Abstract
The extent to which the benefits of bariatric surgery may be maintained by lifestyle changes after surgery is unclear. Our hypothesis is that exercise may sustain some metabolic benefits and counteract some of the adverse effects of surgery. In this review, we present findings supporting the proposition that exercise is key to improving overall health in patients after bariatric surgery.
Keywords: obesity, weight loss, muscle mass, bone mass, physical training.
Summary:
Exercise training is key to improving overall health in patients who have bariatric surgery.
Introduction
Bariatric surgery is considered the treatment of choice for severe obesity (BMI> 40 kg/m2 or 35 kg/m2 with associated comorbidities) as this procedure yields substantial weight loss and reverses cardiovascular risk factors, thereby reducing comorbidities and all-cause mortality (1–8). There is now convincing data that patients who have bariatric surgery live longer than matched non-surgical controls (3, 9, 10). Eliasson et al. (11), for instance, found a 58% reduction in the relative risk for overall mortality and a 59% reduction in the risk of cardiovascular death in patients who had surgery when compared with controls after a median follow-up of 3.5 years.
Bariatric surgery can also be effective in treating patients with type 2 diabetes who fail to respond to lifestyle and medication changes (2, 12). Five-year outcome data from the Surgical Treatment and Medications Potentially Eradicate Diabetes Efficiently (STAMPEDE) trial showed that, among patients with type 2 diabetes and a BMI between 27 and 43 kg/m2, bariatric surgery along with intensive medical therapy was more effective than intensive medical therapy alone in decreasing, or in some cases resolving, hyperglycemia (12). Furthermore, patients with severe obesity and type 2 diabetes undergoing bariatric surgery were shown to experience massive reduction in body weight (~60%); glycated hemoglobin and fasting glucose were rescued to normal levels in ~80% of the sample after ~20 months of surgery (13). When the surgical procedure is specifically prescribed for treating type 2 diabetes – and not obesity per se – the intervention is called metabolic surgery (1).
Notwithstanding, despite the well-reported cardiometabolic benefits brought about by bariatric surgery, it cannot be seen as a “silver bullet” in the management of obesity or diabetes for two main reasons. First, some immediate effects of bariatric surgery tend to dissipate over time. There is some weight regain, especially after the gastric banding procedure (12, 14, 15) and this can contribute to increased cardiometabolic risk factors (e.g., impaired endothelial function, increased inflammation). This suggests that some of the beneficial effects of bariatric surgery may be, to a certain extent, transient. Second, bariatric surgery is associated with some unfavorable health outcomes. Losses in bone and muscle, for example, are well-known undesirable events following these surgical procedures (16, 17).
While bariatric surgery alone has an important, but potentially reversable effect on weight loss and metabolic status, particularly in the long run, the adoption of a healthy lifestyle remains highly relevant for post-operative patients.
In this context, emerging evidence suggests that exercise has the potential to enhance some of the benefits of bariatric surgery, as well as to alleviate some of its undesirable effects (Figure 1). This narrative review summarizes cutting-edge research supporting the hypothesis that exercise is key to improving overall health in patients who have bariatric surgery.
Figure 1.
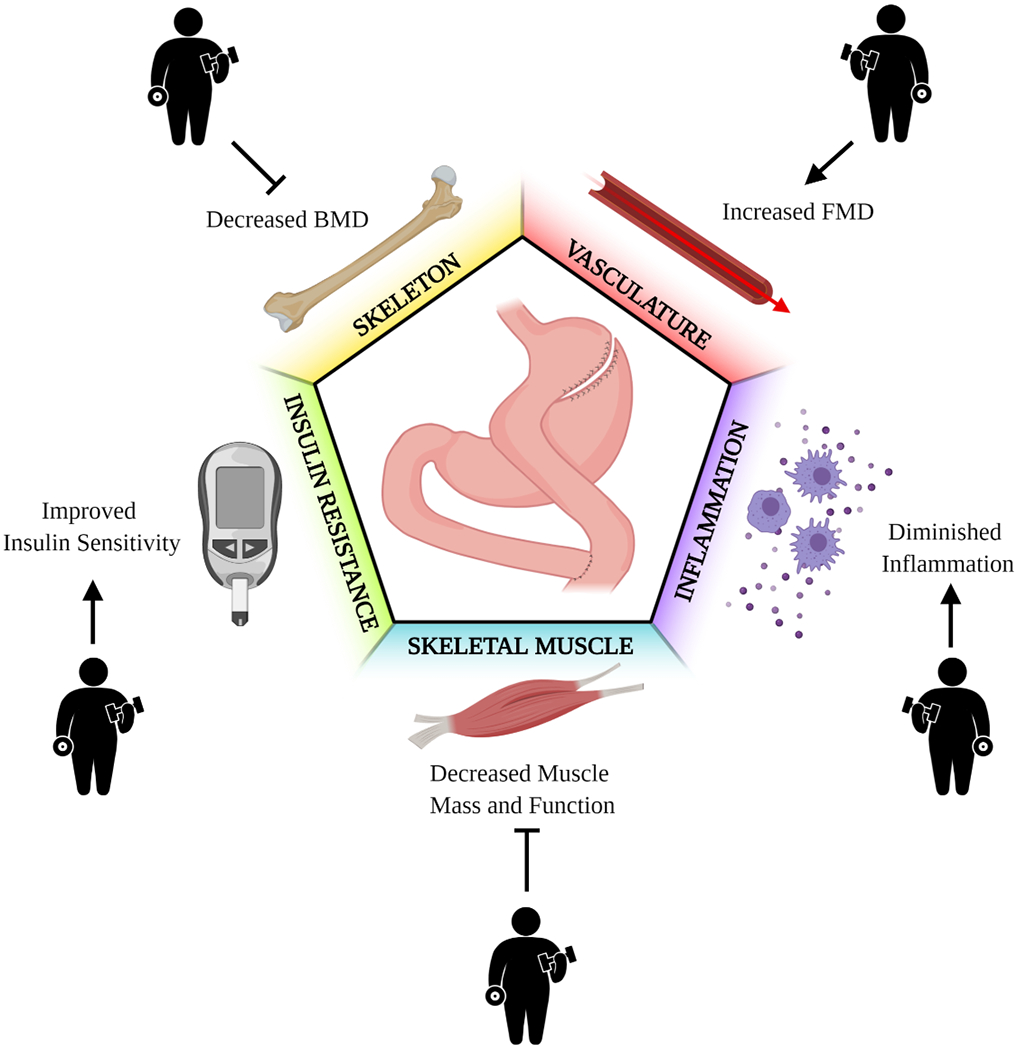
The rationale for prescribing exercise after bariatric surgery. Pointed arrows indicate the potential additive effects of exercise and bariatric surgery, whereas blunt arrows indicate the potential for exercise to counteract adverse physiological and metabolic effects that occur after bariatric surgery. In brief, exercise can potentially sustain, or even enhance, the cardiometabolic effects of bariatric surgery, and these include improvements in insulin sensitivity, inflammatory markers and endothelial function. In addition, exercise may also prevent the loss of muscle and bone mass and functionality, that is commonly observed after bariatric surgery.
