Abstract
Salivary amylase binds specifically to a number of oral streptococcal species. This interaction may play an important role in dental plaque formation. Recently, a 585-bp gene was cloned and sequenced from Streptococcus gordonii Challis encoding a 20.5-kDa amylase-binding protein (AbpA). The goal of this study was to determine if related genes are present in other species of oral streptococci. Biotinylated abpA was used in Southern blot analysis to screen genomic DNA from several strains representing eight species of oral streptococci. This probe hybridized with a 4.0-kb HindIII restriction fragment from all 13 strains of S. gordonii tested. The probe did not appear to bind to any restriction fragments from other species of amylase-binding oral streptococci including Streptococcus mitis (with the exception of 1 of 14 strains), Streptococcus crista (3 strains), Streptococcus anginosus (1 strain), and Streptococcus parasanguinis (1 strain), or to non-amylase-binding oral streptococci including Streptococcus sanguinis (3 strains), Streptococcus oralis (4 strains), and Streptococcus mutans (1 strain). Primers homologous to sequences within the 3′ and 5′ ends of abpA yielded products of 400 bp following PCR of genomic DNA from the Southern blot-positive strains. Several of these PCR products were cloned and sequenced. The levels of similarity of these cloned products to the abpA of S. gordonii Challis ranged from 91 to 96%. These studies reveal that the abpA gene appears to be specific to S. gordonii and differs from genes encoding amylase-binding proteins from other species of amylase-binding streptococci.
The oral viridans group streptococci are a genetically diverse population of bacteria which share many phenotypic traits (1, 12, 31). These species have been found to sort into three larger groups, the mutans group streptococci, the salivarius group streptococci, and the mitis group streptococci (11). Each species is genetically distinct, but they are heterogeneous with respect to expression of phenotypic traits. This phenotypic heterogeneity used to distinguish each species of oral streptococci has made classification of these bacteria by traditional methods difficult. Genetic approaches for identifying members of the viridans group streptococci have therefore been developed, including DNA-DNA hybridization (2–4, 14) and genetic probe hybridization (29), restriction endonuclease-fragment polymorphism analysis of genomic DNA (23) and rRNA (20, 21), analysis of 16S rRNA sequences (11), and PCR analysis (17, 22).
Amylase, the most abundant enzyme in saliva, binds specifically and with a high affinity to several species of oral streptococci (5, 6, 25, 27). Amylase-binding streptococci (ABS) are present in significant numbers in developing human dental plaque (26, 30). It is possible that amylase in oral salivary pellicles serves as an adhesion receptor for ABS (27, 28). ABS colonization appears to be restricted to animal hosts that secrete salivary amylase, suggesting that amylase binding to ABS is essential for their colonization of the oral cavity (26). Biochemical studies have demonstrated that amylase binding to ABS is mediated by specific proteins with variable molecular masses (9). Recent studies demonstrate that an amylase receptor on the surface of Streptococcus gordonii Challis involves a specific protein of 20.5 kDa encoded by a 585-bp gene, abpA (19). Other studies have suggested that amylase binding may serve as a discriminator for the mitis group streptococci (7, 13, 25).
PCR-based assays that use arbitrary or specific gene sequence or sequences have been suggested to be useful for the detection and/or identification of oral bacteria in clinical samples, including Actinomyces species (15), Porphyromonas gingivalis (18), and some members of the viridans group streptococci (8, 10, 16, 17, 22). A genetic assay based on the sequence of abpA may be useful for the identification and/or discrimination of members of the viridans group streptococci.
The present study was performed to determine if abpA is present in other species or strains of the viridans group streptococci and to determine the genetic similarity between identified abpA homologs.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
The streptococcal strains used in this study were the generous gifts of Alan L. Coykendall, University of Connecticut, or Mogens Kilian, University of Aarhus, Aarhus, Denmark, or were from our own culture collection. Streptococci were Gram stained, and their identities were verified with the Rapid STREP system according to the manufacturer's instructions (API System, S.A., Montalieu, France). Streptococcal strains were routinely cultured on sheep blood agar, in tryptic soy broth supplemented with 0.5% yeast extract (Difco, Detroit, Mich.), or in brain heart infusion (Difco) and were grown for 16 to 18 h at 37°C in a candle jar. Escherichia coli DH5α cells used in all transformations were routinely cultured on Luria-Bertani (LB) agar or in LB broth supplemented with 100 μg of ampicillin per ml and grown aerobically for 16 to 18 h at 37°C with shaking. The plasmid pGEM-T (Promega, Madison, Wis.) was used for the cloning of putative abpA homologs.
