Abstract
Due to the continuous rise in conventional plastic production and the deficient management of plastic waste, industry is developing alternative plastic products made of biodegradable or biobased polymers. The challenge nowadays is to create a new product that combines the advantages of conventional plastics with environmentally friendly properties. This study focuses on the assessment of the potential impact that polyvinyl alcohol (PVA)-based polymers may have once they are released into the marine environment, in terms of biodegradation in seawater (assessed by the percentage of the Theoretical Oxygen Demand, or % ThOD, of each compound) and aquatic toxicity, according to the standard toxicity test using Paracentrotus lividus larvae. We have tested three different materials: two glycerol-containing PVA based ones, and another made from pure PVA. Biodegradation of PVA under marine conditions without an acclimated inoculum seems to be negligible, and it slightly improves when the polymer is combined with glycerol, with a 5.3 and 8.4% ThOD achieved after a period of 28 days. Toxicity of pure PVA was also negligible (<1 toxic units, TU), but slightly increases when the material included glycerol (2.2 and 2.3 TU). These results may contribute to a better assessment of the behavior of PVA-based polymers in marine environments. Given the low biodegradation rates obtained for the tested compounds, PVA polymers still require further study in order to develop materials that are truly degradable in real marine scenarios.
Keywords: poly(vinyl alcohol), glycerol, microplastics, biodegradation, toxicity, marine water
1. Introduction
1.1. Marine Plastic Pollution
Plastics are one of the most used materials nowadays due to their low cost, light weight, high durability and excellent isolation properties. The annual production in 2019 reached almost 370 million tons [1]. Nevertheless, this high demand and long environmental persistence caused their widespread accumulation into the environment. Plastic is the predominant component of marine litter; it is estimated that around 8 million tons of mismanaged plastic are released into the ocean every year [2]. Environmental weathering of plastics leads to fragmentation into smaller particles, and fractions less than 5 mm are called microplastics [3]. Microplastics pose a potential risk not only for filter feeders, which can end up consuming plastic debris [4] but also for all trophic levels [5]. As particle size decreases, the bioavailability, translocation and toxicity to organisms increases [6].
United Nations Sustainable Development Goals 2015 (SDGs) include, as part of Goal 14, a call to reduce impacts from plastics [7]. Efforts from all around the world are now developing new synthetic materials innocuous for the environment according to the different end-of-life scenarios. This includes regulatory efforts such as the EU initiative to phase out some single-use plastics (Directive 2019/904). Materials based on renewable components and biodegradable options are already being developed as alternatives to conventional polymers. Nowadays, the production of plastics from renewable raw materials is 1% of total plastic production [8]. However, although these new materials are promising, they present new challenges, such as the potential toxicity of the degradation products, or their rapid fragmentation into microplastics [9].
1.2. Polyvinyl Alcohol
Poly(vinyl alcohol) (PVA) is a synthetic water-soluble biopolymer that is generally prepared by the saponification of polyvinyl acetate [10]. Remarkable advantages of this potentially biodegradable material are transparency, good mechanical and thermal properties and resistance to oxygen permeation [11].
Products made by PVA constitute the largest volume of water-soluble polymer produced this century [12], with 650,000 tons of PVA per year around the globe [13]. PVA can be found in a wide range of items, including food packaging (31.4% of the demand), construction, electronics, coatings, printing, textile, cosmetics and paper [14]. Likewise, the use of PVA in pharmaceutical and medical applications, including tablets, contact lenses and surgical threads, have been reported [15]. Most of these materials can reach the environment without passing through any integrated system of waste treatment [12]. Another less known use of PVA that implies direct input into the ocean is the equipment for sports fishing, such as bags with dry fishing bait attached to the hook that are eventually released [16].
PVA is perhaps the only fully synthetic C-chain polymer biodegradable in aerobic [17] and anaerobic conditions [18], although in laboratory trials, acclimation in order to allow growth of selected microorganisms is usually essential to achieve rapid degradation [17]. Certain bacteria and fungi contain specific oxidases and dehydrogenases that promote an oxidative reaction of the tertiary carbon atoms of PVA chain forming hydrolyzable b-hydroxylketone and 1,3-diketone groups along the polymer backbone [19,20]. Nevertheless, these microorganisms are not present in most environmental scenarios where degradation rates are very low [21].
1.3. Plastic Biodegradation
Biodegradability is a plastic end-of-life option that uses the microorganisms present in a particular environment to completely remove plastic products by mineralization to CO2, water and biomass. This process depends on the chemical structure of the polymer, the additives that it contains and the environmental conditions. Usually, the biodegradation of plastics is carried out by scission of the polymer backbone by hydrolysis or enzymatic cleavage initiated by microorganisms that can digest the polymer [22]. Biodegradable plastics can be decomposed on industrial composting facilities [23,24] but many of them are not suitable for biodegradation in natural environments. Previous studies tested the biodegradability of potentially biodegradable plastics in seawater by recording weight loss [25,26,27], oxygen consumption as biological oxygen demand (BOD) or CO2 evolution [28,29].
