Abstract
The trefoil peptide intestinal trefoil factor (ITF) plays a critical role in the protection of colonic mucosa and is essential to restitution after epithelial damage. These functional properties are accomplished through coordinated promotion of cell migration and inhibition of apoptosis. ITF contains a unique three-looped trefoil motif formed by intrachain disulfide bonds among six conserved cysteine residues, which is thought to contribute to its marked protease resistance. ITF also has a seventh cysteine residue, which permits homodimer formation. A series of cysteine-to-serine substitutions and a C-terminally truncated ITF were made by PCR site-directed mutagenesis. Any alteration of the trefoil motif or truncation resulted in loss of protease resistance. However, neither an intact trefoil domain nor dimerization was required to promote cell migration. This pro-restitution activity correlated with the ability of the ITF mutants to activate mitogen-activated protein (MAP) kinase independent of phosphorylation of the epidermal growth factor (EGF) receptor. In contrast, only intact ITF retained both phosphatidylinositol 3-kinase and the EGF receptor-dependent antiapoptotic effect in HCT116 and IEC-6 cells. The inability to block apoptosis correlated with a loss of trefoil peptide-induced transactivation of the EGF receptor or Akt kinase in HT-29 cells. In addition to defining structural requirements for the functional properties of ITF, these findings demonstrate that distinct intracellular signaling pathways mediate the effects of ITF on cell migration and apoptosis.
Despite constant exposure to potentially injurious agents, the integrity of the gastrointestinal mucosa is maintained by both its intrinsic defenses and its capability for repair (30). The three members of the trefoil peptide family, pS2, SP, and ITF, are secreted in association with mucins in a region-specific fashion throughout the gastrointestinal tract (36). ITF is a predominant secreted product of the intestinal goblet cell and has been demonstrated in in vitro and in vivo studies to play an important role in mucosal homeostasis of the intestinal mucosa (2, 9). Restitution, the rapid sealing of mucosal breaches through spreading and migration of surviving epithelial cells (8) of artificially wounded intestinal epithelial monolayers is accelerated by ITF (9). In addition to facilitating mucosal repair, trefoil peptides protect monolayers of intestinal epithelial cells against a variety of injurious agents including bile salt and Clostridium difficile toxin A (20). Mucin glycoproteins, the other major products of goblet cells, enhance these effects through synergistic interaction with trefoil peptides (9, 20, 25). Furthermore, mice made ITF deficient by homologous recontribution are markedly susceptible to colonic injury induced by standard agents, and restitution is virtually absent (25). This restitution defect can be rescued by topical administration of recombinant ITF. Collectively, these findings indicate that ITF is essential for normal intestinal repair and epithelial homeostasis.
Recent studies have provided insights into intracellular signaling events that mediate these effects. Addition of ITF to the human colon carcinoma-derived cell line HT-29 (22) and the gastric human carcinoma-derived cell line AGS (46) causes tyrosine phosphorylation of the EGF-R and activation of mitogen-activated protein kinases (MAPK). A functional consequence of activation of this pathway is cross-regulation of trefoil peptide expression at the transcriptional level (17, 46). In addition, serine phosphorylation of Akt kinase in an ITF-overexpressing HT-29 line (48) indicates activation of PI3K. Furthermore, ITF was found to prevent apoptosis in a number of gastrointestinal cell lines after serum starvation and ceramide and etoposide addition (48). Of interest, ITF-null mice exhibit an increase in crypt cell apoptoses consistent with the inference that ITF limits apoptosis in the normal intestinal epithelium. Both tyrphostin, an inhibitor of EGF-R tyrosine kinase, and wortmannin, an inhibitor of PI3K, abrogated the protective effects of ITF against apoptosis, suggesting that the protective mechanism of ITF involves activation of both EGF-R and PI3K-Akt pathways (4, 14, 18, 27, 51, 54).
However, the mechanism through which ITF accelerates mucosal restitution, and the role of EGF-R and PI3K signaling in this process, has not been directly determined. ITF secretion is apically directed and has been presumed to act on colonocytes at the luminal aspect, whereas the EGF-R resides predominantly basolaterally in polarized cells (26, 55). This distinguishes ITF from a number of regulatory peptides including cytokines and growth factors (e.g., EGF, transforming growth factor α, and hepatocyte growth factor), which stimulate restitution acting at the basolateral epithelial surface via a transforming growth factor β-dependent mechanism (7, 10–12, 31). ITF has been reported to downregulate E-cadherin–catenin complex formation, which may contribute to the decreased cell-cell and cell-extracellular matrix adhesion that facilitates cell migration (13). Although findings of potential binding proteins have been reported, the presence of specific trefoil receptors mediating these effects has not been proven (5, 45, 54). For this reason, clues to the nature of the interaction between ITF and the cell surface may best be achieved through analysis of the structural requirements for these properties.
Trefoil peptides are characterized by the presence of one or two copies of a distinctive trefoil motif composed of six conserved cysteine residues in the configuration: CX9CX9CX4CCX9WCF (51, 56). This motif forms a three-loop secondary structure with three cysteine-cysteine bonds in the configuration 1-5, 2-4, and 3-6. SP, a two-domain trefoil peptide, is highly resistant to thermal and proteolytic degradation (16). It has been suggested that these properties result from a compact tertiary structure allowed by the trefoil domain and might therefore be common to all members of the family. Solution of the SP (3) and pS2 (35) structures appears to confirm this view. ITF and pS2 also have a seventh cysteine residue that may permit the formation of homodimer. Notably, dimerized pS2 is reported to be more effective than monomer in conferring protection against indomethacin-induced gastric injury and promoting the migration of wounded epithelial monolayers (24).
In aggregate, the above studies suggest that structural features of trefoil peptides enable these factors to play a pivotal role in the maintenance and repair of mucosal integrity. The present studies were undertaken to define the structural requirements for biochemical and functional properties of the representative trefoil peptide ITF. This analysis yields evidence that two key functional effects of trefoil peptides, enhanced migration and protection from apoptosis, are mediated through separable pathways with distinct structural requirements.
MATERIALS AND METHODS
Abbreviations used in this paper.
ITF, intestinal trefoil factor; EGF, epidermal growth factor; EGF-R, epidermal growth factor receptor; MAPK, mitogen-activated protein kinase; ERK, extracellular signal-regulated kinase; SP, spasmolytic polypeptide; PARP, poly(ADP-ribose) polymerase; BSA, bovine serum albumin; PI3K, phosphatidylinositol 3-kinase; TRX, thioredoxin; β-ME, β-mercaptoethanol; SDS-PAGE, sodium dodecyl sulfate-polyacrylamide gel electrophoresis; EDC, 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride; MES, 2(N-morpholino) ethanesulfonic acid; PVDF, polyvinylidene difluoride; Ac-DEVD-pNA, N-acetyl-Asp-Glu-Val-Asp–p-nitroaniline.
Site-directed mutagenesis and construction of TRX-ITF fusion protein expressing vectors.
Site-directed mutagenesis and UDG cloning by the method of Rashtchian et al. (37) were performed using the Gibco Life Technologies (Gaithersburg, Md.) PCR site-directed mutagenesis system as specified by the manufacturer. Briefly, this method introduces a site-specific mutation by amplifying two single-stranded products by PCR with specific mutagenic primers containing the desired mutation. The targeted base change can reside in one or both of the mutagenic primers. The upper strand was amplified using upper-strand primer (USP1-6) and the dU-LacZ forward primer (dU-LacFWD), while the lower strand was amplified using lower-strand primer (LSP1-6) and the dU-LacZ reverse primer (dU-LacREV). The dU-Lac primers annealed to lacZα sequences flanking the multiple-cloning sites of the template. The overlapping internal primers contained 5′-end uracil rather than thymidine, allowing for directional cloning of the amplified DNA by UDG cloning (29). Between 8 and 10 cycles of PCR were performed using 5 ng of human ITF (HITF) in PCR1000 (33) as the template. Methylated (template) DNA was then eliminated by digestion with DpnI. The single-stranded products were treated with UDG to create complementary 3′ overhangs and annealed to the shuttle vector pAMP (Gibco). dU-containing primers were as follows: USP1, 5′-gcuugcaaaucaguctgccgtgtggccaag-3′; LSP1, 5′-ucauuuhcuahcahhcccachtactcctc-3′; USP2, 5′-aaggauaggguggacuccggctacccccatgt-3′; LSP2, 5′-aguccacccuauccuuggccggcacggcaca-3′; USP3, 5′-ucacucuuaaggagcccaacaaccggggcug-3′; LSP3, 5′-acuccuuaagagugacatgggggtagccgc-3′; USP4, 5′-agugcaauaaucggggctcctgctttgactccag-3′; LSP4, 5′-gauuauugcacuccttgggggtgacat-3′; USP5, 5′-aauaaucggggcugcucctttgactccaggatc-3′; LSP5, 5′-agcagccccgauuauagcactccttgggggtg-3′; USP6, 5′-aggagugccuugguctttcaagcccctgac-3′; LSP6, 5′-accauggcacuccugggatcctggagtcaa-3′; KpnI-ended primer to generate the 5′ end for subcloning into pTRX-fus, 5′-aaggtacccgaggagtacgtgggcctgcttgcaaaccag-3′; SalI-ended primer to generate the 3′ end, 5′-ccaggtcgactagaaggtgcattctgcttc-3′; and SalI-ended primer for C57-S57 mutation (using HITF in PCR1000 as the template), 5′-ccaggtcgactagaaggtggattctgcttcctg-3′. All oligonucleotides were synthesized by the Molecular Biology Core of The Center for the Study of Inflammatory Bowel Disease, Massachusetts General Hospital. Sequences of all constructs used were confirmed by dideoxy chain termination sequencing (Sequenase; Amersham).
