Abstract
Objective:
To quantify the bio-accessibility of phosphorus (P) from amino acid-based formulas (AAFs) under different digestive conditions.
Methods:
We developed in vitro batch digestion models with stomach digestion at different pH mimicking normal digestive condition and conditions representing use of acid suppressive medication. To validate bio-accessibility findings we devised a low P murine model to test P bio-availability under compromised digestive conditions using proton pump inhibitors (PPI) to neutralize stomach pH.
Results:
In vitro P bio-accessibility of AAFs Neocate® Infant and Neocate® Junior ranged between 57% and 65% under normal digestive conditions for infants (stomach pH 3.5) and between 38% and 46% under conditions that simulate bypass of stomach acidification, which is comparable to control diet and two EleCare® AAFs. In vivo bioavailability analysis showed that both Neocate® formulas were able to normalize plasma P levels when administered to low P mice along with PPI (control diet+PPI 8.0±0.4, Neocate® Infant 10.1±0.9, Neocate® Junior 9.2±0.6, EleCare® Infant 8.6±0.4, EleCare® Junior 8.7±0.5, n=8–10, p<0.0001 vs. baseline 3.4±0.2 mg/dl). In comparison, plasma P levels remained lower on low P diet (5.7±0.2 mg/dl). Furthermore, urinary P/creatinine and intact fibroblast growth factor 23 (iFGF23) were significantly lowered by low P diet. In contrast, intact parathyroid hormone (iPTH) and 1,25-dihydroxy vitamin D (1,25-D) decreased and increased, respectively, and these parameters likewise normalized in mice administered AAFs.
Conclusion:
Our findings indicate that P bio-accessibility in the in vitro batch digestion model translates well into P bio-availability in mice even under compromised digestive conditions that bypass gastric acidification.
Keywords: Neocate®, EleCare®, phosphorus, bio-accessibility, bio-availability, batch digestion model, hypophosphatemic mouse model
Introduction
Preserving mineral status within healthy ranges is important, particularly for developing infants and children. Calcium and phosphorus (P) serve many different functions in human physiology, including bone mineralization, energy production, and cellular signaling [1]. Most of the body’s calcium (99%) and P (85%) is stored in bone. Plasma levels of both minerals are tightly regulated by parathyroid hormone (PTH),1,25 dihydroxyvitamin D (1,25(OH)2D), and fibroblast growth factor 23 (FGF23) balancing uptake from the diet, redistribution towards and from different body compartments, and renal excretion [2, 3].
Mineral absorption from the diet depends on bio-accessibility and bio-availability (Fig. S1) [4]. Bio-accessibility, i.e., the release of P from the diet, is influenced by many different factors including, but not limited to, food matrix and viscosity, food processing, mineral composition (chemical source of salt and concentration), co-ingestion of other food items, route of administration (e.g., oral intake vs. post-pyloric tube feeding), as well as physiological factors like mineral status, functional state of the gastrointestinal tract, age and use of medication (e.g., proton-pump inhibitors) [5, 6]. Bio-availability, i.e., the fraction of bio-accessible P absorbed in the gastrointestinal tract and available in the systemic circulation, then is affected by the intestinal barrier, which consists of 30% 1,25(OH)2D-dependent transcellular and 70% passive paracellular absorption [7]. 1,25(OH)2D upregulates the type II sodium-phosphate co-transporter NPT2b [8]. Intestinal Pi absorption is highest in infancy and childhood and declines with age. However, it remains robust at approximately 50–70% of bioavailable phosphorus [9].
Dietary management of severe or complex immunoglobulin E (IgE)- and non-IgE-mediated cow’s milk allergy (CMA), and allergy-related gastrointestinal diseases in formula-fed infants [10], with Neocate®, has been proven to be well-tolerated, safe, effective in reducing signs and symptoms of CMA, and support adequate mineral status of CMA infants (including those using acid-suppressive medication) [11, 12]. Despite this, a retrospective chart review [13] reported cases of hypophosphatemia in infants and children with complex medical conditions and extensive medical histories while fed with elemental formula. Two subsequent case studies identified similar reports [14, 15] and also hypothesized that P bio-availability was reduced in these patients. In many of the infants and children described in the case series [13–15] gastric acidification was bypassed either by post-pyloric feeding and/or acid-suppressive medication (90–100% of described cases). The underlying etiology is likely multifactorial, and other factors like PPI use are associated with hypophosphatemia as well [16, 17]. Ultimately bio-availability is individual and condition specific and as such trace element and vitamin status should be monitored [18].
Nevertheless, out of an abundance of caution, the sources of calcium and P in the Neocate® product range were modified in an attempt to optimize the solubility of the mineral salts further. Many types of mineral salts and sources, and combinations of different mixtures of calcium and phosphate salts, were evaluated to optimize mineral solubility further. Preferred product concepts were evaluated for palatability and stability of the emulsion and selected based on bio-accessibility testing in in vitro batch digestion models, which correlated well with bio-accessibility in the TNO in vitro model of the stomach and small intestine (TIM-1) [6, 19–21].
Bio-availability of P is challenging to study in infants and children. To be able to screen a large number of different salt formulas, we developed a simple dialysis-based in vitro batch digestion method, and devised a murine model to validate whether the results obtained in vitro translated into bio-availability of P in vivo.
Materials and Methods
Diets
Control Diet - Teklad #TD.09803 (egg-white based with 0.6% Ca, 0.3% P) and Low P Diet – Teklad #TD.140659 (egg-white based with 0.02% P, 0.6% Ca), were purchased as pellets and powders from Harlan Laboratories, Inc. Madison, USA. Neocate® Infant updated with mineral salts dicalcium phosphate, dipotassium phosphate, tricalcium phosphate, calcium carbonate, tricalcium citrate, and calcium glycerophosphate and Neocate® Junior with mineral salts dipotassium phosphate, tricalcium phosphate, calcium carbonate, magnesium hydrogen phosphate, calcium chloride, were provided by Nutricia, Liverpool, UK. EleCare® Infant and EleCare® Junior were purchased from Abbott Nutrition, Abbott Laboratories, Columbus, Ohio, USA (see Table 1).