Exercise sustains the benefits induced by bariatric surgery
There are several well-documented, clinical benefits induced by bariatric surgery (3, 9). However, how long they persist is still a matter of debate and may depend on several factors, especially the potential adherence to healthy behaviors (i.e., diet and physical activity).
One case in point is endothelial function, commonly assessed through flow-mediated dilation (FMD). As endothelial dysfunction precedes atherosclerotic disease progression, it is widely used as a predictor of cardiovascular events beyond traditional cardiovascular risk factors (18). While short-term assessments have consistently shown improvements in FMD following bariatric surgery (19, 20), less robust effects have been seen in the long term (21). For instance, patients who underwent bariatric surgery experienced a 3.8% increase in FMD 18 months following surgery; however, a 5-year follow-up assessment showed a subsequent 1.4% decline in this outcome (21).
Recently, we showed that FMD improvements following Roux-en-Y gastric bypass were even lesser durable than we predicted: within 9 months, FMD tended to return to pre-surgery values (22). The impairment in FMD was accompanied by an increase in retrograde shear rate, a pattern of vascular flow that is associated with pro-atherogenic effects. Of relevance, however, a 6-month, 3 times a week, supervised, combined aerobic and resistance training program, which started 3 months after surgery, was capable of enhancing the improvements in FMD and retrograde shear rate brought about by bariatric surgery in diabetic and non-diabetic participants (22).
In the same study, we also assessed systemic inflammatory markers across time. Two pro-inflammatory cytokines, interleukin 1 beta (IL-1β) and tumor necrosis factor α (TNF-α), were substantially reduced 3 months after surgery (22). Nonetheless, they rose back to near baseline values 9 months after surgery among non-exercised patients. Patients who exercised, nonetheless, did not experience such an elevation in these pro-inflammatory markers, leading to the conclusion that exercise is important for preserving the anti-inflammatory effect induced by surgery.
Cardiac autonomic function can also be positively modulated by exercise following the surgical procedure. We recently showed that chronotropic response (i.e., the ability to increase heart rate in response to a high metabolic demand) and post-exercise heart rate recovery – two markers for autonomic function – were further improved by exercise in patients who had bariatric surgery (23). The number of participants showing blunted heart recovery, a well-known surrogate of dysautonomia, decreased in the exercise trained group vs. standard of care (non-exercised control) (Figure 2). These findings suggest that exercise training can improve the cardiac autonomic regulation, which may be reflected in overall improvements in cardiovascular health and, therefore, reduced cardiovascular events among patients undergoing bariatric surgery (23).
Figure 2.
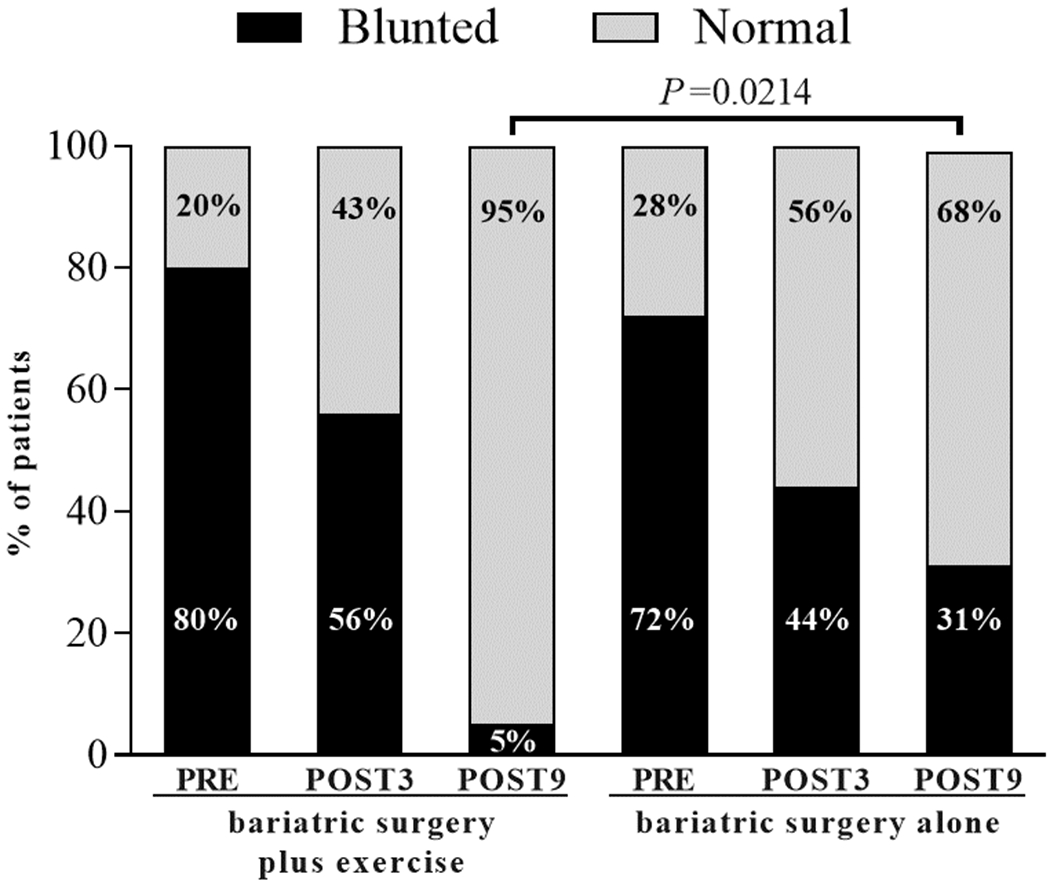
Post-exercise heart rate recovery in patients who had bariatric surgery plus exercise or bariatric surgery alone (control). Following the intervention (POST9), only 5% of participants had blunted hearth rate recovery (defined as a heart rate after 60 seconds of recovery to maximal exercise ≤ 12 bpm) vs. 31% in the non-exercised group. These data suggest that exercise training modulates cardiac autonomic function. PRE = before surgery; POST3 = 3 months after surgery (beginning of the 6-month, exercise training program). Data adapted from Gil et al. (23).
