Abstract
Citrus species of plants are among the most commercially cultivated crops, mainly for their fruit. Besides, the generally consumed flesh inside the fruit, the peel is quite important too. Essential oils extracted from the peel have a history of being used by humankind for centuries. These essential oils are rich in antioxidants and antimicrobial agents. Comparative investigation of volatile constituents, and antioxidant and antimicrobial activities were undertaken. The essential oils were evaluated through gas chromatography-mass spectrometry (GC–MS), and enantiomeric composition by chiral GC–MS. Similarly, the antioxidant properties were evaluated by 2,2-diphenyl-1-picrylhydrazyl scavenging assay, and antimicrobial activities were assayed using the disk diffusion method. The highest extraction yield of 1.83% was observed in Citrus sinensis Osbeck. GC–MS analysis showed limonene (63.76–89.15%), γ-terpinene (0.24–6.43%), β-pinene (0.15–6.09%), linalool (0.35–3.5%), sabinene (0.77–2.17%), myrcene (0.74–1.75%), α-terpineol (0.28–1.15%), and α-pinene (0.2–0.58%) as the major constituents of the essential oil of the Citrus species studied. For the first time, through our study, chiral terpenoids have been observed from Citrus grandis Osbeck essential oil. The order of antioxidant activity is as follows: Citrus grandis Osbeck red flesh > Citrus reticulata Blanco > Citrus sinensis Osbeck > Citrus grandis Osbeck white flesh. Except for Citrus grandis Osbeck white flesh (52.34 µL/mL), all samples demonstrated stronger antioxidant activities than those of the positive control, quercetin (5.60 µL/mL). Therefore, these essential oils can be used as a safe natural antioxidant to prevent product oxidation. Likewise, citrus peel essential oil showed antimicrobial activity against tested bacterial strains, albeit marginal.
Keywords: limonene, antioxidant, enantiomeric distribution, antibacterial activity
1. Introduction
Citrus species belong to the family Rutaceae and are among the most commercially significant crops cultivated in tropical and subtropical climate regions [1]. The peel of citrus fruit is a valuable raw material for the production of essential oils. Such oils have a history dating to very ancient times of being used in human society. At present, these oils find use in the perfume, food, and beverage industries. Likewise, there are cases of the use of such oils in folk and traditional medicines as well [2]. The extraction of these oils can be done through techniques such as hydrodistillation, cold pressing, and solvent extraction [3]. In addition, other techniques such as supercritical fluid extraction (SFE), pressurized liquid extraction (PLE), and microwave-assisted extraction (MAE) are also prevalent [4]. A high proportion (~93%) of citrus peel essential oil is commercially extracted by traditional methods such as steam distillation [5]. Citrus essential oils (CEOs) are particularly fascinating among essential oils since they can be used as antioxidants because of their ability to protect organisms and tissues from damage inflicted by reactive oxygen species, and also as flavoring agents [6,7]. These essential oils can be used as an alternative to synthetic preservatives since they are observed to display their antimicrobial and antioxidant activities with a broad spectrum of biological activities [8,9]. Further adding to their benefits, these essential oils are non-toxic, which is the reason explorations and studies on the antioxidant potential of essential oil are raising. However, it has been found that the emission of volatile components such as limonene, α/β-pinene, and camphene from citrus orchards contribute to ozone formation [10] in the troposphere and the deposition of ozone in the environment, contributing to the greenhouse effect [11].
CEOs are a complex mixture of volatile compounds belonging to terpenes and oxygenated terpenes [12]. Patterns of chemical composition in Citrus species differ with the origin, genotype, environmental factors, and method of extraction [13]. Moreover, volatile oil from Citrus species such as C. reticulata, C. sinensis, C. paradisi, C. grandis, C. limon, and C. medica mostly contain volatile chemicals such as limonene, α/β-pinene, sabinene, β-myrcene, limonene, linalool, α-humulene, and α-terpineol, which are responsible for antioxidant, anti-inflammatory, antifungal, antimicrobial, and wound-healing activities [1,14].
Multidrug-resistant pathogens are distributed globally and have directed the necessity of new antimicrobial agents, but the production has been delayed recently [15,16]. Essential oil, particularly from Citrus species, could be a possible candidate against such pathogens, particularly owing to their promising activity against pathogenic bacteria such as Listeria spp. [17], Salmonella spp. [18], Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa, and Bacillus subtilis [19]. This is why CEOs can be linked to their high antimicrobial efficacy against various pathogens [20,21].