Expression and purification of TRX-ITF fusion proteins.
Wild-type and mutated ITF plasmids were each propagated in GI724 competent cells (Invitrogen, San Diego, Calif.) by chemical transformation. Transformed cells were incubated at 30°C for 3 h and then induced with 100 μg of tryptophan per ml at 37°C for 3 h. The cells were collected by centrifugation, resuspended in phosphate buffer containing 1 mM β-ME, lysed by sonication (cell disruptor 185; Branson Sonic Power Co., Danbury, Conn.), and further centrifuged. Supernatants were incubated with activated equilibrated ThioBond resin (Invitrogen) at 4°C overnight and eluted from the resin with phosphate buffer containing increasing β-ME concentrations. Fractions containing 50, 100, and 200 mM β-ME were concentrated by Centriprep 10 and Centricon 10 concentrators (Amicon, Beverly, Mass.) and applied to a Superose 12 gel filtration column (Pharmacia Biotech, Piscataway, N.J.). Fractions (1 ml) were analyzed by SDS-PAGE.
Chemical cross-linking the wild-type and mutant ITFs.
The cross-linking of wild type, C21S, and C57S fusion proteins was carried out for 2 h at room temperature with 1 mg of EDC (Pierce). Before cross-linking of the peptides, they were treated with a 10-fold molar excess of citraconic anhydride (Pierce) in 0.5 M sodium phosphate buffer (pH 8.5) at room temperature overnight to cover the all the amines in the TRX fusion protein. The cross-linking reactions occurred in the presence of 0.1 M MES buffer plus 15 mg of bovine serum albumin(BSA) per ml for 2 h at room temperature. The conjugates were purified by gel filtration using D-Salt dextran plastic desalting columns (Pierce). The products of the reactions were then run on native-PAGE gels using Tris-glycine running buffer.
Protease digestion of fusion proteins.
Protease digestion of purified recombinant fusion proteins was performed by a modification of previously described methods (49). For assessment of trypsin resistance, 0.01 to 10 μg of aqueous bovine trypsin (pH 7.8) (Sigma) per ml was mixed with 10 μl of fusion proteins (1 mg/ml in 0.1 M Tris-HCl [pH 7.8]) and incubated at 37°C for 4 h. Porcine pepsin (Sigma) was diluted to 1 mg/ml in 0.1 M acetic acid, and 0.01 to 10 μl was added to 10 μl of fusion proteins (1 mg/ml in 0.1 M Tris-HCl [pH 2.0]) and incubated at 37°C for 4 h. The reactions were stopped by heating at 95°C for 5 min, and the products were subjected to SDS–10 to 20% PAGE (Tricine gel; Novex, San Diego, Calif.). The gels were stained with Coomassie blue and scanned with a laser densitometer (Pharmacia LKB Biotechnology). The percentages of the undigested residual amounts of fusion proteins were calculated as the ratio of the residual amounts to the amounts of peptide on gels incubated without proteases.
Synthesis of oligopeptides.
Three decapeptides (wild type, control, and Cys 57 mutated peptide) with comparable biochemical characteristics (see Fig. 5B) were synthesized using Fmoc chemistry (Peptide Core Facility, Massachusetts General Hospital).
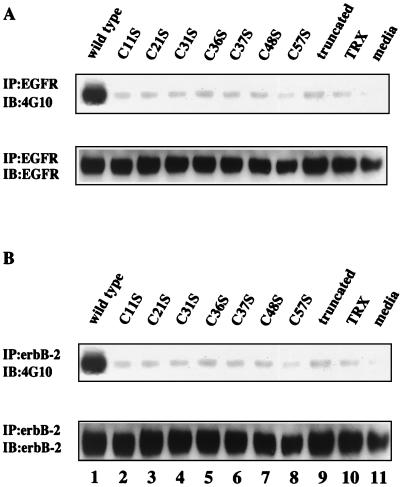
FIG. 5.
Effects of alteration of the tertiary structure of ITF on transactivation of human EGF-R. Cell lysates from HT-29 cells stimulated for 5 min with 100 μg of wild-type and mutated ITF fusion proteins per ml were subjected to immunoprecipitation using anti-human EGF-R or anti-human erbB-2 antibodies. (A) EGF-R immunoprecipitates were subjected to Western blotting using antiphosphotyrosine antibody 4G10 (upper panel). Membranes were stripped and reblotted with anti-human EGF-R antibody (lower panel). (B) erbB-2 immunoprecipitates were subjected to Western blotting using antiphosphotyrosine antibody 4G10 (upper panel). Membranes were stripped and reblotted with anti-human erbB-2 antibody (lower panel).
Cell culture.
The human colon cancer cell lines HT-29 and HCT116 and the rat nontransformed intestinal epithelial cell line IEC-6 were obtained from the American Type Culture Collection, Rockville, Md. IEC-6 cells, used in passages 16 to 19, were grown in Dulbecco's modified Eagle's medium (Mediatech, Herndon, Va.) supplemented with 0.1 U of bovine insulin (Sigma) per ml and 5% fetal calf serum. HT-29 and HCT116 cells were grown in Dulbecco's modified Eagle's medium containing 10% fetal calf serum. All media were supplemented with 4 mM l-glutamine, 50 U of penicillin per ml, and 50 μg of streptomycin per ml. The cells were grown in 5% CO2 at 37°C.
Restitution (migration) in an in vitro model of wounding.
Wound assays were performed essentially as previously described (9). Briefly, confluent monolayers of IEC-6 cells in 24-well multiwell plates were preincubated with 0.1% fetal calf serum-containing medium overnight. Wounds were made with a razor blade designed to make a 4- to 5-mm by 1-cm wound in each well. Cells were washed with fresh serum-free medium to remove residual cell debris, and wounded monolayers were cultured for a further 24 h in fresh serum-free medium in the presence or absence of proteins: TRX (Calbiochem Biochemicals, La Jolla, Calif.), TRX–wild-type ITF fusion protein, TRX-mutated ITF fusion proteins, and bovine serum albumin (BSA; Sigma). For experiments investigating the inhibition of migration, cells were further cocultured with either 30 μM tyrphostin 25 (Sigma) or 25 μM PD98059 (New England Biolabs). Migration of IEC-6 cells was assessed (by an observer blinded to the treatment type) by cell counting and expressed as the mean number of cells present across the wound border in a standardized length using photomicrographs obtained at a magnification of ×100 with an inverted microscope (Nikon Diaphor TMS; Nikon N6006 camera). Duplicate wells were used in each experiment, and data were obtained from at least three separate experiments.
Immunoprecipitation and immunoblotting for EGF-R and erbB-2 phosphorylation.
Confluent monolayers (90 to 95% confluent) of HT-29 cells in six-well plates were incubated for at least 12 h in serum-free medium before being stimulated with 100 μg of TRX-ITF fusion proteins per ml or 66.7 μg of recombinant TRX per ml. The cells were rinsed in cold phosphate-buffered saline and lysed on ice in 0.5% NP-40 lysis buffer, and immunoprecipitates were isolated as previously described (46). Sheep anti-human EGF-R or anti-c-erbB-2 antibodies were purchased from Upstate Biotechnology (Lake Placid, N.Y.). Immunoprecipitates were washed three times in lysis buffer, sample buffer was added, and samples were subjected to SDS-PAGE (4 to 12% Bis-Tris gel; Novex) and then transferred to PVDF membranes (Immobilon-P; Millipore, Bedford, Mass.). Immunoblotting with antiphosphotyrosine antibody 4G10 (Upstate Biotechnology) was also performed as previously described (46). Each membrane was reblotted with anti-human EGF-R or anti-human c-erbB-2 antibody after stripping of antiphosphotyrosine antibodies to confirm equal loading of samples.
Effect of ITF and mutated peptides on apoptosis.
Confluent monolayers of HCT116 and IEC-6 colonic epithelial cells in six-well plates were maintained in 0.1% fetal calf serum-containing medium for 16 h and then further incubated with 3 mg of various fusion proteins per ml, 1 mg of BSA per ml, 2 mg of TRX per ml, or without any additives for 24 h. Etoposide (1 mM) or 50 μM C2-ceramide was added to HCT116 cells and IEC-6 cells, respectively, and the cells were incubated for a further 24 h. Apoptosis was assessed by measuring the cleavage of the caspase substrate p116-PARP (Biomol Research Laboratories, Plymouth Meeting, Pa.) as previously described (46) and by an assay for caspase-3 activity itself. Activation of caspase-3 was detected with the Caspase-3 Cellular Activity Assay Plus kit (Biomol), which uses Ac-DEVD-pNA as a substrate. The assays were conducted as specified by the manufacturer. In brief, cells after induction of apoptosis by 10 h of treatment with either etoposide or C2-ceramide were lysed and supernatants were obtained after centrifugation. Cell lysates were incubated with the substrate at a final concentration of 0.2 mM for 2 h, and the absorbance was read at 405 nm in a microplate reader (7520 microplate reader; Cambridge Technology Inc., Mass.). Caspase activity was expressed as picomoles of pNA per minute per microgram of protein.
Detection of phosphorylation of Akt and ERK.
