Please provide a synopsis (of no more than two sentences) for inclusion in the Contents listing of the journal.
Keywords: copper(I), η 2-interaction, pyridine-2-thiol, allyl derivative, crystal structure
Abstract
The title compounds, di-μ-chlorido-bis({2-[(η-2,3)-(prop-2-en-1-yl)sulfanyl]pyridine-κN}copper(I)), [Cu2Cl2(C8H9NS)2], and di-μ-bromido-bis({2-[(η-2,3)-(prop-2-en-1-yl)sulfanyl]pyridine-κN}copper(I)), [Cu2Br2(C8H9NS)2], were obtained by alternating-current electrochemical synthesis starting from an ethanolic solution of 2-[(prop-2-en-1-yl)sulfanyl]pyridine (Psup) and the copper(II) halide. The isostructural crystals are built up from centrosymmetric [Cu2 Hal 2(Psup)2] dimers, which are formed due to the π,σ-chelating behavior of the organic ligand. In the crystals, the dimers are linked by C—H⋯Hal hydrogen bonds as well as by aromatic π–π stacking interactions into a three-dimensional network.
Chemical context
Cu-containing complexes have been found very promising regarding their catalytic activities in organic syntheses, non-linear optical properties and fluorescent activity (Wang et al., 2005 ▸; Yoshikai & Nakamura, 2012 ▸; Slyvka et al., 2018a ▸; Fedorchuk et al., 2020 ▸). Copper complexes also exhibit considerable biochemical activities, ranging from antibacterial and anti-inflammatory properties to cytostatic and enzyme inhibitory (Iakovidis et al., 2011 ▸; Tisato et al., 2010 ▸). Some of these compounds have been tested in vitro as potential anticancer drugs and found to be effective against A549 adenocarcinoma cells that are resistant to the widely used anticancer drug cisplatin (Marzano et al., 2006 ▸). It is worth noting that copper is an essential trace element with vital roles in many metalloenzymes participating in intracellular processes under normal and pathological conditions (Iakovidis et al., 2011 ▸).
Over the last two decades, increased interest has also been devoted to the crystal engineering of copper(I)–olefin complexes with allyl derivatives of heterocyclic compounds (Goreshnik et al., 2011 ▸; Slyvka et al., 2013 ▸; Hordiichuk et al., 2019 ▸). The presence of a C=C olefin bond in a substituent attached to the heterocyclic ring may serve as a key feature for the selective coordination of transition-metal ions due to metal–olefin π-bonding (Rourke, 2006 ▸; Slyvka et al., 2013 ▸; Kowalska et al., 2021 ▸). Allyl derivatives of some heterocyclic compounds were found to be suitable for the preparation of π-coordination compounds with CuI salts that are unknown (or less stable) in the free state. For instance, the first examples of Cu(C6H5SO3), Cu(p-CH3C6H4SO3) or CuHSO4 π-complexes as well as the direct CuI⋯F(SiF6
2–) interaction have been observed in copper(I) π-compounds with allyl derivatives of triazole and thiadiazole (Goreshnik et al., 2016 ▸; Ardan et al., 2017 ▸; Slyvka et al., 2018
b
▸; Fedorchuk et al., 2020 ▸). N-Allyl derivatives of pyridine were found to be suitable ligands for the crystal engineering of CuI coordination compounds with inorganic fragments of different complexibility and related to the pK
a values of the initial pyridine bases (Goreshnik et al., 2003 ▸; Pavlyuk et al., 2005 ▸). Taking into account the fact that allylsulfanyl derivatives of pyridine have not been investigated for their coordination behavior regarding copper(I), in this work we present the synthesis and structural characterization of two novel copper(I) halide π-coordination compounds [Cu2Cl2(Psup)2] (I) & [Cu2Br2(Psup)2] (II) with 2-[(prop-2-en-1-yl)sulfanyl]pyridine (Psup), C8H9NS.
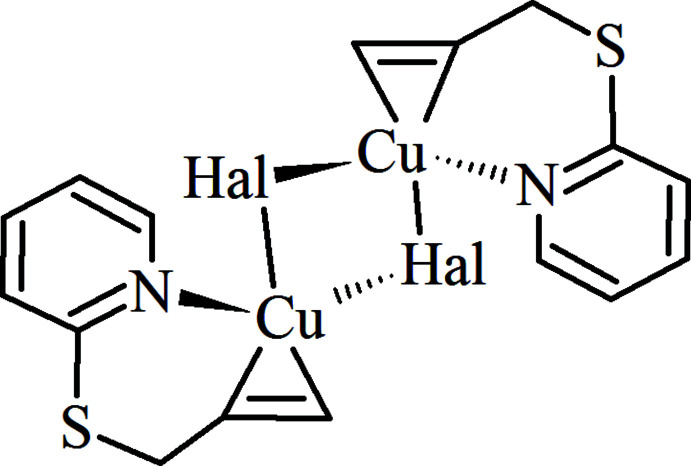
Structural commentary
The title compounds are isostructural and crystallize in the centrosymmetric space group P21/c with one Psup organic molecule, one copper(I) ion and one halide ion in the asymmetric unit. As shown in Figs. 1 ▸ and 2 ▸, these structures are constructed from centrosymmetric [Cu2 Hal 2(Psup)2] [Hal = Cl (I) or Br (II)] dimers, which are formed due to the chelating behavior of the organic ligand. A close to trigonal–pyramidal coordination environment of the CuI cation includes the η 2 allylic C=C bond, the pyridine N atom and a Hal1 ion in the basal plane (Tables 1 ▸ and 2 ▸). The apical position of the CuI polyhedron is occupied by the Hal1i [symmetry code: (i) −x + 1, −y + 1, −z + 1) ion at 2.6186 (9) Å in I and at 2.7113 (6) Å in II. The corresponding four-coordinate geometry indices τ 4 (Yang et al., 2007 ▸) are 0.81 (I) and 0.83 (II). For comparison, in the structures of previously studied CuCl and CuBr π,σ-complexes with allylacetoneoxime, the Cu—Hal ap distances are slightly higher at 2.719 (5) and 2.778 (4) Å (Filinchuk et al., 1998 ▸).