Streptococcal genomic DNA preparation.
Streptococcal genomic DNA was isolated from all strains by a standard method (2), modified by first incubating the bacteria for 1 h at 37°C in the presence of 150 μg each of lysozyme and mutanolysin (Sigma Chemical Co. St. Louis, Mo.) per ml.
Southern blotting.
Genomic DNA (10 μg) from each strain was digested for 15 h with HindIII at 37°C according to the manufacturer's instructions (Gibco-BRL, Grand Island, N.Y.). The restricted DNA was electrophoresed in a 1.0% (wt/vol) agarose gel with a Minnie the Gel-Cicle electrophoresis unit (Hoefer Pharmacia Biotech, San Francisco, Calif.) in TAE buffer (40 mM Tris-acetate, 1 mM EDTA [pH 8.4]) for 15 h at a constant 15 V. Before blotting, the gels were immersed and were gently agitated at room temperature in 250 mM HCl (10 min), 1.5 M NaCl–0.5 M NaOH (25 min), and 1.5 M NaCl–0.5 M Tris HCl (pH 7.5) (30 min), with a water rinse between each treatment. DNA was transferred from the agarose gel to a Hybond-N+ (Amersham, Arlington Heights, Ill.) membrane by capillary blotting and was cross-linked to the membrane by using a UV cross-linker (Stratalinker; Stratagene, La Jolla, Calif.). Hybridization of a PCR-amplified biotinylated abpA probe (19) prepared with the BioPrime DNA Labeling system (Gibco BRL) was performed with the Photogene Nucleic Acid Detection system, version 2.0 (Gibco BRL), according to the manufacturer's instructions, with the following modification. After hybridization of the probe, the membrane was washed twice in prewarmed (65°C) 5× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)–0.5% (wt/vol) sodium dodecyl sulfate (SDS) at 65°C with gentle agitation for approximately 5 min per wash. The membrane was then washed with either prewarmed (50°C) 0.1× SSC–1% (wt/vol) SDS at 50°C for 30 min with gentle agitation (high-stringency Southern blotting) or prewarmed (37°C) 0.5× SSC–0.1% (wt/vol) SDS at 37°C for 30 min with gentle agitation (low-stringency Southern blotting). Finally, the membrane was washed once with 2 ml of 2× SSC per cm2 for 5 min at room temperature with gentle agitation.
Visualization of the Southern blots was performed with the Photogene Nucleic Acid Detection system, version 2.0 (Gibco BRL), according to the manufacturer's instructions. Each membrane was exposed to X-ray film in a cassette for 2 min in order to visualize the location of the hybridized probe.
PCR.
Two primers homologous to abpA from S. gordonii Challis (19), XabpA-2 (5′-TGATGAAGCTACTGATGC-3′) and CabpAR-2 (5′-TAACAACGCTGCAGAAGACAA-3′), were designed to initially screen genomic DNA preparations for the presence of abpA homologs. These primers yielded a product without the putative signal sequence identified in abpA (19). After initial screening, two additional primers, XabpA-1 (5′-AGGAGATAAAACGATGAAA-3′) and PabpAR-1 (5′-GCCATTGGTTCAGTGAT-3′), located just outside the open reading frame of abpA, were designed to amplify the entire putative abpA homolog for cloning. Following sequence analysis of the cloned abpA homologs, two primers which flanked the region of greatest homology between these homologs, XabpA-3 (5′-GGCTCAACATGATGGTG-3′) and CabpAR-3 (5′-CAAGTAACGAGCGTTAGC-3′), were designed. These primers were used to screen selected genomic preparations of strains of oral streptococci to determine whether the abpA gene was present or absent for each species. A standard “touchdown” thermocycler program was used for all screening PCRs. Following an initial denaturation at 94°C for 5 min, four cycles were performed as follows: 1 min at 94°C, 1 min at 64°C, and 1.5 min at 72°C. Four cycle units were repeated in this manner with the annealing temperature lowered by 2°C for each unit, with a final annealing temperature of 50°C. This was followed by 10 min of extension at 72°C.