Table 1:
Calcium and phosphorus levels and mineral salt in two animal diets and four commercially available amino acid-based medical nutrition formulas.
| Diet | P in diet (g/kg powder) |
Ca in diet (g/kg powder) |
Ca/P ratio | Type of P/Ca salts added |
|---|---|---|---|---|
| Control diet: Teklad #TD.09803 | 3 | 6 | 2 | monosodium phosphate, monopotassium phosphate, calcium carbonate |
| Low phosphate diet: Teklad #TD.140659 | 0.2 | 6 | 30 | calcium carbonate |
| Neocate® Infant | 3.97 | 5.61 | 1.41 | dicalcium phosphate, dipotassium phosphate, tricalcium phosphate, calcium carbonate, calcium glycerophosphate, calcium pantothenate |
| Neocate® Junior | 3.82 | 5.65 | 1.48 | dipotassium phosphate, tricalcium phosphate, calcium carbonate, magnesium hydrogen phosphate, calcium chloride, calcium pantothenate |
| EleCare® Infant | 4.00 | 5.50 | 1.38 | calcium phosphate, potassium phosphate, calcium carbonate, calcium pantothenate |
| EleCare® Junior | 3.95 | 5.43 | 1.37 | calcium phosphate, potassium phosphate, calcium carbonate, magnesium phosphate, calcium pantothenate |
ICP-MS
Samples were diluted 15-fold with 5% HNO3:HCl (4:1) and analyzed on a Perkin Elmer Titan MPS with autosampler ESI prepFAST M5-4DX (Perkin Elmer). The limit of detection (LOD) and limit of quantification (LOQ) of the method was 0.037 mg/ml and 0.124 mg/ml for P, respectively. Concentrations in mg/ml were multiplied with the total volume of the succinate buffer (750 ml) and expressed as absolute amounts (in mg).
Bio-accessibility batch digestion using dialysis tubing
To determine mineral bio-accessibility from the diets, we used the dialyzability of minerals as surrogate based on batch digestion described by Bollinger and workers (Fig. S2, [22]). To model the use of PPI and determine its impact on bio-accessibility, we applied two different digestive conditions mimicking:
Normal digestion: saliva, stomach pH 3.5 followed by intestinal digestion and dialysis at neutral pH
PPI digestion: saliva, stomach pH 7.0 followed by intestinal digestion and dialysis at neutral pH
Premixed diets (see composition in Table 1) were subjected to simulated gastric digestion for one hour at 37°C followed by intestinal digestion for three hours at 37°C. Gastric digestion was started by adding simulated saliva (0.6 g/L α-amylase, Sigma-Aldrich) and gastric juice (succinate buffered saline, stomach electrolytes, 0.125 g/L lipase (DF Amano) and 0.05 g/L pepsin (Sigma-Aldrich)) to the diets at pH 3.5 [23] or pH 7.0. Intestinal digestion was started by adding intestinal juice (succinate buffered saline pH 6.5, small intestine electrolytes, 5 g/L porcine bile extract (Sigma-Aldrich), and pancreatin supernatant after centrifugation for 20 min at 9000 rpm (pancreatin from Sigma-Aldrich)) after 1 h of gastric digestion. Twenty-five ml of the mixture was transferred into a dialysis tube (12.4 kD molecular weight cut-off) and placed into succinate buffered saline (as in [22]; for schematic representation, see Fig. S2). At times 0, 1, 2 and 3 hours samples were taken from the succinate buffered saline and analyzed for phosphate or phosphorus and calcium concentration using QuantiChromTM Phosphate Assay Kit (DIPI-500, BioAssay Systems), ICP-MS and QuantiChromTM Calcium Assay Kit (DICA-500, BioAssay Systems) respectively. We calculated bio-accessibility as the proportion of the mineral dialyzed from the product and expressed as a percentage. The bio-accessible amount was the amount dialyzable mineral from 100 g of product in mg.
Bio-availability testing in mice
Male C57Bl6/6NCrl (strain 27) mice were purchased from Charles River Laboratories (Watertown, MA, USA) at 45 days of age. These mice were reared by dams and weaned at 21 days of age on LabDiet 5L79 (0.61% P, 0.85% calcium, St. Louis, MO, USA). Upon arrival, groups comprised each of 5 male mice/individually vented cage were placed on low P diet pellets ad-libitum for two weeks. Sterile corn cob bedding material (Harlan Teklad #7092) and iso-Pads nesting material (Harlan Teklad #6105) was changed twice weekly and mice had free access to water through an automated system that was hyperchlorinated to inhibit bacterial growth. For overnight fasting mice were placed into fresh cages to minimize coprophagy and solid food was removed at 6 PM allowing for an overnight fast until baseline blood & urine were collected as described below between 8 and 10 AM the next morning and final experimental groups of 8–10 mice (5 mice/cage) placed on ad-libitum feeding with control diet, low P diet, or calorically matched feeding for all experimental group groups respectively. For calorically matched feeding, approximately 140 g powdered diet was prepared in advance supplemented with or without 56 mg pantoprazole (final treatment dose 40 mg/kg; Nycomed GmbH, Konstanz, Germany) [24]. Powdered diets were stored at 4°C until use in aliquots of approximately 10 g (weight varied slightly to supply 9.3 kcal/mouse/day). On the day of use, the diet was dissolved with one cc distilled water, shaped into a soft doughy ball, and added to the cage between 8 and 10 AM in a paper tray to be able to monitor complete intake.
Retro-orbital blood (50 ul) was collected using heparinized capillaries at baseline (day 60). Urination was induced by gentle restraint of the animal and 50–100 ul urine was collected at baseline on day 60, and days 64, 67 70 and 74, followed by retro-orbital exsanguination under deep isoflurane anesthesia to collect terminal heparinized and EDTA plasma and serum for biochemical measurements [25]. Weight of mice was recorded at baseline on day 60, and days 64, 67 70 and 74. Retroorbital blood and urine were collected at the same time of the day between 8 and 10 AM to avoid circadian bias.