1.4. Toxicity of Plastics
Marine pollution analysis was commonly based on the performance of chemical analysis. Nonetheless, due to the high complexity of pollutant mixtures and their interactions with environmental factors, this does not offer sufficient information on their potential effects on organisms in the natural environment. Therefore, the evaluation of toxicity in marine waters must integrate conventional analyses with biological methods such as bioassays [30].
The toxicity of common plastic in the marine environment was previously studied [31,32,33], and frequently associated to chemical additives [34] or sorbed environmental pollutants [33]. Nonetheless, biopolymer toxicity is an emerging issue, since adverse effects of biodegradation products must be taken into account. Some components of water-soluble biopolymers such as ethylene released during degradation can have negative effects on surrounding organisms [35]. On the other hand, some biopolymers, e.g., PHB, seemed to impact aquatic organisms through different mechanisms associated with the higher abundance of plastic particles within the nanometric range found in these resins and absent in other materials [34,36].
The main objective of this research is to provide new insights about the potential risks of microplastics from PVA materials for the marine environment. With that aim, biodegradation studies and ecotoxicological tests were performed in order to have a complete insight into the potential impact of these materials.
2. Materials and Methods
2.1. Tested Materials
PVA samples were provided by the Plastic Technology Centre AIMPLAS (Spain) from three different stocks (Table 1). Two of them consisted of a mixture of PVA and glycerol, a common plasticizer used in the production of bioplastic [37], and the third one was a plain PVA resin. All the materials were micronized by a ZM200 ultracentrifuge mill (Retsch, Verder Scientific) and sieved through a <250 µm metallic mesh in order to standardize particle size for further testing. A biodegradable material, PHB powder (<250 µm), purchased from Helian Polymers, was used as a reference material.
Table 1.
Characteristics of the materials tested.
| Reference | Brand | Hydrolysis, Mole % | Viscosity (cps) | % PVA | % Glycerol |
|---|---|---|---|---|---|
| PVA.029 | Selvol™ Polyvinyl Alcohol 205 | 87.00–89.00 | 5.2–6.2 | 85 | 15 |
| PVA.030 | Exceval HR 3010 | 99.0–99.4 | 12.0–16.0 | 85 | 15 |
| PVA.031 | PVA KURARAY POVALTM | 98.0–99.0 | 3.2–3.8 | 100 | 0 |
2.2. Biodegradation Study
Biodegradation of the materials was tested following the UNE-EN ISO 14851:2019 [38] for the oxygen demand measurement in a close respirometer, adapted to seawater as follows. NaNO3, Na2HPO4·2H2O, and FeCl3·6H2O purchased from Merck were used as sources of N, P and Fe, and the nutrient formulation was calculated to fit the well-known Redfield ratio 106:16:1:0.1 for C:N:P:Fe [39,40].
The tests were carried out with a set of 500 mL amber glass incubation bottles closed by respirometer units with piezoresistive electronic pressure sensors OxiTop®-i IS 6-WTW. The experiments included blanks (with no sample), PHB positive controls and the tested plastic materials, all treatments per duplicate. The medium for the experiment included 0.8 µm filtered seawater sterilized with UV light, enriched with nutrients as explained above, and 1% v. of marine inoculum. The inoculum used was sediment interstitial water collected the same testing day from the beach of Canido (Vigo, NW Iberian Peninsula) in a location under the influence of a freshwater course submitted to frequent discharges of urban wastewater. Then, 250 mL of the medium and 25 mg of microplastic sample were added to each bottle with a magnetic stirrer for continuous mixing. Before closing the bottles, a rubber with KOH pearls was placed in the neck in order to capture the evolved CO2. Biological oxygen demand (expressed as mg O2/L) was recorded every day for a 28-day period.
Blank-corrected BOD values are expressed as % of the Theoretical Oxygen Demand (ThOD), defined as the theoretical amount of oxygen required to fully degrade the whole organic carbon content of the polymer to CO2, as described in UNE-EN ISO 14851:2019 [38], and as percentage of the BOD recorded in the positive control (%C+). The latter is more suited to biopolymers, since the main purpose is to detect relevant biodegradation compared to a well-known biodegradable polymer, and because the actual atomic composition of tested polymers is not always disclosed.