The analytical method used for the investigation of volatile chemistry in citruses is GC–MS, which helps in discrete complex mixtures of secondary metabolites produced by aromatic plants [14]. Along with GC–MS, an advanced technique of chiral GC–MS is used to obtain the chirality of the secondary metabolites. This is crucial for verifying the authenticity of the molecules and similarly aids in the identification of contaminants.
Despite the substantial data on peel oils of citrus from different geological locations of the world, there is a lack of information on the chemical composition and biological properties of Nepalese citrus. Therefore, this study sought to profile volatile compounds present in the epicarp of mandarin (Citrus reticulata Blanco), sweet orange (Citrus sinensis Osbeck), and pummelo (Citrus grandis Osbeck) from different geographical regions of Nepal, and access their antioxidant and antimicrobial activity alongside the enantiomeric distribution of chiral terpenoids, which may provide useful information regarding their authentication and associated impurities.
2. Results and Discussion
2.1. Isolation of Essential Oil and Yields
The extraction yield of essential oils from Citrus species varied from 0.5 to 1.83% in the study (Table 1), which depends on different factors such as regulation of biosynthetic genes, climatic variation, and expression of metabolites. Bourgou et al. reported 0.46 to 2.70% yield from Citrus species such as mandarin (2.7%) and orange (0.74%) [20]. Citrus peel grinding degree, hydrodistillation time, and added salts had an impact on Citrus reticulata Blanco peel reported from China [22]. Moreover, Tran et al. using the hydrodistillation to extract the essential oil from Vietnamese powdered mandarin (Citrus reticulata Blanco) at a temperature of 110–120 °C, reported the greatest yield of 5%, with a peel-to-solvent ratio of 1:4 (g/mL), and an extraction duration of 150 min indicating the size of the peel, the water-to-peel ratio, the temperature extraction, and the time extraction affecting extraction by hydrodistillation [23].
Table 1.
Extraction yield of essential oil in citrus peel.
| Plant | Extraction Method | GPS Coordinates | Extraction Yield (v/w%) |
|---|---|---|---|
| Mandarin Citrus reticulata Blanco (C1) |
Hydrodistillation | 27°16′59.0″ N, 85°58′46.5″ E | 0.5 |
| Sweet Orange Citrus sinensis Osbeck (C2) |
Hydrodistillation | 27°16′59.0″ N, 85°58′46.5″ E | 1.83 |
| Pummelo Citrus grandis Osbeck red flesh (C3) |
Hydrodistillation | 27°38′14.5″ N, 85°22′04.7″ E | 1.0 |
| Pummelo Citrus grandis Osbeck white flesh (C4) |
Hydrodistillation | 27°38′14.2″ N, 85°22′04.6″ E | 1.5 |
Note: Citrus grandis Osbeck (red flesh) and Citrus grandis Osbeck (white flesh) are cultivars of Citrus grandis Osbeck.
2.2. Chemical Composition of CEOs
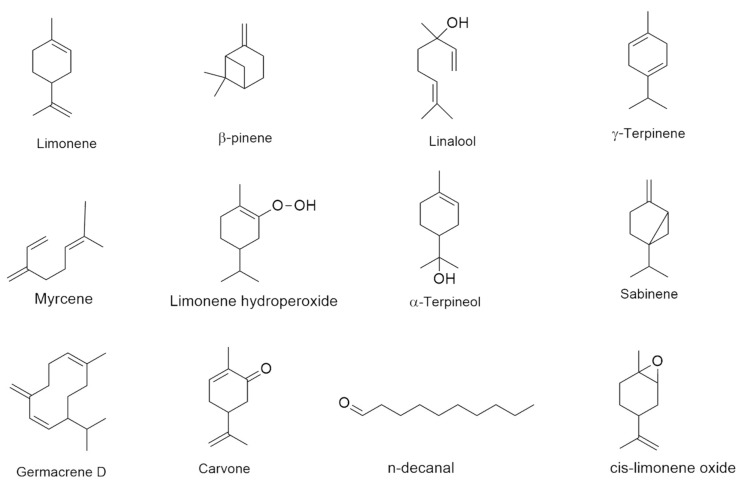
The volatile constituents in CEOs were identified by GC–MS. The identified compounds are presented in Table 2 with their respective percentages. The result revealed that 50 components were present in sample Citrus reticulata Blanco (C1); among the major components were limonene (83.67%), γ-terpinene (6.09%), and linalool (2.65%). A previous study reported 53 components and the major compound was limonene. Thus, our finding is similar to a previous study on the essential oil of Citrus reticulata [22]. A total of 47 components were identified in Citrus sinensis Osbeck (C2) accounting for the total percentage of essential oil. Limonene (86.59%) and linalool (3.50%) were the main components. Previous studies showed that limonene was the main constituent of Citrus sinensis Osbeck essential oil [24]. Analysis by GC–MS identified, in the essential oil of Citrus grandis Osbeck red flesh (C3), 55 components, and limonene (63.76%), β-pinene (6.09%), and limonene hydroperoxide 1 (2.66%) were the main components. Analysis of Citrus grandis Osbeck white flesh (C4) essential oil relieved 53 components where limonene (89.15%) and β-pinene (2.51%) were the most abundant. This is comparable to the results of earlier research on Citrus grandis Osbeck, in which a total of 33 compounds, with the major component limonene (87.5%), were identified [25]. From a previous report, a similar range of limonene was observed [26]. Extraction methods used can influence the number of volatile components present in the CEOs [27]. In this study, most secondary metabolites belong to the monoterpene class followed by oxygenated monoterpene. Major compounds in the Citrus species are shown in Figure 1.