Animal research for this study was first approved on October 22, 2014, by the Yale Institutional Animal Care and Use Committee (protocol 2014-11635), was renewed on September 7, 2016, and is valid through September 30, 2020. Yale University has an approved Animal Welfare Assurance (#A3230-01) on file with the NIH Office of Laboratory Animal Welfare. The Assurance was approved on May 5, 2015. All methods for the study were carried out in accordance with relevant guidelines and according to the 2013 AVMA guidelines for the euthanasia of animals. For anesthesia, 30% isoflurane (open drop method) was used. However, most blood and tissue collections were performed as terminal procedures.
Blood and urine parameters
Blood and spot urines were collected as described above. Serum creatinine levels were measured by HPLC/MS/MS. The COBAS Mira Plus automated chemistry analyzer (Roche Diagnostics, Pleasanton, CA, USA) and Phospho-Liqui-UV kit #830 (Stanbio, Boerne, TX, USA) were used to determine plasma and urine inorganic phosphorus. Urinary creatinine was measured by colorimetric assay (DICT-500; BioAssay Systems, Hayward, CA, USA). Concentrations of plasma intact parathyroid hormone (PTH) and plasma intact fibroblast growth factor (FGF)-23 protein were determined using ELISA kits 60-2305 and 60-6500 (Quidel/Immutopics, San Clemente, CA, USA), respectively. Concentrations of serum 25-hydroxyvitamin D (25(OH)D) and 1,25-dihydroxyvitamin D (1,25(OH)2D) were determined using Immunodiagnostic Systems (IDS, Fountain Hills, AZ, USA) kits I-2500 and I-2430, respectively. Measurements of blood P and urine for P and creatinine permit calculation of the tubular reabsorption of P using the formula %TRP=100*1-((U-P*S-creatinine)/(S-P*U-creatinine)).
Statistical and image analysis
Data are expressed as means±SEM and were analyzed as previously described [26] in Prism 8.0 (GraphPad Software, Inc., La Jolla, CA, USA). Differences between groups were considered significant if p-values obtained with Student’s t-test were <0.05. The Mann-Whitney U test was used for comparisons when there was evidence by the Shapiro-Wilk normality test that the data were not normally distributed. Two-way ANOVA and Dunnett’s or Tukey’s test for multiple comparisons was used to determine significant differences between more than two treatment groups with a significance threshold of p<0.05.
Results
Bio-accessibility of phosphorus in different digestive conditions
In vitro P bio-accessibility using a dialysis-based in vitro batch digestion method (Fig. S2) in the amino acid-based formulas ranged between 57% and 65% for P under normal digestive conditions for infants (stomach pH 3.5), and ranged between 38% and 46% for P under conditions that simulate gastric bypass or PPI use (stomach pH neutralized to pH7.0) (Fig. 1). Whereas the salts used in these formulas were different (Table 1), bio-accessibility was quite similar for P.
Figure 1: Bio-accessibility of P under two different digestive conditions.
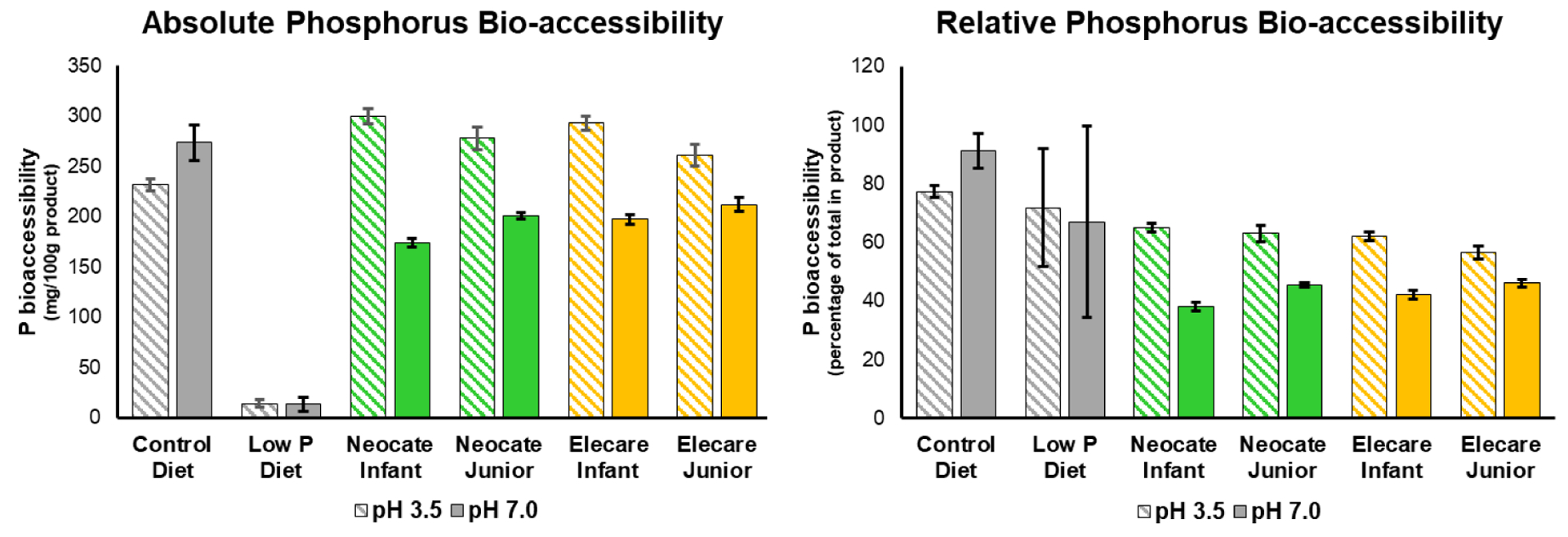
A: Absolute bio-accessible amount of P dialyzed from the diets. B: Relative bio-accessibility of P dialyzed from the diets. (N=3±SD)
Devising a dietary protocol that renders mice hypophosphatemic and hypophosphaturic
Chronic adaptation to a low P diet in wild type mice appears to rely on the up-regulation of Npt2b in the duodenum [27, 28] and upregulation of Npt2a in the proximal tubules [29], and results in normalization of blood P after a couple of weeks. To avoid this chronic adaptation, we devised a scheme, whereby mice are placed on test diets for two weeks following a run-in with low P (low P diet, 0.02% P, 0.6% calcium) for two weeks (Fig. S3). Mice that were on low P diet maintained hypophosphatemia and hypophosphaturia during these two test weeks. However, plasma P was significantly higher by day 74 when compared to day 60 (Fig. S4A, B). Mice fed control diet ad libitum normalized plasma P and urinary P/creatinine when compared to mice that remained on low P diet ad libitum (Fig. S4.1A, B). Since there were detectable differences, when compared to mice that were fed control diet ad libitum compared to control diet after overnight fasting (Fig. S4), we standardized the model. In subsequent experiments, we collected blood and urine parameters after overnight fasting between 8 and 10 AM the next day. The addition of pantoprazole raised stomach pH as expected (Fig. S5A). Although no significant increase in urinary P/creatinine was observed, plasma P was significantly lower in mice receiving control diet with PPI, and urinary P/creatinine was significantly higher (Fig. S4.1A, B). Similarly, urinary P/creatinine was lower with ad libitum feeding and higher with PPI treatment at day 64, 67, and 70 (Fig. S5A, B).