Figure 1.
The molecular structure of I with displacement ellipsoids drawn at the 50% probability level. Symmetry code: (i) −x + 1, −y + 1, −z + 1.
Figure 2.
The molecular structure of II with displacement ellipsoids drawn at the 50% probability level. Symmetry code: (i) −x + 1, −y + 1, −z + 1.
Table 1. Selected bond lengths (Å) for I .
| Cu1—Cl1 | 2.2691 (9) | Cu1—C8 | 2.037 (3) |
| Cu1—Cl1i | 2.6186 (9) | Cu1—C9 | 2.052 (3) |
| Cu1—N1 | 2.026 (2) |
Symmetry code: (i) -x+1, -y+1, -z+1.
Table 2. Selected bond lengths (Å) for II .
| Cu1—Br1 | 2.4097 (6) | Cu1—C8 | 2.048 (4) |
| Cu1—Br1i | 2.7113 (6) | Cu1—C9 | 2.065 (4) |
| Cu1—N1 | 2.025 (3) |
Symmetry code: (i) -x+1, -y+1, -z+1.
Being π-connected to the metal center, the C8=C9 bond of the ligand is elongated due to back-donation from an occupied 3d metal orbital to a low-lying empty π*-orbital of the olefin to 1.364 (4) Å (I) and to 1.354 (6) Å (II) in comparison with an uncoordinated allylic C=C bond (Slyvka et al., 2021 ▸). The allylsulfanyl group in (I) and (II) has synclinal conformation relative to the S1—C7 bond and an antiperiplanar conformation relative to the C7—C8 bond [the corresponding torsion angles C2—S1—C7—C8 and S1—C7—C8—C9 are 68.1 (3) and −152.1 (3)°, respectively, in I and 68.3 (3) and −151.7 (3)°(II)].
Supramolecular features
As shown in Fig. 3 ▸ and listed in Tables 3 ▸ and 4 ▸, the crystal structures of (I) and (II) features several weak intermolecular interactions. The hydrogen atom H6 of the pyridine ring participates in an intramolecular C—H⋯Hal bond with the Hal ion of the inorganic subunit. The other hydrogen atom H6 of the pyridine ring and the methylene hydrogen atom H7B of the allylsulfanyl substituent are involved in intermolecular C—H⋯Hal bonding, linking the dimeric moieties into a three-dimensional network. The pyridine rings of adjacent dimers are also involved in face-to-face π–π stacking interactions with a centroid–centroid separation of 3.680 (4) Å in I and 3.693 (4) Å in II. The unit-cell packing for (I) is shown in Fig. 4 ▸.
Figure 3.
Fragment of the extended structure of I with hydrogen bonds shown as dashed lines. Symmetry codes: (i) −x + 1, −y + 1, −z + 1; (ii) x + 1, y, z; (iii) −x + 1, y +
 , −z +
, −z +
 ; (iv) x, −y +
; (iv) x, −y +
 , −z +
, −z +
 . The packing for II is essentially identical.
. The packing for II is essentially identical.
Table 3. Hydrogen-bond geometry (Å, °) for I .
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C3—H3⋯Cl1ii | 0.95 | 2.91 | 3.581 (3) | 129 |
| C6—H6⋯Cl1 | 0.95 | 2.80 | 3.447 (3) | 126 |
| C7—H7B⋯Cl1iii | 0.99 | 2.89 | 3.676 (3) | 137 |
Symmetry codes: (ii) x+1, y, z; (iii) -x+1, y+{\script{1\over 2}}, -z+{\script{1\over 2}}.
Table 4. Hydrogen-bond geometry (Å, °) for II .
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C3—H3⋯Br1ii | 0.95 | 3.02 | 3.696 (4) | 129 |
| C6—H6⋯Br1 | 0.95 | 2.94 | 3.576 (4) | 126 |
| C7—H7B⋯Br1iii | 0.99 | 2.94 | 3.744 (4) | 139 |
Symmetry codes: (ii) x+1, y, z; (iii) -x+1, y+{\script{1\over 2}}, -z+{\script{1\over 2}}.
Figure 4.
A view along the a-axis direction of the crystal packing of I.
Database survey
The most closest related compounds to the title compounds, containing a similar {Cu2 Hal 2} dimer in which a π,σ-chelating ligand is bound to copper(I) are: di-μ-chlorobis[(1-allyl-3,5-dimethylpyrazole)copper(I)] (III) [Cambridge Structural Database (Version 2021.1; Groom et al., 2016 ▸) refcode ALMPCU; Fukushima et al., 1976 ▸], bis(μ 2-chloro)-bis(η 2-allylacetoneoxime-N)dicopper(I) (IV) (GOKYAG; Filinchuk et al., 1998 ▸), bis(μ 2-bromo)-bis(η 2-allylacetoneoxime-N)dicopper(I) (V) (GOKYEK; Filinchuk et al., 1998 ▸), bis[(μ 2-bromo)(η 2-2-(allylthio)benzimidazole-N)copper(I)] (VI) (WUCRAN; Goreshnik et al., 2002 ▸) and bis{(μ 2-iodo)[(η 2-allyl)(2-pyridyl)dimethylsilane]copper} (VII) (XAZGIP; Kamei et al., 2005 ▸).