2.3. Toxicity Test
Marine aquatic toxicity of PVA leachates was tested using the Paracentrotus lividus sea-urchin embryo test (SET) according to [41]. The leachates were obtained in artificial seawater [42] at 10 g/L and tested using geometrical serial dilutions (×1, ×1/3, ×1/10…) until the dilution was found, with no toxic effect (NOEC). Four replicates per treatment and eight controls were added. Sea urchins were provided by the Marine Culture Unit of ECIMAT (CIM-University of Vigo) the same day the tests were performed. Eggs were fertilized with a few µL of sperm in a 50 mL measuring cylinder and transferred before the first division into glass vials with 4 mL of the experimental solution and a density of 40 individuals per mL. Vials were closed with Teflon-lined caps and kept at 20 °C in the dark for 48 h. Afterwards, the incubation time vials were fixed with 40% formalin for ulterior observation under an inverted microscope (Leica DMI 4000B). The length as a maximum linear dimension was measured on 35 larvae per vial using Leica Application Suite LAS image analysis software version 4.12.0 (Leica Microsystems, Germany). The acceptability criteria for this test were the percentage of fertilized eggs (>98%) and size increase in controls >253 µm [43].
Statistical analyses were carried out using IBM SPSS (v. 24). Normal distribution and homoscedasticity were checked using the Shapiro–Wilk and Levene’s tests, respectively. Leachate dilutions that produced larval growths significantly different to the control (p < 0.05) were identified using Dunnett’s post hoc test or, when the variances were not homogeneous, Dunnett’s T3 test in order to find the lowest no observed adverse effects concentration (NOEC) and the lowest observed adverse effect concentration (LOEC). The leachate dilutions that produced a 50% decrease in larval growth with respect to the control (EC50) was also calculated and their 95% confidence intervals (CIs) were calculated adjusting the data obtained to the Probit dose-response model. Toxic Units (TU) were calculated as TU= 1/EC50, and materials were classified following the assessment criteria shown in Table 2.
Table 2.
Toxicity classification based on the sea-urchin embryo test Toxic Units (TU).
| EC50 (mg/L) | TU | Toxicity |
|---|---|---|
| >10,000 | <1 | None |
| 2000–10,000 | 1 ≤ TU< 5 | Slight |
| 400–2000 | 5 ≤ TU< 25 | Relevant |
| <400 | ≥25 | High |
3. Results and Discussion
3.1. Biodegradability Test
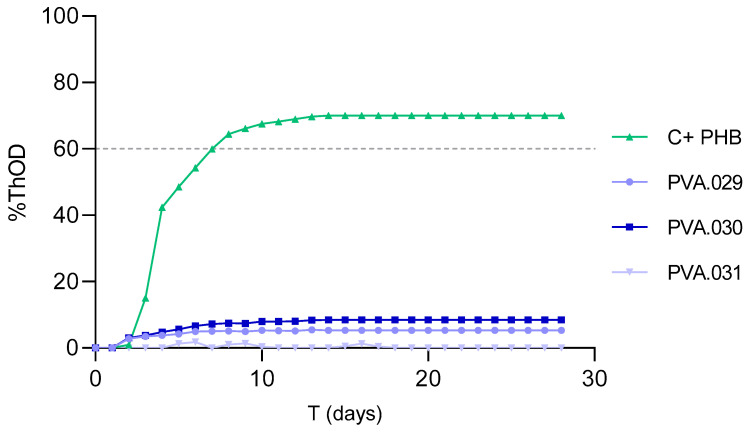
Figure 1 shows the biodegradation results for the 28 days of incubation obtained for each material. According to UNE-EN ISO 14851:2019 guidelines [38], a substance is considered biodegradable if the BOD is higher than 60% ThOD. PHB was used as positive control due to its well-known biodegradability in seawater, as shown by Tachibana et al. who reported 80% biodegradation in 25 days [28]. Similar percentages of biodegradation were found in the present study, where the PHB control achieved 70% degradation after 28 days. Some standards propose an extended incubation period of 60 days, and up to 180 days (ISO 19679, ISO 23977-2, ASTM D7991-15) [44,45]. This extension considerably increases the technical complexity of the experimental setup and increases the chances of failures during the execution of the tests. Moreover, even though we may need to consider a longer period for certain materials, it was observed that the degradation became stable after 28 days for both positive control and tested materials. Materials PVA.029 and PVA.030, including 15% glycerol in their composition, showed a final biodegradation percentage of 5.3 and 8.4% ThOD, respectively, and are thus classified as slightly biodegradable. For the PVA.031 sample, composed by PVA only, we observed that the biodegradation was negligible. However, studies about degradability of PVA on freshwater inoculated with municipal sewage sludge reported percentages of biodegradation of 13% during 21 days [12]. The marine medium used in the present studies (pH 8.3) may lack microbial strains present in wastewater. Additionally, seawater shows a pH > 8, which may have retarded degradation, since biodegradation of PVA was reported to be higher in acidic aqueous solutions than in alkaline ones [46].
Figure 1.
Biodegradation expressed by %ThOD for the different materials and the positive control (C+ PHB). Discontinuous line: 60% biodegradability threshold.