A separate program was used for PCR-mediated cloning reactions. After initial denaturation for 3 min at 94°C, 30 cycles were performed as follows: 1 min at 94°C, 2 min at 55°C, and 3 min at 72°C. This was followed by 10 min of extension at 72°C.
Ligation and transformation of abpA into pGEM-T and E. coli DH5α.
PCR-amplified abpA was ligated into pGEM-T (Promega) and then transformed into E. coli DH5α according to the manufacturer's instructions. The cells were plated onto LB agar containing 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (40 μg/ml; Sigma), isopropyl-β-d-thiogalactopyranoside (22 μg/ml; Sigma), and ampicillin (100 μg/ml; Sigma), and the plates were incubated at 37°C overnight. The plates were transferred to 4°C for 2 to 3 h, and positive clones identified by selection of blue and white colonies. The presence of abpA was confirmed by PCR with the XabpA-2 and CabpAR-2 primers and the thermocycler program described above. Plasmids were isolated for sequencing as described previously (24).
DNA sequencing.
Plasmid preparations were sequenced by the Nucleic Acid Sequencing Facility at the State University of New York at Buffalo. T7 and M13r sequencing primers complementary to the pGEM-T vector were provided by the facility.
DNA sequence analysis.
Sequence analysis was performed on a Macintosh personal computer with DNAsis software (Hitachi Software Engineering, San Bruno, Calif.). Basic alignment parameters and homology analysis were applied to the sequences. Comparison of the sequences with GenBank sequences was performed with the BLAST search engine.
Amylase binding assays.
The binding of amylase to all strains of streptococci included in this study was confirmed by previously described methods (6, 25). The sizes of the amylase-binding proteins (ABPs) produced by streptococcal strains were determined by SDS-polyacrylamide gel electrophoresis and Western blotting as described previously (9, 19).
RESULTS
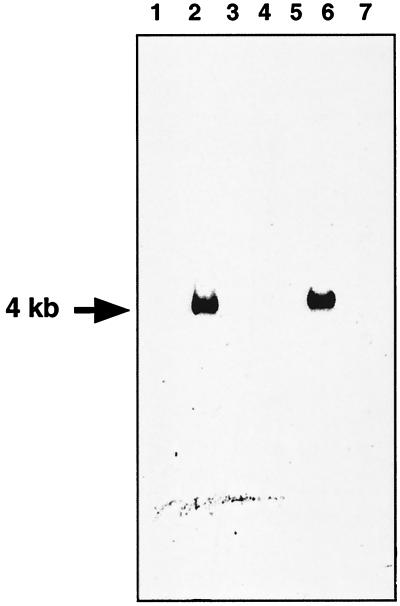
Laboratory strains of oral streptococci, assigned to various species by using the most recent knowledge of their taxonomic status, were tested for their ability to bind salivary amylase. Consistent with previous results, strains assigned to the species S. gordonii, S. mitis, S. crista, and S. parasanguinis bound amylase, while strains assigned to S. sanguis, S. mutans, and S. oralis did not bind amylase. DNA extracted from these strains was subjected to Southern blotting and PCR experiments to search for the abpA gene. Initial Southern blotting experiments in which DNA from representative strains from eight species of oral streptococci was cut with HindIII and probed with the biotinylated abpA gene revealed a 4.0-kb restriction fragment from all strains considered to be S. gordonii (Fig. 1). The probe, even when incubated with DNA preparations under low-stringency conditions, did not appear to bind to any restriction fragments from other species of oral streptococci including the amylase-binding species S. mitis, S. crista, and S. parasanguinis. Indeed, Southern blotting results were identical under both high- and low-stringency conditions. The only exception was found with S. mitis 10712, whose genomic DNA hybridized with the abpA probe to yield a 4.0-kb restriction fragment.