Compounds (III) and (VII) crystallize in the triclinic crystal system in space group P
 . Compounds (IV), (V) and (VI) crystallize in the monoclinic crystal system in space group P21/c (settings P21/a, P21/c and P21/n, respectively). Structures (III), (IV), (V) and (VI) are built up from centrosymmetric [Cu2
Hal
2(Ligand)2] dimers. In the compounds bis[(μ
2-chloro)chloro(η
2-1-allyl-2-aminopyridinium)copper(I)] (XIII) (BEBFOE) and bis[(μ
2-chloro)bromo(η
2-1-allyl-2-aminopyridinium)copper(I)] (IX) (BEBGAR; Goreshnik et al., 2003 ▸), the 1-allyl-2-aminopyridinium cation acts as a monodentate π-ligand, being attached to the centrosymmetic anionic {Cu2Hal4}2− part through the allylic C=C bond. An analogous monodentate 1-allylpyridinium cation in the structure of catena-[bis(μ
3-chloro)bis(μ
2-chloro)bis(η
2-1-allylpyridinium)dichlorotetracopper(I)] (X) (YAPQIQ; Pavlyuk et al., 2005 ▸) forces the realization of an infinite {Cu4Cl4}
n
inorganic chain.
. Compounds (IV), (V) and (VI) crystallize in the monoclinic crystal system in space group P21/c (settings P21/a, P21/c and P21/n, respectively). Structures (III), (IV), (V) and (VI) are built up from centrosymmetric [Cu2
Hal
2(Ligand)2] dimers. In the compounds bis[(μ
2-chloro)chloro(η
2-1-allyl-2-aminopyridinium)copper(I)] (XIII) (BEBFOE) and bis[(μ
2-chloro)bromo(η
2-1-allyl-2-aminopyridinium)copper(I)] (IX) (BEBGAR; Goreshnik et al., 2003 ▸), the 1-allyl-2-aminopyridinium cation acts as a monodentate π-ligand, being attached to the centrosymmetic anionic {Cu2Hal4}2− part through the allylic C=C bond. An analogous monodentate 1-allylpyridinium cation in the structure of catena-[bis(μ
3-chloro)bis(μ
2-chloro)bis(η
2-1-allylpyridinium)dichlorotetracopper(I)] (X) (YAPQIQ; Pavlyuk et al., 2005 ▸) forces the realization of an infinite {Cu4Cl4}
n
inorganic chain.
Synthesis and crystallization
Crystals of the title compounds were obtained under conditions of alternating-current electrochemical synthesis (Slyvka et al., 2018a ▸) starting from an ethanolic solution of 2-[(prop-2-en-1-yl)sulfanyl]pyridine (Psup) and the copper(II) halide. For this, a solution of Psup (1.5 mmol, 0.227 g) in 2.0 ml of 96% ethanol was added to a solution of CuCl2·2H2O (1.6 mmol, 0.273 g) or CuBr2 (1.6 mmol, 0.357 g) in 3.0 ml of 96% ethanol. The mixture was carefully stirred and then was placed into a small 5.5 ml test tube. A copper wire was wrapped into a spiral of 1 cm diameter. A straight copper wire was placed inside the spiral. These copper electrodes were inserted in a cork and immersed in the aforementioned mixture. The mixture was subjected to alternating current reduction (frequency 50 Hz, voltage 0.45 V) and after 3–4 days, good-quality slightly yellowish crystals of the title compounds appeared on the copper wire electrodes. Compound I: yield 12%, m.p. 413 K; compound II: yield 8%, m.p. 407 K.
Refinement
Crystal data, data collection and structure refinement details are summarized in Table 5 ▸. All H atoms were positioned geometrically with C—H = 0.95–0.99 Å and refined as riding atoms. The constraint U iso(H) = 1.2U eq(C) was applied in all cases.
Table 5. Experimental details.
| I | II | |
|---|---|---|
| Crystal data | ||
| Chemical formula | [Cu2Cl2(C8H9NS)2] | [Cu2Br2(C8H9NS)2] |
| M r | 500.42 | 589.34 |
| Crystal system, space group | Monoclinic, P21/c | Monoclinic, P21/c |
| Temperature (K) | 150 | 150 |
| a, b, c (Å) | 9.2729 (16), 9.5740 (13), 11.037 (2) | 9.5009 (6), 9.6022 (5), 11.0936 (8) |
| β (°) | 108.52 (2) | 107.257 (7) |
| V (Å3) | 929.1 (3) | 966.50 (11) |
| Z | 2 | 2 |
| Radiation type | Mo Kα | Mo Kα |
| μ (mm−1) | 2.80 | 6.55 |
| Crystal size (mm) | 0.33 × 0.28 × 0.19 | 0.44 × 0.35 × 0.22 |
| Data collection | ||
| Diffractometer | Rigaku New Gemini, Dual, Atlas | Rigaku New Gemini, Dual, Atlas |
