger G, Vliegenthart JF, Schachner M. Structure of the HNK-1 carbohydrate epitope on bovine peripheral myelin glycoprotein P0. J Biol Chem. 1996;271(38):22957-22960. [DOI] [PubMed] [Google Scholar]
- 4.Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system. Nat Rev Immunol. 2007;7(4):255-266. [DOI] [PubMed] [Google Scholar]
- 5.Kelm S, Pelz A, Schauer R, et al. Sialoadhesin, myelin-associated glycoprotein and CD22 define a new family of sialic acid-dependent adhesion molecules of the immunoglobulin superfamily. Curr Biol. 1994;4(11):965-972. [DOI] [PubMed] [Google Scholar]
- 6.Latov N, Braun PE, Gross RB, Sherman WH, Penn AS, Chess L. Plasma cell dyscrasia and peripheral neuropathy: identification of the myelin antigens that react with human paraproteins. Proc Natl Acad Sci U S A. 1981;78(11):7139-7142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dalakas MC. Pathogenesis of immune-mediated neuropathies. Biochim Biophys Acta. 2015;1852(4):658-666. [DOI] [PubMed] [Google Scholar]
- 8.Dalakas MC. Advances in the diagnosis, immunopathogenesis and therapies of IgM-anti-MAG antibody-mediated neuropathies. Ther Adv Neurol Disord. 2018;11:1756285617746640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Magy L, Kaboré R, Mathis S, et al. Heterogeneity of polyneuropathy associated with anti-MAG antibodies. J Immunol Res. 2015;2015:450391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vallat JM, Magy L, Ciron J, Corcia P, Le Masson G, Mathis S. Therapeutic options and management of polyneuropathy associated with anti-MAG antibodies. Expert Rev Neurother. 2016;16(9):1111-1119. [DOI] [PubMed] [Google Scholar]
- 11.Mygland A, Monstad P. Chronic polyneuropathies in vest-agder, Norway. Eur J Neurol. 2001;8(2):157-165. [DOI] [PubMed] [Google Scholar]
- 12.Mahdi-Rogers M, Hughes RA. Epidemiology of chronic inflammatory neuropathies in southeast England. Eur J Neurol. 2014;21(1):28-33. [DOI] [PubMed] [Google Scholar]
- 13.Briani C, Visentin A, Salvalaggio A, Cacciavillani M, Trentin L. Obinutuzumab, a new anti-CD20 antibody, and chlorambucil are active and effective in anti-myelin-associated glycoprotein antibody polyneuropathy. Eur J Neurol. 2019;26(2):371-375. [DOI] [PubMed] [Google Scholar]
- 14.Rakocevic G, Martinez-Outschoorn U, Dalakas MC. Obinutuzumab, a potent anti-B-cell agent, for rituximab-unresponsive IgM anti-MAG neuropathy. Neurol Neuroimmunol Neuroinflamm. 2018;5(4):e460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lunn MP, Nobile-Orazio E. Immunotherapy for IgM anti-myelin-associated glycoprotein paraprotein-associated peripheral neuropathies. Cochrane Database Syst Rev. 2016;10(10):CD002827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Codron P, Cousin M, Subra JF, et al. Therapeutic plasma exchange in chronic dysimmune peripheral neuropathies: a 10-year retrospective study. J Clin Apher. 2017;32(6):413-422. [DOI] [PubMed] [Google Scholar]
- 17.Svahn J, Petiot P, Vial C, et al. Anti-MAG antibodies in 202 patients: clinicopathological and therapeutic features. J Neurol Neurosurg Psychiatry. 2018;89(5):499-505. [DOI] [PubMed] [Google Scholar]
- 18.Dyck PJ, Low PA, Windebank AJ, et al. Plasma exchange in polyneuropathy associated with monoclonal gammopathy of undetermined significance. N Engl J Med. 1991;325(21):1482-1486. [DOI] [PubMed] [Google Scholar]