FIG. 1.
Example of Southern blot hybridization of HindIII-restricted streptococcal genomic DNA probed with abpA. Lane 1, S. mitis OP51; lane 2, S. mitis NCTC 10712; lane 3, S. crista CR3; lane 4, S. crista CR311; lane 5, S. sanguinis 10556; lane 6, S. gordonii FAS 4; lane 7, S. mutans 10449.
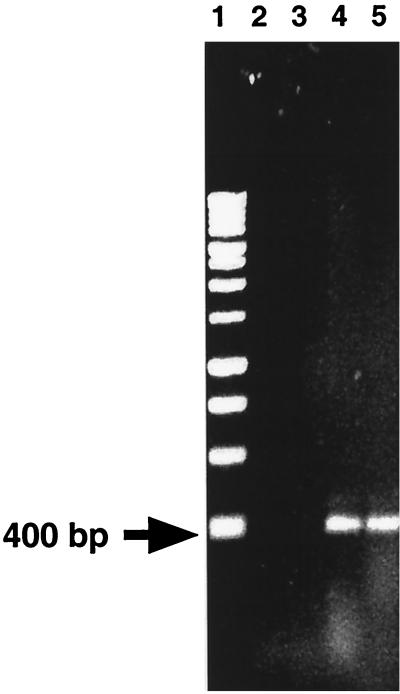
To confirm these results, genomic DNA preparations from the streptococcal strains were subjected to PCR with primers XabpA-2 and CabpAR-2 (Fig. 2). Of all of the strains examined, only strains classified as S. gordonii yielded an approximately 400-bp product, which conformed to the predicted size of the product (Fig. 2). Again, the only exception noted was S. mitis 10712, from which a 400-bp PCR product was obtained. A summary of the results obtained is provided in Table 1.
FIG. 2.
Example of products obtained by PCR with primers XabpA-2 and CabpAR-2. Lane 1, molecular size standards; lane 2, S. mutans 10449; lane 3, S. mitis OP51; lane 4, S. gordonii Challis; lane 5, S. gordonii NCTC 7865.
TABLE 1.
Characterization of streptococcal strains
| Species or strain | Source of strains | Amylase binding | Esculin hydrolysis | Arginine hydrolysis | Size(s) (kDa) of ABP(s)a | Size (kb) of hybridization band with abpA probe | Size (bp) of abpA PCR product |
|---|---|---|---|---|---|---|---|
| S. gordonii | |||||||
| Challis | Our laboratory | + | + | + | 82, 20 | 4 | 400 |
| Blackburn | Our laboratory | + | + | + | 82, 20 | 4 | 400 |
| SPED3 | A. Coykendall | + | + | + | 82, 20 | 4 | 400 |
| LGR2 | A. Coykendall | + | + | + | 82, 20 | 4 | 400 |
| M5 | A. Coykendall | + | + | + | 82, 20 | 4 | 400 |
| GEO2 | A. Coykendall | + | + | + | 82, 20 | 4 | 400 |
| 7865 | A. Coykendall | + | + | + | 82, 20 | 4 | 400 |
| CN2814 | A. Coykendall | + | + | + | 82, 20 | 4 | 400 |
| JF2 | A. Coykendall | + | + | + | 82, 20 | 4 | 400 |
| MJ2 | A. Coykendall | + | + | + | 82, 20 | 4 | 400 |
| G9B | Our laboratory | + | + | + | 82, 20 | 4 | 400 |
| NCTC 7865 | Our laboratory | + | + | + | 82, 20 | 4 | 400 |
| FAS4 | Our laboratory | + | + | + | 82, 20 | 4 | 400 |
| S. mitis | |||||||
| 10712 | Our laboratory | + | + | + | 87, 20 | 4 | 400 |
| UC 5873 | A. Coykendall | + | − | − | 30 | ||
| NS 51 | A. Coykendall | + | − | − | 36 | ||
| UC 921A | A. Coykendall | + | − | − | ND | ||
| NS 51 | A. Coykendall | + | − | − | ND | ||
| OP51 | A. Coykendall | + | − | − | 31, 26 | ||
| UC 2948 | A. Coykendall | + | − | − | ND | ||
| UC 6950A | A. Coykendall | + | − | − | ND | ||
| OT 25 | M. Kilian | + | − | − | ND | ||
| SK 92 | M. Kilian | + | − | − | ND | ||
| SK 137 | M. Kilian | + | − | − | ND | ||
| SK 145 | M. Kilian | + | − | − | ND | ||
| SK 141 | M. Kilian | + | − | − | ND | ||
| Col 85/1862 | A. Coykendall | + | − | − | ND | ||
| S. crista | |||||||
| CC5A | A. Coykendall | + | − | − | 82, 30 | − | − |
| CR311 | A. Coykendall | + | − | + | 82, 30 | − | − |
| CR3 | A. Coykendall | + | − | − | 82, 30 | − | − |
| S. anginosus 10708 | A. Coykendall | + | − | + | ND | − | − |
| S. parasanguinis MGH413 | A. Coykendall | + | − | + | 87, 21 | − | − |
| S. mutans 10449 | A. Coykendall | − | + | − | − | − | − |