Bio-availability of phosphate in mice corresponds well to the observed in vitro bio-accessibility
Next, we compared the Neocate® products Neocate® Infant and Junior following further optimization of the solubility of the mineral salts and bio-accessibility of P in vitro to unmodified commercial products Elecare Infant and Elecare Junior in hypophosphatemic and hypophosphaturic mice.
Mice fed low P diet ad libitum for two weeks showed a significantly reduced baseline (day 60) urinary P/creatinine when compared to the normal range for mice (Fig. 2). Urinary P/creatinine remained lower on the low P diet on day 64, 67, 70 and 74, but normalized on a control diet with PPI and exceeded this level on Neocate® Infant with PPI (albeit not significantly when analyzing Neocate® Junior, EleCare® Infant, EleCare® Junior with PPI as endpoint results for Fig. 3B).
Figure 2: Urine phosphate excretion.
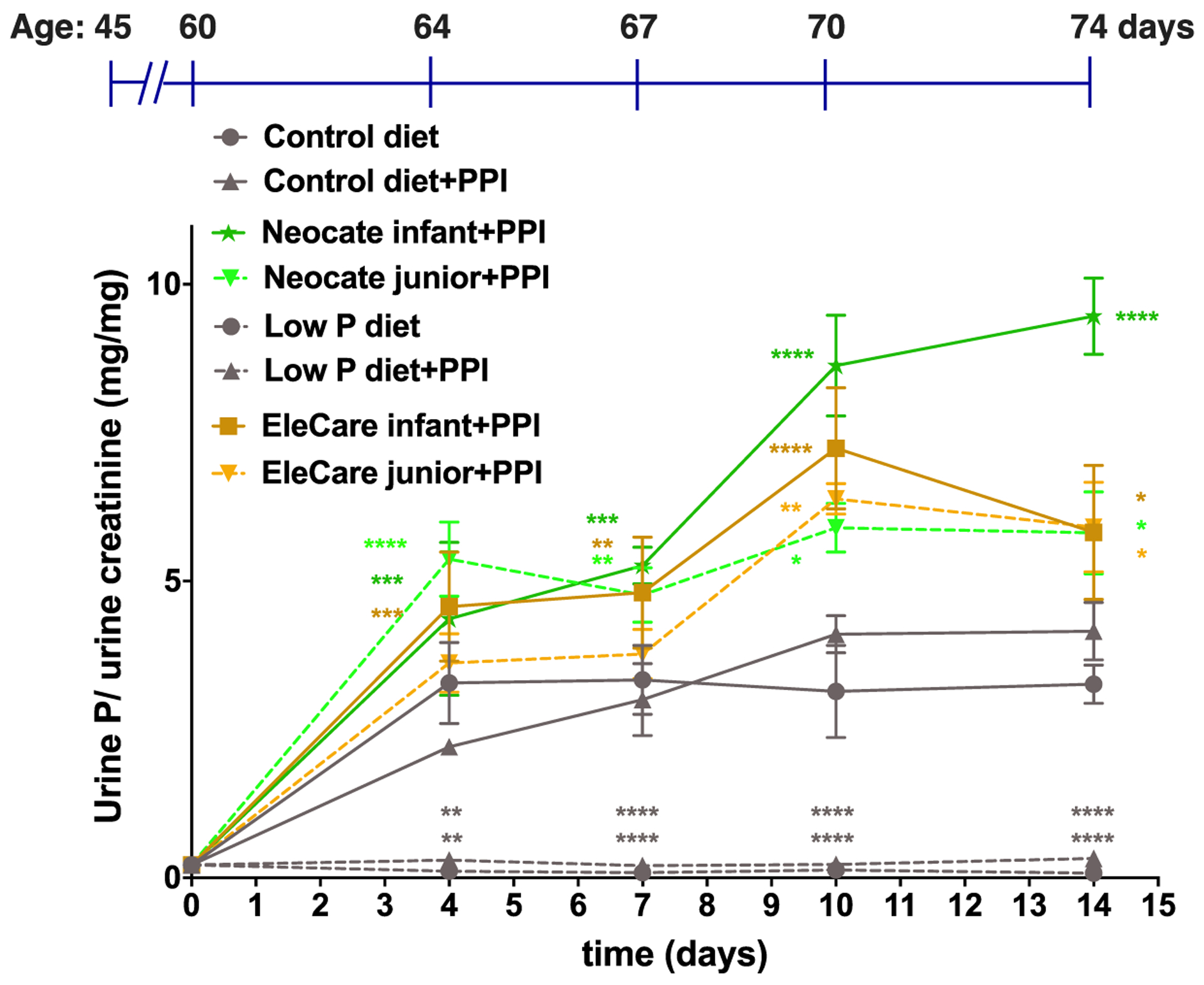
Spot urine samples were collected at 60, 64, 67, 70 days of age, and at day 74, mice were sacrificed for urine and terminal blood collections (see Fig. S3). Shown are means±SEM one-way ANOVA and Dunnett’s test for multiple comparisons was used to determine significant differences between groups, p vs. Control diet+PPI, ****p<0.00002, ***p=0.0002, **p=0.002, *p=0.03.