- 19.Oksenhendler E, Chevret S, Léger JM, Louboutin JP, Bussel A, Brouet JC. Plasma exchange and chlorambucil in polyneuropathy associated with monoclonal IgM gammopathy. IgM-associated Polyneuropathy Study Group. J Neurol Neurosurg Psychiatry. 1995;59(3):243-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Comi G, Roveri L, Swan A, et al. A randomised controlled trial of intravenous immunoglobulin in IgM paraprotein associated demyelinating neuropathy. J Neurol. 2002;249(10):1370-1377. [DOI] [PubMed] [Google Scholar]
- 21.Noronha V, Fynan TM, Duffy T. Flare in neuropathy following rituximab therapy for Waldenstrom's macroglobulinemia. J Clin Oncol. 2006;24(1):e3. [DOI] [PubMed] [Google Scholar]
- 22.Rudnicki SA, Harik SI, Dhodapkar M, Barlogie B, Eidelberg D. Nervous system dysfunction in Waldenström's macroglobulinemia: response to treatment. Neurology. 1998;51(4):1210-1213. [DOI] [PubMed] [Google Scholar]
- 23.Ellie E, Vital A, Steck A, Boiron JM, Vital C, Julien J. Neuropathy associated with "benign" anti-myelin-associated glycoprotein IgM gammopathy: clinical, immunological, neurophysiological pathological findings and response to treatment in 33 cases. J Neurol. 1996;243(1):34-43. [DOI] [PubMed] [Google Scholar]
- 24.Pestronk A, Florence J, Miller T, Choksi R, Al-Lozi MT, Levine TD. Treatment of IgM antibody associated polyneuropathies using rituximab. J Neurol Neurosurg Psychiatry. 2003;74(4):485-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levine TD, Pestronk A. IgM antibody-related polyneuropathies: B-cell depletion chemotherapy using Rituximab. Neurology. 1999;52(8):1701-1704. [DOI] [PubMed] [Google Scholar]
- 26.Wilson HC, Lunn MP, Schey S, Hughes RA. Successful treatment of IgM paraproteinaemic neuropathy with fludarabine. J Neurol Neurosurg Psychiatry. 1999;66(5):575-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doneddu PE, Kazmi M, Samuel M, Mahdi-Rogers M, Hadden RDM. Deterioration of tremor after treatment with rituximab in anti-MAG neuropathy. J Neurol Sci. 2017;373:344-345. [DOI] [PubMed] [Google Scholar]
- 28.Zara G, Zambello R, Ermani M. Neurophysiological and clinical responses to rituximab in patients with anti-MAG polyneuropathy. Clin Neurophysiol. 2011;122(12):2518-2522. [DOI] [PubMed] [Google Scholar]
- 29.Sherman WH, Olarte MR, McKiernan G, Sweeney K, Latov N, Hays AP. Plasma exchange treatment of peripheral neuropathy associated with plasma cell dyscrasia. J Neurol Neurosurg Psychiatry. 1984;47(8):813-819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dalakas MC, Rakocevic G, Salajegheh M, et al. Placebo-controlled trial of rituximab in IgM anti-myelin-associated glycoprotein antibody demyelinating neuropathy. Ann Neurol. 2009;65(3):286-293. [DOI] [PubMed] [Google Scholar]
- 31.Gruson B, Ghomari K, Beaumont M, et al. Long-term response to rituximab and fludarabine combination in IgM anti-myelin-associated glycoprotein neuropathy. J Peripher Nerv Syst. 2011;16(3):180-185. [DOI] [PubMed] [Google Scholar]
- 32.Weiss MD, Becker P. Paradoxical worsening of anti–myelin-associated glycoprotein polyneuropathy following rituximab. Muscle Nerve. 2014;49(3):457-458. [DOI] [PubMed] [Google Scholar]
- 33.Sala E, Robert-Varvat F, Paul S, Camdessanché JP, Antoine JC. Acute neurological worsening after Rituximab treatment in patients with anti-MAG neuropathy. J Neurol Sci. 2014;345(1-2):224-227. [DOI] [PubMed] [Google Scholar]
- 34.Baron M, Lozeron P, Harel S, et al. Plasma exchanges for severe acute neurological deterioration in patients with IgM anti-myelin-associated glycoprotein (anti-MAG) neuropathy. J Neurol. 2017;264(6):1132-1135. [DOI] [PubMed] [Google Scholar]