Expressed as a percentage of the positive control, the biodegradation rate of the sample PVA.029 was 7.8 and 12.4% for the 030.PVA one, values that still represent a low degree of biodegradation. As expected, the material with the higher degree of hydrolysis (030.PVA) results as the most biodegradable, as reduced molecular weight is a precondition for microbial attack, and the hydrolytic mechanisms enhance the biodegradation processes [47]. Moreover, PVA materials can reach up to 60% degradation in 32 days depending on the degree of solubility of the polymer [48].
The detected increase of 6.85% on average in the biodegradation rate for glycerol-containing PVA may be due to the changes in the hydrophilic characteristics of the glycerol that reduce internal hydrogen bonds in the polymer chain and decrease the residual mass as described by Abdullah and Dong (2019) [49]. They observed an increase of 23.33% in biodegradation rates when adding glycerol. Moreover, raw glycerol has biodegradation on natural water between 68 and 78% [50].
It should be noted that due to the lack of research about biodegradability on microparticles from bioplastics, this study can be only compared to studies using larger fragments (films, pellets, etc.). There is a gap of information in this area that needs to be covered, given that the final faith of microplastics and their degradation products is not well known [51].
Some labels have been developed by industry to distinguish plastics that can biodegrade in the environment. For instance, the label created by Vinçotte OK Biodegradable WATER applies the standard BS EN ISO 14851:2019 [38,52]. Nevertheless, as Harrison et al. pointed out, there is no agreement on which standards to use for plastic biodegradation [53], and a more realistic point of view unifying all the criteria required for these specific materials is needed. It is also important to bear in mind that biodegradability is an intrinsic property of a material, but performed biodegradation will necessarily depend on environmental conditions, and thus no single standard will be able to be representative of the multiple end-of-life scenarios present in the aquatic environments.
3.2. Toxicity Test
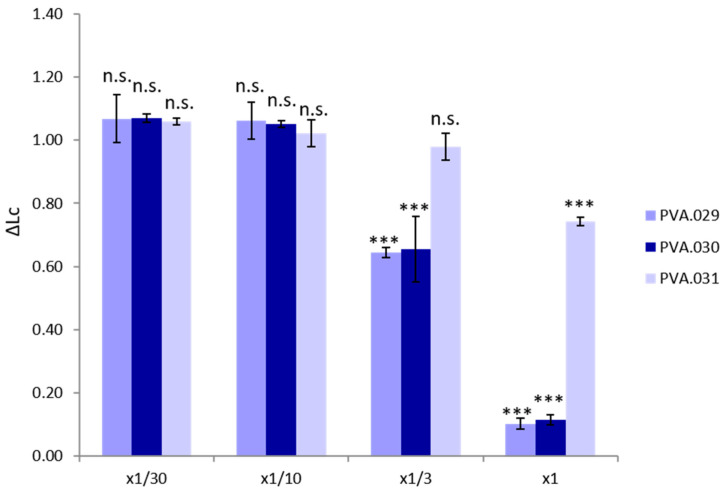
Toxicity of the materials using the standard sea urchin model with a concentration of 10 g/L is reflected in Table 3. According to the current EU classification of chemicals, based on their short-term aquatic toxicity [54], all samples are classified as harmless (EC50 > 100 mg/L). Following the TU classification (see Table 2), the polymer composed of plain PVA showed a total absence of toxicity with a TU < 1. This result is supported by toxicity bioassays in seven other aquatic species including marine water individuals Hyalella azteca and Leptocheirus plumulosus that showed a low toxicity for the PVA material [55]. The materials PVA.029 and PVA.030 presented a certain degree of aquatic toxicity (2.3 and 2.2 TU, respectively) with a larval growth lower than the PVA.031 sample (Figure 2). Consequently, PVA.029 and PVA.030 could be classified as slightly toxic.
Table 3.
Toxicity of different materials expressed as no observed adverse effects concentration (NOEC) and the lowest observed adverse effect concentration (LOEC); toxic units (TU); 50% decrease in larval growth with respect to the control (EC50).
| Sample | NOEC (g/L) | LOEC (g/L) | TU | CE50 (mg/L) |
|---|---|---|---|---|
| PVA.029 | 1 | 3.33 | 2.3 | 4285 (1585–12164) |
| PVA.030 | 1 | 3.33 | 2.2 | 4382 (3750–5017) |
| PVA.031 | 3.33 | 10 | <1 | 2403.8 (1814.8–3773.5) |
Figure 2.
Paracentrotus lividus larvae size increase ratio compared to control treatment (ΔLc) for the different sample dilutions. * p < 0.05, ** p < 0.01, *** p < 0.001.
ECHA [56] shows a CE50 for glycerol on freshwater invertebrate organisms at 48 h of 1995 (1851 to 2068) mg/L, corresponding to a TU < 1. According to studies carried out on three different aquatic bioindicators, glycerol ethers can be classified as harmless for the environment in acute exposure [57]. Glycerol has been presented as a sustainable solvent for its good biodegradability and low toxicity on certain organisms [58], but its effects when blended with polymers and other materials need further research in order to confirm that these compounded materials do not pose a threat for the environment. In fact, we have found a slight but significant increase in toxicity for glycerol-PVA blends, compared to plain PVA.