One of the most recognized effects of bariatric surgery is the reversal of insulin resistance, and this is one of the reasons the procedure has been re-named as metabolic surgery, especially in the context of type 2 diabetes treatment (1). Nevertheless, in the absence of exercise, the improvements in insulin sensitivity may be quite transient. This is supported by data from our study (24), in which several surrogates of insulin resistance – specially influenced by insulin levels – were impaired from 3 to 9 months after surgery. Once again, exercise training abrogated such an effect, sustaining the benefits of surgery throughout the 9-month follow-up period. These findings support previous data showing that exercise can potentiate insulin sensitivity in patients who underwent Roux-en-Y gastric bypass (25). However, in our study (24), the magnitude of the effect of exercise appeared to be more pronounced, as compared to those of Coen et al.’s study (25). Differences in training models (e.g., supervised, 3 x week, aerobic plus resistance exercises in ours vs. semi-supervised, 3-5 x week, aerobic exercises in Coen et al.’s) and in the assessments of insulin sensitivity (e.g., oral glucose tolerance test in ours vs. hyperinsulinemic-euglycemic clamp in Coen et al.’s) could partially explain the discrepancy in the magnitude of the effects seen in these studies.
The underlying mechanisms that explain the cardiometabolic benefits induced by exercise training prescribed for post-operative patients are largely underexplored. So far, the limited number of mechanistic studies have primarily focused on elucidating the synergistic role of exercise and bariatric surgery on insulin resistance.
Coen et al. (26) were the first to provide insights into the mechanisms by which exercise improves insulin sensitivity following bariatric surgery. They elegantly showed that exercise-induced improvements in insulin sensitivity were associated with an increased mitochondrial oxidative capacity and a reduction in specific lipid species that are known to impair insulin signaling.
More recently, we conducted a series of experiments that provide compelling evidence that exercise also acts through muscle extracellular matrix remodeling to improve insulin resistance in patients who have bariatric surgery (24). An abnormal muscle extracellular matrix phenotype is known to impair cell-to-cell and cell-to-extracellular matrix interactions, potentially diminishing insulin’s access to myocytes (27). In our study, bariatric surgery was only partially able to remodel the extracellular matrix. Conversely, exercised patients went through a more pronounced extracellular matrix remodeling, with consistent evidence – from the muscle ultrastructure to the gene – confirming a robust change in extracellular remodeling towards a lean, healthy phenotype (Figure 3).
Figure 3.
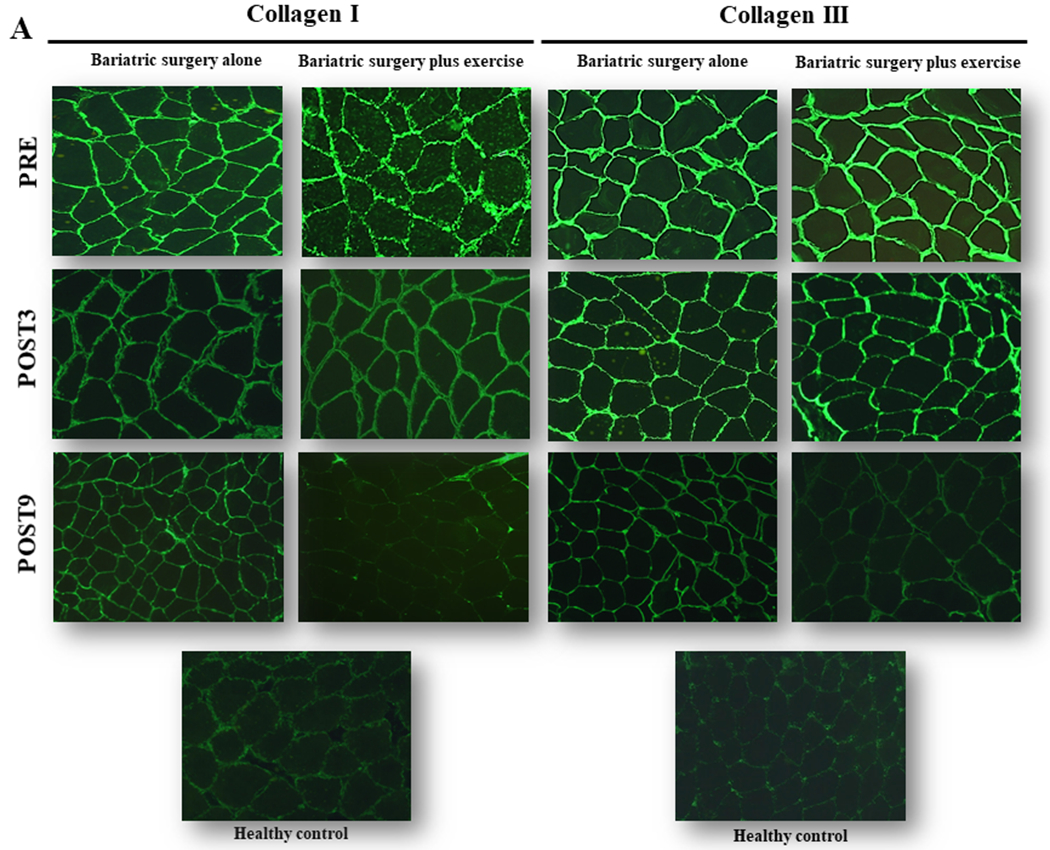
Skeletal muscle extracellular matrix immunohistochemistry showing representative images of collagen I (Panel A) and III (Panel B) staining in patients who underwent bariatric surgery and engaged or not in exercise training. Muscle biopsies were taken at baseline (PRE) and 3 (POST3) and 9 (POST9) months after the surgery. The 6-month exercise intervention started at POST3. A healthy lean group served as controls. Note that exercise training was able to reduce both collagen I and III, as compared with surgery alone. In the group who exercised, collagen profiles resembled those of healthy, lean controls. These data are well-aligned with other phenotyping and genotyping characterization that collectively showed substantial extracellular remodeling following exercise, but not by bariatric surgery alone. For further details, the readers may refer to Dantas et al. (24), from where this figure was adapted.