| Absorption correction | Analytical (CrysAlis PRO; Rigaku OD, 2021 ▸) | Analytical (CrysAlis PRO; Rigaku OD, 2021 ▸) |
| T min, T max | 0.546, 0.693 | 0.191, 0.368 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 8088, 2161, 1730 | 6837, 2162, 1854 |
| R int | 0.058 | 0.044 |
| (sin θ/λ)max (Å−1) | 0.686 | 0.682 |
| Refinement | ||
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.036, 0.077, 1.08 | 0.034, 0.079, 1.08 |
| No. of reflections | 2161 | 2162 |
| No. of parameters | 109 | 109 |
| H-atom treatment | H-atom parameters constrained | H-atom parameters constrained |
| Δρmax, Δρmin (e Å−3) | 0.51, −0.64 | 0.82, −0.75 |
Supplementary Material
Crystal structure: contains datablock(s) I, II, publication_text. DOI: 10.1107/S2056989021011002/hb7993sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989021011002/hb7993Isup2.hkl
Structure factors: contains datablock(s) II. DOI: 10.1107/S2056989021011002/hb7993IIsup3.hkl
Additional supporting information: crystallographic information; 3D view; checkCIF report
supplementary crystallographic information
Di-µ-chlorido-bis({2-[(η-2,3)-(prop-2-en-1-yl)sulfanyl]pyridine-κN}copper(I)) (I) . Crystal data
| [Cu2Cl2(C8H9NS)2] | F(000) = 504 |
| Mr = 500.42 | Dx = 1.789 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation, λ = 0.71073 Å |
| a = 9.2729 (16) Å | Cell parameters from 3255 reflections |
| b = 9.5740 (13) Å | θ = 3.8–28.9° |
| c = 11.037 (2) Å | µ = 2.80 mm−1 |
| β = 108.52 (2)° | T = 150 K |
| V = 929.1 (3) Å3 | Irregular, yellowish |
| Z = 2 | 0.33 × 0.28 × 0.19 mm |
Di-µ-chlorido-bis({2-[(η-2,3)-(prop-2-en-1-yl)sulfanyl]pyridine-κN}copper(I)) (I) . Data collection
| New Gemini, Dual, Cu at home/near, Atlas diffractometer | 1730 reflections with I > 2σ(I) |
| Detector resolution: 10.6426 pixels mm-1 | Rint = 0.058 |
| ω scans | θmax = 29.2°, θmin = 2.9° |
| Absorption correction: analytical (CrysalisPro; Rigaku OD, 2021) | h = −12→12 |
| Tmin = 0.546, Tmax = 0.693 | k = −12→11 |
| 8088 measured reflections | l = −15→15 |
| 2161 independent reflections |
Di-µ-chlorido-bis({2-[(η-2,3)-(prop-2-en-1-yl)sulfanyl]pyridine-κN}copper(I)) (I) . Refinement
| Refinement on F2 | Primary atom site location: dual |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.036 | H-atom parameters constrained |
| wR(F2) = 0.077 | w = 1/[σ2(Fo2) + (0.0228P)2 + 0.6316P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.08 | (Δ/σ)max = 0.001 |
| 2161 reflections | Δρmax = 0.51 e Å−3 |
| 109 parameters | Δρmin = −0.64 e Å−3 |
| 0 restraints |
Di-µ-chlorido-bis({2-[(η-2,3)-(prop-2-en-1-yl)sulfanyl]pyridine-κN}copper(I)) (I) . Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
Di-µ-chlorido-bis({2-[(η-2,3)-(prop-2-en-1-yl)sulfanyl]pyridine-κN}copper(I)) (I) . Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Cu1 | 0.57740 (4) | 0.64590 (4) | 0.46958 (4) | 0.01225 (12) | |
| Cl1 | 0.36070 (8) | 0.54060 (8) | 0.34752 (7) | 0.01340 (18) | |
| S1 | 0.91865 (9) | 0.82046 (8) | 0.47781 (8) | 0.01693 (19) | |
| N1 | 0.7401 (3) | 0.5908 (2) | 0.3922 (2) | 0.0098 (5) | |
| C2 | 0.8718 (3) | 0.6558 (3) | 0.4035 (3) | 0.0113 (6) | |
| C3 | 0.9837 (3) | 0.5968 (3) | 0.3594 (3) | 0.0142 (7) | |
| H3 | 1.075431 | 0.645998 | 0.368256 | 0.017* | |
| C4 | 0.9603 (4) | 0.4679 (3) | 0.3036 (3) | 0.0175 (7) | |
| H4 | 1.036450 | 0.425281 | 0.275400 | 0.021* | |
| C5 | 0.8239 (4) | 0.4003 (3) | 0.2888 (3) | 0.0158 (7) | |
| H5 | 0.803694 | 0.311139 | 0.249195 | 0.019* | |
| C6 | 0.7184 (3) | 0.4656 (3) | 0.3331 (3) | 0.0145 (7) | |
| H6 | 0.624182 | 0.419633 | 0.321361 | 0.017* | |
| C7 | 0.7410 (4) | 0.8972 (3) | 0.4778 (3) | 0.0145 (7) | |
| H7A | 0.759319 | 0.996020 | 0.504812 | 0.017* | |
| H7B | 0.669754 | 0.896375 | 0.389294 | 0.017* | |
| C8 | 0.6665 (4) | 0.8249 (3) | 0.5633 (3) | 0.0137 (7) | |
| H8 | 0.729407 | 0.776905 | 0.636305 | 0.016* | |
| C9 | 0.5133 (4) | 0.8248 (3) | 0.5412 (3) | 0.0198 (8) | |