Similarly, it is necessary to include the assessment of the potential toxicity of degradation products in the scheme of evaluation of biopolymers before they can be presented as more environmentally friendly alternatives compared to conventional plastics.
4. Conclusions
This study characterized the biodegradation and ecotoxicity of PVA polymers in marine environments. The results obtained, though limited, support the lack of biodegradability of PVA materials under conditions representative of a natural marine environment. The slight biodegradability of the blended materials was attributable to the glycerol component of the blend. Since plastics of different nature are increasingly found in marine environments, standards with a view to assess biodegradation under realistic marine end-of-life scenarios are urgently needed.
On the other hand, none of the tested polymers pose a relevant risk to the model marine organism used, the sensitive Sea urchin Embryo Test (SET), but slight toxicity arises for glycerol-containing blends. These tests should be conducted with a broader group of aquatic species. These standards should take into account not only biodegradability in terms of mineralization to CO2, but also mechanical degradation, potential release of microplastics and lack of toxicity of additives and biodegradation products.
Acknowledgments
The authors thank the Plastic Technology Centre AIMPLAS (Valencia, Spain) for supplying the different PVA materials. Funding for open access charge: Universidade de Vigo/CISUG.
Author Contributions
Conceptualization, O.A.-L., S.L.-I. and R.B.; data curation, S.L.-I.; formal analysis, O.A.-L.; funding acquisition, R.B.; investigation, O.A.-L., S.L.-I. and R.B.; methodology, O.A.-L., S.L.-I. and R.B.; project administration, R.B.; resources, O.A.-L., S.L.-I. and R.B.; supervision, R.B.; writing—original draft, O.A.-L.; writing—review and editing, O.A.-L., S.L.-I. and R.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the RisBioPlas Project, financed by the Ministry of Science and Innovation—Spanish Agency of Research (AEI: 10.13039/501100011033); by LABPLAS Project (LAnd-Based Solutions for PLAStics in the Sea, Grant Agreement Nr.: 101003954, financed by the EU H2020 program); by the RESPONSE Project (toward a risk-based assessment of microplastic pollution in marine ecosystems, financed by the Joint Programming Initiative, Healthy and Productive Seas and Oceans, JPI Oceans-Spanish National Research funding Agency: PCI2020-112110). S.L.I. was supported by the Ministry of Universities of Spain (FPU grant reference FPU19/02280).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.PlasticsEurope Plastics—The Facts 2020. An Analysis in European Plastic Production, Demand and Waste Data. PlasticEurope. 2020:1–64. [Google Scholar]
- 2.Napper I.E., Thompson R.C. Plastic Debris in the Marine Environment: History and Future Challenges. Glob. Chall. 2020;1900081:1–9. doi: 10.1002/gch2.201900081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrady A.L. Microplastics in the Marine Environment. Mar. Pollut. Bull. 2011;62:1596–1605. doi: 10.1016/j.marpolbul.2011.05.030. [DOI] [PubMed] [Google Scholar]
- 4.Tanaka K., Takada H. Microplastic Fragments and Microbeads in Digestive Tracts of Planktivorous Fish from Urban Coastal Waters. Sci. Rep. 2016;6:1–8. doi: 10.1038/srep34351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barboza L.G.A., Dick Vethaak A., Lavorante B.R.B.O., Lundebye A.K., Guilhermino L. Marine Microplastic Debris: An Emerging Issue for Food Security, Food Safety and Human Health. Mar. Pollut. Bull. 2018;133:336–348. doi: 10.1016/j.marpolbul.2018.05.047. [DOI] [PubMed] [Google Scholar]
- 6.Beiras R., Schönemann A.M. Currently Monitored Microplastics Pose Negligible Ecological Risk to the Global Ocean. Sci. Rep. 2020;10:1–9. doi: 10.1038/s41598-020-79304-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walker T.R. (Micro) Plastics and the UN Sustainable Development Goals. Curr. Opin. Green Sustain. Chem. 2021;30:100497. doi: 10.1016/j.cogsc.2021.100497. [DOI] [Google Scholar]