Figure 3: Endpoint blood and urine biochemistry.
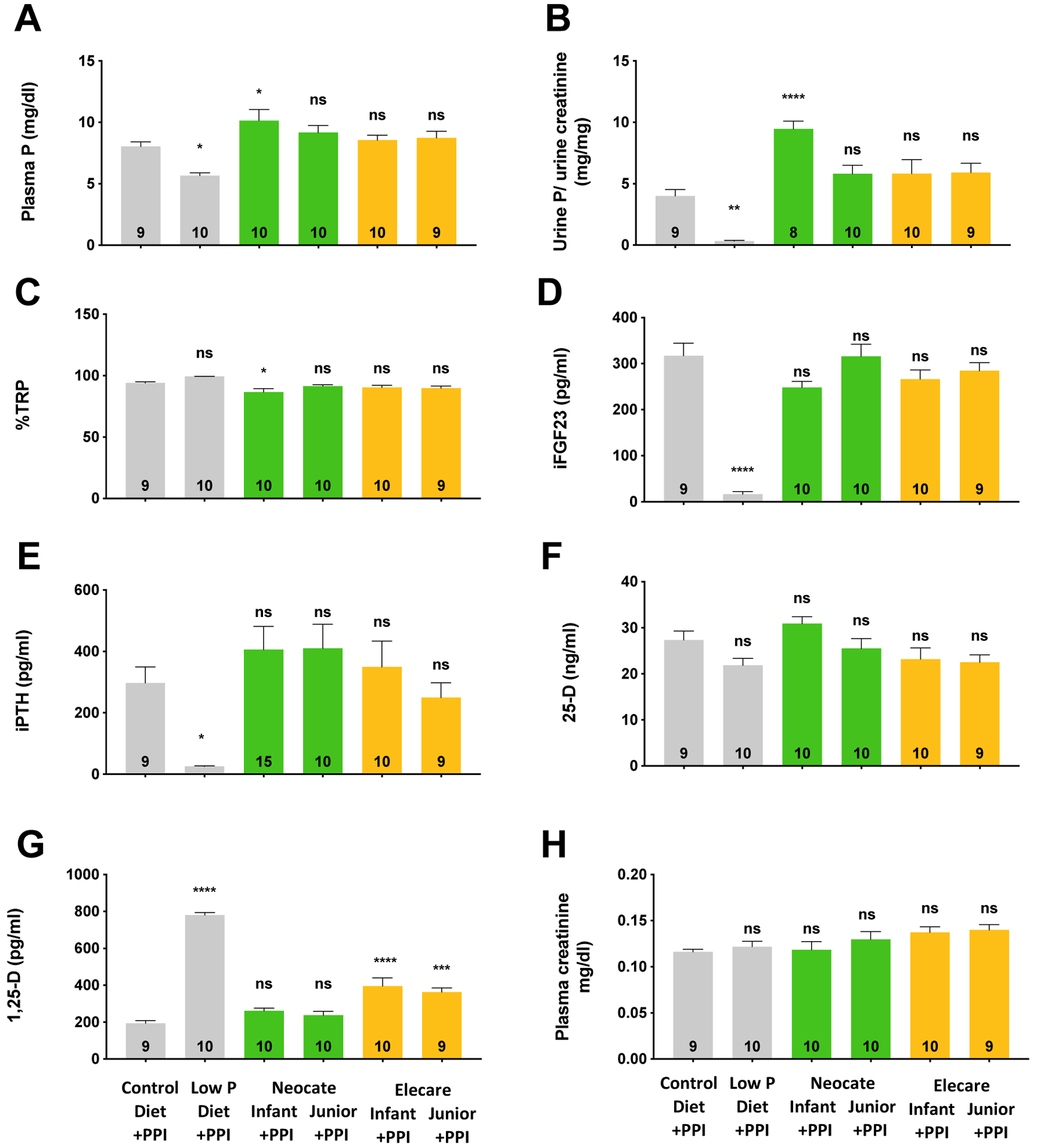
Spot urine samples were collected at day 74 mice, followed by sacrifice and terminal blood collections (see also Fig. 3). Shown are means±SEM, one-way ANOVA and Dunnett’s test for multiple comparisons was used to determine significant differences between groups, n per group is represented in the bars, p vs. Control diet+PPI, ****p<0.00002, ***p=0.0002, **p=0.002, *p=0.03, ns=not significant.
Likewise, at baseline (day 60), plasma P levels were significantly lower, compared to the normal range for mice, and remained lowered on the low P diet on day 74 (Fig. 3A). Plasma P levels normalized on a control diet with PPI and on all four amino acid-based formulas with PPI. Urinary P/creatinine (Fig. 3B), %TRP (Fig. 3C), intact fibroblast growth factor 23 (iFGF23, Fig. 3D) and intact parathyroid hormone (iPTH, Fig. 3E) were significantly reduced on low P diet down to lower limits of quantification, whereas 1,25-dihydroxy vitamin D (1,25-D, Fig. 3G) was elevated, respectively, in low P diet groups compared to control diet with PPI. Urinary creatinine (Fig. 3H) and body weight (Fig. S5B) did not differ among the groups.
