Abstract
Background
Accumulated evidence has indicated that a high‐normal FT4 level is an independent risk factor for the clinical progression of AF. However, the association between elevated FT4 concentration within the normal range and AF recurrence after cryoballoon ablation in China is unknown.
Methods
This retrospective and observational study included 453 AF patients who underwent cryoballoon ablation from January 2016 to August 2018. Patients were classified into quartiles based on preprocedural serum FT4 concentration. The clinical characteristics of the patients and the long‐term rate of AF recurrence after ablation were assessed.
Results
After a mean follow‐up period of 17.4 ± 9.0 months, 91 (20.1%) patients suffered from AF recurrence. The AF recurrence rate by FT4 quartile was 17.7%, 19.0%, 21.4%, and 22.3% for participants with FT4 in quartile 1, 2, 3, and 4, respectively (p < .001). On multivariate Cox regression, FT4 concentration (HR: 1.187, 95% CI: 1.093–1.290, p < .001) and left atrial diameter (HR: 1.052, 95% CI: 1.014–1.092, p = .007) were significant predictors of AF recurrence. When stratifying for AF type, the rate of postoperative recurrence was independently increased as FT4 concentration increased in paroxysmal AF, but not in persistent AF (p < .001 in paroxysmal AF and p = .977 in persistent AF).
Conclusion
Higher FT4 level within the normal range predicted the outcome of cryoballoon ablation in Chinese paroxysmal AF patients without structural heart disease.
Keywords: atrial fibrillation, cryoballoon ablation, free thyroxine, recurrence
1. INTRODUCTION
Atrial fibrillation (AF), the most common cardiac arrhythmia, is frequently associated with ischemic stroke and heart failure (Markides & Schilling, 2003). Epidemic data have shown that the incidence of AF is increasing rapidly worldwide, and the overall prevalence of community‐based AF is estimated to range from 0.37% to 3.56% in China (Bai et al., 2017). Catheter ablation with pulmonary vein isolation (PVI), including cryoballoon ablation and radiofrequency ablation, has been widely acknowledged as an effective method of AF treatment, especially in paroxysmal AF (Parameswaran et al., 2020). Compared with antiarrhythmic drugs, it significantly improves the postoperative recurrence rate and patient quality of life (Jaïs et al., 2008). However, the approximately 20–30% recurrence rate remains unsatisfactory, and additional noninvasive and accurate risk factors for the early prediction of AF recurrence are needed (Buiatti et al., 2017).
Thyroid hormones are well established to play critical roles in cardiovascular function (Cappola et al., 2006). They can regulate systemic vascular resistance, blood volume, and inotropic and chronoscopic effects (Yen et al., 2006). Moreover, they can increase the propensity for atrial arrhythmias, particularly AF, through shortening of the action potential (AP) duration in the atrial myocardium (Marrakchi et al., 2015). Overt hyperthyroidism and subclinical hyperthyroidism have been well demonstrated as risk factors in AF patients and as markers of worse cardiovascular adverse outcomes, including dementia, coronary heart disease, and heart failure (Marrakchi et al., 2015). In patients undergoing AF catheter ablation, prior data showed that overt hyperthyroidism and subclinical hyperthyroidism were associated with an increased risk of AF recurrence (Wongcharoen et al., 2015). A recently published retrospective study reported that even higher free thyroxine (FT4) levels within the reference range were associated with an increased prevalence and incidence of AF (Anderson et al., 2019), which indicated that elevated FT4 levels within the reference range may be a potential target in the pathophysiology of AF and may consequently contribute to the low clinical success rates after PVI.
Cryoballoon ablation shares comparable success rates and safety with radiofrequency ablation and is characterized by its shorter learning curve and procedure duration (Kuck et al., 2016). Nevertheless, to the best of our knowledge, there are no available data regarding the effect of normal thyroid function on the clinical outcomes of cryoballoon ablation. Most observational studies examining catheter ablation outcomes in patients in relation to thyroid function used radiofrequency ablation and focused primarily on thyroid‐stimulating hormone (TSH) and less on FT4 (Morishima et al., 2018; Sousa et al., 2015; Tang et al., 2010). Therefore, the objective of this study was to evaluate the potential effects of FT4 concentration on AF‐free survival after cryoballoon ablation and to assess the free triiodothyronine (FT3) and TSH concentrations to identify which thyroid hormone subtype is the best biomarker for predicting AF recurrence in Chinese euthyroid patients.
2. METHODS
2.1. Studied population
This retrospective and observational study included 453 consecutive symptomatic drug‐refractory paroxysmal or persistent AF patients who underwent cryoballoon ablation procedures in the Department of Cardiology, Shanghai Tenth People's Hospital (China) from January 2016 to August 2018. The inclusion criteria were in accordance with diagnostic criteria for symptomatic drug‐refractory nonvalvular AF and indications for AF ablation (January et al., 2014). Paroxysmal AF was defined as AF episodes that were self‐terminating and lasted for up to 7 days, and persistent AF was defined as continuous AF that lasted longer than 7 days. The exclusion criteria were as follows: (1) left atrial diameter (LAD) >55 mm; (2) myocarditis or cardiomyopathies; (3) structural heart disease; (4) intracardiac thrombi; (5) left atrial ablation; (6) heart failure with NYHA class IV; (7) acute or chronic infections or inflammatory diseases, severe liver or renal dysfunction, and history of cerebral infarction or transient cerebral ischemia within 6 months. In addition, participants with abnormal TSH, FT3, or FT4 levels, thyroid medication, prior or current uncontrolled thyroid or parathyroid disease and patients who had been on amiodarone for 3 months prior to admission were excluded from the analysis. Patients were classified into quartiles based on preprocedural serum FT4 concentration. All subjects provided written informed consent before participating in the study. The current study complied with the Declaration of Helsinki and was approved by the Shanghai Tenth Hospital Ethics Committee.
2.2. Clinical data collection
Upon patient hospitalization, detailed data regarding medical history and clinical and demographic characteristics at baseline were collected. Diabetes was diagnosed according to the WHO criteria (American Diabetes, 2018). Hypertension was defined as blood pressure higher than 140/90 mmHg or the use of antihypertensive medications. Heart failure patients were those with New York Heart Association class IV heart failure or left ventricular ejection fraction (LVEF) <40%. The risk score for stroke was evaluated by CHA2DS2‐VASc score (Lip et al., 2010). Euthyroidism in our study was defined as TSH, FT4, and FT3 levels within the reference range while not taking any thyroid medication.