- 8.European Bioplastics Bioplastics Market Data 2019. [(accessed on 1 September 2021)]. Available online: https://www.european-bioplastics.org/market/
- 9.Hidalgo-Ruz V., Gutow L., Thompson R.C., Thiel M. Microplastics in the Marine Environment: A Review of the Methods Used for Identification and Quantification. Environ. Sci. Technol. 2012;46:3060–3075. doi: 10.1021/es2031505. [DOI] [PubMed] [Google Scholar]
- 10.Finch C.A. Polyvinyl Alcohol, Properties and Applications. J. Polym. Sci. Polym. Lett. Ed. 1974;12:105–106. doi: 10.1002/pol.1974.130120212. [DOI] [Google Scholar]
- 11.Kanatt S.R., Makwana S.H. Development of Active, Water-Resistant Carboxymethyl Cellulose-Poly Vinyl Alcohol-Aloe Vera Packaging Film. Carbohydr. Polym. 2020;227:115303. doi: 10.1016/j.carbpol.2019.115303. [DOI] [PubMed] [Google Scholar]
- 12.Chiellini E., Corti A., Solaro R. Biodegradation of Poly(Vinyl Alcohol) Based Blown Films under Different Environmental Conditions. Polym. Degrad. Stab. 1999;64:305–312. doi: 10.1016/S0141-3910(98)00206-7. [DOI] [Google Scholar]
- 13.Xu S., Malik M.A., Qi Z., Huang B.T., Li Q., Sarkar M. Influence of the PVA Fibers and SiO2 NPs on the Structural Properties of Fly Ash Based Sustainable Geopolymer. Constr. Build. Mater. 2018;164:238–245. doi: 10.1016/j.conbuildmat.2017.12.227. [DOI] [Google Scholar]
- 14.DeMerlis C.C., Schoneker D.R. Review of the Oral Toxicity of Poly Vinyl Alcohol (PVA) Food Chem. Toxicol. 2003;41:319–326. doi: 10.1016/S0278-6915(02)00258-2. [DOI] [PubMed] [Google Scholar]
- 15.Muppalaneni S. Polyvinyl Alcohol in Medicine and Pharmacy: A Perspective. J. Dev. Drugs. 2013;2:2329–6631. doi: 10.4172/2329-6631.1000112. [DOI] [Google Scholar]
- 16.PVOH Polymers LTD Soluble Polymers For Innovation. [(accessed on 27 October 2021)]. Available online: https://www.pvohpolymers.co.uk/fisheries/
- 17.Porter J.J., Snider E.H. Long Term Biodegradability of Textile Chemicals. J. Water Pollut. Control Fed. 1976;48:2198–2210. [PubMed] [Google Scholar]
- 18.Wu H.F., Yue L.Z., Jiang S.L., Lu Y.Q., Wu Y.X., Wan Z.Y. Biodegradation of Polyvinyl Alcohol by Different Dominant Degrading Bacterial Strains in a Baffled Anaerobic Bioreactor. Water Sci. Technol. 2019;79:2005–2012. doi: 10.2166/wst.2019.202. [DOI] [PubMed] [Google Scholar]
- 19.Watanabe Y., Morita M., Hamada N., Tsujisaca Y. Formation of Hydrogen Peroxide by a Polyvinyl Degrading Alcohol Enzyme. Agr. Biol. 1975;39:2447–2448. [Google Scholar]
- 20.Suzuki T., Ichihara Y., Yamada M., Tonomura K. Some Characteristics of Pseudomonas O–3 Which Utilizes Polyvinyl Alcohol. Agric. Biol. Chem. 1973;37:747–756. doi: 10.1271/bbb1961.37.747. [DOI] [Google Scholar]
- 21.Chiellini E., Corti A., D’Antone S., Solaro R. Biodegradation of Poly (Vinyl Alcohol) Based Materials. Prog. Polym. Sci. 2003;28:963–1404. doi: 10.1016/S0079-6700(02)00149-1. [DOI] [Google Scholar]
- 22.Yamatsu A., Matsumi R., Atomi H., Imanaka T. Isolation and Characterization of a Novel Poly (Vinyl Alcohol)-Degrading Bacterium, Sphingopyxis Sp. PVA3. Appl. Microbiol. Biotechnol. 2006;72:804–811. doi: 10.1007/s00253-006-0351-4. [DOI] [PubMed] [Google Scholar]
- 23.Rujnić Havstad M., Juroš L., Katančić Z., Pilipović A. Influence of Home Composting on Tensile Properties of Commercial Biodegradable Plastic Films. Polymers. 2021;13:2785. doi: 10.3390/polym13162785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lambert S., Wagner M. Environmental Performance of Bio-Based and Biodegradable Plastics: The Road Ahead. Chem. Soc. Rev. 2017;46:6855–6871. doi: 10.1039/C7CS00149E. [DOI] [PubMed] [Google Scholar]
- 25.Bagheri A.R., Laforsch C., Greiner A., Agarwal S. Fate of So-Called Biodegradable Polymers in Seawater and Freshwater. Glob. Chall. 2017;1:1700048. doi: 10.1002/gch2.201700048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thellen C., Coyne M., Froio D., Auerbach M., Wirsen C., Ratto J.A. A Processing, Characterization and Marine Biodegradation Study of Melt-Extruded Polyhydroxyalkanoate (PHA) Films. J. Polym. Environ. 2008;16:1–11. doi: 10.1007/s10924-008-0079-6. [DOI] [Google Scholar]