These findings collectively support the idea that the improvements in metabolic outcomes brought about by surgery are progressively reduced in the absence of exercise (24). Thus, exercise strategies should be incorporated into the therapeutic regimen of patients who have bariatric surgery to help sustain, or even enhance, the metabolic benefits from the surgical procedure.
Exercise mitigates some undesirable effects of bariatric surgery
Notwithstanding the prominent benefits of bariatric surgery on weight reduction and metabolism, literature seems unequivocal in reporting important negative physiological and metabolic consequences of this procedure, with severe bone and muscle mass losses constituting clinically relevant points of concern (17, 28–31).
A number of studies have reported increased bone turnover after surgery, evidenced by alterations in bone turnover markers, such as urinary N-telopeptide cross-linked collagen type 1 (NTx) and serum osteocalcin, responsible for bone resorption and formation, respectively. This may manifest early after surgery (~3 months) (17, 28) and persist several years after the procedure (32). Importantly, perturbations in bone metabolism have been paralleled with significant bone mass losses at various body sites that are detectable within a few months up to years following surgery (16, 17, 28, 32). Recently, we added to the literature by showing that bariatric surgery-induced increases in several bone turnover biomarkers, including collagen type I C-telopeptide (CTX), procollagen type I N-propeptide (P1NP), sclerostin, dickkopf-1 (DKK1), and osteopontin. These responses were accompanied by attenuation in BMD loss at the femoral neck (−4.4% vs. −7.3% in controls), total hip (−5.0% vs. −7.3% in controls), and distal radius (−2.7% vs. −4.6% in controls). Detrimental effects on bone microarchitecture markers were also noted (33).
Multiple mechanisms have been put forward to help explain bone loss following bariatric surgery, including nutrient malabsorption, muscle wasting, and weight loss-induced mechanical unloading of the skeleton (34–36). Physical exercise has been considered as a potential strategy to offset bone loss in different populations, including patients with obesity under energy restriction (37). Despite this, the effects of exercise on bariatric surgery patients remained unexplored until recently. Campanha-Versiani et al. (38) were the first to demonstrate the role of exercise training (supervised, aerobic plus resistance exercises, 2 x week for 36 weeks) in mitigating the reduction in total (−5.7% vs. −11.3% in controls), lumbar spine (−1.7% vs. −6.9% in controls) and hip (−2.7% vs. −7.4%) BMD one year following Roux-en-Y gastric bypass, evidencing the ability of bone tissue to remodel in response to exercise stimuli even under the massive physiological perturbations imposed by surgery.
In our recent clinical trial (33), we shed light on this subject by providing more robust evidence on the potential role of exercise training in mitigating bariatric surgery-induced bone loss as well as by exploring possible mechanisms underpinning this response. We showed that exercise, as compared to standard of care, was effective in attenuating bone loss resulting from bariatric surgery. Additionally, exercise was able to reduce sclerostin levels, which is known to downregulate the bone formation-related WNT signaling (39). Sclerostin, thus, emerges as a potential candidate for explaining the protective effect of exercise on bone mass in these patients.
Similarly to what has been observed in bone, bariatric surgery also has a negative impact on muscle. Muscle mass, strength and functionality losses are commonly reported among obese patients undergoing bariatric surgery (29–31). These adverse effects may limit the patients’ activities of daily living and increase morbidity, in spite of the drastic weight loss. Additionally, skeletal muscle mass is the largest glucose disposal site in the organism, so that muscle waste may be detrimental for the regulation of glucose metabolism, particularly in patients at risk for, or with, insulin resistance or type 2 diabetes. Despite unresolved issues related to the mechanisms underpinning the often thought interplay between skeletal muscle and bone tissue, muscle wasting may negatively impact bone and vice-versa (40). This has implications for the management of patients after bariatric surgery, a procedure that can simultaneously impair bone and muscle mass.
Resistance exercise is classically recognized as a potent stimulus capable of increasing muscle strength and mass; however, its effects aligned to bariatric surgery have been underexplored. The few studies that are available are mostly restricted to the investigation of the effect of exercise on fat-free mass (e.g., using dual-energy X-ray absorptiometry or bioelectrical impedance) (38, 41), which provides a limited insight into the actual effect of this intervention on skeletal muscle adaptations. Moreover, the potential mechanisms related to the exercise-induced muscle remodeling among patients who have bariatric surgery remain largely unknown.
Our recent trial also addressed this topic. We demonstrated that exercise attenuated the loss in fat-free mass of approximately 3 kg, and reversed the loss in muscle strength (e.g., difference between groups for leg press maximal strength, 83.2 kg) 9 months after surgery (42). We collected muscle samples from our patients at baseline, 3 and 9 months after surgery. Immunohistochemistry analysis showed that exercise was able to reverse surgery-induced muscle atrophy at the fiber level. Interestingly, this was accompanied by increases in capillarization and satellite cell content. It has been proposed that muscle fiber remodeling depends on the pool of satellite cells, which are localized between basal lamina and sarcolemma. These multipotent cells can be activated by exercise, and then proliferate and differentiate to supply additional myonuclei to the fiber, thereby supporting the hypertrophic process (43, 44). From a mechanistic point of view, we also found that exercise was able to suppress the ubiquitin-proteasome system via the downregulation of Atrogin-1 (45), a pathway involved in muscle protein breakdown (46). These findings provide a putative molecular mechanism underpinning muscle wasting following bariatric surgery and help to explain how exercise may offset such an effect.
Clinically, exercise appears to be a useful tool to prevent losses of muscle mass, strength and bone mass when prescribed to post-operative patients.
Perspectives and conclusions
Nowadays, it is undisputable that bariatric surgery can protect against severe obesity and its comorbidities, specially type 2 diabetes (1–3). However, an in-depth analysis of the literature suggests that the beneficial effects of bariatric surgery may not be completely sustainable in the long run in the absence of behavior and lifestyle changes. Simply put, bariatric surgery alone should not be viewed as a “silver bullet” in the treatment of obesity.
A growing but limited number of publications show that exercise can preserve, or even potentiate, the cardiometabolic benefits of bariatric surgery (26). The systemic effects of exercise in this condition include improvements in insulin sensitivity, vascular function, systemic inflammation, and functionality. Of relevance, exercise also plays a role in partially or fully preventing the negative effects of surgery on bone metabolism and muscle mass, which may confer long-term protection against osteopenia/osteoporosis and frailty/sarcopenia, a hypothesis that still remains to be tested.