2.3. Preprocedural preparation
Standard 12‐lead electrocardiogram (ECG) and Holter examination were recorded in all patients. Routine chest X‐ray and transthoracic echocardiography (TTE) evaluations were performed to assess left atrial diameter and left ventricular ejection fraction in all patients within 1 week prior to ablation. To exclude the presence of thrombi in the left atrium and left atrial appendage, all patients underwent TEE the day before the procedure.
Prior to ablation, all antiarrhythmic drugs (AADs) were withdrawn for at least five half‐lives. All patients needed to be anticoagulated with warfarin or new oral anticoagulants (NOACs) at least 8 weeks before the ablation procedure. For patients taking warfarin, ablation was performed if their INR was maintained within 2.0–3.0 without interruption prior to ablation. Commonly used oral anticoagulants, such as dabigatran, rivaroxaban, and warfarin, were discontinued 24 h before the procedure.
2.4. Laboratory tests
Baseline fasting blood samples were obtained from all patients on the first morning of hospitalization. Serum concentrations of N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) and C‐reactive protein (CRP); plasma levels of glucose, high‐density lipoprotein cholesterol (HDL), and low‐density lipoprotein cholesterol (LDL‐C); and the international normalized ratio (INR) were assessed by colorimetric enzymatic assay systems (Roche MODULAR P‐800, Switzerland). Preprocedural serum thyroid function was assessed with a commercial assay (ADVIA Centaur XP Immunoassay System; Siemens Healthcare Diagnostic Inc.,). The laboratory reference ranges of FT4, FT3, and TSH concentrations were 10.5–24.4 pmol/L, 2.8–6.3 pmol/L and 0.38–4.34 mIU/L, respectively.
2.5. Cryoballoon ablation procedure
Circumferential pulmonary vein isolation following cryoballoon ablation was performed by experienced electrophysiologists, as described previously in detail (Liu et al., 2019). In short, cryoballoon ablation procedures were performed with patients under local anesthesia and conscious sedation. All patients had groin‐entry venous‐route catheter introduction with entry into the left atrium via transseptal puncture under X‐ray guidance. Selective PV angiography was used to identify the PVs. A standard commercially available balloon catheter (Arctic Front Advance™ 10.5‐F shaft, 23‐ or 28 mm diameter, Medtronic Inc.,) was introduced into the left atrium via a sheath (FlexCath Advance™ Steerable Sheath, 15 F, Medtronic Inc.,). The choice of balloon size was based on the patients’ LA and PV anatomy. An 8‐pole circular mapping catheter (Achieve™, Medtronic Inc.,) with a shaft diameter of 1.1 mm and a 15 mm loop was advanced through the lumen of the cryoballoon catheter, replacing the guide wire. PV electrograms were recorded at baseline using a circular mapping catheter to compare with analogous recordings after ablation. The acute procedural endpoint was defined as the absence or dissociation of all PV potentials, as confirmed by bidirectional block using a circular mapping catheter after a 30 min waiting period (subsequent to the last ablation).
2.6. Postablation management
After the cryoballoon ablation procedure, all participants were monitored with ECG telemetry for at least 24 h and reevaluated by TTE to rule out pericardial effusion. Oral anticoagulation, such as dabigatran, rivaroxaban, or warfarin, was initiated in the evening after ablation. It continued for at least 3 months after the procedure and thereafter based on the CHA2DS2‐VASc score. Antiarrhythmic drugs such as sotalol were continued for at least 3 months as well.
2.7. Follow‐up and study outcome
After hospital discharge, the follow‐up information of patients was obtained from telephone interviews or each visit in the outpatient clinics at 1, 3, 6, and 12 months and then every 6 months thereafter, or earlier if symptoms consistent with recurrent AF developed after the ablation. The primary outcome was defined as AF recurrence, which was any recording of AF on ECG or an episode longer than 30 s on 24 h ECG Holter registration after a blanking period of 3 months. Patients who experienced a second ablation at any time were also classified as having recurrence.
2.8. Statistical analysis
Continuous variables are presented as the mean ± standard deviation (SD). Categorical variables are expressed as numbers and percentages. For continuous variables, one‐way ANOVA was used to compare FT4 quartiles. Categorical variables were compared by the chi‐square test or Fisher's exact test, as appropriate. FT4 level was analyzed as both a continuous and a categorical variable. Recurrence‐free survival over time regarding the FT4 quartiles was calculated using Kaplan–Meier curves, and differences in AF‐free survival among groups were compared by the log‐rank test. Independent predictors of AF recurrence after the cryoballoon ablation procedure were identified using a Cox proportional hazards model. Variables in the stepwise multivariate analysis only included covariates with a p value < .05 in univariate analysis. Specific subgroup analysis by AF type was also performed using Cox regression analysis. The hazard ratio (HR) and 95% confidence interval (CI) are reported. A two‐tailed P value of less than 0.05 was considered statistically significant. All analyses were performed using Statistical Package for Social Sciences (SPSS) for Windows 10.
3. RESULTS
3.1. Subjects' characteristics
The present study comprised 453 symptomatic AF patients who underwent cryoballoon ablation. The mean age of the participants was 65.5 ± 9.4 years, and 286 (63.1%) were male. Of them, the mean CHA2DS2‐VASC score was 2.3 ± 1.6. There were 57 (12.6%) persistent and 396 (87.4%) paroxysmal AF patients. The mean LAD was 41.2 ± 5.4 mm, and LVEF was 60.9% ± 7.2%. The mean concentrations of FT4, FT3 and TSH were 17.0 ± 2.8 pmol/l, 4.6 ± 0.6 pmol/l, and 2.1 ± 1.1 mIU/ml, respectively (Table 1). All patients were further divided into quartiles based on preprocedural FT4 level, and their clinical characteristics and laboratory data are shown in Table 2. Participants in the highest FT4 quartile were less likely to be smokers and alcohol drinkers than those in the lower quartiles. FT3 concentrations increased with increasing FT4 quartile (4.47 ± 0.51 pmol/l in quartile 1 vs. 4.59 ± 0.59 pmol/l in quartile 2 vs. 4.66 ± 0.56 pmol/l in quartile 3 vs. 4.70 ± 0.70 pmol/l in quartile 4, p < .05). There were no other significant differences in baseline characteristics among the FT4 quartile groups.