| H9A | 0.447951 | 0.872113 | 0.468765 | 0.024* | |
| H9B | 0.471792 | 0.777530 | 0.598143 | 0.024* |
Di-µ-chlorido-bis({2-[(η-2,3)-(prop-2-en-1-yl)sulfanyl]pyridine-κN}copper(I)) (I) . Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Cu1 | 0.0103 (2) | 0.0106 (2) | 0.0177 (2) | −0.00093 (15) | 0.00702 (17) | −0.00293 (16) |
| Cl1 | 0.0082 (4) | 0.0164 (4) | 0.0146 (4) | −0.0007 (3) | 0.0022 (3) | 0.0002 (3) |
| S1 | 0.0130 (4) | 0.0140 (4) | 0.0249 (5) | −0.0034 (3) | 0.0076 (4) | −0.0022 (3) |
| N1 | 0.0066 (12) | 0.0092 (12) | 0.0125 (13) | 0.0009 (10) | 0.0013 (11) | 0.0005 (11) |
| C2 | 0.0131 (15) | 0.0132 (15) | 0.0063 (14) | 0.0031 (13) | 0.0013 (13) | 0.0022 (12) |
| C3 | 0.0097 (15) | 0.0187 (17) | 0.0160 (16) | 0.0024 (13) | 0.0064 (13) | 0.0057 (14) |
| C4 | 0.0147 (16) | 0.0270 (19) | 0.0129 (16) | 0.0101 (14) | 0.0073 (14) | 0.0026 (15) |
| C5 | 0.0192 (17) | 0.0145 (16) | 0.0128 (16) | 0.0050 (14) | 0.0037 (14) | −0.0029 (13) |
| C6 | 0.0117 (15) | 0.0160 (16) | 0.0162 (16) | 0.0010 (13) | 0.0049 (14) | 0.0018 (13) |
| C7 | 0.0176 (17) | 0.0091 (15) | 0.0184 (16) | −0.0004 (13) | 0.0079 (14) | −0.0004 (13) |
| C8 | 0.0182 (17) | 0.0080 (15) | 0.0154 (16) | −0.0003 (13) | 0.0059 (14) | −0.0017 (13) |
| C9 | 0.0245 (19) | 0.0091 (16) | 0.031 (2) | 0.0003 (14) | 0.0164 (16) | −0.0026 (14) |
Di-µ-chlorido-bis({2-[(η-2,3)-(prop-2-en-1-yl)sulfanyl]pyridine-κN}copper(I)) (I) . Geometric parameters (Å, º)
| Cu1—Cl1 | 2.2691 (9) | C4—H4 | 0.9500 |
| Cu1—Cl1i | 2.6186 (9) | C4—C5 | 1.383 (4) |
| Cu1—N1 | 2.026 (2) | C5—H5 | 0.9500 |
| Cu1—C8 | 2.037 (3) | C5—C6 | 1.375 (4) |
| Cu1—C9 | 2.052 (3) | C6—H6 | 0.9500 |
| S1—C2 | 1.766 (3) | C7—H7A | 0.9900 |
| S1—C7 | 1.804 (3) | C7—H7B | 0.9900 |
| N1—C2 | 1.340 (4) | C7—C8 | 1.503 (4) |
| N1—C6 | 1.349 (4) | C8—H8 | 0.9500 |
| C2—C3 | 1.397 (4) | C8—C9 | 1.364 (4) |
| C3—H3 | 0.9500 | C9—H9A | 0.9500 |
| C3—C4 | 1.366 (4) | C9—H9B | 0.9500 |
| Cl1—Cu1—Cl1i | 95.20 (3) | C4—C5—H5 | 120.9 |
| N1—Cu1—Cl1i | 97.91 (7) | C6—C5—C4 | 118.1 (3) |
| N1—Cu1—Cl1 | 105.77 (7) | C6—C5—H5 | 120.9 |
| N1—Cu1—C8 | 101.34 (11) | N1—C6—C5 | 124.2 (3) |
| N1—Cu1—C9 | 136.50 (12) | N1—C6—H6 | 117.9 |
| C8—Cu1—Cl1i | 103.19 (9) | C5—C6—H6 | 117.9 |
| C8—Cu1—Cl1 | 144.63 (9) | S1—C7—H7A | 108.7 |
| C8—Cu1—C9 | 38.96 (12) | S1—C7—H7B | 108.7 |
| C9—Cu1—Cl1i | 106.96 (10) | H7A—C7—H7B | 107.6 |
| C9—Cu1—Cl1 | 106.81 (10) | C8—C7—S1 | 114.4 (2) |
| Cu1—Cl1—Cu1i | 84.80 (3) | C8—C7—H7A | 108.7 |
| C2—S1—C7 | 105.89 (15) | C8—C7—H7B | 108.7 |
| C2—N1—Cu1 | 128.2 (2) | Cu1—C8—H8 | 93.7 |
| C2—N1—C6 | 116.7 (3) | C7—C8—Cu1 | 105.2 (2) |
| C6—N1—Cu1 | 114.72 (19) | C7—C8—H8 | 118.4 |
| N1—C2—S1 | 122.7 (2) | C9—C8—Cu1 | 71.15 (18) |
| N1—C2—C3 | 122.3 (3) | C9—C8—C7 | 123.2 (3) |
| C3—C2—S1 | 115.0 (2) | C9—C8—H8 | 118.4 |
| C2—C3—H3 | 120.2 | Cu1—C9—H9A | 105.0 |
| C4—C3—C2 | 119.5 (3) | Cu1—C9—H9B | 94.9 |
| C4—C3—H3 | 120.2 | C8—C9—Cu1 | 69.90 (18) |
| C3—C4—H4 | 120.5 | C8—C9—H9A | 120.0 |
| C3—C4—C5 | 119.0 (3) | C8—C9—H9B | 120.0 |
| C5—C4—H4 | 120.5 | H9A—C9—H9B | 120.0 |
| Cu1—N1—C2—S1 | −7.6 (4) | C2—C3—C4—C5 | 1.8 (5) |
| Cu1—N1—C2—C3 | 171.1 (2) | C3—C4—C5—C6 | −1.0 (5) |
| Cu1—N1—C6—C5 | −171.2 (2) | C4—C5—C6—N1 | −1.1 (5) |
| S1—C2—C3—C4 | 178.2 (2) | C6—N1—C2—S1 | 179.9 (2) |
| S1—C7—C8—Cu1 | −75.0 (2) | C6—N1—C2—C3 | −1.4 (4) |
| S1—C7—C8—C9 | −152.1 (3) | C7—S1—C2—N1 | −19.4 (3) |
| N1—C2—C3—C4 | −0.6 (5) | C7—S1—C2—C3 | 161.8 (2) |
| C2—S1—C7—C8 | 68.1 (3) | C7—C8—C9—Cu1 | 96.2 (3) |
| C2—N1—C6—C5 | 2.3 (4) |
Symmetry code: (i) −x+1, −y+1, −z+1.
Di-µ-chlorido-bis({2-[(η-2,3)-(prop-2-en-1-yl)sulfanyl]pyridine-κN}copper(I)) (I) . Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C3—H3···Cl1ii | 0.95 | 2.91 | 3.581 (3) | 129 |
| C6—H6···Cl1 | 0.95 | 2.80 | 3.447 (3) | 126 |
| C7—H7B···Cl1iii | 0.99 | 2.89 | 3.676 (3) | 137 |
Symmetry codes: (ii) x+1, y, z; (iii) −x+1, y+1/2, −z+1/2.