The scientific agenda on the topic (summarized in Table 1) should also comprise the investigation of the impact of different types of training among patients who have bariatric surgery, including not only physiological assessments, but also sociocultural aspects, such as patients’ preferences and barriers, autonomy, and long-term adherence to exercise. This is key knowledge for health care professionals to better tailor their exercise programs, particularly considering that people referred to bariatric surgery may be more refractory to any physical activities. Observational data suggest that bariatric surgery itself can potentially increase physical activity levels, but controversial data on this subject exist (47). In order to tackle inactivity and sedentariness, therefore, behavioral interventions with particular focus on increasing spontaneous activity should be considered.
Table 1.
Research gaps in the context of exercise interventions following bariatric surgery.
| i. | Could exercise prevent major poor outcomes (frailty/sarcopenia, osteoporosis, mortality)? |
| ii. | In which aspect or condition exercise intervention could replace bariatric surgery in the management of severe obesity? |
| iii. | What would be the optimal exercise training model (resistance, aerobic, combined) and its delivery method (supervised, semi-supervised, home-based, etc) to elicit the greatest benefits? |
| iv. | What would be the barriers and facilitators to ensure an optimal adherence in the long run? |
| v. | Could exercise be effective and safe in adolescents and older adults who have bariatric surgery? |
| vi. | How distinct surgery techniques (sleeve gastrectomy, Roux-en-Y gastric bypass) may influence exercise-related outcomes? |
| vii. | How patients’ pre-surgery conditions (presence of type 2 diabetes or any other comorbidities) may influence exercise-related outcomes? |
| viii. | What would be the underlying physiological and molecular mechanisms of exercise? |
| ix. | Could the implementation of exercise interventions reduce the costs related to bariatric surgery failure, reversal of cardiometabolic improvements or any adverse outcomes? |
Furthermore, pragmatic, head-to-head studies comparing exercise interventions with bariatric surgery are lacking. As the widespread application of bariatric surgery as a public health strategy to combat the obesity pandemic is difficult to envision, non-inferiority trials involving exercise combined or not with other lifestyle interventions may provide alternative treatment options, and which are perhaps more feasible on a larger scale.
Another underexplored area is the utility and consequences of bariatric surgery in youth and older adults. It seems that the surgical procedure may confer cardiometabolic benefits in both populations (48, 49); however, the sustainability of such effects as well as the potential risk of adverse events secondary to surgery, such as losses in bone and muscle mass, makes exercise a plausible co-prescription, pending trials to investigate its safety, efficacy, and feasibility.
Whether patients’ pre-surgery features (e.g., type 2 diabetes or any other comorbidities) mediate the exercise outcomes is also unknown. Likewise, it is unclear whether different types of surgery (sleeve gastrectomy, Roux-en-Y gastric bypass) interact differently with post-surgery exercise, modulating its responses. Studies involving large samples are necessary to allow sensitivity analyses comparing, for instance, patients with type 2 diabetes vs. normoglycemic, or the training responses between patients undergoing sleeve gastrectomy vs. Roux-en-Y gastric bypass.
It is difficult to envision other interventions capable of promoting such profound and systemic physiological adaptations as those ensued by bariatric surgery and exercise. For this reason, it will be a complicated task to elucidate all the underlying mechanisms of those interventions separately, and even more so, in combination. The use of emerging “omics” techniques may represent a useful starting-point to identify genes and pathways through which exercise and bariatric surgery may act. Using RNA-sequencing, a transcriptomics approach, we were able to identify molecular candidates that could partially explain the superimposed benefits of exercise on bariatric surgery. Namely, we observed that exercise, but not bariatric surgery, suppressed transforming growth factor-beta 1/SMAD 2/3 pathway (directly associated with extracellular matrix expansion), while increasing its antagonist follistatin (involved in the upregulation of muscle mass and insulin sensitivity) (24). This helps to understand how exercise in conjunction with bariatric surgery induces greater extracellular matrix remodeling and better insulin sensitivity than surgery alone. The search for novel molecular mechanisms is of paramount relevance to unravel all of the therapeutic potential of exercise strategies along with bariatric surgery, which may ultimately become therapeutic targets for treating obesity and its comorbidities in a more generalizable context.
One striking observation from our serial studies is that the cardiometabolic protection promoted by exercise appears to be independent of changes in body weight, fat mass, visceral fat, maximal oxygen consumption or lipid profile. This raises the possibility that exercise may confer health benefits by ameliorating non-traditional risk factors, such as endothelial dysfunction, as previously reported by our laboratories (22). Further investigation should look at more comprehensive outcomes to test this hypothesis.
Finally, efforts are needed to develop, implement and test exercise interventions in the post-bariatric recovery in relation to their cost-effectiveness, with the working hypothesis that exercise could reduce the costs related to surgery failure (commonly defined as achieving or maintaining less than 50% of excess weight loss over 18 to 24 months or a BMI > 35), reversal of several cardiometabolic improvements, or major adverse events.
In conclusion, a solid body of work shows that bariatric surgery can alleviate severe obesity and induce remission of type 2 diabetes in a large group of patients. Nonetheless, failure to adopt a healthier lifestyle may compromise the success of the surgical procedure. We and others have recently produced data that support exercise training to, on one hand, i) sustain, or even enhance, the cardiometabolic effects of bariatric surgery, and, on another hand, ii) prevent muscle and bone detrimental effects that accompany the surgical procedure (Figure 4). Thus, we argue that exercise is a simple, cost-effective, and safe tool that should be incorporated into post-operative care of patients following bariatric surgery so as to ensure optimal gains in overall health.
Figure 4.