TABLE 1.
Baseline Characteristics of the Study Population
| Clinical characteristics | All patients (Total n = 453) |
|---|---|
| Demographics | |
| Age, years | 65.5 ± 9.4 |
| Sex, male, N(%) | 286(63.1%) |
| Clinical parameters | |
| CHA2DS2‐vasc score | 2.3 ± 1.6 |
| Heart rate (beats/min) | 80.8 ± 16.8 |
| Paroxysmal AF, N(%) | 396(87.4%) |
| Smoker, N(%) | 80(17.7%) |
| Alcohol, N(%) | 49(10.8%) |
| Diabetes mellitus, N(%) | 78(17.2%) |
| Hypertension, N(%)) | 258(57.0%) |
| Stroke/TIA, N(%) | 126(27.8%) |
| Dyslipidemia, N(%) | 28(6.2%) |
| ACE inhibitor/ARB, N (%) | 154(34.0%) |
| β‐Blockers, N (%) | 140(30.9%) |
| Biochemical parameters | |
| HDL, mmol/L | 1.2 ± 0.3 |
| LDL, mmol/L | 2.4 ± 0.8 |
| NT‐proBNP, pg/ml | 597.6 ± 785.6 |
| CRP (mg/L) | 4.0 ± 3.5 |
| Echocardiography | |
| LAD, (mm) | 41.2 ± 5.4 |
| LVEF, (%) | 60.9 ± 7.2 |
| Thyroid function | |
| FT3, pmol/L | 4.6 ± 0.6 |
| FT4, pmol/L | 17.0 ± 2.8 |
| TSH, mIU/L | 2.1 ± 1.1 |
Data are reported as means ± SD for continuous variables and n (%) for categorical variables.
Abbreviations: ACEI, angiotensin‐converting‐enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin receptor blocker; CRP, C‐reactive protein; FT3, free triiodothyronine; FT4, free thyroxine; HDL, high‐density lipoprotein; LAD, left atrial diameter; LDL, low‐density lipoprotein; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; TIA, transient ischemic attack; TSH, thyroid‐stimulating hormone.
TABLE 2.
Baseline characteristics of the participants according to the quartile of FT4 (pmol/L)
| Characteristic | Serum FT4, pmol/L | ||||
|---|---|---|---|---|---|
|
Quartile 1 (10.68–14.71) |
Quartile 2 (14.71–16.88) |
Quartile 3 (16.88–18.83) |
Quartile 4 (18.83–24.25) |
p value | |
| No. of patients | 113 | 116 | 112 | 112 | |
| Age, years | 67.00 ± 8.96 | 65.30 ± 9.40 | 64.72 ± 8.92 | 64.83 ± 10.25 | .237 |
| Sex, male, N(%) | 72 (63.7%) | 76 (65.5%) | 69 (61.6%) | 69 (61.6%) | .914 |
| CHA2DS2‐VASc | 2.31 ± 1.56 | 2.39 ± 1.80 | 2.20 ± 1.46 | 2.44 ± 1.46 | .684 |
| Heart rate (beats/min) | 78.50 ± 17.11 | 80.54 ± 16.37 | 84.15 ± 17.95 | 80.13 ± 15.43 | .079 |
| Type of AF: persistent (%) | 20 (17.7%) | 12 (10.3%) | 14 (12.5%) | 11 (9.8%) | .262 |
| Smoker, N(%) | 29 (25.7%) | 27 (23.3%) | 14 (12.5%) | 10 (8.9%) | .001* |
| Alcohol, N(%) | 18 (15.9%) | 16 (13.8%) | 8 (7.1%) | 7 (6.3%) | .044* |
| Diabetes mellitus, N(%) | 18 (15.9%) | 24 (20.7%) | 12 (10.7%) | 24 (21.4%) | .120 |
| Hypertension, N(%)) | 56 (49.6%) | 65 (56.0%) | 64 (57.1%) | 73 (65.2%) | .130 |
| Stroke/TIA, N(%) | 31 (27.4%) | 35 (30.2%) | 31 (27.7%) | 29 (25.9%) | .911 |
| Dyslipidemia, N(%) | 11 (9.7%) | 6 (5.2%) | 5 (4.5%) | 6 (5.4%) | .339 |
| ACE inhibitor/ARB, N (%) | 36 (31.9%) | 41 (35.3%) | 36 (32.1%) | 41 (36.6%) | .841 |
| β‐blockers, N (%) | 34 (30.1%) | 40 (34.5%) | 29 (25.9%) | 37 (33.0%) | .515 |
| HDL, mmol/L | 1.14 ± 0.30 | 1.14 ± 0.28 | 1.18 ± 0.27 | 1.19 ± 0.30 | .410 |
| LDL, mmol/L | 2.44 ± 0.83 | 2.30 ± 0.82 | 2.40 ± 0.82 | 2.29 ± 0.85 | .412 |
| NT‐proBNP, pg/ml | 506.8 ± 598.2 | 592.8 ± 746.8 | 697.5 ± 1020.2 | 594.6 ± 717.4 | .345 |
| CRP (mg/L) | 3.42 ± 1.03 | 3.90 ± 3.07 | 4.27 ± 4.12 | 4.40 ± 4.78 | .164 |
| LAD, (mm) | 40.83 ± 5.03 | 41.77 ± 5.61 | 41.22 ± 5.89 | 41.07 ± 5.13 | .603 |
| LVEF, (%) | 61.74 ± 5.22 | 60.72 ± 7.87 | 60.70 ± 7.29 | 60.44 ± 8.17 | .546 |
| FT3, pmol/L | 4.47 ± 0.51 | 4.59 ± 0.59 | 4.66 ± 0.56 | 4.70 ± 0.70 | .019* |
| TSH, mIU/L | 2.25 ± 1.19 | 2.17 ± 1.06 | 1.94 ± 0.91 | 1.99 ± 1.10 | .092 |
Data are reported as means ± SD for continuous variables and n (%) for categorical variables.