Discussion
Mineral bio-accessibility from amino acid-based medical nutrition formulas can be influenced by the route of administration, specific digestive conditions such as stomach pH, and type of mineral salt and concentration of minerals in the formulation. We here combined in vitro batch digestion methodology for rapid screening of salt formulas with validation in a mouse model rendered hypophosphatemic and hypophosphaturic condition. This procedure enabled us to evaluate phosphate bio-availability from selected powdered Neocate® products. Our findings suggest that P bio-accessibility measured in our in vitro batch digestion model translates well into P bio-availability in mice since the two further optimized amino acid-based formulas tested could restore parameters of P homeostasis, even with neutralized stomach pH to the same degree as two control amino acid-based nutrition products. These data may support the use of the further optimized Neocate® product line for patients with complex gastrointestinal medical conditions necessitating use of proton-pump inhibitors and administration into the small intestine.
P bio-accessibility from the amino acid- based formulas were diminished to 46% using in vitro batch digestion with gastric digestion at pH 7.0, to model in vivo conditions where PPIs are used, and gastric acidification was absent. This setting nicely reproduced findings reported earlier with an experimental setup where gastric digestion was omitted to mimic post-pyloric feeding [6]. Conversely, in vitro batch digestion with the gastric phase at pH 3.5 that models normal digestive conditions increased P bio-accessibility up to 65%, and resembles P bio-accessibility obtained in the TIM-1 model [6] and in line with the general values for intestinal absorption of P in humans of 50–70% [9]. These data are consistent with the well-recognized fact that reducing pH improves P bio-accessibility [5].
Our mice rendered hypophosphatemic and hypophosphaturic condition on low P diet and mirrored similar metabolic changes as observed in a small number of children with chronic diseases with complex gastrointestinal conditions under nutrition management with powdered infant and junior Neocate in addition to diverse medical and drug interventions, i.e., suppressed iPTH, iFGF23 and increased 1,25-D along with hypophosphatemia and hypophosphaturia [13]. Furthermore, consistent with the finding in complex patients that substitution by human milk, alternate formula, or phosphate supplementation along with Neocate® was able to reverse the hypophosphatemia [13]; our mice normalized blood and urine P rapidly, when fed a control diet. Blood P in mice was higher following two weeks of nutrition with the control diet, albeit non-significantly, when compared to control diet+PPI. Consequently, iFGF23 was significantly increased in the control diet, when compared to control diet+PPI. These findings suggest that P bio-availability is improved by stomach acidification, as shown in the in vitro batch digestion model. Finally, the updated powdered Neocate® products Neocate® Infant & Junior were able to restore normal iPTH, iFGF23, 1,25-D and blood, and urine P levels comparable to the two EleCare® control products. These results were found even in the presence of PPI that reduces gastric acidification and despite varying phosphate salts and concentrations of calcium, phosphorus, and amino acids in the studied Neocate® and Elecare® products. Our findings in this P recovery model in mice are in line with the bio-equivalence of the two product lines, which we recently showed in healthy volunteers receiving gastric acid-suppressive medication [30].
Chronic adaptation to a low P diet in wild type mice appears to rely on the up-regulation of Npt2b in the duodenum and upregulation of Npt2a in the proximal tubules [29]. As a result, we observed some improvement in hypophosphatemia between days 60 and 74. Since urine P excretion did not change over this period, intestinal absorption as a result of increased 1,25-D synthesis and reduced phosphaturia due to suppressed PTH is likely causing this compensation by upregulating Npt2b in the intestine and by upregulating Npt2a in the kidneys, respectively. Interestingly, ad-lib feeding slightly decreased plasma P and urinary P/creatinine, presumably due to glucose-induced insulin-mediated shift of P into cells [31–35]. Also, PPI supplementation slightly increased urinary P excretion, which in light of the discordant reduction of blood P is likely due to a PPI-induced renal tubular P leak, since 1,25-D and iFGF23 were unchanged. It is also possible that the up-regulation of iPTH at least in the setting of the control diet contributed to the observed PPI-induced phosphaturia. These data in mice are consistent with the observation that proton pump inhibitor therapy was found to cause vitamin D and mineral deficiencies [36] and contribute to fracture risk [37, 38].
Our study is limited by the short treatment period of two weeks, which prevents us from detecting and drawing conclusions on long term changes in bone turnover in mice fed a low P diet and then given one of these formulas, so future long term studies will need to be designed to address this question in the mice. Dietary calcium and calcium bio-accessibility is an essential factor that determines the bio-accessibility of P. We previously showed that gastric acidification improves calcium bio-accessibility, which in turn, may affect P bio-accessibility and reduce secondary hyperparathyroidism and hypocalcemia. Differences in calcium content may be the reason for subtle differences observed between formulas for PTH and 1,25-D (Fig. 3E, G). It is also unknown whether hypochlorhydria and different salt formulas modify intestinal and renal P-transporter expression, which could be studied in, for example, immunoblot analysis of intestinal and renal brush border membrane preparations. Mouse models lacking Npt2b expression in the intestine [7, 28, 39] or Npt2a [29, 40–42] in the proximal tubules could be used to define the contribution of these transporters further.
Conclusions
In summary, our findings indicate that P bio-accessibility measured in the in vitro batch digestion models translates well into P bio-availability in mice. These in vitro batch digestion models can be used to further optimize the solubility of phosphate salt selection for amino acid-based formulas used in patients with altered digestive conditions. Our results show that phosphate salts in the Neocate® product range have P bio-accessibility and bio-availability that is comparable to control formulas even under compromised digestive conditions that bypass gastric acidification.
Supplementary Material
Figure S1: Relationship between bio-accessibility and bio-availability.
Figure S2: Experimental design of the in vitro digestion and dialysis study.
Figure S3: Experimental design of the murine bio-availability study.
Figure S4: Blood and urine biochemistry.
Figure S5: Stomach pH and weights.
Figure S6: Urine phosphate excretion.
Highlights:
A murine model to test phosphorus (P) bio-availability is able to validate in vitro bio-accessibility of P.
In vitro P bio-accessibility of amino-acid based formulas (AAFs) ranged between 57% and 65% under normal digestive conditions for infants (stomach pH 3.5).
In vitro P bio-accessibility of AAFs ranged between 38% and 46% under conditions that simulate bypass of stomach acidification.
In vivo bioavailability analysis showed that AAFs were able to normalize plasma P levels when administered to low P mice under conditions that simulate bypass of stomach acidification.