Di-µ-bromido-bis({2-[(η-2,3)-(prop-2-en-1-yl)sulfanyl]pyridine-κN}copper(I)) (II) . Crystal data
| [Cu2Br2(C8H9NS)2] | F(000) = 576 |
| Mr = 589.34 | Dx = 2.025 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation, λ = 0.71073 Å |
| a = 9.5009 (6) Å | Cell parameters from 3535 reflections |
| b = 9.6022 (5) Å | θ = 3.1–29.0° |
| c = 11.0936 (8) Å | µ = 6.55 mm−1 |
| β = 107.257 (7)° | T = 150 K |
| V = 966.50 (11) Å3 | Irregular, yellowish |
| Z = 2 | 0.44 × 0.35 × 0.22 mm |
Di-µ-bromido-bis({2-[(η-2,3)-(prop-2-en-1-yl)sulfanyl]pyridine-κN}copper(I)) (II) . Data collection
| New Gemini, Dual, Cu at home/near, Atlas diffractometer | 1854 reflections with I > 2σ(I) |
| Detector resolution: 10.6426 pixels mm-1 | Rint = 0.044 |
| ω scans | θmax = 29.0°, θmin = 2.9° |
| Absorption correction: analytical (CrysalisPro; Rigaku OD, 2021) | h = −12→12 |
| Tmin = 0.191, Tmax = 0.368 | k = −10→12 |
| 6837 measured reflections | l = −12→13 |
| 2162 independent reflections |
Di-µ-bromido-bis({2-[(η-2,3)-(prop-2-en-1-yl)sulfanyl]pyridine-κN}copper(I)) (II) . Refinement
| Refinement on F2 | 0 restraints |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.034 | H-atom parameters constrained |
| wR(F2) = 0.079 | w = 1/[σ2(Fo2) + (0.0328P)2 + 1.3651P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.08 | (Δ/σ)max = 0.001 |
| 2162 reflections | Δρmax = 0.82 e Å−3 |
| 109 parameters | Δρmin = −0.74 e Å−3 |
Di-µ-bromido-bis({2-[(η-2,3)-(prop-2-en-1-yl)sulfanyl]pyridine-κN}copper(I)) (II) . Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
Di-µ-bromido-bis({2-[(η-2,3)-(prop-2-en-1-yl)sulfanyl]pyridine-κN}copper(I)) (II) . Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Br1 | 0.36045 (4) | 0.54960 (3) | 0.34080 (3) | 0.01538 (12) | |
| Cu1 | 0.58645 (5) | 0.64663 (4) | 0.47542 (4) | 0.01478 (13) | |
| S1 | 0.91726 (10) | 0.82315 (9) | 0.48313 (10) | 0.0201 (2) | |
| N1 | 0.7467 (3) | 0.5936 (3) | 0.3976 (3) | 0.0135 (6) | |
| C2 | 0.8735 (4) | 0.6601 (3) | 0.4079 (3) | 0.0127 (7) | |
| C3 | 0.9838 (4) | 0.6033 (4) | 0.3631 (3) | 0.0176 (8) | |
| H3 | 1.072777 | 0.652922 | 0.372109 | 0.021* | |
| C4 | 0.9622 (4) | 0.4747 (4) | 0.3060 (4) | 0.0191 (8) | |
| H4 | 1.036808 | 0.433779 | 0.276414 | 0.023* | |
| C5 | 0.8301 (4) | 0.4056 (4) | 0.2920 (3) | 0.0186 (8) | |
| H5 | 0.811443 | 0.317584 | 0.251361 | 0.022* | |
| C6 | 0.7270 (4) | 0.4681 (4) | 0.3387 (4) | 0.0168 (8) | |
| H6 | 0.636629 | 0.420650 | 0.329185 | 0.020* | |
| C7 | 0.7453 (4) | 0.8986 (4) | 0.4850 (4) | 0.0171 (8) | |
| H7A | 0.762903 | 0.996856 | 0.512413 | 0.021* | |
| H7B | 0.677805 | 0.898701 | 0.397736 | 0.021* | |
| C8 | 0.6700 (4) | 0.8262 (4) | 0.5692 (4) | 0.0192 (8) | |
| H8 | 0.729668 | 0.778274 | 0.641149 | 0.023* | |
| C9 | 0.5222 (5) | 0.8253 (4) | 0.5484 (4) | 0.0247 (9) | |
| H9A | 0.459626 | 0.872317 | 0.477213 | 0.030* | |
| H9B | 0.481069 | 0.777660 | 0.605052 | 0.030* |
Di-µ-bromido-bis({2-[(η-2,3)-(prop-2-en-1-yl)sulfanyl]pyridine-κN}copper(I)) (II) . Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Br1 | 0.01321 (19) | 0.0189 (2) | 0.0130 (2) | −0.00013 (14) | 0.00236 (14) | 0.00135 (13) |