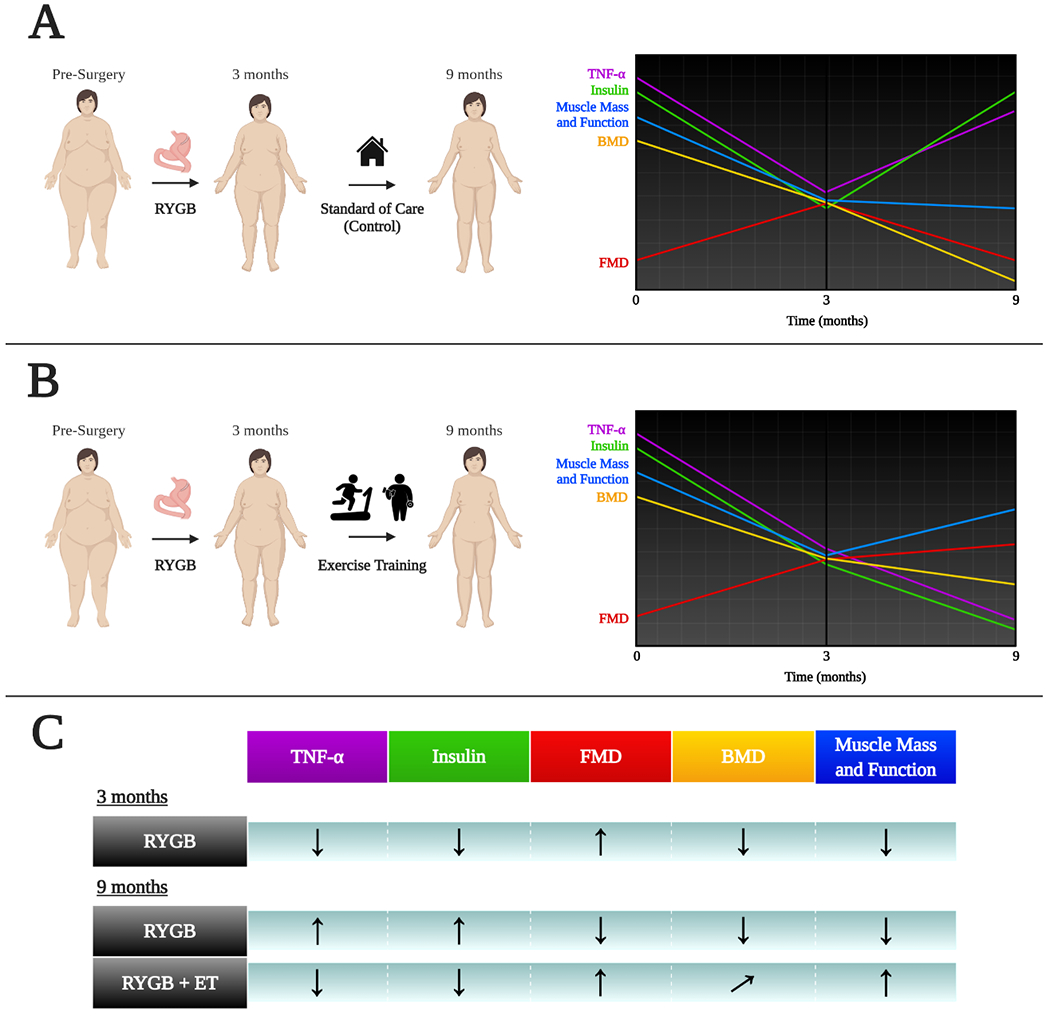
Schematic view of the effects of bariatric surgery alone or combined with exercise training in multiple health biomarkers. Outcomes were assessed at baseline, 3 (POST3), and 9 (POST9) months after the surgery. The 6-month exercise intervention started at POST3. In summary, bariatric surgery (Panel A) induced transient improvements in TNF-α, flow-mediated dilation (FMD), and insulin levels, which were not sustained 9 months after surgery in the absence of exercise. The inclusion of exercise in the post-operative period (Panel B) sustained, or even enhanced, the improvements in these variables brought about by surgery alone. In addition, as seen in Panel A, there were detrimental effects of surgery on bone and muscle mass and strength across time. Exercise following surgery (Panel B) reversed (or attenuated, in case of bone mineral density [BMD]) these responses. Panel C summarizes the outcomes for surgery alone (RYGB) or surgery plus exercise training (RYGB + ET). Upward arrows indicate increases; downward arrows indicate decreases; diagonal arrow indicates partial attenuation. Data derived from (22, 24, 33, 42, 45).
Key Points.
Bariatric surgery is the treatment of choice for severe obesity.
However, certain cardiometabolic benefits of bariatric surgery may be less durable in the absence of exercise.
Exercise can also counteract the adverse effects of bariatric surgery on bone and muscle mass.
As bariatric surgery alone is not a “silver bullet” against obesity and its comorbidities, exercise should be part of post-operative care to ensure the best health outcomes.
Acknowledgments
We are thankful to Igor Longobardi for the assistance in creating the figures (using BioRender.com). The Lab of Applied Physiology & Nutrition is supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Conselho Nacional de Pesquisa e Tecnologia (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). The Kirwan Lab is supported in part by NIH grants R01 DK114156, R01 DK108089, U01 DK114154 and U54 GM104940.
Disclosure of funding:
Fundação de Amparo à Pesquisa do Estado de São Paulo to BG and HR (2016/10993-5 and 2017/13552-2); National Institutes of Health, U01 DK114156 and U54 GM104940 to JPK.
Footnotes
Conflicts of interest: The authors declare no conflict of interests.