Funding:
This research was supported by Danone Nutricia Research. We are grateful to the Yale O’Brien Center NIH (NIH/NIDDK P30DK079310) for mouse serum and urine creatinine analyses, and to the Yale Mineral Metabolism Laboratory for mouse serum 25-hydroxyvitamin D (25(OH)D) and 1,25-dihydroxyvitamin D (1,25(OH)2D) measurements.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest:
Sampada Chande declares no conflict of interest.
Jonathan Fetene declares no conflict of interest
Francina Dijk is employed by Danone Nutricia Research, Utrecht, NL
Steven Yannicelli is employed by Nutricia North America, Bethesda, MD, USA
Thomas O. Carpenter has received research support from Ultragenyx, Novato, CA, USA
Ardy van Helvoort is employed by Danone Nutricia Research, Utrecht, NL
Clemens Bergwitz has received research support from Nutricia North America, Bethesda, MD, USA
The funders contributed to the design of the study, analyses, interpretation of data and in the writing of the manuscript, and in the decision to publish the results.
References
- [1].Shaker JLD, L.. Calcium and Phosphate Homeostasis. South Dartmouth (MA): Endotext; [Internet]; 2000. [Google Scholar]
- [2].Bansal VK. Clinical Methods: The History, Physical, and Laboratory Examinations: Serum Inorganic Phosphorus. 3rd edition. ed. Boston: Butterworths; 1990. [PubMed] [Google Scholar]
- [3].Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. Dietary Reference Intakes for Calcium and Vitamin D. Washington (DC): National Academies Press (US); 2011. [PubMed] [Google Scholar]
- [4].Collins CD, Craggs M, Garcia-Alcega S, Kademoglou K, Lowe S. ‘Towards a unified approach for the determination of the bioaccessibility of organic pollutants’. Environ Int. 2015;78:24–31. [DOI] [PubMed] [Google Scholar]
- [5].MacKay M, Jackson D, Eggert L, Fitzgerald K, Cash J. Practice-based validation of calcium and phosphorus solubility limits for pediatric parenteral nutrition solutions. Nutr Clin Pract. 2011;26:708–13. [DOI] [PubMed] [Google Scholar]
- [6].Venema K, Verhoeven J, Verbruggen S. Calcium and phosphorus bioaccessibility from different amino acid-based medical nutrition formulas for infants and children under in vitro digestive conditions Clinical Nutrition Experimental. 2020;32:20–8. [Google Scholar]
- [7].Sabbagh Y, Giral H, Caldas Y, Levi M, Schiavi SC. Intestinal Phosphate Transport. Advances in Chronic Kidney Disease. 2011;18:85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Sabbagh Y, O’Brien SP, Song W, Boulanger JH, Stockmann A, Arbeeny C, et al. Intestinal npt2b plays a major role in phosphate absorption and homeostasis. J Am Soc Nephrol. 2009;20:2348–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Carpenter TO, Bergwitz C, Insogna KL. Chapter 20 - Phosphorus homeostasis and related disorders. In: Bilezikian JP, Martin TJ, Clemens TL, Rosen CJ, editors. Principles of Bone Biology (Fourth Edition): Academic Press; 2020. p. 469–507. [Google Scholar]
- [10].Meyer R, Groetch M, Venter C. When Should Infants with Cow’s Milk Protein Allergy Use an Amino Acid Formula? A Practical Guide. The journal of allergy and clinical immunology In practice. 2018;6:383–99. [DOI] [PubMed] [Google Scholar]
- [11].Harvey BM, Eussen S, van Helvoort A, Harthoorn LF. Cow’s Milk Allergic Infants on Elemental Formula Maintain Adequate Mineral Status Despite Using Acid-suppressive Drugs. J Pediatr Gastroenterol Nutr. 2019;69:e147–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Harvey BM, Eussen SRBM, Harthoorn LF, Burks AW. Mineral Intake and Status of Cow’s Milk Allergic Infants Consuming an Amino Acid-based Formula. J Pediatr Gastroenterol Nutr. 2017;65:346–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Gonzalez Ballesteros LF, Ma NS, Gordon RJ, Ward L, Backeljauw P, Wasserman H, et al. Unexpected widespread hypophosphatemia and bone disease associated with elemental formula use in infants and children. Bone. 2017;97:287–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Creo AL, Epp LM, Buchholtz JA, Tebben PJ. Prevalence of Metabolic Bone Disease in Tube-Fed Children Receiving Elemental Formula. Horm Res Paediatr. 2018;90:291–8. [DOI] [PubMed] [Google Scholar]
- [15].Uday S, Sakka S, Davies JH, Randell T, Arya V, Brain C, et al. Elemental formula associated hypophosphataemic rickets. Clin Nutr. 2018. [DOI] [PubMed] [Google Scholar]
- [16].Bacchetta J, Salusky IB. Evaluation of hypophosphatemia: lessons from patients with genetic disorders. Am J Kidney Dis. 2012;59:152–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wolfe-Wylie M, Mouzaki M, Sochett E, Harrington J. Chronic high dose protonpump inhibitors as a cause of hypophosphatemic rickets Endocrine Society’s 98th AnnualMeeting and Expo, April 1–4, 2016 Boston 2016. [Google Scholar]
- [18].Braegger C, Decsi T, Dias JA, Hartman C, Kolacek S, Koletzko B, et al. Practical approach to paediatric enteral nutrition: a comment by the ESPGHAN committee on nutrition. J Pediatr Gastroenterol Nutr. 2010;51:110–22. [DOI] [PubMed] [Google Scholar]
- [19].Mulet-Cabero AI, Egger L, Portmann R, Menard O, Marze S, Minekus M, et al. A standardised semi-dynamic in vitro digestion method suitable for food - an international consensus. Food Funct. 2020;11:1702–20. [DOI] [PubMed] [Google Scholar]
- [20].Minekus M The tno gastro-intestinal model (TIM). In: Verhoeckx K, Cotter P, Lopez-Exposito I, Kleiveland C, Lea T, Mackie A, et al. , editors. The impact of food bioactives on health: In vitro and ex vivo models. CH: Cham; 2015. p. 37–46. [Google Scholar]