Confluent monolayers of HCT116 or IEC-6 cells were treated with 3 mg of various fusion proteins per ml, 1 mg of BSA per ml, 2 mg of TRX per ml, or without any additives for 16 h, washed with phosphate-buffered saline, and immediately harvested on ice in sample buffer. Lysates were obtained after sonication and heat treatment at 95°C for 5 min followed by centrifugation. SDS-PAGE and immunoblotting with antibody to serine-phosphorylated Akt or ERK antibody (both from New England Biolabs) were performed as previously described (46). For inhibition of the antiapoptotic effects of wild-type fusion protein, cells were cultured with 9 mg of the peptide per ml in the presence of either 100 nM wortmannin (Sigma) or 30 μM tyrphostin 25.
RESULTS
Expression and purification of the TRX-ITF fusion proteins.
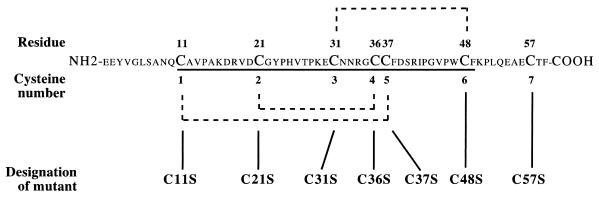
To examine the structural features necessary for the functional properties of human ITF, we designed seven N-terminal fusion point mutated proteins (Fig. 1). Each mutation replaced one of the seven cysteine residues with a serine residue. Substitution of any of the cysteine residues 1 through 6 with serine can be expected to disrupt the formation of the complete trefoil domain. Mutation of the cysteine 7 to serine was expected to prevent the formation of peptide dimers. In addition to these seven point mutations, we produced a truncated ITF protein in which the C-terminal 10 amino acids were deleted immediately after Phe49. Expression plasmids encoding each mutation or truncated human ITF as fusion proteins with TRX were transformed into GI724 Escherichia coli competent cells. From these bacterial cell lysates (Fig. 2, lane 1), fusion proteins were first separated using TRX affinity columns in phosphate buffer containing β-ME (lanes 2 and 3). Subsequent two-step gel filtration methods produced fusion proteins that were more than 95% pure by SDS-PAGE (lanes 4 and 5). Under reducing conditions, wild-type ITF fusion protein (Fig. 3A, lane 1) and each of the seven mutated proteins (lanes 2 to 8) migrated at a molecular mass of 21 kDa, corresponding to the combined molecular mass of ITF (7 kDa [lane 10]) and TRX (14 kDa [lane 11]). The C-terminally truncated fusion protein (lane 9, arrow) yielded a protein with a lower molecular mass, consistent with deletion of the C-terminal 10 amino acids.
FIG. 1.
Amino acid sequence of human ITF and designation of mutations. The primary sequence of ITF is shown, with the single trefoil domain underlined. Disulfide bonds between paired cysteine residues contributing to the trefoil structure are indicated by dashed lines. The seventh cysteine residue (C57) permits homodimer formation.
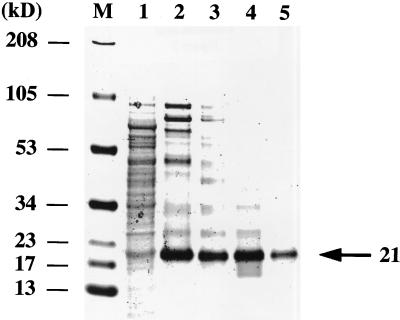
FIG. 2.
Biosynthesis and purification of wild-type ITF-TRX fusion proteins. GI724 cells were chemically transformed with a plasmid encoding wild-type ITF-TRX fusion protein. After induction with tryptophan, cells were lysed by sonication in phosphate buffer. Proteins from cell lysates (lane 1) were purified twice through TRX resin affinity columns. Partially purified lysates (lanes 2 and 3) were purified twice by gel filtration (lanes 4 and 5), separated by SDS-PAGE, and stained with Coomassie brilliant blue. Wild-type ITF fusion protein, >95% purified, migrated on the gel at the predicted molecular size of 21 kDa (arrow), as assessed by densitometry. M, molecular mass markers.
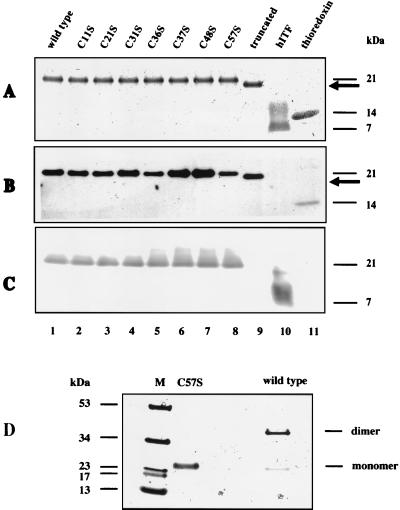
FIG. 3.
Detection of ITF fusion proteins. (A) Wild-type and mutated ITF fusion proteins were expressed in GI724 cells and purified. Wild-type ITF fusion protein, mutated ITF fusion proteins containing individual Cys-Ser substitutions, truncated ITF fusion protein (lane truncated), recombinant purified human ITF expressed in yeast (lane hITF), and positive control for thioredoxin were separated by gradient SDS-PAGE under reducing conditions and visualized with Coomassie brilliant blue. The arrow indicates the migration position of the C-terminal truncation product. (B) SDS-PAGE was performed as in panel A, proteins were transferred to PVDF, and immunoblotting was performed with monoclonal anti-TRX antibody. (C) The PVDF membrane from panel B was stripped and reblotted with rabbit polyclonal antiserum raised against the C terminus of ITF; this antibody did not recognize the C-terminal deletion product. (D) Mutated ITF C57S and wild-type ITF fusion protein were separated by gradient SDS-PAGE under non-reducing conditions, and the gel was stained with Coomassie blue. All C57S protein migrated at 21 kDa, indicating the corresponding monomeric fusion protein (faint band in the wild-type lane).
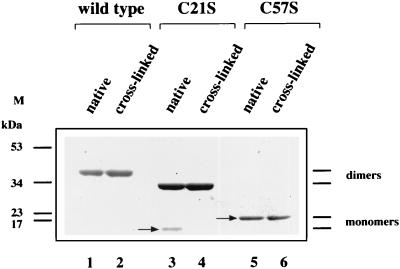
Fusion proteins were then subjected to Western blotting using a monoclonal antibody to TRX (Fig. 3B). This antibody recognized a fusion protein species of the same mass (Fig. 3B, lanes 1 to 9) as seen by Coomassie staining (Fig. 3A). Reblotting with rabbit anti-ITF polyclonal antibody raised against the C-terminal peptide (33) also confirmed immunoreactive ITF of both wild-type (Fig. 3C, lane 1) and mutated (lanes 2 to 8) fusion proteins at the same molecular mass. As predicted, the anti-ITF antibody directed against the C-terminal peptide of ITF did not recognize the truncated ITF fusion protein (lane 9). Wild-type and C57S proteins were then electrophoresed on a gradient SDS-PAGE gel under nonreducing conditions and stained with Coomassie blue. Loss of the seventh cysteine residue prevented homodimer formation compared to wild-type ITF fusion protein (Fig. 3D).
Protease resistance.
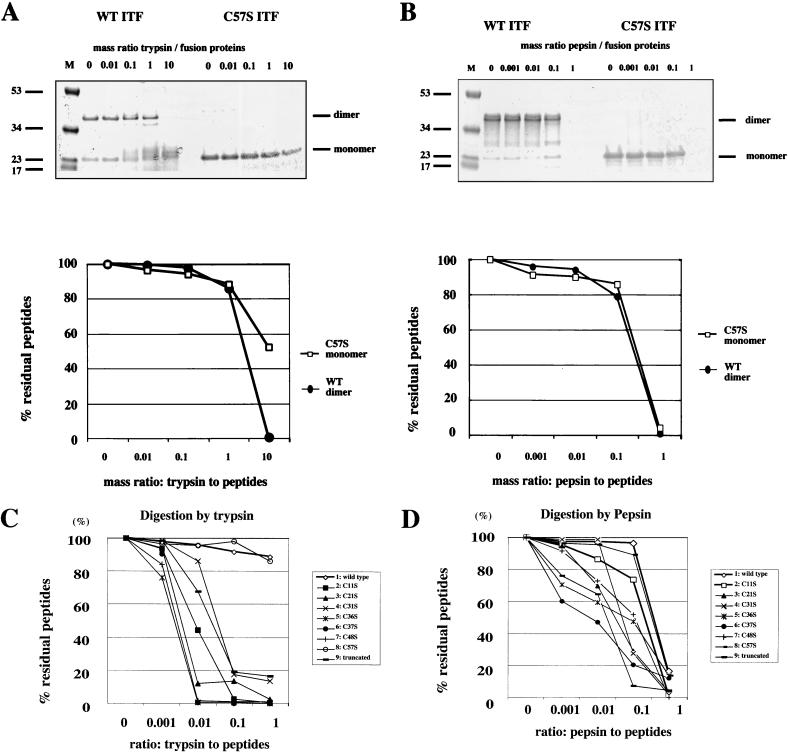
Trefoil peptides including ITF are highly resistant to the extracellular proteases trypsin and chymotrypsin (16; H. Kindon, K. Devaney, L. Thim, and D. K. Podolsky, submitted for publication), although the structural basis of this resistance has not been defined. To examine the importance of the presence of an intact trefoil motif or dimerization to protease resistance, ITF fusion proteins were incubated with either trypsin or pepsin at several concentrations and subjected to SDS-PAGE under nonreducing conditions. Both wild-type and C57S mutant peptides were substantially resistant to these proteases (Fig. 4A and B). In contrast, as seen in Fig. 4C and D, mutated fusion proteins designated C11S, C21S, C31S, C36S, C37S, and C48S mutations and a C-terminal truncation of ITF were readily cleaved by both trypsin and pepsin. These results indicate that protease resistance may require an intact trefoil domain but not dimerization.