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin receptor blocker; CRP, C‐reactive protein; FT3, free triiodothyronine; FT4, free thyroxine; HDL, high‐density lipoprotein; LAD, left atrial diameter; LDL, low‐density lipoprotein; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; TIA, transient ischemic attack; TSH, thyroid‐stimulating hormone.
p < .05.
3.2. Patients with higher FT4 levels presented higher rates of AF recurrence after the cryoballoon ablation procedure
After a mean follow‐up period of 17.4 ± 9.0 months, 91 patients (20.1%) experienced recurrence after cryoballoon ablation. Kaplan–Meier survival curves showed the cumulative incidence of AF recurrence among FT4 quartiles. Patients with higher FT4 levels had an increased risk of AF recurrence after cryoballoon ablation (Figure 1). The recurrence rate of AF in FT4 quartile 4 (22.3%) was significantly higher than that in FT4 quartile 3 (21.4%), FT4 quartile 2 (19.0%), and FT4 quartile 1 (17.7%) (p < .001).
FIGURE 1.
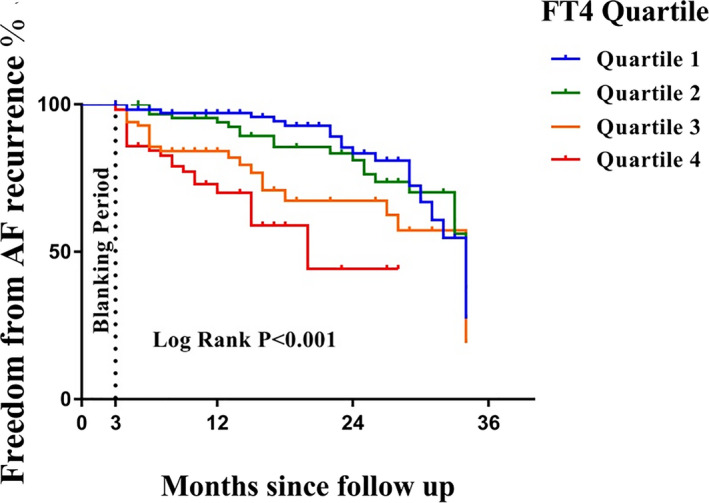
Kaplan–Meier curve for AF‐free survival after cryoballoon ablation, according to serum FT4 quartile within the reference range (log‐rank p < .001). AF, atrial fibrillation; PVI, pulmonary vein isolation; FT4, free thyroxine
3.3. Ablation complications
Regarding safety, the overall incidence of phrenic nerve paralysis (PNP) was 8 (1.8%). Pericardial tamponade occurred in one patient and was relieved after pericardial drainage. There were no other complications, such as PV stenosis, esophageal fistula, gastroparesis, or air embolism, noted in our study.
3.4. Higher FT4 is independently associated with AF recurrence in paroxysmal but not in persistent AF patients
Subgroup analysis in terms of AF type was also performed using Cox proportional hazards regression analysis after adjusting for sex, age, diabetes mellitus, hypertension, dyslipidemia, smoking, alcohol use, CHA2DS2‐vasc score, and LAD, as shown in Figure 2. The lowest FT4 quartile (ranging from 10.68–14.71 pmol/L) was chosen as the reference group. The higher risk of AF recurrence with higher concentrations of FT4 remained significant among paroxysmal AF patients only. The HR for patients with persistent AF did not reach statistical significance.
FIGURE 2.
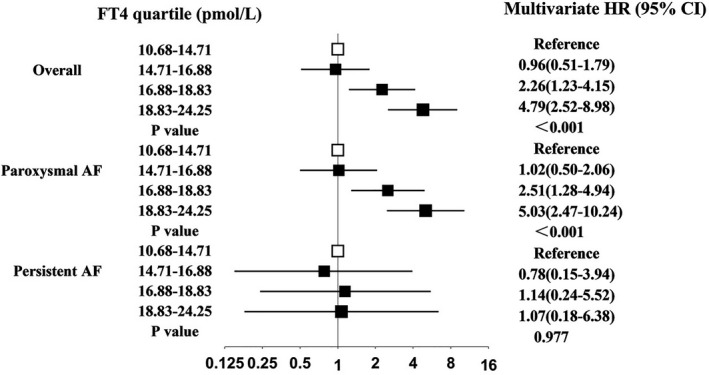
Associations between FT4 quartiles within the reference range and the risk of AF recurrence after cryoballoon ablation in the overall patients and stratified by AF type
3.5. Predictive value of higher FT4 levels within the normal range
In the univariate Cox proportional hazards analysis, FT4 concentration, LAD, and diabetes mellitus were associated with AF recurrence. However, no significant associations between FT3 or TSH level and AF recurrence were observed in univariate analysis or in multivariable models. In multivariable Cox regression analysis, adjustment for other potential risk factors, FT4 concentration (HR: 1.187, 95% CI: 1.093–1.290, p < .001), and LAD (HR: 1.052, 95% CI: 1.014–1.092, p = .007) remained significant and independent predictors for AF recurrence after cryoballoon ablation (Table 3).
TABLE 3.
Univariate and multivariate Cox regression analysis for independent predictors of recurrence after cryoballoon ablation in patients with atrial fibrillation
| Variable | Univariate Cox regression analysis | Multivariate Cox regression analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p‐Value | HR (95% CI) | p‐Value | |
| Age (years) | 1.027 (0.987–1.068) | .191 | ||
| Sex: male (%) | 1.090 (0.588–2.021) | .783 | ||
| CHA2DS2‐vasc score | 0.856 (0.581–1.260) | .430 | ||
| Heart rate (beats/min) | 1.010 (.997–1.023) | .120 | ||
| Type of AF: persistent (%) | 1.193 (0.638–2.232) | .580 | ||
| Smoking (%) | 1.180 (0.548–2.539) | .672 | ||
| Alcohol consumption (%) | 0.500 (0.206–1.217) | .127 | ||
| Diabetes mellitus (%) | 2.181 (1.041–4.571) | .039* | 1.366 (0.794–2.352) | .260 |
| Hypertension (%) | 0.667 (0.358–1.241) | .201 | ||
| History of stroke/TIA (%) | 1.331 (0.720–2.463) | .362 | ||
| Dyslipidemia (%) | 0.594 (0.244–1.447) | .251 | ||
| NT‐proBNP, pg/ml | 1.000 (1.000–1.000) | .828 | ||
| LDL, mmol/L | 1.005 (0.768–1.314) | .973 | ||
| CRP (mg/L) | 0.961 (0.882–1.048) | .370 | ||
| FT3, pmol/L | 1.926 (0.649–1.321) | . 671 | ||
| FT4, pmol/L | 1.199 (1.092–1.315) | <.001* | 1.187 (1.093–1.290) | <.001* |
| TSH, mIU/L | 0.822 (0.649–1.040) | .103 | ||
| LAD, (mm) | 1.043 (1.000–1.088) | . 048* | 1.052 (1.014–1.092) | .007* |
| LVEF, (%) | .997 (0.969–1.025) | .817 | ||
Abbreviations: HR: hazard ratio. CI: confidence interval. Other abbreviations as inTable 1.