- 27.Accinelli C., Saccà M.L., Mencarelli M., Vicari A. Deterioration of Bioplastic Carrier Bags in the Environment and Assessment of a New Recycling Alternative. Chemosphere. 2012;89:136–143. doi: 10.1016/j.chemosphere.2012.05.028. [DOI] [PubMed] [Google Scholar]
- 28.Tachibana K., Urano Y., Numata K. Biodegradability of Nylon 4 Film in a Marine Environment. Polym. Degrad. Stab. 2013;98:1847–1851. doi: 10.1016/j.polymdegradstab.2013.05.007. [DOI] [Google Scholar]
- 29.Tosin M., Weber M., Siotto M., Lott C., Innocenti F.D. Laboratory Test Methods to Determine the Degradation of Plastics in Marine Environmental Conditions. Front. Microbiol. 2012;3:1–9. doi: 10.3389/fmicb.2012.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beiras R., Durán I., Parra S., Urrutia M.B., Besada V., Bellas J., Viñas L., Sánchez-Marín P., González-Quijano A., Franco M.A., et al. Linking Chemical Contamination to Biological Effects in Coastal Pollution Monitoring. Ecotoxicology. 2012;21:9–17. doi: 10.1007/s10646-011-0757-3. [DOI] [PubMed] [Google Scholar]
- 31.Cole M., Lindeque P., Fileman E., Halsband C., Galloway T.S. The Impact of Polystyrene Microplastics on Feeding, Function and Fecundity in the Marine Copepod Calanus Helgolandicus. Environ. Sci. Technol. 2015;49:1130–1137. doi: 10.1021/es504525u. [DOI] [PubMed] [Google Scholar]
- 32.Burns E.E., Boxall A.B.A. Microplastics in the Aquatic Environment: Evidence for or against Adverse Impacts and Major Knowledge Gaps. Environ. Toxicol. Chem. 2018;37:2776–2796. doi: 10.1002/etc.4268. [DOI] [PubMed] [Google Scholar]
- 33.Cormier B., Gambardella C., Tato T., Perdriat Q., Costa E., Veclin C., Le Bihanic F., Grassl B., Dubocq F., Kärrman A., et al. Chemicals Sorbed to Environmental Microplastics Are Toxic to Early Life Stages of Aquatic Organisms. Ecotoxicol. Environ. Saf. 2021;208:111665. doi: 10.1016/j.ecoenv.2020.111665. [DOI] [PubMed] [Google Scholar]
- 34.Beiras R., Verdejo E., Campoy-López P., Vidal-Liñán L. Aquatic Toxicity of Chemically Defined Microplastics Can Be Explained by Functional Additives. J. Hazard. Mater. 2021;406:124338. doi: 10.1016/j.jhazmat.2020.124338. [DOI] [PubMed] [Google Scholar]
- 35.Druege U. Ethylene Action in Plants. Springer; Berlin/Heidelberg, Germany: 2006. Ethylene and Plant Responses to Abiotic Stress; pp. 81–118. [Google Scholar]
- 36.González-Pleiter M., Tamayo-Belda G., Pulido-Reyes G.A., Leganés F., Rosal R., Fernández-Piñas F. Secondary Nanoplastics Released from a Biodegradable Microplastic Severely Impact Freshwater Environments. Environ. Sci. Process. Impacts. 2019;6:1382–1392. doi: 10.1039/C8EN01427B. [DOI] [Google Scholar]
- 37.Tarique J., Sapuan S.M., Khalina A. Effect of Glycerol Plasticizer Loading on the Physical, Mechanical, Thermal, and Barrier Properties of Arrowroot (Maranta Arundinacea) Starch Biopolymers. Sci. Rep. 2021;11:1–17. doi: 10.1038/s41598-021-93094-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.ISO (International Organization for Standardization) ISO 14851: Determination of the Ultimate Aerobic Biodegradability of Plastic Materials in an Aqueous Medium—Method by Measuring the Oxygen Demand in a Closed Respirometer. ISO; Geneva, Switzerland: 2019. [Google Scholar]
- 39.Redfield A.C. The biological control of chemical factors in the environment. Am. Sci. 1958;46:205–221. [PubMed] [Google Scholar]
- 40.Tortell P.D., Maldonado M.T., Granger J., Price N.M. Marine bacteria and biogeochemical cycling of iron in the oceans. FEMS Microbiol. Ecol. 1999;29:1–11. doi: 10.1111/j.1574-6941.1999.tb00593.x. [DOI] [Google Scholar]
- 41.Beiras R., Tato T., López-Ibáñez S. A 2-Tier standard method to test the toxicity of microplastics in marine water using Paracentrotus lividus and Acartia clausi larvae. Environ Toxicol Chem. 2018;38:630–637. doi: 10.1002/etc.4326. [DOI] [PubMed] [Google Scholar]