FIG. 4.
Protease resistance of wild-type and mutated ITF peptides. (A) Wild-type (WT) and C57S ITF were digested with various concentrations of trypsin (0 to 10 mg of trypsin/mg of fusion protein) and electrophoresed on nonreducing SDS gels. The gels were stained with Coomassie brilliant blue (top panel), and proteins were quantitated by densitometry. The residual percentage was calculated from the residual dimerized wild-type ITF or residual mutated ITF monomer (lower panel). (B) Wild-type (WT) and C57S ITF were digested with various concentrations of pepsin (0 to 1 mg of pepsin/mg of fusion protein). The residual percentage was calculated in the same way as in panel A. (C) Wild-type protein and the indicated mutated ITF fusion proteins were digested with various concentrations of trypsin (0 to 1 mg of protease/mg of fusion protein) and electrophoresed on reducing SDS gels. The residual percentage was calculated from the residual wild-type ITF or residual mutated ITF monomers. (D) Wild-type and mutated ITF fusion proteins were digested with various concentrations of pepsin and electrophoresed on reducing SDS gels. The residual percentage was calculated in the same way as in panel C.
EGF-R and erbB-2 phosphorylation.
We previously observed that ITF induced tyrosine phosphorylation of the EGF-R in a colonic epithelial cell line (HT-29) and a gastric cancer cell line (AGS) (46). To assess the effect of various mutations on the ability of ITF to activate this pathway, HT-29 cells were stimulated with ITF fusion proteins for 5 min. Cell extracts were immunoprecipitated with anti-human EGF-R antibody and blotted with antiphosphotyrosine antibody. In contrast to treatment with wild-type ITF fusion protein (Fig. 5A, lane 1), EGF-R phosphorylation was virtually undetectable when cells were treated with any of the mutated ITF fusion proteins (lanes 2 to 9). Wild-type ITF fusion protein also stimulated the phosphorylation of c-erbB-2 in HT-29 cells (Fig. 5C, lane 1). As seen with EGF-R, no significant phosphorylation of c-erbB-2 was detected in cells treated with any of the mutated ITF fusion proteins (lanes 2 to 9). These findings indicate that both an intact trefoil domain and dimerization are required to effect tyrosine phosphorylation of both EGF-R and erbB-2.
Structural requirement for cell migration in association with ERK phosphorylation.
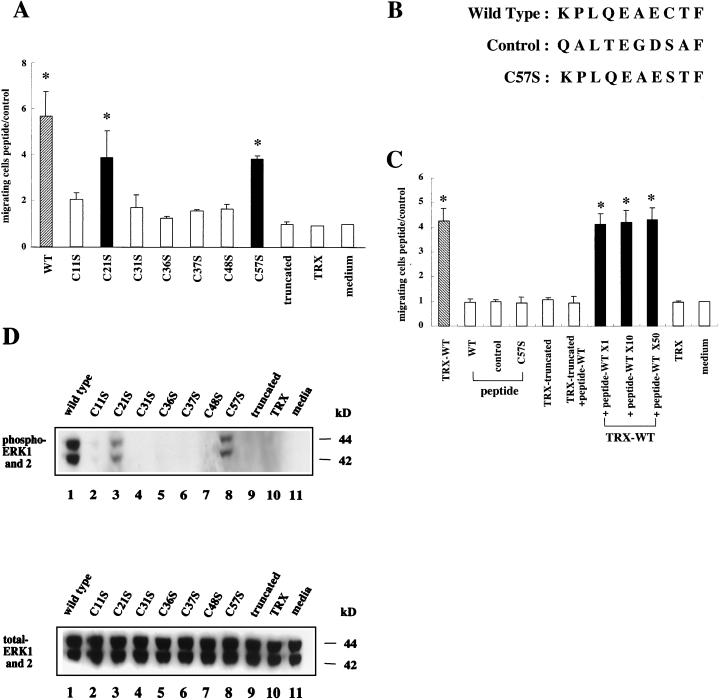
The ability to promote mucosal cell restitution is a key functional property of trefoil peptides. Mutated ITF fusion proteins were assessed to determine whether structural alterations affected the ability to promote cell migration, using wounded intestinal epithelial monolayers. Consistent with previous studies, wild-type ITF fusion protein enhanced migration activity four- to sixfold compared to control medium- or TRX-treated cells (Fig. 6A). No enhancement was seen when ITF fusion protein was replaced with BSA (data not shown). Mutated ITF fusion proteins C11S, C31S, C36S, C37S, and C48S were unable to significantly promote cell migration. However, C21S and C57S retained significant promigration activity (Fig. 6A). The latter findings indicate that an intact trefoil domain per se or trefoil dimerization may not be required to promote cell migration.
FIG. 6.
Effects of mutated ITF fusion proteins and wild-type or mutated peptides on restitution. (A) Wounds were established in confluent monolayers of IEC-6 cells as described in the text, and wounded monolayers were cultured for 24 h after the addition of control medium, TRX (2 mg/ml), or medium containing wild-type or mutated ITF fusion proteins (3 mg/ml). Cells migrating across the wound margin were counted by an observer blinded to the treatment group and quantitated as the ratio of the number of migrating cells after treatment to the number of cells migrating after the addition of medium alone. Results from three separate experiments are presented as mean and standard deviation. ∗, P < 0.05 compared with medium-treated cells. (B) Amino acid sequences of wild-type, control, and mutated peptides. (C) Wounds were established in confluent monolayers of IEC-6 cells as described in the text, and wounded monolayers were cultured for 24 h after the addition of control media, peptides (1×, 0.5 mg/ml; 10×, 5 mg/ml; 50×, 25 mg/ml), TRX (2 mg/ml), or medium containing wild-type or truncated ITF fusion proteins (3 mg/ml). Cell migration was quantified as above. ∗, P < 0.05 compared with medium-treated cells. (D) Confluent IEC-6 cells were preincubated with 3 mg of wild-type (lane 1) or mutated and truncated (lanes 2 to 9) ITF fusion proteins per ml, 2 mg of TRX per ml (lane 10), or medium alone (lane 11) for 2 h. Cell lysates were subjected to Western blotting and hybridized with antiphosphorylated ERK antibody (upper panel) or anti-total ERK antibody (lower panel).
The C-terminally truncated ITF fusion protein also lacked the ability to promote cell migration. The latter observation suggested that the C-terminal peptide of ITF might alone induce cell migration. To address this possibility, three decapeptides (wild type, control, and Cys57 mutated peptide) with comparable biochemical characteristics (depicted in Fig. 6B) were synthesized and examined for cell migration activity. However, as shown in Fig. 6C, neither wild-type nor Cys57 mutated peptides, when added at a comparable molar ratio (0.5 mg/ml), enhanced migration activity compared to a control “nonspecific” peptide. Furthermore, pretreatment with a 50-fold excess molar ratio of wild-type peptide did not competitively inhibit the promigration activity of wild-type ITF fusion protein. These results suggest that the inability of the truncated ITF to promote migration results from an alteration in the tertiary structure of the residual ITF or the corequirement for structural elements in the C-terminal tail and the trefoil domain.
ITF fusion proteins were then evaluated for their ability to promote activation of the MAPK pathway. IEC-6 cells were serum starved and stimulated with wild-type or mutated ITF fusion proteins. Cell lysates were assessed for the presence of phosphorylated ERK1 and ERK2 using antiphosphorylated and total ERK antibodies. As shown in Fig. 6D, wild-type ITF fusion protein stimulated the phosphorylation of ERK1 and ERK2, as previously demonstrated with AGS cells (46) and HT-29 cells. No significant phosphorylation of ERK1 and ERK2 was observed following stimulation with mutated ITF fusion proteins C11S, C31S, C36S, C37S, and C48S. However, the same mutated peptides (C21S and C57S) which were able to stimulate migration also retained the ability to stimulate ERK activation (Fig. 6D). Interestingly, stimulation of phosphorylation of ERK1 and ERK2 by these two mutated proteins was proportional to their ability to stimulate cell migration.
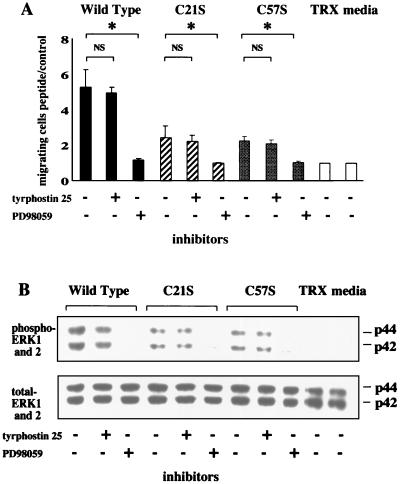
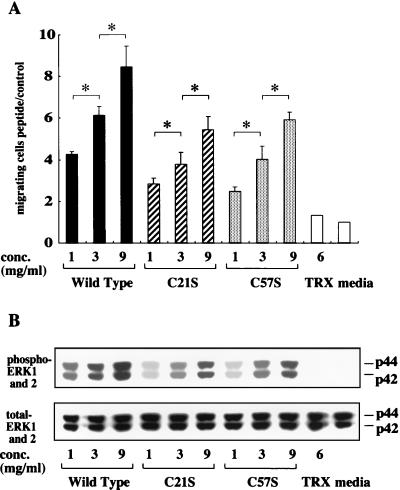
To determine whether ERK phosphorylation induced by wild-type, C21S, and C57S peptides occurs downstream of EGF-R signaling, these peptides were added to IEC-6 cells with or without either tyrphostin 25, an inhibitor of EGF-R phosphorylation, or PD98059, a MEK1 inhibitor. Interestingly, pretreatment with tyrphostin 25 did not affect promigratory activity (Fig. 7A) or the phosphorylation of ERK1 and ERK2 (Fig. 7B) in cells stimulated with both wild-type protein and these two mutants. In contrast, PD98059 completely blocked both migration and phosphorylation. Figure 8 shows the dose dependency of both cell migration (Fig. 8A) and ERK phosphorylation (Fig. 8B) mediated by wild-type and C21S and C57S mutant peptides. These results suggest that the ability to stimulate migration is contingent on the ability of the peptide to stimulate ERK activation independently of EGF-R phosphorylation, which appears not to have an absolute requirement for intact trefoil secondary structure or trefoil peptide dimerization.