p < .05.
4. DISCUSSION
This was the first study to investigate the impact of FT4 within the normal range on the efficacy of cryoballoon for nonvalvular AF in a Chinese population. The main findings were as follows: (1) Higher FT4 levels were associated with higher rates of AF recurrence, and participants in the highest quartile of the normal range of serum FT4 had a higher risk of AF recurrence than those who were in the lowest quartile; (2) FT4 within the normal range but not TSH or FT3 was a significant and independent predictor for AF recurrence after cryoballoon ablation during long‐term follow‐up; (3) The impact of higher FT4 concentration on AF recurrence after cryoballoon ablation is more pronounced in patients with paroxysmal AF than in those with persistent AF.
Attention to good thyroid function control is increasingly critical, as higher thyroid hormone even within the euthyroid range has been associated with an increased risk of cardiovascular adverse events, particularly AF (Heeringa et al., 2008). In 2015, a multicenter study including 2843 participants investigated the relationships between thyroid function within the euthyroid range and the incidences of atrial fibrillation, coronary heart disease, heart failure, hip fracture, dementia, and all‐cause death in older adults. That study found that higher FT4 was associated with an increased risk of AF, higher TSH was associated with a lower incidence of dementia, and total T3 was not associated with any outcome (Cappola et al., 2015). Similar findings have also recently been reported by a large observational cohort study in which higher FT4 levels within the reference range were associated with an increased prevalence and incidence of AF. Higher thyroid hormone is correlated with more severe cardiac fibrosis and could shorten the action potential duration and decrease the speed of repolarization in atrial and ventricular myocytes, as well as in PV cardiomyocytes (Davis et al., 2008), ultimately facilitating the maintenance of multiple reentrant circuits in the heart, which in turn contributes to the electrical and structural remodeling in the pathophysiology of AF (Selmer et al., 2012). However, little is known about whether high‐normal thyroid function influences the risk of AF recurrence in euthyroid participants.
Several studies have assessed the effects of high‐normal thyroid function on AF recurrence after catheter ablation. In 2010, a prospective study by Tang et al first showed that a high‐normal level of FT4 is an independent predictor of recurrence of AF after catheter ablation of paroxysmal AF in both univariate and multivariate analyses (Tang et al., 2010). Subsequently, a larger European sample study of 1095 patients with AF assessed TSH and FT4 as predictors of arrhythmia relapse. Sousa et al found even within the normal range, FT4 levels influenced the success rate of LA ablation procedures. Higher FT4 quartiles were associated with a higher relapse rate (Sousa et al., 2015). Nevertheless, these studies had specific limitations because they neither strictly restricted the FT3 concentration to within the normal range nor specifically investigated the association between normal thyroid function and AF recurrence following cryoballoon ablation. In our study, we restricted the FT3 concentration within the reference range to that classified as euthyroid. Our data demonstrated that high‐normal FT4 levels were associated with a higher risk of AF recurrence after cryoballoon ablation. The risk of AF recurrence is mainly associated with increased FT4 levels, but not with TSH or FT3. Furthermore, we found that when stratifying for AF type, the highest FT4 quartiles were associated with a higher risk of AF recurrence than the lowest FT4 quartile in paroxysmal AF patients. However, in persistent AF patients, this increasing risk trend was nonsignificant. One explanation could be that persistent AF was associated with more advanced atrial structural remodeling and more non‐PV triggers than paroxysmal AF (Wong et al., 2017). Thus, thyroid hormone may be less able to modulate arrhythmogenic effects after cryoballoon‐based PVI ablation than in other scenarios (Chen et al., 2002).
Cryoballoon ablation has shown significantly reduced hospitalization and lower complication rates compared with radiofrequency ablation since its launch on the market (Osório et al., 2019). The one‐year freedom from AF after a single procedure was reported to be 85.8% in patients with paroxysmal AF and 61.3% in patients with persistent AF (Irfan et al., 2016). To further reduce the rate of AF recurrence and improve cardiovascular outcomes, the latest 2020 ESC AF Guidelines recommend the integrated ABC pathway on AF patient management, where C represents cardiovascular risk factors and concomitant diseases (detection and management) (Hindricks et al., 2020). In previous studies, Giacomo et al. demonstrated that obesity, duration of symptoms before ablation, and higher LA diameter can successfully predict AF recurrence after cryoballoon ablation over a 5 years follow‐up period (Mugnai et al., 2020). Giuseppe et al also identified that early recurrence of AF was a significant and independent predictor of recurrence in patients undergoing cryoballoon ablation in a large multicenter study (Stabile et al., 2020). In the present study, the overall recurrence rate was 20.1% after a mean follow‐up of 17.4 ± 9.0 months, which was in accordance with previous studies. Our findings showed that FT4 concentration and LAD were independent predictors of AF recurrence after cryoballoon ablation. Notably, no association was found between the risk of AF recurrence and different levels of TSH or FT3 within the reference range in our study. It could be hypothesized that the cardiac function responses to thyroid hormone are mainly mediated by the concentrations of specific receptors within the heart. Although the affinity of the receptor for the metabolically active form of T3 is much higher than that for T4 (Davis et al., 2008), the majority of released TH is in the form of T4, which exceeds serum FT3, and T3 is generated by the peripheral conversion of T4. TSH secretion is commonly regarded to reflect the negative feedback of thyroid function. However, TSH secretion can be regulated not only by thyroid hormone but also by other hormones, such as somatostatin and dopamine, from the hypothalamus (Yen, 2001). As a result, FT4 may play a more sensitive role in predicting arrhythmia recurrence after cryoballoon ablation.