Table 2.
Essential oil compositions of citrus essential oil.
| Retention Index | Compounds | Mandarin Citrus reticulata Blanco (C1) % |
Sweet Orange Citrus sinensis Osbeck (C2) % |
Pummelo Citrus grandis Osbeck Red Flesh (C3) % |
Pummelo Citrus grandis Osbeck White Flesh (C4) % |
|---|---|---|---|---|---|
| 924 | α-Thujene | 0.1 | 0.04 | 0.02 | 0.04 |
| 931 | α-Pinene | 0.57 | 0.2 | 0.58 | 0.42 |
| 948 | Camphene | 0.04 | 0.03 | 0.06 | 0.05 |
| 972 | Sabinene | 1.3 | 0.77 | 2.17 | 2.17 |
| 978 | β-Pinene | 0.58 | 0.15 | 6.09 | 2.51 |
| 983 | 6-Methyl-5-hepten-2-one | - | - | 0.15 | - |
| 989 | Myrcene | 1.59 | 1.18 | 0.74 | 1.75 |
| 1002 | n-Octanal | 0.19 | 0.28 | 0.06 | - |
| 1003 | Para-mentha-1(7),8-diene | - | - | - | 0.01 |
| 1008 | α-Phellandrene | 0.05 | 0.04 | - | 0.03 |
| 1008 | δ-3-Carane | 0.03 | 0.04 | - | 0.03 |
| 1016 | α-Terpinene | 0.13 | 0.04 | - | 0.11 |
| 1024 | p-Cymene | 0.24 | 0.03 | 0.32 | 0.03 |
| 1028 | Limonene | 83.67 | 86.59 | 63.76 | 89.15 |
| 1029 | β-phellandrene | - | 0.35 | - | - |
| 1031 | 1,8-Cineole | - | 0.07 | 0.54 | - |
| 1034 | Cis-β-ocimene | 0.04 | - | - | 0.04 |
| 1044 | Trans-β-ocimene | 0.24 | 0.13 | - | 0.43 |
| 1058 | γ-Terpinene | 6.43 | 0.64 | - | 0.24 |
| 1062 | 3-Methyl decane | - | - | - | 0.01 |
| 1067 | Cis-linalool oxide | - | - | 0.53 | 0.08 |
| 1069 | n-Octanol | 0.1 | 0.19 | - | - |
| 1073 | Pinol | - | - | 0.02 | - |
| 1086 | Terpinolene | 0.34 | 0.08 | - | 0.06 |
| 1086 | Trans-linalool oxide | - | 0.04 | 0.37 | 0.04 |
| 1091 | p-Cymenene | 0.01 | - | - | 0.01 |
| 1098 | Perillene | - | - | 0.07 | - |
| 1099 | Linalool | 2.65 | 3.5 | 1.87 | 0.35 |
| 1105 | n-Nonanal | 0.08 | 0.14 | - | - |
| 1112 | 2-Methyl-6-methylen-octa-1,7-dien-3-one | 0.02 | 0.05 | - | - |
| 1122 | Trans-p-Mentha-2,8-dien-1-ol | 0.01 | - | 0.34 | 0.02 |
| 1124 | Cyclooctanone | - | - | 0.07 | - |
| 1124 | Cis-para-menth-2-en-1-ol | - | - | - | 0.01 |
| 1130 | Limona ketone | - | - | 0.05 | - |
| 1133 | Cis-limonene oxide | 0.01 | - | 2.02 | - |
| 1137 | Trans-limonene oxide | 0.02 | - | 1.31 | - |
| 1137 | Cis-para-mentha-2,8-dien-1-ol | 0.01 | - | - | 0.02 |
| 1138 | Geijerene | 0.03 | - | - | - |
| 1140 | Trans-pinocarveol | - | - | 0.11 | - |
| 1145 | Camphor | 0.01 | - | 0.08 | - |
| 1152 | Citronellal | 0.1 | 0.05 | - | - |
| 1163 | Pinocarvone | - | - | 0.22 | - |
| 1166 | 6-Methyl-bicyclo [3.3.0] oct-2-en-7-one | - | - | 0.12 | - |
| 1175 | Menthol | - | 0.05 | - | - |
| 1178 | Naphthalene | 0.01 | - | - | 0.01 |
| 1179 | Para-1,8-menthadien-4-ol | 0.01 | - | 0.12 | - |
| 1180 | Terpinen-4-ol | 0.2 | 0.51 | 0.33 | 0.13 |
| 1184 | Para-Methyl acetophenone | - | - | 0.02 | - |
| 1186 | Cryptone | - | - | 0.17 | - |
| 1191 | Methyl salicylate | 0.01 | 0.14 | 0.76 | - |