FIG. 7.
Restitution and ERK phosphorylation do not require EGF-R phosphorylation. Confluent monolayers of IEC-6 cells were cultured after addition of control medium, TRX (2 mg/ml), or medium containing wild-type or mutated ITF fusion proteins (3 mg/ml) in the presence or absence of 30 μM tyrphostin 25 or 25 μM PD98059. Migrating cells (A) and ERK phosphorylation (B) were examined as described in legend of Fig. 6. Results from three separate experiments are presented as mean and standard deviation. ∗, P < 0.05 compared with PD98059-treated cells. NS, not significant.
FIG. 8.
ITF shows restitution activity and ERK in a dose-dependent manner. Confluent monolayers of IEC-6 cells were cultured after the addition of control medium, TRX (6 mg/ml), or medium containing wild-type or mutated ITF fusion proteins (1, 3, or 9 mg/ml). Migrating cells (A) and ERK phosphorylation (B) were examined as previously described. Results from three separate experiments are presented as mean and standard deviation. ∗, P < 0.05; shows a significant difference between columns.
The results described above indicate that C21S and C57S mutants have some characteristics distinguishing them from the other mutants. To further assess these characteristics reflecting the functional importance of the mutants, wild-type protein and both C21S and C57S mutants were chemically cross-linked using cross-linking reagents which react with a carboxyl group of ITF peptides and the reacted peptides were run on native-PAGE gels. As shown in Fig. 9, the C57S mutant (lane 5) did not form homodimers even after chemical cross-linking (lane 6), indicating that the C57S mutant does not exist as homodimer under native conditions. Furthermore, the C21S peptide (arrow in lane 3) migrated on the native gel faster than did the wild-type or C57S (arrow in lane 5) mutant peptide and formed homodimers (lane 4), indicating that C21S mutant has a more compact tertiary structure than do the wild-type and C57S mutant peptides. These results suggest again that cell migration does not require homodimerization of the trefoil peptide. It is also suggested that C21S mutant peptide might be able to access the entry of this peptide more easily than other mutant peptides, presumably due to its compact tertiary structure.
FIG. 9.
Chemical cross-linking of wild-type and C21S and C57S mutant fusion proteins. Chemical cross-linking analysis was carried out as described in Materials and Methods. Cross-linking products were run on native conditioned Tris-glycine gels with non-cross-linking native fusion proteins. The total protein volume was 10 μg in each lane.
Structural requirement for the antiapoptotic effect of ITF.
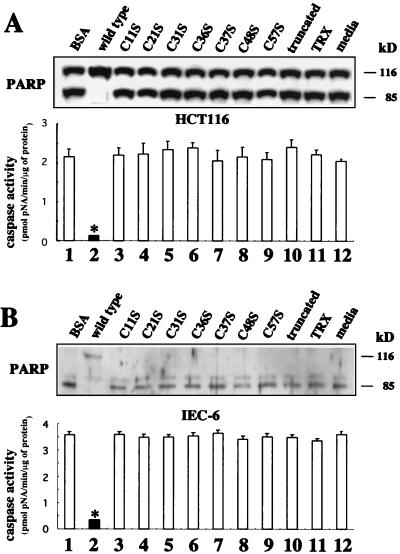
Previous studies showed that ITF has a cytoprotective function against injury by various agents. In addition, we found that ITF has protective effects against etoposide- or ceramide-induced apoptosis in colonic epithelial cells (48). HCT116 cells and IEC-6 cells were preincubated with ITF fusion proteins or BSA overnight and further incubated for 24 h with 1 mM etoposide or 50 μM C2-ceramide for induction of apoptosis. Cell lysates were evaluated for degradation of the caspase substrate PARP (p116-PARP) by immunoblotting. Both etoposide (Fig. 10A, upper panel) and ceramide (Fig. 10B, upper panel) induced p116 cleavage to a predominant 85-kDa species in the presence of BSA (lane 1) or medium alone (lane 12). In contrast, only the uncleaved 116-kDa band was detected in cells to which wild-type ITF was added, indicating that wild-type ITF protected cells from apoptosis (lane 2). However, 85-kDa fragments were detected in cells to which any of the mutated (lanes 3 to 9) or truncated (lane 10) ITF fusion proteins were added. To quantify the antiapoptotic effect of these mutant peptides, the cellular proteolytic activity of the caspase 10 h after induction of apoptosis was measured using the colorimetric substrate Ac-DEVD-pNA. Similar to previous reports showing that both etoposide and C2-ceramide treatment induce caspase-3 (-like) activation (38, 58), BSA-pretreated HCT116 and IEC-6 cell lysates showed high caspase-3 activity (Fig. 10, lower panels). In contrast, only wild-type peptide treatment resulted in very low activity, while cell lysate treated with the other mutants showed high activity similar to that observed in cells treated with BSA.
FIG. 10.
Mutated ITF fusion proteins lose antiapoptotic activity. (A) Confluent HCT116 cells were preincubated with either 3 mg of BSA per ml (lane 1), wild-type (lane 2) and mutated and truncated (lanes 3 to 10) ITF fusion proteins, 2 mg of TRX per ml (lane 11), or medium alone (lane 12) for 16 h. Apoptosis of HCT116 cells was induced by further incubation of the cells for 24 h with etoposide (1 mM). Cells lysates were separated by SDS-PAGE, transferred to PVDF membranes, and immunoblotted with anti-PARP antibody (upper panel). Caspase-3 activity was assayed using the Caspase-3 Cellular Activity Assay Kit Plus (Biomol), which uses Ac-DEVD-pNA as a substrate, as described in Materials and Methods. The data are expressed as picomoles per minute per microgram of protein with standard deviation in duplicated experiments (lower panel). (B) IEC-6 cells were treated in the same way as HCT116 cells, and apoptosis was induced by addition of C2-ceramide (50 μM).
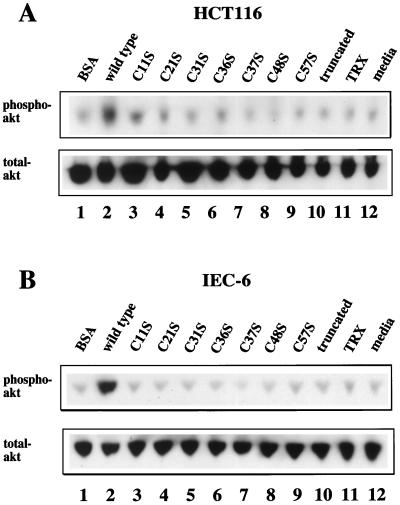
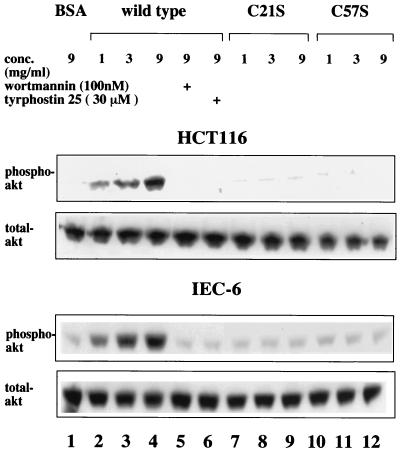
Previous studies demonstrated that the antiapoptotic effects of ITF are associated with activation of the PI3K-Akt signaling pathway (48). Accordingly, effects of the variant ITFs on Akt activation were determined. Phosphorylated Akt was abundant in wild-type ITF-treated HCT116 and IEC-6 cell lines, as expected (Fig. 11, lanes 2). In contrast, mutated ITF fusion proteins (lanes 3 to 10) did not induce significant serine phosphorylation of Akt. In addition, to examine the dose dependency of this activity, wild-type and C21S and C57S mutant peptides were added to both HCT116 and IEC-6 cells at various concentration and the cell lysates were assessed for Akt phosphorylation. As shown in Fig. 12, only wild-type ITF affected dose-dependent Akt phosphorylation, which was completely blocked by the addition of either wortmannin or tyrphostin 25, as previously observed (Fig. 11). These results suggest that the antiapoptotic effects of ITF require both an intact trefoil domain and dimer formation while cell migration requires neither ERK phosphorylation nor transactivation of EGF-R/c-erbB-2.
FIG. 11.
Detection of serine phosphorylation of Akt kinase in HCT116 and IEC-6 cells. (A) Confluent HCT116 cells were preincubated with 3 mg of BSA per ml (lane 1), wild-type (lane 2) and mutated and truncated (lanes 3 to 10) ITF fusion proteins, 2 mg of TRX per ml (lane 11), or medium alone (lane 12) for 16 h. Cell lysates were subjected to SDS-PAGE, transferred to PVDF membranes, and immunoblotted with a specific phosphorylated Akt antibody (upper panel) or anti-total Akt antibody (lower panel). (B) Confluent IEC-6 cells were treated in the same way as the HCT116 cells in panel A. Western blotting was performed as above (upper panel, antiphosphorylated Akt; lower panel, anti-total Akt antibody).