References
- 1.Rubino F, Nathan DM, Eckel RH, Schauer PR, Alberti KG, Zimmet PZ, et al. Metabolic Surgery in the Treatment Algorithm for Type 2 Diabetes: A Joint Statement by International Diabetes Organizations. Diabetes Care. 2016;39(6):861–77. doi: 10.2337/dc16-0236. [DOI] [PubMed] [Google Scholar]
- 2.Dixon JB, O’Brien PE, Playfair J, Chapman L, Schachter LM, Skinner S, et al. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA. 2008;299(3):316–23. doi: 10.1001/jama.299.3.316. [DOI] [PubMed] [Google Scholar]
- 3.Adams TD, Gress RE, Smith SC, Halverson RC, Simper SC, Rosamond WD, et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357(8):753–61. doi: 10.1056/NEJMoa066603. [DOI] [PubMed] [Google Scholar]
- 4.Adams TD, Davidson LE, Litwin SE, Kim J, Kolotkin RL, Nanjee MN, et al. Weight and Metabolic Outcomes 12 Years after Gastric Bypass. N Engl J Med. 2017;377(12):1143–55. doi: 10.1056/NEJMoa1700459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292(14):1724–37. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 6.Buchwald H, Estok R, Fahrbach K, Banel D, Jensen MD, Pories WJ, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009;122(3):248–56 e5. doi: 10.1016/j.amjmed.2008.09.041. [DOI] [PubMed] [Google Scholar]
- 7.Sjostrom L, Peltonen M, Jacobson P, Sjostrom CD, Karason K, Wedel H, et al. Bariatric surgery and long-term cardiovascular events. JAMA. 2012;307(1):56–65. doi: 10.1001/jama.2011.1914. [DOI] [PubMed] [Google Scholar]
- 8.Bhatt DL, Aminian A, Kashyap SR, Kirwan JP, Wolski K, Brethauer SA, et al. Cardiovascular Biomarkers After Metabolic Surgery Versus Medical Therapy for Diabetes. J Am Coll Cardiol. 2019;74(2):261–3. doi: 10.1016/j.jacc.2019.04.058. [DOI] [PubMed] [Google Scholar]
- 9.Perry CD, Hutter MM, Smith DB, Newhouse JP, McNeil BJ. Survival and changes in comorbidities after bariatric surgery. Ann Surg. 2008;247(1):21–7. doi: 10.1097/SLA.0b013e318142cb4b. [DOI] [PubMed] [Google Scholar]
- 10.Carlsson LMS, Sjoholm K, Jacobson P, Andersson-Assarsson JC, Svensson PA, Taube M, et al. Life Expectancy after Bariatric Surgery in the Swedish Obese Subjects Study. N Engl J Med. 2020;383(16):1535–43. doi: 10.1056/NEJMoa2002449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eliasson B, Liakopoulos V, Franzen S, Naslund I, Svensson AM, Ottosson J, et al. Cardiovascular disease and mortality in patients with type 2 diabetes after bariatric surgery in Sweden: a nationwide, matched, observational cohort study. Lancet Diabetes Endocrinol. 2015;3(11):847–54. doi: 10.1016/S2213-8587(15)00334-4. [DOI] [PubMed] [Google Scholar]
- 12.Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Aminian A, Brethauer SA, et al. Bariatric Surgery versus Intensive Medical Therapy for Diabetes - 5-Year Outcomes. N Engl J Med. 2017;376(7):641–51. doi: 10.1056/NEJMoa1600869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schauer PR, Burguera B, Ikramuddin S, Cottam D, Gourash W, Hamad G, et al. Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann Surg. 2003;238(4):467–84; discussion 84–5. doi: 10.1097/01.sla.0000089851.41115.1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Brethauer SA, Navaneethan SD, et al. Bariatric surgery versus intensive medical therapy for diabetes−-3-year outcomes. N Engl J Med. 2014;370(21):2002–13. doi: 10.1056/NEJMoa1401329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sjostrom L, Narbro K, Sjostrom CD, Karason K, Larsson B, Wedel H, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357(8):741–52. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 16.Lindeman KG, Greenblatt LB, Rourke C, Bouxsein ML, Finkelstein JS, Yu EW. Longitudinal 5-Year Evaluation of Bone Density and Microarchitecture After Roux-en-Y Gastric Bypass Surgery. J Clin Endocrinol Metab. 2018;103(11):4104–12. doi: 10.1210/jc.2018-01496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coates PS, Fernstrom JD, Fernstrom MH, Schauer PR, Greenspan SL. Gastric bypass surgery for morbid obesity leads to an increase in bone turnover and a decrease in bone mass. J Clin Endocrinol Metab. 2004;89(3):1061–5. doi: 10.1210/jc.2003-031756. [DOI] [PubMed] [Google Scholar]
- 18.Halcox JP, Schenke WH, Zalos G, Mincemoyer R, Prasad A, Waclawiw MA, et al. Prognostic value of coronary vascular endothelial dysfunction. Circulation. 2002;106(6):653–8. doi: 10.1161/01.cir.0000025404.78001.d8. [DOI] [PubMed] [Google Scholar]
- 19.Gokce N, Vita JA, McDonnell M, Forse AR, Istfan N, Stoeckl M, et al. Effect of medical and surgical weight loss on endothelial vasomotor function in obese patients. Am J Cardiol. 2005;95(2):266–8. doi: 10.1016/j.amjcard.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 20.Saleh MH, Bertolami MC, Assef JE, Taha MI, de Freitas W Jr., Petisco AC, et al. Improvement of atherosclerotic markers in non-diabetic patients after bariatric surgery. Obes Surg. 2012;22(11):1701–7. doi: 10.1007/s11695-012-0706-0. [DOI] [PubMed] [Google Scholar]
- 21.Tschoner A, Sturm W, Gelsinger C, Ress C, Laimer M, Engl J, et al. Long-term effects of weight loss after bariatric surgery on functional and structural markers of atherosclerosis. Obesity (Silver Spring). 2013;21(10):1960–5. doi: 10.1002/oby.20357. [DOI] [PubMed] [Google Scholar]
- 22.Dantas WS, Gil S, Murai IH, Costa-Hong V, Pecanha T, Merege-Filho CAA, et al. Reversal of Improved Endothelial Function After Bariatric Surgery Is Mitigated by Exercise Training. J Am Coll Cardiol. 2018;72(18):2278–9. doi: 10.1016/j.jacc.2018.07.094. [DOI] [PubMed] [Google Scholar]