| S. sanguinis | |||||||
| 804 | A. Coykendall | − | − | + | − | − | − |
| MPC1 | A. Coykendall | − | + | + | − | − | − |
| 10556 | Our laboratory | − | + | + | − | − | − |
| S. oralis | |||||||
| CR834 | A. Coykendall | − | − | − | − | − | − |
| BU174 | A. Coykendall | − | − | − | − | − | − |
| 16532AR | A. Coykendall | − | + | − | − | − | − |
| 9811 | A. Coykendall | − | − | − | − | − | − |
Determined in our laboratory as described in Materials and Methods and as reported by Gwynn and Douglas (9). ND, not determined.
The putative abpA homologs from S. gordonii NCTC 7865, FAS4, G9B, and 10712 were cloned into E. coli and sequenced. All cloned PCR fragments were determined to encode a protein of 20 kDa. The extent of homology between the cloned PCR fragments and abpA from S. gordonii Challis was found to range from 91 to 96%.
DISCUSSION
The present study was performed to determine if homologs of abpA exist in other species or strains of the viridans group streptococci and to determine the genetic similarity between identified abpA homologs. To accomplish these goals, HindIII-digested genomic DNAs from multiple strains of known amylase-binding and non-amylase-binding reference strains were first probed by southern blotting with biotinylated abpA from S. gordonii Challis. Next, strains were screened by PCR with primers homologous to abpA from S. gordonii Challis. Several putative abpA genes were then cloned and sequenced, and the extent of their homology with abpA from S. gordonii Challis was determined. Primers based on the region of greatest homology between abpA homologs were used in a PCR to test the ability of an abpA-based assay to identify members of the viridans group streptococci. Finally, the sizes of the ABPs of the streptococcal strains were determined and were correlated with the presence of abpA.
The results of the present study suggest that abpA is essentially unique to S. gordonii. It is interesting that S. gordonii is the only species that consistently produces a 20-kDa ABP, the size of the protein encoded by abpA. The only exception to this finding was seen with S. mitis 10712, whose genomic DNA hybridized with the abpA probe and from which a 400-bp PCR product was obtained. It is, however, also curious that this strain of S. mitis was the only strain of this species to hydrolyze esculin and arginine and to produce an ABP of 20 kDa. This finding may be explained by the possibility that the abpA gene was transferred to S. mitis via a horizontal gene transfer mechanism. Alternatively, it is also possible that our copy of this strain is not authentic and/or is contaminated with S. gordonii, thus yielding the positive test for the abpA gene.
While it was somewhat surprising that the abpA probe did not hybridize with fragments from other amylase-binding species, including most S. mitis and all S. crista strains, such a finding is not unprecedented. For example, although most oral streptococci produce related glucosyltransferases (gtf) that share at least some sequence homology, DNA probes based on gtf from S. mutans do not hybridize with gtf from other species of mutans group streptococci (29).