FIG. 12.
Requirement of phosphorylation of both PI3K and EGFR for antiapoptotic effect of ITF. Confluent HCT116 and IEC-6 cells were preincubated with 9 mg of BSA per ml (lane 1) or 1 to 9 mg of wild-type (lanes 2 to 6), C21S (lanes 7 to 9), and C57S (lanes 10 to 12) ITF fusion protein per ml for 16 h in the presence or absence of 100 nM wortmannin or 25 μM tyrphostin 25. Cell lysates were subjected to SDS-PAGE, transferred to PVDF membranes, and immunoblotted with a specific phosphorylated Akt antibody (upper panel) or anti-total Akt antibody (lower panel).
DISCUSSION
Expression and purification of TRX-ITF fusion proteins.
Trefoil proteins comprise a family of secreted proteins expressed by gastrointestinal mucus cells with common structural features (40). Their characteristic structural motif is designated a trefoil or P domain and is composed of three intrachain loops formed by six cysteine residues. Trefoil peptides are stable in the gastrointestinal lumen and resistant to proteases such as trypsin, chymotrypsin, pepsin, and carboxypeptidase (16; Kindon et al., submitted). Because their primary amino acid sequence should confer susceptibility to cleavage by extracellular proteases, the stability of the trefoil peptides is presumably a reflection of their secondary structure and/or the related compact tertiary structure (34). In this study, contributions of key structural features of the trefoil peptide ITF to biochemical stability and functional properties were evaluated. Wild-type and mutated ITF proteins were produced as fusions with TRX. TRX fusion proteins have been extensively used for expression and purification of large amounts of heterologous protein from E. coli (6, 23). A wild-type TRX-ITF was shown to bind to cells at same sites as “native” ITF did (46). N-terminal fusions were chosen to preserve structural elements and the conformation of the C terminus, particularly the ability to dimerize in the presence of cysteine 57 (50).
Protease resistance requires an intact trefoil domain but not dimerization.
Initial characterization of these proteins demonstrated similar migration on SDS-PAGE. The effect of cysteine substitutions on secondary structure was then assessed by performing tryptic cleavage at pH 7.8 and peptic cleavage at pH 2.0. Modulation of any of the cysteines (1 through 6) which participate in formation of the three interchain loops of the trefoil domain led to loss of resistance to trypsin and pepsin. The C57S mutation showed a similar resistance to proteolytic degradation to that of wild-type ITF, indicating that dimer formation was not necessary for the physiologic stability of ITF, because the C57S mutant was shown not to be dimerized even after the chemical cross-linking. Unexpectedly, ITF with a C-terminal truncation was vulnerable to protease digestion. This deletion may prevent the formation of the third trefoil loop; alternatively, a more subtle conformational change may have been generated. In general, cleavage of ITF mutations indicated a significant change in secondary structure, allowing the enzyme to access active sites. The nature of these structural alterations requires formal analysis by circular dichroism and nuclear magnetic resonance spectroscopy. Nevertheless, these findings confirm the assumption that the trefoil domain confers protease resistance. This contrasts with growth factors of the EGF family, which possess three intracellular loops formed by disulfide bonding yet are rapidly cleaved by acid pepsin (32).
Cell migration does not require an intact trefoil domain or dimer formation: association with retained ERK phosphorylation.
Trefoil peptides are motogens that are upregulated at the sites of mucosal injury and participate in mucosal repair by stimulating the migration of cells at the mucosal wounding edge (1, 57). In the present study, cell migration of the rat nontransformed colonic cell line IEC-6 was assessed after wounding and the addition of wild-type and mutated ITF proteins. Two mutated proteins, C21S and C57S, yielded significant cell migration compared to the control. The results of the cross-linking study of the C57 mutant show that this mutant cannot form homodimers because of the lack of this cysteine residue, suggesting that cell migration does not require dimerization of the peptide. Because the C21S mutant migrates more rapidly than the wild-type peptide under the native gel condition, it is possible that the C21S mutation adopts a low-energy state approximating a trefoil domain, even in the absence of the first disulfide bond. Significantly, mutation of the fourth cysteine, Cys36, which participates with Cys21 in formation of the second trefoil loop, showed no promigratory activity. Indeed, negligible cell migration was seen after addition of all other mutations of the trefoil domain compared to control.
A mutated ITF with a C-terminal deletion also lacked promigratory activity. A synthetic peptide representing the deleted residues was unable to rescue this activity, and a 50-fold molar excess of this peptide could not inhibit migration induced by wild-type ITF. This point has important functional and possibly therapeutic consequences. The high luminal activity and stability of trefoil peptides, as demonstrated in a range of in vivo and in vitro studies, has suggested therapeutic uses in conditions such as inflammatory bowel disease and in the prevention of radiotherapy- or chemotherapy-induced intestinal damage (48, 52). The functional analysis presented here indicates that biological activity depends on the conformation of the trefoil domain.
In the present studies, we also found a strong correlation between the ability of peptides to initiate cell migration and activation of ERK phosphorylation in IEC-6 cells. Growth factor-, cytokine-, and integrin-mediated motogenic signaling has been defined in a range of transformed and nontransformed cell lines in which activation of Ras, MEK, Erks, and myosin light-chain kinase are critical and sequential intermediates (21, 28). Myosin light-chain kinase functions downstream of Ras/ERK to promote the migration of urokinase-type plasminogen activator-stimulated cells in an integrin-selective manner (28, 59). Several lines of evidence indicate that a common motogenic signal requires multiple convergent signals, which may include activation of receptor tyrosine kinases, rho PI3K, and phosphorylation of the focal adhesion proteins focal adhesion kinase (FAK) and paxillin (15, 19, 39, 41, 42, 44). In the present study, stimulation of migration correlated with stimulation of ERK phosphorylation independent of EGF-R phosphorylation by wild-type and mutated ITFs. Thus, while the cell migration induced by C21S may have been unexpected, this was confirmed by ERK activation by this peptide. Furthermore, ERK activation does not require dimerized ITF, since C57S-ITF addition induced ERK phosphorylation in IEC-6 cells.
EGF-R, erbB-2, and Akt phosphorylation, together with protection against apoptosis, requires intact ITF dimer.
The distinction between MAPK-regulated motogenic signaling and the signaling pathways downstream from EGF receptor and erbB2 activation is highlighted by experiments with the mutated ITFs. While C21S and C57S ITF induced MAPK activation and cell migration, no EGF-R or erbB-2 phosphorylation was seen after the addition of mutant ITF. Nor was apoptotic cell death prevented by the addition of C21S or C57S ITF. Previously, we have shown that wild-type ITF induces trans-activation of the human pS2 promoter. MAPK activation was an absolute requirement for this effect, which was blocked by either pharmacological MEK inhibition or overexpression of the dual-specificity MAPK phosphatase PAC1 (46). Overexpression of a dominant-negative EGF-R, HER653 (53), partially inhibited hpS2 trans-activation by ITF. Moreover, and in agreement with the results of the present study, either HER653 expression or pharmacological EGF-R inhibition with tyrphostin 25 completely prevented the antiapoptotic affect of ITF in HT-29 and AGS cells (48). While there are multiple candidates for cell surface ITF substrates responsible for MAPK activation in parallel with the EGF-R, the C21S and C57S mutants should provide useful tools for their detection. Use of these mutants should also complement studies performed in epithelial lines rendered deficient in the EGF-R.
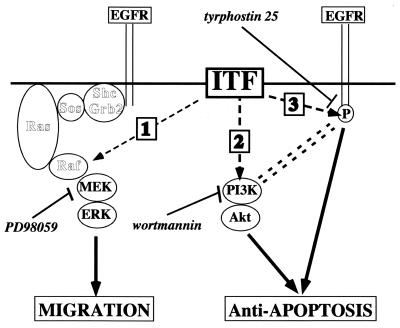
The observed utilization of several pathways by ITF to effect cell motility may provide insights into an apparent paradox. Although secreted ITF is present at the apical aspect of polarized colonocytes, EGF-R are present predominantly in basolateral surfaces (26, 55). EGF-R distribution is cell density dependent in vitro (43). It is therefore possible that initiation of cell movement in an EGF-R-independent fashion allows EGF-R redistribution and consequent association of ITF and EGF-R. In aggregate, the present studies are consistent with the notion that trefoil peptides promote migration and block apoptosis through distinct pathways. As indicated in Fig. 13, migration depends on ERK stimulation but not EGF-R stimulation (pathway 1), while the latter is required (pathway 3) in parallel with PI3K (pathway 2) to effect modulation of apoptosis.
FIG. 13.
Hypothetical pathways involved in ITF signaling. According to this model, ITF signaling utilizes MEK-ERK pathway 1 for cell migration, which does not require intact trefoil peptide. In contrast, for antiapoptotic effect, ITF requires intact dimerized peptide in the activation of both PI3K (pathway 2) and EGF-R (pathway 3) signal pathways.
ITF is essential for normal colonocyte homeostasis (25, 48). Our results confirm the functional importance of the conserved trefoil domain for protease resistance and initiation of critical signaling events in epithelial migration. Detailed structural analysis of wild-type and key mutated ITF proteins should help further define the nature of the interactions between ITF and the colonocyte surface. The delineation of EGF-R-independent signaling that mediates cell migration in response to ITF should provide insight into cell response to injury.