- 23.Gil S, Peçanha T, Murai IH, Merege-Filho C, Sa Pinto AL, Pereira RMR, et al. Exercise enhances the effect of bariatric surgery in markers of cardiac autonomic function. Obesity Surgery. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dantas WS, Roschel H, Murai IH, Gil S, Davuluri G, Axelrod CL, et al. Exercise-Induced increases in insulin sensitivity after bariatric surgery are mediated by muscle extracellular matrix remodeling. Diabetes. 2020;69(8):1675–91. doi: 10.2337/db19-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coen PM, Tanner CJ, Helbling NL, Dubis GS, Hames KC, Xie H, et al. Clinical trial demonstrates exercise following bariatric surgery improves insulin sensitivity. J Clin Invest. 2015;125(1):248–57. doi: 10.1172/JCI78016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coen PM, Menshikova EV, Distefano G, Zheng D, Tanner CJ, Standley RA, et al. Exercise and Weight Loss Improve Muscle Mitochondrial Respiration, Lipid Partitioning, and Insulin Sensitivity After Gastric Bypass Surgery. Diabetes. 2015;64(11):3737–50. doi: 10.2337/db15-0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williams AS, Kang L, Wasserman DH. The extracellular matrix and insulin resistance. Trends Endocrinol Metab. 2015;26(7):357–66. doi: 10.1016/j.tem.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fleischer J, Stein EM, Bessler M, Della Badia M, Restuccia N, Olivero-Rivera L, et al. The decline in hip bone density after gastric bypass surgery is associated with extent of weight loss. J Clin Endocrinol Metab. 2008;93(10):3735–40. doi: 10.1210/jc.2008-0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hue O, Berrigan F, Simoneau M, Marcotte J, Marceau P, Marceau S, et al. Muscle force and force control after weight loss in obese and morbidly obese men. Obes Surg. 2008;18(9):1112–8. doi: 10.1007/s11695-008-9597-5. [DOI] [PubMed] [Google Scholar]
- 30.Lyytinen T, Liikavainio T, Paakkonen M, Gylling H, Arokoski JP. Physical function and properties of quadriceps femoris muscle after bariatric surgery and subsequent weight loss. J Musculoskelet Neuronal Interact. 2013;13(3):329–38. [PubMed] [Google Scholar]
- 31.Pereira AZ, Marchini JS, Carneiro G, Arasaki CH, Zanella MT. Lean and fat mass loss in obese patients before and after Roux-en-Y gastric bypass: a new application for ultrasound technique. Obes Surg. 2012;22(4):597–601. doi: 10.1007/s11695-011-0538-3. [DOI] [PubMed] [Google Scholar]
- 32.Yu EW, Bouxsein ML, Putman MS, Monis EL, Roy AE, Pratt JS, et al. Two-year changes in bone density after Roux-en-Y gastric bypass surgery. J Clin Endocrinol Metab. 2015;100(4):1452–9. doi: 10.1210/jc.2014-4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murai IH, Roschel H, Dantas WS, Gil S, Merege-Filho C, de Cleva R, et al. Exercise Mitigates Bone Loss in Women With Severe Obesity After Roux-en-Y Gastric Bypass: A Randomized Controlled Trial. J Clin Endocrinol Metab. 2019;104(10):4639–50. doi: 10.1210/jc.2019-00074. [DOI] [PubMed] [Google Scholar]
- 34.Brzozowska MM, Sainsbury A, Eisman JA, Baldock PA, Center JR. Bariatric surgery, bone loss, obesity and possible mechanisms. Obes Rev. 2013;14(1):52–67. doi: 10.1111/j.1467-789X.2012.01050.x. [DOI] [PubMed] [Google Scholar]
- 35.Stein EM, Silverberg SJ. Bone loss after bariatric surgery: causes, consequences, and management. Lancet Diabetes Endocrinol. 2014;2(2):165–74. doi: 10.1016/S2213-8587(13)70183-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu EW. Bone metabolism after bariatric surgery. J Bone Miner Res. 2014;29(7):1507–18. doi: 10.1002/jbmr.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Villareal DT, Aguirre L, Gurney AB, Waters DL, Sinacore DR, Colombo E, et al. Aerobic or Resistance Exercise, or Both, in Dieting Obese Older Adults. N Engl J Med. 2017;376(20):1943–55. doi: 10.1056/NEJMoa1616338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Campanha-Versiani L, Pereira DAG, Ribeiro-Samora GA, Ramos AV, de Sander Diniz MFH, De Marco LA, et al. The Effect of a Muscle Weight-Bearing and Aerobic Exercise Program on the Body Composition, Muscular Strength, Biochemical Markers, and Bone Mass of Obese Patients Who Have Undergone Gastric Bypass Surgery. Obes Surg. 2017;27(8):2129–37. doi: 10.1007/s11695-017-2618-5. [DOI] [PubMed] [Google Scholar]
- 39.Baron R, Kneissel M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med. 2013;19(2):179–92. doi: 10.1038/nm.3074. [DOI] [PubMed] [Google Scholar]
- 40.Laurent MR, Dubois V, Claessens F, Verschueren SM, Vanderschueren D, Gielen E, et al. Muscle-bone interactions: From experimental models to the clinic? A critical update. Mol Cell Endocrinol. 2016;432:14–36. doi: 10.1016/j.mce.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 41.Herring LY, Stevinson C, Carter P, Biddle SJH, Bowrey D, Sutton C, et al. The effects of supervised exercise training 12–24 months after bariatric surgery on physical function and body composition: a randomised controlled trial. Int J Obes (Lond). 2017;41(6):909–16. doi: 10.1038/ijo.2017.60. [DOI] [PubMed] [Google Scholar]
- 42.Gil S, Dantas WS, Murai IH, Filho CM, Santo MA, de Cleva R, et al. Exercise Mitigates The Loss In Muscle Mass And Functionality In Obese Women Undergoing Bariatric Surgery. American College of Sports Medicine’s 66th Annual Meeting; Orlando, USA. Medicine & Science in Sports & Exercise; 2019. p. 418–9. [Google Scholar]
- 43.Yin H, Price F, Rudnicki MA. Satellite cells and the muscle stem cell niche. Physiological reviews. 2013;93(1):23–67. doi: 10.1152/physrev.00043.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Joanisse S, Nederveen JP, Snijders T, McKay BR, Parise G. Skeletal Muscle Regeneration, Repair and Remodelling in Aging: The Importance of Muscle Stem Cells and Vascularization. Gerontology. 2017;63(1):91–100. doi: 10.1159/000450922. [DOI] [PubMed] [Google Scholar]
- 45.Gil S, Gualano B, Dantas WS, Murai IH, Ghosh S, Shinjo SK, et al. Exercise Suppresses The Ubiquitin-proteasome System In The Skeletal Muscle Of Obese Women Following Bariatric Surgery. American College of Sports Medicine’s 67th Annual Meeting; San Francisco, USA. Medicine & Science in Sports & Exercise. 2020. p. 642. [Google Scholar]
- 46.Cohen S, Nathan JA, Goldberg AL. Muscle wasting in disease: molecular mechanisms and promising therapies. Nature reviews Drug discovery. 2015;14(1):58–74. doi: 10.1038/nrd4467. [DOI] [PubMed] [Google Scholar]
- 47.Jacobi D, Ciangura C, Couet C, Oppert JM. Physical activity and weight loss following bariatric surgery. Obes Rev. 2011;12(5):366–77. doi: 10.1111/j.1467-789X.2010.00731.x. [DOI] [PubMed] [Google Scholar]
- 48.O’Keefe KL, Kemmeter PR, Kemmeter KD. Bariatric surgery outcomes in patients aged 65 years and older at an American Society for Metabolic and Bariatric Surgery Center of Excellence. Obes Surg. 2010;20(9):1199–205. doi: 10.1007/s11695-010-0201-4. [DOI] [PubMed] [Google Scholar]
- 49.Inge TH, Courcoulas AP, Jenkins TM, Michalsky MP, Helmrath MA, Brandt ML, et al. Weight Loss and Health Status 3 Years after Bariatric Surgery in Adolescents. N Engl J Med. 2016;374(2):113–23. doi: 10.1056/NEJMoa1506699. [DOI] [PMC free article] [PubMed] [Google Scholar]