The present results also suggest that ABPs of other species of streptococci are encoded by distinct ABP genes. Previous studies demonstrated that S. gordonii was the most homogeneous species, since all of the strains tested produced proteins migrating with molecular masses of 82 and 20 kDa (9). Other species are more heterogeneous, producing ABPs between 82 and 87 kDa and/or between 20 and 36 kDa. Binding of amylase to the 82- to 87-kDa proteins on ligand blots is prevented by amylase inhibitors, amylase substrates, and periodate treatment, but these treatments have no effect on amylase binding to 20- to 36-kDa proteins (9). These results suggest the presence of two classes of ABPs, those of 20 to 36 kDa and those of 82 to 87 kDa. Within the 20- to 36-kDa class of ABPs, the results of the present study suggest that abpA appears to be unique to S. gordonii (with the exception of S. mitis 10712).
Progress in the development of molecular biological approaches to classification of the oral streptococci has been made over the past several years. For example, DNA fingerprint analysis compared the genotypes of 21 reference strains of oral streptococci representing S. gordonii, S. sanguinis, S. oralis, S. parasanguinis, and S. crista (23). Fingerprint patterns for most strains examined were found to be unique, suggesting that the diversity of strains within these streptococcal species was too great to permit species identification by DNA fingerprint patterns. Further studies were performed by using restriction fragment polymorphisms of rRNA genes, in which DNA fragments obtained following restriction enzyme digestions were hybridized with a cDNA probe obtained by reverse transcription of E. coli 16S and 23S rRNAs. S. oralis, S. mitis, and S. parasanguis showed bands that were absent from S. gordonii, S. sanguinis, and S. crista, while the last three groups showed species-specific bands, and S. oralis could be distinguished from S. mitis and S. parasanguinis. S. mitis and S. parasanguinis could not be distinguished, since they shared multiple bands (20). Ribotyping allowed identification of 48 of 53 unknown isolates to the species level (21), suggesting that this technique can be used for genotypic identification of S. sanguinis, S. oralis, and S. gordonii isolates. This analysis, however, can be somewhat complex and requires the use of band-matching software to obtain reproducible results.
More promising results have been obtained by AP-PCR, which allows identification of unknown oral isolates to the species level (22). PCR was also used to amplify an internal fragment of the gene encoding the streptococcal manganese-dependent superoxide dismutase from the type strains of 29 species of streptococci (17). Following cloning of the amplimers, sequencing and sequence analysis allowed accurate identification of the strains to the species level. These techniques, which require the use of sophisticated computer analysis, may limit the utility of these approaches for rapid identification of species. PCR for the identification of bacteria to the species level may best be used if specific primers that amplify a single gene sequence unique to each species can be designed. Such a test would be relatively easy to perform and would yield unambiguous results. The amplification of specific gene targets (such as abpA) that yield products specific to each species that can be easily discerned by gel electrophoresis would obviate the need for gene sequencing and complex band-matching software analysis. The abpA gene of S. gordonii may thus be a useful target gene in this regard.
In summary, the present data suggest that the abpA gene is unique to S. gordonii. Other ABS appear to have ABPs encoded by genes distinct from abpA. These data also suggest that once the sequences for all ABP genes are identified, it may be possible to use these genes as the basis for PCR-based assays for identification of the ABS to the species level.
ACKNOWLEDGMENTS
This study was supported in part by grants DE 09838 (to F.A.S.) and Dentist Scientist Award DE 00158 (to J.D.R.) from the National Institute of Dental Research and by a Howard Hughes Summer Scholars Fellowship (to A.E.B.) from Bates College, Lewiston, Maine.