ACKNOWLEDGMENTS
We are grateful to Ian Rosenberg for critical support for cross-linking experiments and also thank Kathryn Lynch-Devaney for technical support of cell migration assays.
This work was supported by grants from the Crohn's and Colitis Foundation of America (to D.T.) and the National Institutes of Health (DK43351 and DK46906 to D.K.P.).
REFERENCES
- 1.Alison M R, Chinery R, Poulsom R, Ashwood P, Longcroft J M, Wright N A. Experimental ulceration leads to sequential expression of spasmolytic polypeptide, intestinal trefoil factor, epidermal growth factor, and transforming growth factor alpha mRNAs in rat stomach. J Pathol. 1995;175:405–414. doi: 10.1002/path.1711750408. [DOI] [PubMed] [Google Scholar]
- 2.Babyatsky M W, deBeaumont M, Thim L, Podolsky D K. Oral trefoil peptide protect against ethanol- and indomethacin-induced gastric injury in rats. Gastroenterology. 1996;105:1323–1332. doi: 10.1053/gast.1996.v110.pm8566596. [DOI] [PubMed] [Google Scholar]
- 3.Carr M, Bauer C J, Gradwell M J, Feeney J. Solution structure of a trefoil-motif-containing cell growth factor, porcine spasmolytic protein. Proc Natl Acad Sci USA. 1994;91:2206–2210. doi: 10.1073/pnas.91.6.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen P, Xie H, Sekar M, Gupta K, Wells A. Epidermal growth factor protects epithelial cells against Fas-induced apoptosis. J Biol Chem. 1994;274:17612–17618. doi: 10.1074/jbc.274.25.17612. [DOI] [PubMed] [Google Scholar]
- 5.Chinery R, Cox H M. Immunoprecipitation and characterization of a binding protein specific for the peptide, intestinal trefoil factor. Peptides. 1995;16:749–755. doi: 10.1016/0196-9781(95)00045-l. [DOI] [PubMed] [Google Scholar]
- 6.Chung D C, Brand S J, Tillotson L G. Mutually exclusive interactions between factors binding to adjacent Sp1 and AT-rich elements regulate gastrin gene transcription in insulinoma cells. J Biol Chem. 1995;270:8829–8836. doi: 10.1074/jbc.270.15.8829. [DOI] [PubMed] [Google Scholar]
- 7.Ciacci C, Lind S E, Podolsky D K. Transforming growth factor beta regulation of migration in wounded rat intestinal epithelial monolayers. Gastroenterology. 1993;105:93–101. doi: 10.1016/0016-5085(93)90014-4. [DOI] [PubMed] [Google Scholar]
- 8.Dieckgraefe B K, Stenson W F, Alpers D H. Gastrointestinal epithelial response to injury. Curr Opin Gastroenterol. 1996;12:109–114. [Google Scholar]
- 9.Dignass A U, Lynch-Devaney K, Kindon H, Thim L, Podolsky D K. Trefoil peptides promote epithelial migration through a TGF-β independent pathway. J Clin Investig. 1994;94:376–383. doi: 10.1172/JCI117332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dignass A U, Lynch-Devaney K, Podolsky D K. Hepatocyte growth factor/scatter factor modulate intestinal epithelial cell proliferation and migration. Biochem Biophys Res Commun. 1994;202:701–709. doi: 10.1006/bbrc.1994.1987. [DOI] [PubMed] [Google Scholar]
- 11.Dignass A U, Podolsky D K. Cytokine modulation of intestinal epithelial cell restitution: central role of transforming growth factor-β. Gastroenterology. 1993;105:1323–1332. doi: 10.1016/0016-5085(93)90136-z. [DOI] [PubMed] [Google Scholar]
- 12.Dignass A U, Tsunekawa S, Podolsky D K. Fibroblast growth factors modulate intestinal epithelial cell growth and migration. Gastroenterology. 1994;106:1254–1262. doi: 10.1016/0016-5085(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 13.Efstathiou J A, Noda M, Rowan A, Dixon C, Chinery R, Jawhari A, Hattori T, Wright N A, Bodmer W F, Pignatelli M. Intestinal trefoil factor controls the expression of the adenomatous polyposis coli-catenin and the E-cadherin-catenin complexes in human colon carcinoma cells. Proc Natl Acad Sci USA. 1998;95:3122–3127. doi: 10.1073/pnas.95.6.3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gibson S, Tu S, Oyer R, Anderson S M, Johnson G L. Epidermal growth factor protects epithelial cells against Fas-induced apoptosis. J Biol Chem. 1999;274:17612–17618. doi: 10.1074/jbc.274.25.17612. [DOI] [PubMed] [Google Scholar]
- 15.Gilmore A P, Romer L H. Inhibition of focal adhesion kinase (FAK) signaling in focal adhesions decreases cell motility and proliferation. Mol Cell Biol. 1996;7:1209–1224. doi: 10.1091/mbc.7.8.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jørgensen K H, Thim L, Jacobsen H E. Pancreatic spasmolytic polypeptide (PSP). I. Preparation and initial chemical characterization of a new polypeptide from porcine pancreas. Regul Pept. 1982;3:207–219. doi: 10.1016/0167-0115(82)90126-4. [DOI] [PubMed] [Google Scholar]
- 17.Kanai M, Podolsky D K. Intestinal trefoil factor induces inactivation of extracellular signal-related protein kinases in intestinal epithelial cells. Proc Natl Acad Sci USA. 1997;95:178–182. doi: 10.1073/pnas.95.1.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kennedy S G, Wagner A J, Conzen S D, Jordan J, Bellacosa A, Tsichlis P N, Hay N. The PI 3-kinase/Akt signaling pathway delivers an anti-apoptotic signal. Genes Dev. 1997;11:701–713. doi: 10.1101/gad.11.6.701. [DOI] [PubMed] [Google Scholar]
- 19.Khwaja A, Lehmann K, Marte B M, Downward J. Phosphoinositide 3-kinase induces scattering and tubulogenesis in epithelial cells through a novel pathway. J Biol Chem. 1998;273:18793–18801. doi: 10.1074/jbc.273.30.18793. [DOI] [PubMed] [Google Scholar]
- 20.Kindon H, Pothoulakis C, Thim L, Lynch-Devaney K, Podolsky D K. Trefoil peptide protection of intestinal epithelial barrier function: cooperative interaction with mucin glycoprotein. Gastroenterology. 1995;109:516–523. doi: 10.1016/0016-5085(95)90340-2. [DOI] [PubMed] [Google Scholar]
- 21.Klemke R L, Cai S, Giannini A L, Gallagher P J, deLanerolle P, Cheresh D A. Regulation of cell motility by mitogen-activated protein kinase. J Cell Biol. 1997;137:481–492. doi: 10.1083/jcb.137.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu D, El-Hariry I, Karayiannakis A J, Wilding J, Chinery R, Kmiot W, McCrea P D, Gullick W J, Pignatelli M. Phosphorylation of β-catenin and epidermal growth factor receptor by intestinal growth factor. Lab Investig. 1997;77:557–563. [PubMed] [Google Scholar]
- 23.Lunn C A, Kathju S, Wallace B J, Kushner S R, Pigiet V. Amplification and purification of plasmid-encoded thioredoxin from Escherichia coli K12. J Biol Chem. 1984;259:10469–10474. [PubMed] [Google Scholar]
- 24.Marchbank T, Westley B R, May F E B, Calman D P, Playford R J. Dimerization of human pS2 (TFF1) plays a key role in its protective/healing effects. J Pathol. 1998;185:153–158. doi: 10.1002/(SICI)1096-9896(199806)185:2<153::AID-PATH87>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 25.Mashimo H, Wu D-C, Podolsky D K, Fishman M C. Impaired defense of intestinal mucosa in mice lacking intestinal trefoil factor. Science. 1996;274:262–265. doi: 10.1126/science.274.5285.262. [DOI] [PubMed] [Google Scholar]
- 26.Menard D, Pothier P. Radioautographic localization of epidermal growth factor receptors in human fetal gut. Gastroenterology. 1991;101:640–649. doi: 10.1016/0016-5085(91)90520-u. [DOI] [PubMed] [Google Scholar]
- 27.Moro L, Venturino M, Bozzo C, Silengo L, Altruda F, Beguinot L, Tarone G, Defilippi P. Integrins induce activation of EGF receptor: role in MAP kinase induction and adhesion-dependent cell survival. EMBO J. 1998;17:6622–6633. doi: 10.1093/emboj/17.22.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nguyen D H, Catling A D, Webb D J, Sankovie M, Walker L A, Somlyo A V, Weber M J, Gonias S L. Myosin light chain kinase functions downstream of Ras/ERK to promote migration of urokinase-type plasminogen activator-stimulated cells in an integrin-selective manner. J Cell Biol. 1999;146:149–164. doi: 10.1083/jcb.146.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nisson P E, Rashtchain A, Watkins P C. Rapid and efficient cloning of Alu-PCR products using uracil DNA glycosylase. PCR Methods Appl. 1991;1:120–123. doi: 10.1101/gr.1.2.120. [DOI] [PubMed] [Google Scholar]