- [21].Minekus M, Marteau P, Havenaar R, Huisintveld J. A multicompartmental dynamic computer-controlled model simulating the stomach and small-intestine. Atla-Altern Lab Anim 1995;23:197–209. [Google Scholar]
- [22].Bollinger DW, Tsunoda A, Ledoux DR, Ellersieck MR, Veum TL. A comparison of the test tube and the dialysis tubing in vitro methods for estimating the bioavailability of phosphorus in feed ingredients for swine. J Agric Food Chem. 2005;53:3287–94. [DOI] [PubMed] [Google Scholar]
- [23].Havenaar R, Anneveld B, Hanff LM, de Wildt SN, de Koning BA, Mooij MG, et al. In vitro gastrointestinal model (TIM) with predictive power, even for infants and children? Int J Pharm. 2013;457:327–32. [DOI] [PubMed] [Google Scholar]
- [24].Schwarz P, Kubler JA, Strnad P, Muller K, Barth TF, Gerloff A, et al. Hepcidin is localised in gastric parietal cells, regulates acid secretion and is induced by Helicobacter pylori infection. Gut. 2012;61:193–201. [DOI] [PubMed] [Google Scholar]
- [25].Li Y, Caballero D, Ponsetto J, Chen A, Zhu C, Guo J, et al. Response of Npt2a knockout mice to dietary calcium and phosphorus. PLoS One. 2017;12:e0176232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Chande S, Ho B, Fetene J, Bergwitz C. Transgenic mouse model for conditional expression of influenza hemagglutinin-tagged human SLC20A1/PIT1. PLoS One. 2019;14:e0223052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ohi A, Hanabusa E, Ueda O, Segawa H, Horiba N, Kaneko I, et al. Inorganic phosphate homeostasis in sodium-dependent phosphate cotransporter Npt2b(+)/(−) mice. Am J Physiol Renal Physiol. 2011;301:F1105–13. [DOI] [PubMed] [Google Scholar]
- [28].Schiavi SC, Tang W, Bracken C, O’Brien SP, Song W, Boulanger J, et al. Npt2b deletion attenuates hyperphosphatemia associated with CKD. J Am Soc Nephrol. 2012;23:1691–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Giral H, Caldas Y, Sutherland E, Wilson P, Breusegem S, Barry N, et al. Regulation of rat intestinal Na-dependent phosphate transporters by dietary phosphate. Am J Physiol Renal Physiol. 2009;297:F1466–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Bergwitz C, Eussen S, Janssens P, Visser M, Carpenter TO, van Helvoort A. Different elemental infant formulas show equivalent phosphorus and calcium bioavailability in healthy volunteers. Nutr Res. 2021;85:71–83. [DOI] [PubMed] [Google Scholar]
- [31].Trautvetter U, Kiehntopf M, Jahreis G. Postprandial effects of calcium phosphate supplementation on plasma concentration-double-blind, placebo-controlled cross-over human study. Nutrition journal. 2013;12:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Berner YN, Shike M. Consequences of phosphate imbalance. Annu Rev Nutr. 1988;8:121–48. [DOI] [PubMed] [Google Scholar]
- [33].Stadie WC. The relation of insulin to phosphate metabolism. Yale Journal of Biology and Medicine. 1944;16:539–59. [PMC free article] [PubMed] [Google Scholar]
- [34].Khattab M, Abi-Rashed C, Ghattas H, Hlais S, Obeid O. Phosphorus ingestion improves oral glucose tolerance of healthy male subjects: a crossover experiment. Nutrition journal. 2015;14:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].MacLeod DB, Montoya DR, Fick GH, Jessen KR. The effect of 25 grams i.v. glucose on serum inorganic phosphate levels. Ann Emerg Med. 1994;23:524–8. [DOI] [PubMed] [Google Scholar]
- [36].Heidelbaugh JJ. Proton pump inhibitors and risk of vitamin and mineral deficiency: evidence and clinical implications. Therapeutic advances in drug safety. 2013;4:125–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Nassar Y, Richter S. Proton-pump Inhibitor Use and Fracture Risk: An Updated Systematic Review and Meta-analysis. J Bone Metab. 2018;25:141–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Thong BKS, Ima-Nirwana S, Chin KY. Proton Pump Inhibitors and Fracture Risk: A Review of Current Evidence and Mechanisms Involved. International journal of environmental research and public health. 2019;16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Sabbagh Y, O’Brien SP, Song W, Stockmann A, Arbeeny C, Schiavi SC. Importance of Npt2b in Phosphate Homeostasis: Characterization of the Conditional Npt2b Knockout Mouse. J Am Soc Nephrol. Philadelphia 2008. p. [SA-FC343]. [Google Scholar]
- [40].Chau H, El-Maadawy S, McKee MD, Tenenhouse HS. Renal calcification in mice homozygous for the disrupted type IIa Na/Pi cotransporter gene Npt2. J Bone Miner Res. 2003;18:644–57. [DOI] [PubMed] [Google Scholar]
- [41].Tenenhouse HS, Gauthier C, Chau H, St-Arnaud R. 1alpha-Hydroxylase gene ablation and Pi supplementation inhibit renal calcification in mice homozygous for the disrupted Npt2a gene. Am J Physiol Renal Physiol. 2004;286:F675–81. [DOI] [PubMed] [Google Scholar]
- [42].Tenenhouse HS, Martel J, Gauthier C, Segawa H, Miyamoto K. Differential effects of Npt2a gene ablation and X-linked Hyp mutation on renal expression of Npt2c. Am J Physiol Renal Physiol. 2003;285:F1271–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Relationship between bio-accessibility and bio-availability.
Figure S2: Experimental design of the in vitro digestion and dialysis study.
Figure S3: Experimental design of the murine bio-availability study.
Figure S4: Blood and urine biochemistry.
Figure S5: Stomach pH and weights.
Figure S6: Urine phosphate excretion.