REFERENCES
- 1.Coykendall A. Classification and identification of the viridans streptococci. Clin Microbiol Rev. 1989;2:315–328. doi: 10.1128/cmr.2.3.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coykendall A L, Gustafson K B. Deoxyribonucleic acid hybridizations among strains of Streptococcus salivarius and Streptococcus bovis. Int J Syst Bacteriol. 1985;35:274–280. [Google Scholar]
- 3.Coykendall A L, Specht P A. DNA base sequence homologies among strains of Streptococcus sanguis. J Gen Microbiol. 1975;91:92–98. doi: 10.1099/00221287-91-1-92. [DOI] [PubMed] [Google Scholar]
- 4.Coykendall A L, Wesbecher P M, Gustafson K B. “Streptococcus milleri,” Streptococcus constellatus, and Streptococcus intermedius are later synonyms of Streptococcus anginosus. Int J Syst Bacteriol. 1987;37:222–228. [Google Scholar]
- 5.Douglas C W I. The binding of human salivary α-amylase by oral strains of streptococcal bacteria. Arch Oral Biol. 1983;28:567–573. doi: 10.1016/0003-9969(83)90003-1. [DOI] [PubMed] [Google Scholar]
- 6.Douglas C W I. Characterization of the α-amylase receptor of Streptococcus gordonii NCTC 7868. J Dent Res. 1990;69:1746–1752. doi: 10.1177/00220345900690110701. [DOI] [PubMed] [Google Scholar]
- 7.Douglas C W I, Pease A A, Whiley R A. Amylase-binding as a discriminator among oral streptococci. FEMS Microbiol Lett. 1990;66:193–198. doi: 10.1016/0378-1097(90)90281-t. [DOI] [PubMed] [Google Scholar]
- 8.Garnier F, Gerbaud G, Courvalin P, Galimand M. Identification of clinically relevant viridans group streptococci to the species level by PCR. J Clin Microbiol. 1997;35:2337–2341. doi: 10.1128/jcm.35.9.2337-2341.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gwynn J P, Douglas C W I. Comparison of amylase-binding proteins in oral streptococci. FEMS Microbiol Lett. 1994;124:373–380. doi: 10.1111/j.1574-6968.1994.tb07311.x. [DOI] [PubMed] [Google Scholar]
- 10.Igarashi T, Yamamoto A, Goto N. Direct detection of Streptococcus mutans in human dental plaque by polymerase chain reaction. Oral Microbiol Immunol. 1996;11:294–298. doi: 10.1111/j.1399-302x.1996.tb00184.x. [DOI] [PubMed] [Google Scholar]
- 11.Kawamura Y, Hou X, Sultana F, Miura H, Ezaki T. Determination of the 16S rRNA sequences of Streptococcus mitis and Streptococcus gordonii and phylogenetic relationships among members of the genus Streptococcus. Int J Syst Bacteriol. 1995;45:406–408. doi: 10.1099/00207713-45-2-406. [DOI] [PubMed] [Google Scholar]
- 12.Kilian M, Mikkelsen L, Henrichsen J. Taxonomic study of viridans streptococci: description of Streptococcus gordonii sp. nov. and emended descriptions of Streptococcus sanguis (White and Niven 1946), Streptococcus oralis (Bridge and Sneath 1982), and Streptococcus mitis (Andrewes and Horder 1906) Int J System Bacteriol. 1989;39:471–484. [Google Scholar]
- 13.Kilian M, Nyvad B. Ability to bind salivary α-amylase discriminates certain viridans group streptococcal species. J Clin Microbiol. 1990;28:2576–2577. doi: 10.1128/jcm.28.11.2576-2577.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kilpper-Balz R, Wenzig P, Schleifer K H. Molecular relationships and classification of some viridans streptococci as Streptococcus oralis and emended description of Streptococcus oralis (Bridge and Sneath 1982) Int J Syst Bacteriol. 1985;35:482–488. [Google Scholar]
- 15.Kiyama M, Hiratsuk K, Saito S, Shiroza T, Takiguchi H, Abiko Y. Detection of Actinomyces species using nonradioactive riboprobes coupled with polymerase chain reaction. Biochem Mol Med. 1996;58:151–155. doi: 10.1006/bmme.1996.0043. [DOI] [PubMed] [Google Scholar]
- 16.Li Y, Caufield P W. Arbitrarily primed polymerase chain reaction fingerprinting for the genotypic identification of mutans streptococci from humans. Oral Microbiol Immunol. 1998;13:17–22. doi: 10.1111/j.1399-302x.1998.tb00745.x. [DOI] [PubMed] [Google Scholar]