- 35.Renaud S, Gregor M, Fuhr P, et al. Rituximab in the treatment of polyneuropathy associated with anti-MAG antibodies. Muscle Nerve. 2003;27(5):611-615. [DOI] [PubMed] [Google Scholar]
- 36.Renaud S, Fuhr P, Gregor M, et al. High-dose rituximab and anti-MAG-associated polyneuropathy. Neurology. 2006;66(5):742-744. [DOI] [PubMed] [Google Scholar]
- 37.Benedetti L, Briani C, Grandis M, et al. Predictors of response to rituximab in patients with neuropathy and anti-myelin associated glycoprotein immunoglobulin M. J Peripher Nerv Syst. 2007;12(2):102-107. [DOI] [PubMed] [Google Scholar]
- 38.Benedetti L, Briani C, Franciotta D, et al. Long-term effect of rituximab in anti-mag polyneuropathy. Neurology. 2008;71(21):1742-1744. [DOI] [PubMed] [Google Scholar]
- 39.Kilidireas C, Anagnostopoulos A, Karandreas N, Mouselimi L, Dimopoulos MA. Rituximab therapy in monoclonal IgM-related neuropathies. Leuk Lymphoma. 2006;47(5):859-864. [DOI] [PubMed] [Google Scholar]
- 40.Souayah N, Noopur R, Tick-Chong PS. Beneficial effects of Rituximab in patients with anti-MAG (myelin-associated glycoprotein) neuropathy: case reports. Immunopharmacol Immunotoxicol. 2013;35(5):622-624. [DOI] [PubMed] [Google Scholar]
- 41.Léger JM, Viala K, Nicolas G, et al. Placebo-controlled trial of rituximab in IgM anti-myelin-associated glycoprotein neuropathy. Neurology. 2013;80(24):2217-2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iancu Ferfoglia R, Guimarães-Costa R, Viala K, et al. Long-term efficacy of rituximab in IgM anti-myelin-associated glycoprotein neuropathy: RIMAG follow-up study. J Peripher Nerv Syst. 2016;21(1):10-14. [DOI] [PubMed] [Google Scholar]
- 43.Hospital MA, Viala K, Dragomir S, et al. Immunotherapy-based regimen in anti-MAG neuropathy: results in 45 patients. Haematologica. 2013;98(12):e155-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gorson KC, Ropper AH, Weinberg DH, Weinstein R. Treatment experience in patients with anti-myelin-associated glycoprotein neuropathy. Muscle Nerve. 2001;24(6):778-786. [DOI] [PubMed] [Google Scholar]
- 45.Duncombe AS, et al. R-CP chemoimmunotherapy in patients with IgM paraproteinaemic neuropathy produces improvements in functional, electrophysiological and serological outcomes. Hematological Oncol. 2017;35(S2):217-218. [Google Scholar]
- 46.Nobile-Orazio E, Baldini L, Barbieri S, et al. Treatment of patients with neuropathy and anti-MAG IgM M-proteins. Ann Neurol 1988;24(1):93-97. [DOI] [PubMed] [Google Scholar]
- 47.Campagnolo M, Zambello R, Nobile-Orazio E, et al. IgM MGUS and Waldenstrom-associated anti-MAG neuropathies display similar response to rituximab therapy. J Neurol Neurosurg Psychiatry. 2017;88(12):1094-1097. [DOI] [PubMed] [Google Scholar]
- 48.Niermeijer JM, Eurelings M, van der Linden MW, et al. Intermittent cyclophosphamide with prednisone versus placebo for polyneuropathy with IgM monoclonal gammopathy. Neurology. 2007;69(1):50-59. [DOI] [PubMed] [Google Scholar]
- 49.Niermeijer JM, Eurelings M, Lokhorst H, et al. Neurologic and hematologic response to fludarabine treatment in IgM MGUS polyneuropathy. Neurology. 2006;67(11):2076-2079. [DOI] [PubMed] [Google Scholar]
- 50.Kelly JJ, Adelman LS, Berkman E, Bhan I. Polyneuropathies associated with IgM monoclonal gammopathies. Arch Neurol. 1988;45(12):1355-1359. Additional eReferences e1-e31: links.lww.com/NXI/A649. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data and the statistical analysis are available in the manuscript; the supplemental data are reported in the original articles cited in the manuscript.