Our findings underline the great importance of early detection and comprehensive management of thyroid function in AF patients who undergo cryoballoon ablation. These findings have important clinical implications. Thyroid function screening is a routine assessment for patients with newly diagnosed AF to rule out thyroid disease‐related AF. It is easily available in hospital and outpatient clinics and is convenient for patients and cardiologists to monitor during follow‐up. Furthermore, with the advancement of catheter ablation techniques and technologies, complete isolation of pulmonary veins is currently the cornerstone of AF catheter ablation (Parameswaran et al., 2020). However, there are differences between ablation strategies to achieve PVI, as cryoballoon ablation is performed in a single‐shot manner, but radiofrequency ablation is done in a point‐by‐point manner (Buist et al., 2020). Our results provide evidence that euthyroid patients with higher FT4 have a greater tendency to have increased AF recurrence in cryoballoon ablation outcome. Better control of high‐normal thyroid function for AF patients may offer potential benefits, especially in those with high risk of AF recurrence. Regarding the fact that patients with subclinical hyperthyroidism (suppressed TSH level and normal FT4) have an increased risk of developing atrial fibrillation, a special report by Nacar et.al (Nacar et al., 2012) in 30 patients with subclinical hyperthyroidism have demonstrated that used propylthiouracil treatment to restore biochemical euthyroidism can reverse prolonged atrial conduction time and prevent the development of atrial fibrillation. Other population‐based studies also found antithyroid therapy in subclinical hyperthyroidism could reduce the average heart rate and supraventricular arrhythmias and improves diastolic function at rest and systolic performance during exercise (Kaminski et al., 2011; Wiersinga, 2011). Our findings have revealed the fact that higher FT4 levels within the reference range were associated with an increased recurrence of AF, even in biochemically euthyroid subjects. Further studies are warranted to investigate whether antithyroid treatment before and after the ablation procedure prevents or reverses AF recurrence in order to improve the success rate of cryoballoon ablation in patients with higher FT4 concentrations within the reference range.
4.1. Limitations
There are several limitations to our study that need to be considered. First, we did not use extended electrocardiographic monitoring devices such as an implantable cardiac monitor to detect atrial fibrillation recurrence after cryoballoon ablation. Thus, the long‐term recurrence rates may be underestimated because our data do not include asymptomatic or undocumented episodes. Second, we did not compare the differences in electrophysiological characteristics of PVs among FT4 quartiles, and we failed to evaluate the independent effects of individual therapies on the outcome of cryoballoon ablation. Finally, because of the relatively small number of participants with persistent AF who had AF recurrence after cryoballoon ablation, our findings in the research may not be generalizable to broader persistent AF populations. A multicenter study with a larger number of patients needs to be done on this population subset.
5. CONCLUSIONS
Higher FT4 levels within the normal range predicted the outcome of cryoballoon ablation in Chinese paroxysmal atrial fibrillation patients without structural heart disease.
ETHICS STATEMENT
The current study was approved by the local institution review committee of Shanghai Tenth People’s Hospital, and it complied with the precepts of the Declaration of Helsinki.
CONFLICTS OF INTEREST
None.
ACKNOWLEDGMENTS
This work was supported by the Chinese National Natural Science Foundation No. 81700291 and 2016 YFC1301202 from National Key Research and Development Program.
Pei, Y. , Xu, S. , Yang, H. , Ren, Z. , Meng, W. , Zheng, Y. , Guo, R. , Li, S. , Zhao, D. , Tang, K. , Li, H. , & Xu, Y. (2021). Higher FT4 level within the normal range predicts the outcome of cryoballoon ablation in paroxysmal atrial fibrillation patients without structural heart disease. Annals of Noninvasive Electrocardiology, 26, e12874. 10.1111/anec.12874
Yan Pei, Shaojie Xu, Contributed equally to this work
Contributor Information
Kai Tang, Email: doctortangkai@sina.com.
Hailing Li, Email: 1986lihailing@tongji.edu.cn.
Yawei Xu, Email: xuyaweicn@yahoo.com.cn.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- American, D. A. (2018). 2. Classification and diagnosis of diabetes: Standards of medical care in diabetes‐2018. Diabetes Care, 41, S13–S27. 10.2337/dc18-S002 [DOI] [PubMed] [Google Scholar]
- Anderson, J. L. , Jacobs, V. , May, H. T. , Bair, T. L. , Benowitz, B. A. , Lappe, D. L. , Muhlestein, J. B. , Knowlton, K. U. , & Bunch, T. J. (2019). Free thyroxine within the normal reference range predicts risk of atrial fibrillation. Journal of Cardiovascular Electrophysiology, 31, 18–29. 10.1111/jce.14183 [DOI] [PubMed] [Google Scholar]
- Bai, Y. , Wang, Y. L. , Shantsila, A. , & Lip, G. Y. H. (2017). The global burden of atrial fibrillation and stroke: A systematic review of the clinical epidemiology of atrial fibrillation in Asia. Chest, 152, 810–820. 10.1016/j.chest.2017.03.048 [DOI] [PubMed] [Google Scholar]