- 30.Nusrat A, Delp C, Madara J L. Intestinal epithelial restitution: characterization of a cell culture model and mapping of cytoskeletal elements in migrating cells. J Clin Investig. 1992;89:1501–1511. doi: 10.1172/JCI115741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nusrat A, Parkos C, Bacarra A, Godowski P, Delp-Archer C, Rosen C, Madara J. Hepatocyte growth factor/scatter factor effects on epithelial cells: regulation of intercellular junctions in transformed and nontransformed cell lines, basolateral polarization of c-met receptor in transformed and natural intestinal epithelia, and induction of rapid wound repair in a transformed model epithelium. J Clin Investig. 1994;93:2056–2065. doi: 10.1172/JCI117200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Playford R J, Marchbank T, Calnan D P, Calam J, Royston P, Batten J J, Hansen H F. Epidermal growth factor is digested to smaller, less active forms in acidic gastric juice. Gastroenterology. 1995;108:92–101. doi: 10.1016/0016-5085(95)90012-8. [DOI] [PubMed] [Google Scholar]
- 33.Podolsky D K, Lynch-Devaney K, Stow J L, Oates P, Murgue B, deBeaumont M, Sands B E, Mahida Y R. Identification of human intestinal trefoil factor: goblet cell-specific expression of a peptide targeted for apical secretion. J Biol Chem. 1993;268:6694–6702. [PubMed] [Google Scholar]
- 34.Polshakov V I, Frenkiel T A, Westley B, Chadwick M, May F, Carr M D, Feeney J. NMR-based structural studies of the pNR-2/pS2 single domain trefoil peptide. Similarities to porcine spasmolytic peptide and evidence for a monomeric structure. Eur J Biochem. 1995;233:847–855. doi: 10.1111/j.1432-1033.1995.847_3.x. [DOI] [PubMed] [Google Scholar]
- 35.Polshakov V I, Williams M A, Gargaro A R, Frenkiel T A, Westley B R, Chadwick M P, May F E, Feeney J. High-resolution solution structure of human pNR-2/pS2: a single trefoil motif protein. J Mol Biol. 1997;267:418–432. doi: 10.1006/jmbi.1997.0896. [DOI] [PubMed] [Google Scholar]
- 36.Poulsom R, Wright N A. Trefoil peptides: a newly recognized family of epithelial mucin-associated molecules. Am J Physiol. 1993;272:G1540–G1549. doi: 10.1152/ajpgi.1993.265.2.G205. [DOI] [PubMed] [Google Scholar]
- 37.Rashtchian A, Thornton C G, Heidecker G. A novel method for site-directed mutagenesis using PCR and uracil DNA glycosylase. PCR Methods Appl. 1992;2:124–130. doi: 10.1101/gr.2.2.124. [DOI] [PubMed] [Google Scholar]
- 38.Reyland M E, Anderson S M, Matassa A A, Barzen K A, Quissell D O. Protein kinase C δ is essential for etoposide-induced apoptosis in salivary gland acinar cells. J Biol Chem. 1999;274:19115–19123. doi: 10.1074/jbc.274.27.19115. [DOI] [PubMed] [Google Scholar]
- 39.Rodriguez-Viciana P, Warne P H, Khwaja A, Marte B M, Pappin D, Das P, Waterfield M D, Ridley A, Downward J. Role of phosphoinositide 3-OH kinase in cell transformation and control of actin cytoskeleton by Ras. Cell. 1997;89:457–467. doi: 10.1016/s0092-8674(00)80226-3. [DOI] [PubMed] [Google Scholar]
- 40.Sands B E, Podolsky D K. The trefoil peptide family. Annu Rev Physiol. 1996;58:253–273. doi: 10.1146/annurev.ph.58.030196.001345. [DOI] [PubMed] [Google Scholar]
- 41.Santos M F, McCormack S A, Guo Z, Okolicany J, Zheng Y, Johnson L R, Tigyi G. Rho proteins play a critical role in cell migration during the early phase of mucosal restitution. J Clin Investig. 1997;100:216–225. doi: 10.1172/JCI119515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schlaepfer D D, Jones K C, Hunter T. Multiple Grb2-mediated integrin-stimulated signaling pathways to ERK2/mitogen-activated protein kinase: summation of both c-Src- and focal adhesion kinase-initiated tyrosine phosphorylation events. Mol Cell Biol. 1998;18:2571–2585. doi: 10.1128/mcb.18.5.2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suarez-Quian C A, Byers S W. Redistribution of epidermal growth factor receptor as a function of cell density, cell-cell adhesion and calcium in human (A-431) cells. Tissue Cell. 1993;25:1–17. doi: 10.1016/0040-8166(93)90061-o. [DOI] [PubMed] [Google Scholar]
- 44.Takaishi K, Kikuchi A, Kuroda S, Kotani K, Sasaki T, Takai Y. Involvement of rho p21 and its inhibitory GDP/GTP exchange protein (rho GDI) in cell motility. Mol Cell Biol. 1993;13:72–79. doi: 10.1128/mcb.13.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tan X-D, Hsueh W, Chang H, Wei K-R, Gonzalez-Crussi F. Characterization of a putative receptor for intestinal trefoil factor in rat small intestine: identification by in situ binding and ligand blotting. Biochem Biophys Res Commun. 1997;237:673–677. doi: 10.1006/bbrc.1997.7144. [DOI] [PubMed] [Google Scholar]
- 46.Taupin D R, Podolsky D K. Mitogen-activated protein kinase activation regulates intestinal epithelial differentiation. Gastroenterology. 1999;116:1072–1080. doi: 10.1016/s0016-5085(99)70010-7. [DOI] [PubMed] [Google Scholar]
- 47.Taupin D R, Wu D C, Jeon W K, Devaney K, Wang T C, Podolsky D K. The trefoil gene family are coordinately expressed immediate-early genes: EGF receptor-and MAP kinase-dependent interregulation. J Clin Investig. 1999;103:R31–R38. doi: 10.1172/JCI3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Taupin D R, Kinoshita K, Podolsky D K. Intestinal trefoil factor confers colonic epithelial resistance to apoptosis. Proc Natl Acad Sci USA. 2000;97:799–804. doi: 10.1073/pnas.97.2.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thim L. A new family of growth factor-like peptides. 'Trefoil' disulfide loop structures as a common feature in breast cancer associated peptide (pS2), pancreatic spasmolytic polypeptide (PSP), and frog skin peptides (spasmolysins) FEBS Lett. 1989;250:85–90. doi: 10.1016/0014-5793(89)80690-8. [DOI] [PubMed] [Google Scholar]
- 50.Thim L, Thomsen J, Christensen M, Jørgensen K H. The amino acid sequence of pancreatic spasmolytic polypeptide. Biochim Biophys Acta. 1985;827:410–418. doi: 10.1016/0167-4838(85)90226-2. [DOI] [PubMed] [Google Scholar]
- 51.Thim L, Wöldike H F, Nielsen P F, Christensen M, Lynch-Devaney K, Podolsky D K. Characterization of human and rat intestinal trefoil factor produced in yeast. Biochemistry. 1995;34:4757–4764. doi: 10.1021/bi00014a033. [DOI] [PubMed] [Google Scholar]
- 52.Tran C P, Cook G A, Yeomans N D, Thim L, Giraud A S. Trefoil peptide TFF2 (spasmolytic polypeptide) potently accelerates healing and reduces inflammation in a rat model of colitis. Gut. 1999;44:636–642. doi: 10.1136/gut.44.5.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wagner M, Cao T, Lopez M E, Hope C, van Nostrand K, Kobrin M S, Fan H U, Buchler M W, Korc M. Expression of a truncated EGF receptor is associated with inhibition of pancreatic cell growth and enhanced sensitivity to cisplatinum. Int J Cancer. 1996;68:782–787. doi: 10.1002/(SICI)1097-0215(19961211)68:6<782::AID-IJC16>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 54.Walker F, Kato A, Gonez L J, Hibbs M L, Pouliot N, Levitzki A, Burgess A W. Activation of Ras/Mitogen-activated protein kinase pathway by kinase-defective epidermal growth factor receptors results in cell survival but not proliferation. Mol Cell Biol. 1998;18:7192–7204. doi: 10.1128/mcb.18.12.7192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Westermark K, Westermark B, Karlsson F A, Ericson L E. Location of epidermal growth factor receptors on porcine thyroid follicle cells and receptor regulation by thyrotropin. Endocrinology. 1986;118:1040–1046. doi: 10.1210/endo-118-3-1040. [DOI] [PubMed] [Google Scholar]
- 56.Wright N A, Hoffmann W, Otto W R, Rio M C, Thim L. Rolling in the clover: trefoil factor family (TFF)-domain peptides, cell migration and cancer. FEBS Lett. 1997;408:121–123. doi: 10.1016/s0014-5793(97)00424-9. [DOI] [PubMed] [Google Scholar]
- 57.Wright N A, Poulsom R, Stamp G, Vannorden S, Saffaf C, Elia G, Ahnen D, Jeffery R, Longcroft J, Pike C, Rio M C, Chambon P. Trefoil peptide gene expression in gastrointestinal epithelial cells in inflammatory bowel disease. Gastroenterology. 1993;104:12–20. doi: 10.1016/0016-5085(93)90830-6. [DOI] [PubMed] [Google Scholar]
- 58.Yoshimura S, Banno Y, Nakashima S, Takenaka K, Sakai H, Nishimura Y, Sakai N, Shimizu S, Eguchi Y, Tsujimoto Y, Nozawa Y. Ceramide formation leads to caspase-3 activation during hypoxic PC12 cell death. J Biol Chem. 1998;273:6921–6927. doi: 10.1074/jbc.273.12.6921. [DOI] [PubMed] [Google Scholar]
- 59.Zeigler M E, Chi Y, Schmidt T, Varani J. Role of ERK and JNK pathways in regulating cell motility and matrix metalloproteinase 9 production in growth factor-stimulated human epidermal keratinocytes. J Cell Physiol. 1999;180:271–284. doi: 10.1002/(SICI)1097-4652(199908)180:2<271::AID-JCP15>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]