| Cu1 | 0.0151 (2) | 0.0135 (2) | 0.0174 (3) | −0.00122 (17) | 0.00731 (19) | −0.00381 (16) |
| S1 | 0.0185 (5) | 0.0159 (5) | 0.0268 (5) | −0.0045 (4) | 0.0081 (4) | −0.0043 (4) |
| N1 | 0.0154 (15) | 0.0150 (14) | 0.0108 (15) | 0.0022 (12) | 0.0048 (12) | −0.0002 (11) |
| C2 | 0.0152 (18) | 0.0151 (17) | 0.0073 (17) | 0.0006 (14) | 0.0024 (14) | 0.0040 (13) |
| C3 | 0.0165 (19) | 0.0237 (19) | 0.0136 (19) | −0.0004 (15) | 0.0063 (15) | 0.0019 (14) |
| C4 | 0.0192 (19) | 0.023 (2) | 0.016 (2) | 0.0059 (16) | 0.0066 (16) | 0.0024 (15) |
| C5 | 0.021 (2) | 0.0209 (19) | 0.0142 (19) | 0.0041 (15) | 0.0054 (15) | −0.0011 (14) |
| C6 | 0.0167 (19) | 0.0165 (18) | 0.018 (2) | −0.0004 (15) | 0.0058 (15) | −0.0007 (14) |
| C7 | 0.022 (2) | 0.0116 (17) | 0.019 (2) | −0.0006 (15) | 0.0079 (16) | −0.0002 (14) |
| C8 | 0.030 (2) | 0.0103 (17) | 0.019 (2) | −0.0003 (15) | 0.0101 (17) | −0.0027 (14) |
| C9 | 0.033 (2) | 0.0115 (18) | 0.036 (2) | −0.0004 (16) | 0.020 (2) | −0.0065 (15) |
Di-µ-bromido-bis({2-[(η-2,3)-(prop-2-en-1-yl)sulfanyl]pyridine-κN}copper(I)) (II) . Geometric parameters (Å, º)
| Cu1—Br1 | 2.4097 (6) | C4—H4 | 0.9500 |
| Cu1—Br1i | 2.7113 (6) | C4—C5 | 1.387 (5) |
| Cu1—N1 | 2.025 (3) | C5—H5 | 0.9500 |
| Cu1—C8 | 2.048 (4) | C5—C6 | 1.374 (5) |
| Cu1—C9 | 2.065 (4) | C6—H6 | 0.9500 |
| S1—C2 | 1.765 (4) | C7—H7A | 0.9900 |
| S1—C7 | 1.793 (4) | C7—H7B | 0.9900 |
| N1—C2 | 1.338 (5) | C7—C8 | 1.505 (5) |
| N1—C6 | 1.357 (5) | C8—H8 | 0.9500 |
| C2—C3 | 1.397 (5) | C8—C9 | 1.354 (6) |
| C3—H3 | 0.9500 | C9—H9A | 0.9500 |
| C3—C4 | 1.375 (5) | C9—H9B | 0.9500 |
| Cu1—Br1—Cu1i | 82.521 (18) | C4—C5—H5 | 120.9 |
| Br1—Cu1—Br1i | 97.479 (19) | C6—C5—C4 | 118.1 (4) |
| N1—Cu1—Br1i | 98.64 (8) | C6—C5—H5 | 120.9 |
| N1—Cu1—Br1 | 106.43 (9) | N1—C6—C5 | 123.9 (4) |
| N1—Cu1—C8 | 101.60 (14) | N1—C6—H6 | 118.1 |
| N1—Cu1—C9 | 136.30 (14) | C5—C6—H6 | 118.1 |
| C8—Cu1—Br1i | 104.22 (11) | S1—C7—H7A | 108.5 |
| C8—Cu1—Br1 | 141.26 (11) | S1—C7—H7B | 108.5 |
| C8—Cu1—C9 | 38.43 (15) | H7A—C7—H7B | 107.5 |
| C9—Cu1—Br1 | 104.57 (12) | C8—C7—S1 | 115.0 (3) |
| C9—Cu1—Br1i | 107.05 (12) | C8—C7—H7A | 108.5 |
| C2—S1—C7 | 106.04 (17) | C8—C7—H7B | 108.5 |
| C2—N1—Cu1 | 128.0 (2) | Cu1—C8—H8 | 93.7 |
| C2—N1—C6 | 117.2 (3) | C7—C8—Cu1 | 104.9 (2) |
| C6—N1—Cu1 | 114.5 (2) | C7—C8—H8 | 118.0 |
| N1—C2—S1 | 122.9 (3) | C9—C8—Cu1 | 71.5 (2) |
| N1—C2—C3 | 122.3 (3) | C9—C8—C7 | 123.9 (4) |
| C3—C2—S1 | 114.7 (3) | C9—C8—H8 | 118.0 |
| C2—C3—H3 | 120.3 | Cu1—C9—H9A | 104.7 |
| C4—C3—C2 | 119.3 (4) | Cu1—C9—H9B | 95.0 |
| C4—C3—H3 | 120.3 | C8—C9—Cu1 | 70.1 (2) |
| C3—C4—H4 | 120.4 | C8—C9—H9A | 120.0 |
| C3—C4—C5 | 119.1 (4) | C8—C9—H9B | 120.0 |
| C5—C4—H4 | 120.4 | H9A—C9—H9B | 120.0 |
| Cu1—N1—C2—S1 | −6.5 (4) | C2—C3—C4—C5 | 1.2 (5) |
| Cu1—N1—C2—C3 | 171.5 (3) | C3—C4—C5—C6 | −1.3 (6) |
| Cu1—N1—C6—C5 | −172.5 (3) | C4—C5—C6—N1 | 0.1 (6) |
| S1—C2—C3—C4 | 178.3 (3) | C6—N1—C2—S1 | −179.3 (3) |
| S1—C7—C8—Cu1 | −74.2 (3) | C6—N1—C2—C3 | −1.4 (5) |
| S1—C7—C8—C9 | −151.7 (3) | C7—S1—C2—N1 | −20.3 (3) |
| N1—C2—C3—C4 | 0.2 (5) | C7—S1—C2—C3 | 161.7 (3) |
| C2—S1—C7—C8 | 68.3 (3) | C7—C8—C9—Cu1 | 95.9 (3) |
| C2—N1—C6—C5 | 1.3 (5) |
Symmetry code: (i) −x+1, −y+1, −z+1.