- Buiatti, A. , von Olshausen, G. , Barthel, P. , Schneider, S. , Luik, A. , Kaess, B. , Laugwitz, K. L. , & Hoppmann, P. (2017). Cryoballoon vs. radiofrequency ablation for paroxysmal atrial fibrillation: An updated meta‐analysis of randomized and observational studies. Europace, 19, 378–384. 10.1093/europace/euw262 [DOI] [PubMed] [Google Scholar]
- Buist, T. J. , Zipes, D. P. , & Elvan, A. (2020). Atrial fibrillation ablation strategies and technologies: Past, present, and future. Clinical Research in Cardiology, 110(6):775–788. 10.1007/s00392-020-01751-5 [DOI] [PubMed] [Google Scholar]
- Cappola, A. R. , Arnold, A. M. , Wulczyn, K. , Carlson, M. , Robbins, J. , & Psaty, B. M. (2015). Thyroid function in the euthyroid range and adverse outcomes in older adults. Journal of Clinical Endocrinology and Metabolism, 100, 1088–1096. 10.1210/jc.2014-3586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappola, A. R. , Fried, L. P. , Arnold, A. M. , Danese, M. D. , Kuller, L. H. , Burke, G. L. , Tracy, R. P. , & Ladenson, P. W. (2006). Thyroid status, cardiovascular risk, and mortality in older adults. JAMA, 295, 1033–1041. 10.1001/jama.295.9.1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y. C. , Chen, S. A. , Chen, Y. J. , Chang, M. S. , Chan, P. , & Lin, C. I. (2002). Effects of thyroid hormone on the arrhythmogenic activity of pulmonary vein cardiomyocytes. Journal of the American College of Cardiology, 39, 366–372. 10.1016/s0735-1097(01)01731-4 [DOI] [PubMed] [Google Scholar]
- Davis, P. J. , Leonard, J. L. , & Davis, F. B. (2008). Mechanisms of nongenomic actions of thyroid hormone. Front Neuroendocrinol, 29, 211–218. 10.1016/j.yfrne.2007.09.003 [DOI] [PubMed] [Google Scholar]
- Marrakchi, S. , Kanoun, F. , Idriss, S. , Kammoun, I. , & Kachboura, S. (2015). Arrhythmia and thyroid dysfunction. Herz, 40(Suppl. 2), 101–109. 10.1007/s00059-014-4123-0 [DOI] [PubMed] [Google Scholar]
- Heeringa, J. , Hoogendoorn, E. H. , van der Deure, W. M. , Hofman, A. , Peeters, R. P. , Hop, W. C. , den Heijer, M. , Visser, T. J. , & Witteman, J. C. (2008). High‐normal thyroid function and risk of atrial fibrillation: the rotterdam study. Archives of Internal Medicine, 168, 2219–2224. 10.1001/archinte.168.20.2219 [DOI] [PubMed] [Google Scholar]
- Hindricks, G. , Potpara, T. , Dagres, N. , Arbelo, E. , Bax, J. J. , Blomström‐Lundqvist, C. , Boriani, G. , Castella, M. , Dan, G. A. , Dilaveris, P. E. , Fauchier, L. , Filippatos, G. , Kalman, J. M. , La Meir, M. , Lane, D. A. , Lebeau, J. P. , Lettino, M. , Lip, G. Y. H. , Pinto, F. J. , Thomas, G. N. , Valgimigli, M. , van Gelder, I. C. , van Putte, B. P. , & Watkins, C. L. , Group E.S.C.S.D . (2020). 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European association of cardio‐thoracic surgery (EACTS). European Heart Journal, 42(5):373–498. 10.1093/eurheartj/ehaa612 [DOI] [PubMed] [Google Scholar]
- Irfan, G. , de Asmundis, C. , Mugnai, G. , Poelaert, J. , Verborgh, C. , Umbrain, V. , Beckers, S. , Hacioglu, E. , Hunuk, B. , Velagic, V. , Stroker, E. , Brugada, P. , & Chierchia, G. B. (2016). One‐year follow‐up after second‐generation cryoballoon ablation for atrial fibrillation in a large cohort of patients: A single‐centre experience. Europace: European Pacing, Arrhythmias, and Cardiac Electrophysiology: Journal of the Working Groups on Cardiac Pacing, Arrhythmias, and Cardiac Cellular Electrophysiology of the European Society of Cardiology, 18, 987–993. 10.1093/europace/euv365 [DOI] [PubMed] [Google Scholar]
- Jaïs, P. , Cauchemez, B. , Macle, L. , Daoud, E. , Khairy, P. , Subbiah, R. , Hocini, M. , Extramiana, F. , Sacher, F. , Bordachar, P. , Klein, G. , Weerasooriya, R. , Clémenty, J. , & Haïssaguerre, M. (2008). Catheter ablation versus antiarrhythmic drugs for atrial fibrillation: The A4 study. Circulation, 118, 2498–2505. 10.1161/CIRCULATIONAHA.108.772582 [DOI] [PubMed] [Google Scholar]
- January, C. T. , Wann, L. S. , Alpert, J. S. , Calkins, H. , Cigarroa, J. E. , Cleveland, J. C. Jr , Conti, J. B. , Ellinor, P. T. , Ezekowitz, M. D. , Field, M. E. , Murray, K. T. , Sacco, R. L. , Stevenson, W. G. , Tchou, P. J. , Tracy, C. M. , Yancy, C. W. , American College of Cardiology, American Heart Association Task Force on Practice G . (2014). 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American college of cardiology/american heart association task force on practice guidelines and the heart rhythm society. Journal of the American College of Cardiology, 64, e1–76. 10.1016/j.jacc.2014.03.022 [DOI] [PubMed] [Google Scholar]
- Kuck, K. H. , Brugada, J. , Fürnkranz, A. , Metzner, A. , Ouyang, F. , Chun, K. R. , Elvan, A. , Arentz, T. , Bestehorn, K. , Pocock, S. J. , Albenque, J. P. , Tondo, C. , & Fire, I. I. C. E. (2016). Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. New England Journal of Medicine, 374, 2235–2245. 10.1056/NEJMoa1602014 [DOI] [PubMed] [Google Scholar]
- Lip, G. Y. , Nieuwlaat, R. , Pisters, R. , Lane, D. A. , & Crijns, H. J. (2010). Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor‐based approach: The euro heart survey on atrial fibrillation. Chest, 137, 263–272. 10.1378/chest.09-1584 [DOI] [PubMed] [Google Scholar]