| 1195 | α-Terpineol | 0.29 | 0.92 | 1.15 | 0.28 |
| 1197 | γ-Terpineol | - | - | 0.29 | - |
| 1197 | Cis-Piperitol | - | - | 0.1 | 0.03 |
| 1207 | n-Decanal | 0.4 | 1.14 | - | - |
| 1209 | Octyl acetate | - | 0.09 | - | - |
| 1218 | Trans-carveol | 0.01 | 0.07 | 0.86 | 0.01 |
| 1223 | Nerol | 0.02 | 0.03 | - | 0.01 |
| 1226 | Citronellol | 0.06 | - | - | - |
| 1229 | Thymol methyl ether | 0.11 | - | - | - |
| 1232 | Cis-Carveol | - | - | - | 0.03 |
| 1238 | Neral | 0.01 | 0.18 | 0.08 | - |
| 1242 | Carvone | 0.01 | 0.02 | 1.3 | - |
| 1250 | Geraniol | - | - | - | 0.04 |
| 1253 | Piperitone | - | 0.06 | 0.31 | - |
| 1258 | Methyl-dodecane | - | - | - | 0.01 |
| 1266 | n-Decanol | 0.01 | - | - | - |
| 1268 | Geranial | - | 0.32 | 0.13 | - |
| 1270 | Isopiperitenone | - | 0.14 | - | |
| 1278 | Perilla aldehyde | 0.04 | 0.1 | 0.15 | - |
| 1282 | Bornyl acetate | - | - | 0.04 | 0.02 |
| 1285 | Limonene dioxide | - | - | 0.23 | - |
| 1287 | Mentha-dienehydroperoxide | - | - | 2.53 | - |
| 1289 | Thymol | 0.08 | - | - | - |
| 1309 | 4-Vinyl guaiacol | - | 0.08 | - | - |
| 1317 | Limonene hydroperoxide | - | - | 2.39 | - |
| 1329 | Para-mentha-1,8-dien-4-hydroperoxide | - | - | 1.1 | - |
| 1331 | Bicycloelemene | - | - | - | 0.02 |
| 1334 | δ-Elemene | 0.04 | 0.04 | - | 0.07 |
| 1348 | IsoNeryl-acetate | - | - | - | 0.01 |
| 1353 | Terpena-diol | - | - | 0.05 | - |
| 1354 | Limonene hydroperoxide 1 | - | - | 2.66 | - |
| 1357 | Eugenol | - | - | - | 0.01 |
| 1357 | Cis-Carvyl acetate | - | - | - | 0.01 |
| 1359 | Neryl acetate | - | - | - | 0.01 |
| 1373 | Limonene hydroperoxide 2 | - | - | 2.04 | - |
| 1376 | α-Copaene | - | 0.04 | - | 0.01 |
| 1377 | Geranyl acetate | - | 0.03 | - | 0.3 |
| 1391 | β-Elemene | 0.01 | - | - | 0.03 |
| 1405 | Cis-caryophyllene | - | - | 0.09 | - |
| 1409 | Dodecanal | 0.03 | 0.17 | - | - |
| 1418 | β-Caryophyllene | - | 0.07 | 0.16 | 0.06 |
| 1430 | γ-Elemene | 0.02 | - | - | - |
| 1431 | β-Copaene | - | 0.04 | 0.07 | 0.04 |
| 1433 | Perillyl acetate | - | - | - | 0.04 |
| 1484 | Germacrene D | 0.03 | 0.05 | - | 1.05 |
| 1496 | Valencene | - | 0.06 | - | - |
| 1496 | Bicyclogermacrene | - | - | - | 0.08 |
| 1500 | α-Muurolene | - | - | - | 0.01 |
| 1504 | Trans-trans-α-farnesene | - | 1.97 | - | - |
| 1521 | δ-cadinene | - | 0.06 | - | 0.01 |
| 1560 | Germacrene B | 0.03 | - | - | 0.01 |
| 1576 | Spathulenol | - | - | 0.08 | - |
| 1578 | Caryophyllene oxide | - | - | 0.12 | - |
| Monoterpenes | 95.37 | 90.3 | 74.69 | 97.08 | |
| Oxygenated monoterpenes | 3.64 | 5.17 | 23.12 | 1.44 | |
| Sesquiterpene | 0.13 | 2.33 | 0.32 | 1.39 | |
| Oxygenated sesquiterpenes | 0 | 0 | 0.25 | 0.03 | |
| Others | 0.86 | 2.2 | 1.62 | 0.06 |
Figure 1.