- 17.Poyart C, Quesne G, Coulon S, Berche P, Trieu-Cuot P. Identification of streptococci to species level by sequencing the gene encoding the manganese-dependent superoxide dismutase. J Clin Microbiol. 1998;36:41–47. doi: 10.1128/jcm.36.1.41-47.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Riggio M P, Macfarlane T W, Mackenzie D, Lennon A, Smith A J, Kinane D. Comparison of polymerase chain reaction and culture methods for detection of Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in subgingival plaque samples. J Periodontal Res. 1996;31:496–501. doi: 10.1111/j.1600-0765.1996.tb01415.x. [DOI] [PubMed] [Google Scholar]
- 19.Rogers J D, Haase E M, Brown A E, Douglas C W I, Gwynn J P, Scannapieco F A. Identification and analysis of a gene (abpA) encoding a major amylase-binding protein in Streptococcus gordonii. Microbiology. 1998;144:1223–1233. doi: 10.1099/00221287-144-5-1223. [DOI] [PubMed] [Google Scholar]
- 20.Rudney J D, Larson C J. Species identification of oral viridans streptococci by restriction fragment polymorphism analysis of rRNA genes. J Clin Microbiol. 1993;31:2467–2473. doi: 10.1128/jcm.31.9.2467-2473.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rudney J D, Larson C J. Use of restriction fragment polymorphism analysis of rRNA genes to assign species to unknown clinical isolates of oral viridans streptococci. J Clin Microbiol. 1994;32:437–443. doi: 10.1128/jcm.32.2.437-443.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rudney J D, Larson C J. Identification of oral mitis group streptococci by arbitrarily primed polymerase chain reaction. Oral Microbiol Immunol. 1999;14:33–42. doi: 10.1034/j.1399-302x.1999.140104.x. [DOI] [PubMed] [Google Scholar]
- 23.Rudney J D, Neuvar E K, Soberay A H. Restriction endonuclease-fragment polymorphisms of oral viridans streptococci, compared by conventional and field-inversion gel electrophoresis. J Dent Res. 1992;71:1182–1188. doi: 10.1177/00220345920710051001. [DOI] [PubMed] [Google Scholar]
- 24.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 25.Scannapieco F A, Bergey E J, Reddy M S, Levine M J. Characterization of salivary α-amylase binding to Streptococcus sanguis. Infect Immun. 1989;57:2853–2863. doi: 10.1128/iai.57.9.2853-2863.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scannapieco F A, Solomon L, Wadenya R O. Emergence in human dental plaque and host distribution of amylase-binding streptococci. J Dent Res. 1994;73:1627–1635. doi: 10.1177/00220345940730100701. [DOI] [PubMed] [Google Scholar]
- 27.Scannapieco F A, Torres G, Levine M J. Salivary α-amylase: role in dental plaque and caries formation. Crit Rev Oral Biol Med. 1993;4:301–307. doi: 10.1177/10454411930040030701. [DOI] [PubMed] [Google Scholar]
- 28.Scannapieco F A, Torres G I, Levine M J. Salivary amylase promotes adhesion of oral streptococci to hydroxyapatite. J Dent Res. 1995;74:1360–1366. doi: 10.1177/00220345950740070701. [DOI] [PubMed] [Google Scholar]
- 29.Smorawinska M, Kuramitsu H K. DNA probes for detection of cariogenic Streptococcus mutans. Oral Microbiol Immunol. 1992;7:177–181. doi: 10.1111/j.1399-302x.1992.tb00532.x. [DOI] [PubMed] [Google Scholar]
- 30.Tseng C C, Scannapieco F A, Levine M J. Use of a replica-plate assay for the rapid assessment of salivary protein-bacterial interactions. Oral Microbiol Immunol. 1992;7:53–56. doi: 10.1111/j.1399-302x.1992.tb00021.x. [DOI] [PubMed] [Google Scholar]
- 31.Whiley R A, Beighton D. Current classification of the oral streptococci. Oral Microbiol Immunol. 1998;13:195–216. doi: 10.1111/j.1399-302x.1998.tb00698.x. [DOI] [PubMed] [Google Scholar]