- Liu, L. , Zhao, D. , Zhang, J. , Yang, H. , Abdu, F. A. , Guo, R. , Li, S. , Tang, K. , Li, H. , Che, W. , & Xu, Y. (2019). Impact of stable coronary artery disease on the efficacy of cryoballoon ablation for the atrial fibrillation. American Journal of the Medical Sciences, 358, 204–211. 10.1016/j.amjms.2019.06.004 [DOI] [PubMed] [Google Scholar]
- Markides, V. , & Schilling, R. J. (2003). Atrial fibrillation: Classification, pathophysiology, mechanisms and drug treatment. Heart, 89, 939–943. 10.1136/heart.89.8.939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishima, I. , Okumura, K. , Morita, Y. , Kanzaki, Y. , Takagi, K. , Yoshida, R. , Nagai, H. , Ikai, Y. , Furui, K. , Yoshioka, N. , Tsuboi, H. , & Murohara, T. (2018). High‐normal thyroid‐stimulating hormone shows a potential causal association with arrhythmia recurrence after catheter ablation of atrial fibrillation. Journal of the American Heart Association, 7, e009158. 10.1161/JAHA.118.009158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugnai, G. , Paparella, G. , Overeinder, I. , Ströker, E. , Sieira, J. , Bisignani, A. , Iacopino, S. , Boveda, S. , Beckers, S. , Umbrain, V. , Bala, G. , Brugada, P. , de Asmundis, C. , & Chierchia, G. B. (2020). Long‐term clinical outcomes after single freeze cryoballoon ablation for paroxysmal atrial fibrillation: A 5‐year follow‐up. J Interv Card Electrophysiol, 61(1), 87–93. 10.1007/s10840-020-00788-w [DOI] [PubMed] [Google Scholar]
- Osório, T. G. , Coutiño, H. E. , Brugada, P. , Chierchia, G. B. , & De Asmundis, C. (2019). Recent advances in cryoballoon ablation for atrial fibrillation. Expert Review of Medical Devices, 16, 799–808. 10.1080/17434440.2019.1653181 [DOI] [PubMed] [Google Scholar]
- Parameswaran, R. , Al‐Kaisey, A. M. , & Kalman, J. M. (2020). Catheter ablation for atrial fibrillation: current indications and evolving technologies. Nature Reviews Cardiology, 18(3), 210–225. 10.1038/s41569-020-00451-x [DOI] [PubMed] [Google Scholar]
- Selmer, C. , Olesen, J. B. , Hansen, M. L. , Lindhardsen, J. , Olsen, A. M. , Madsen, J. C. , Faber, J. , Hansen, P. R. , Pedersen, O. D. , Torp‐Pedersen, C. , & Gislason, G. H. (2012). The spectrum of thyroid disease and risk of new onset atrial fibrillation: A large population cohort study. BMJ, 345, e7895. 10.1136/bmj.e7895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa, P. A. , Providencia, R. , Albenque, J. P. , Khoueiry, Z. , Combes, N. , Combes, S. , & Boveda, S. (2015). Impact of free thyroxine on the outcomes of left atrial ablation procedures. American Journal of Cardiology, 116, 1863–1868. 10.1016/j.amjcard.2015.09.028 [DOI] [PubMed] [Google Scholar]
- Stabile, G. , Iacopino, S. , Verlato, R. , Arena, G. , Pieragnoli, P. , Molon, G. , Manfrin, M. , Rovaris, G. , Curnis, A. , Bertaglia, E. , Mantica, M. , Sciarra, L. , Landolina, M. , & Tondo, C. (2020). Predictive role of early recurrence of atrial fibrillation after cryoballoon ablation. Europace, 22(12), 1798–1804. 10.1093/europace/euaa239 [DOI] [PubMed] [Google Scholar]
- Tang, R. B. , Liu, D. L. , Dong, J. Z. , Liu, X. P. , Long, D. Y. , Yu, R. H. , Hu, F. L. , Wu, J. H. , Liu, X. H. , & Ma, C. S. (2010). High‐normal thyroid function and risk of recurrence of atrial fibrillation after catheter ablation. Circulation Journal, 74, 1316–1321. 10.1253/circj.cj-09-0708 [DOI] [PubMed] [Google Scholar]
- Wong, C. L. , Tam, H. V. , Fok, C. V. , Lam, P. E. , & Fung, L. M. (2017). Thyrotoxic atrial fibrillation: Factors associated with persistence and risk of ischemic stroke. J Thyroid Res, 2017, 4259183. 10.1155/2017/4259183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wongcharoen, W. , Lin, Y. J. , Chang, S. L. , Lo, L. W. , Hu, Y. F. , Chung, F. P. , Chong, E. , Chao, T. F. , Tuan, T. C. , Chang, Y. T. , Lin, C. Y. , Liao, J. N. , Lin, Y. C. , Chen, Y. Y. , & Chen, S. A. (2015). History of hyperthyroidism and long‐term outcome of catheter ablation of drug‐refractory atrial fibrillation. Heart Rhythm, 12, 1956–1962. 10.1016/j.hrthm.2015.06.004 [DOI] [PubMed] [Google Scholar]
- Yen, P. M. (2001). Physiological and molecular basis of thyroid hormone action. Physiological Reviews, 81, 1097–1142. 10.1152/physrev.2001.81.3.1097 [DOI] [PubMed] [Google Scholar]
- Yen, P. M. , Ando, S. , Feng, X. , Liu, Y. , Maruvada, P. , & Xia, X. (2006). Thyroid hormone action at the cellular, genomic and target gene levels. Molecular and Cellular Endocrinology, 246, 121–127. 10.1016/j.mce.2005.11.030 [DOI] [PubMed] [Google Scholar]
- Nacar, A. B. , Acar, G. , Yorgun, H. , Akçay, A. , Özkaya, M. , Canpolat, U. , Akkoyun, M. , & Tuncer, C. (2012). The effect of antithyroid treatment on atrial conduction times in patients with subclinical hyperthyroidism. Echocardiography, 29, 950–955. 10.1111/j.1540-8175.2012.01718.x [DOI] [PubMed] [Google Scholar]
- Kaminski, G. , Michalkiewicz, D. , Makowski, K. , Podgajny, Z. , Szalus, N. , Ruchala, M. , Szczepanek, E. , & Gielerak, G. (2011). Prospective echocardiographic evaluation of patients with endogenous subclinical hyperthyroidism and after restoring euthyroidism. Clinical Endocrinology‐Oxford, 74, 501–507. 10.1111/j.1365-2265.2010.03957.x [DOI] [PubMed] [Google Scholar]
- Wiersinga, W. M. (2011). Should we treat mild subclinical/mild hyperthyroidism? Yes. European Journal of Internal Medicine, 22, 324–329. 10.1016/j.ejim.2011.03.008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