- 42.Lorenzo J.I., Nieto O., Beiras R. Effect of Humic Acids on Speciation and Toxicity of Copper To Paracentrotus lividus larvae in seawater. Aquat. Toxicol. 2002;58:27–41. doi: 10.1016/S0166-445X(01)00219-3. [DOI] [PubMed] [Google Scholar]
- 43.Saco-Álvarez L., Durán I., Ignacio Lorenzo J., Beiras R. Methodological Basis for the Optimization of a Marine Sea-Urchin Embryo Test (SET) for the Ecological Assessment of Coastal Water Quality. Ecotoxicol. Environ. Saf. 2010;73:491–499. doi: 10.1016/j.ecoenv.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 44.(ISO) International Organization for Standardization . ISO 19679: Determination of Aerobic Biodegradation of Non-Floating Plastic Materials in a Seawater/Sediment Interface—Method by Analysis of Evolved Carbon Dioxide. ISO; Geneva, Switzerland: 2016. [Google Scholar]
- 45.ASTM (American Society for Testing and Materials) D7991–15 Standard Test Method for Determining Aerobic Biodegradation of Plastics Buried in Sandy Marine Sediment under Controlled Laboratory Conditions. ASTM; West Conshohocken, PA, USA: 2015. [Google Scholar]
- 46.Lin C.C., Lee L.T., Hsu L.J. Degradation of Polyvinyl Alcohol in Aqueous Solutions Using UV-365 Nm/S2O82- Process. Int. J. Environ. Sci. Technol. 2014;11:831–838. doi: 10.1007/s13762-013-0280-6. [DOI] [Google Scholar]
- 47.Folino A., Karageorgiou A., Calabrò P.S., Komilis D. Biodegradation of Wasted Bioplastics in Natural and Industrial Environments: A Review. Sustainability. 2020;12:6030. doi: 10.3390/su12156030. [DOI] [Google Scholar]
- 48.Byrne D., Boeije G., Croft I., Hüttmann G., Luijkx G., Meier F., Parulekar Y., Stijntjes G. Biodegradability of Polyvinyl Alcohol Based Film Used for Liquid Detergent Capsules. Tenside Surfactants Deterg. 2021;58:88–96. doi: 10.1515/tsd-2020-2326. [DOI] [Google Scholar]
- 49.Abdullah Z.W., Dong Y. Biodegradable and Water Resistant Poly(Vinyl) Alcohol (PVA)/Starch (ST)/Glycerol (GL)/Halloysite Nanotube (HNT) Nanocomposite Films for Sustainable Food Packaging. Front. Mater. 2019;6:1–17. doi: 10.3389/fmats.2019.00058. [DOI] [Google Scholar]
- 50.Reuschenbach P., Pagga U., Strotmann U. A Critical Comparison of Respirometric Biodegradation Tests Based on OECD 301 and Related Test Methods. Water Res. 2003;37:1571–1582. doi: 10.1016/S0043-1354(02)00528-6. [DOI] [PubMed] [Google Scholar]
- 51.Wei X.F., Bohlén M., Lindblad C., Hedenqvist M., Hakonen A. Microplastics generated from a biodegradable plastic in freshwater and seawater. Water Res. 2021;15:117123. doi: 10.1016/j.watres.2021.117123. [DOI] [PubMed] [Google Scholar]
- 52.TUV AUSTRIA Certification Scheme OK Biodegradable Water. [(accessed on 17 October 2021)]. Available online: https://www.tuv-at.be/green-marks/doc-center/
- 53.Harrison J., Boardman C., O’Callaghan K., Delort A.-M., Song J. Biodegradability standards for carrier bags and plastic films in aquatic environments: A critical review. R. Soc. Open Sci. 2018;5:171792. doi: 10.1098/rsos.171792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.European Commission Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on Classification, Labelling and Packaging of Substances and Mixtures. Off. J. Eur. Union. 2008 December 16;:353. [Google Scholar]
- 55.Arfsten D.P., Burton D.T., Fisher D.J., Callahan J., Wilson C.L., Still K.R., Spargo B.J. Assessment of the aquatic and terrestrial toxicity of five biodegradable polymers. Environ. Res. 2004;94:198–210. doi: 10.1016/S0013-9351(03)00087-2. [DOI] [PubMed] [Google Scholar]
- 56.ECHA Short-Term Toxicity to Aquatic Invertebrates. [(accessed on 27 August 2021)]. Available online: https://echa.europa.eu/es/registration-dossier/-/registered-dossier/14481/6/2/4.
- 57.Perales E., García C.B., Lomba L., García J.I., Pires E., Sancho M.C., Navarro E., Giner B. Comparative Ecotoxicity Study of Glycerol-Biobased Solvents. Environ. Chem. 2017;14:370–377. doi: 10.1071/EN17082. [DOI] [Google Scholar]
- 58.Gu Y., Jérôme F. Glycerol as a sustainable solvent for green chemistry. Green Chem. 2010;12:1127–1138. doi: 10.1039/c001628d. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.