Di-µ-bromido-bis({2-[(η-2,3)-(prop-2-en-1-yl)sulfanyl]pyridine-κN}copper(I)) (II) . Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C3—H3···Br1ii | 0.95 | 3.02 | 3.696 (4) | 129 |
| C6—H6···Br1 | 0.95 | 2.94 | 3.576 (4) | 126 |
| C7—H7B···Br1iii | 0.99 | 2.94 | 3.744 (4) | 139 |
Symmetry codes: (ii) x+1, y, z; (iii) −x+1, y+1/2, −z+1/2.
Funding Statement
This work was funded by Ministry of Education and Science of Ukraine grants 0120U101622 and 0120U102028; Javna Agencija za Raziskovalno Dejavnost RS grant P1–0045.
References
- Ardan, B., Kinzhybalo, V., Slyvka, Y., Shyyka, O., Luk‘yanov, M., Lis, T. & Mys‘kiv, M. (2017). Acta Cryst. C73, 36–46. [DOI] [PubMed]
- Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. (2009). J. Appl. Cryst. 42, 339–341.
- Fedorchuk, A. A., Slyvka, Yu. I., Goreshnik, E. A., Kityk, I. V., Czaja, P. & Mys’kiv, M. G. (2018). J. Mol. Struct. 1171, 644–649.
- Fedorchuk, A., Goreshnik, E., Slyvka, Yu. & Mys’kiv, M. (2020). Acta Chim. Slov. 67, 1148–1154. [PubMed]
- Filinchuk, Ya. E., Mys’kiv, M. G. & Davydov, V. N. (1998). Koord. Khim.(Russ.)(Coord. Chem.). 24, 771–775.
- Fukushima, K., Kobayashi, A., Miyamoto, T. & Sasaki, Y. (1976). Bull. Chem. Soc. Jpn, 49, 143–146.
- Goreshnik, E. A., Schollmeyer, D. & Myskiv, M. G. (2002). ZAAC, 628, 2118–2122.
- Goreshnik, E. A., Slyvka, Yu. I. & Mys’kiv, M. G. (2011). Inorg. Chim. Acta, 377, 177–180.
- Goreshnik, E. A., Veryasov, G., Morozov, D., Slyvka, Yu., Ardan, B. & Mys’kiv, M. G. (2016). J. Organomet. Chem. 810, 1–11.
- Goreshnik, E., Schollmeier, D. & Mys’kiv, M. (2003). Acta Cryst. C59, m478–m481. [DOI] [PubMed]
- Groom, C. R., Bruno, I. J., Lightfoot, M. P. & Ward, S. C. (2016). Acta Cryst. B72, 171–179. [DOI] [PMC free article] [PubMed]
- Hordiichuk, O. R., Slyvka, Yu. I., Kinzhybalo, V. V., Goreshnik, E. A., Bednarchuk, T. J., Bednarchuk, O., Jedryka, J., Kityk, I. & Mys’kiv, M. G. (2019). Inorg. Chim. Acta, 495, 119012.
- Iakovidis, I., Delimaris, I. & Piperakis, S. M. (2011). Mol. Biol. Int. 2011, 1–13. [DOI] [PMC free article] [PubMed]
- Kamei, T., Fujita, K., Itami, K. & Yoshida, J. (2005). Org. Lett. 7, 4725–4728. [DOI] [PubMed]
- Kowalska, D. A., Kinzhybalo, V., Slyvka, Y. I. & Wołcyrz, M. (2021). Acta Cryst.. B77, 241–248. [DOI] [PubMed]
- Marzano, C., Pellei, M., Alidori, S., Brossa, A., Lobbia, G. G., Tisato, F. & Santini, C. (2006). J. Inorg. Biochem. 100, 299–304. [DOI] [PubMed]
- Pavlyuk, O. V., Goreshnik, E. A., Ciunik, Z. & Mys’kiv, M. G. (2005). ZAAC, 631, 793–797.
- Rigaku OD (2021). CrysAlis PRO. Rigaku Oxford Diffraction, Tokyo, Japan.
- Rourke, J. (2006). Appl. Organomet. Chem. 20, 811–811.
- Sheldrick, G. M. (2015a). Acta Cryst. A71, 3–8.
- Sheldrick, G. M. (2015b). Acta Cryst. C71, 3–8.
- Slyvka, Yu., Fedorchuk, A. A., Goreshnik, E., Lakshminarayana, G., Kityk, I. V., Czaja, P. & Mys’kiv, M. (2018b). Chem. Phys. Lett. 694, 112–119.
- Slyvka, Yu., Goreshnik, E., Pavlyuk, O. & Mys’kiv, M. (2013). Open Chem. 11, 1875–1901.
- Slyvka, Y., Kinzhybalo, V., Shyyka, O. & Mys’kiv, M. (2021). Acta Cryst. C77, 249–256. [DOI] [PubMed]
- Slyvka, Yu. I., Fedorchuk, A. A., Pokhodylo, N. T., Lis, T., Kityk, I. V. & Mys’kiv, M. G. (2018a). Polyhedron, 147, 86–93.
- Tisato, F., Marzano, C., Porchia, M., Pellei, M. & Santini, C. (2010). Med. Res. Rev. 30, 708–749. [DOI] [PubMed]
- Wang, X.-S., Zhao, H., Li, Y.-H., Xiong, R.-G. & You, X.-Z. (2005). Top. Catal. 35, 43–61.
- Yang, L., Powell, D. R. & Houser, R. P. (2007). Dalton Trans. pp. 955–964. [DOI] [PubMed]
- Yoshikai, N. & Nakamura, E. (2012). Chem. Rev. 112, 2339–2372. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I, II, publication_text. DOI: 10.1107/S2056989021011002/hb7993sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989021011002/hb7993Isup2.hkl
Structure factors: contains datablock(s) II. DOI: 10.1107/S2056989021011002/hb7993IIsup3.hkl
Additional supporting information: crystallographic information; 3D view; checkCIF report