Major compounds in Citrus species.
2.3. Enantiomeric Composition of CEOs
In total, 8, 6, 8, and 7 chiral compounds were identified in Citrus reticulata Blanco, Citrus sinensis Osbeck, Citrus grandis Osbeck red flesh, and Citrus grandis Osbeck white flesh (C1, C2, C3, and C4), respectively. Relative percentages of the levorotatory and dextrorotatory compounds in the essential oil from different species of citrus are listed in Table 3. Limonene is a major monoterpene that has (+)-limonene in our study. Limonene, linalool, and germacrene D exist in dextrorotatory form. On the other hand, the levorotatory enantiomer predominated for β-pinene, carvone, and trans-carveol while the enantiomeric distribution varied for α-pinene, sabinene, and α-terpineol depending upon the Citrus species. This study reports for the first time chiral terpenoids from Citrus grandis Osbeck (C3 and C4) essential oil.
Table 3.
Enantiomeric distributions of chiral terpenoid of CEOs.
| Chiral Terpenoids | Citrus Peel Essential Oil along with Enantiomeric Composition | |||||||
|---|---|---|---|---|---|---|---|---|
| Mandarin Citrus reticulata Blanco (C1) |
Sweet Orange Citrus sinensis Osbeck (C2) |
Pummelo Citrus grandis Osbeck (Red Flesh) (C3) |
Pummelo Citrus grandis Osbeck (White Flesh) (C4) |
|||||
| − | + | − | + | − | + | − | + | |
| α-Pinene | 29.08 | 70.92 | 0 | 100 | 53.89 | 46.11 | 27.13 | 72.87 |
| Sabinene | 7.32 | 92.68 | 0 | 100 | 40.5 | 59.5 | 18.04 | 81.96 |
| β-Pinene | 34.33 | 65.67 | - | - | 99.15 | 0.85 | 97.14 | 2.86 |
| Limonene | 0.9 | 99.1 | 0.59 | 99.41 | 0.87 | 99.13 | 0.69 | 99.31 |
| Linalool | 3.19 | 96.81 | 11.19 | 88.81 | 31.69 | 68.31 | 38.94 | 61.06 |
| α-Terpineol | 46.11 | 53.89 | 10.12 | 89.88 | 58.64 | 41.36 | 42.61 | 57.39 |
| Germacrene D | - | - | - | - | - | - | 5.86 | 94.14 |
| Carvone | - | - | - | - | 50.86 | 49.14 | - | - |
| trans-Carveol | - | - | - | - | 59.2 | 40.8 | - | - |
| Terpinen-4-ol | 53.97 | 46.03 | 28.69 | 71.31 | - | - | - | - |
| α-Thujene | 76.86 | 23.17 | - | - | - | - | - | - |
2.4. Antioxidant Activity of CEOs
Literature shows that extracts from the peel of citrus had the highest antioxidant activity compared to essential oil. In the present study, DPPH radical scavenging assay was used for the determination of the antioxidant activity of citrus peel essential oil by comparing it with the activity of quercetin. The secondary metabolites of citrus peel essential oil can donate electrons; it changes the purple color of DPPH to yellow (diphenyl-picrylhydrazine) for quantification of antioxidant activity. The IC50 value is a commonly used criterion for determining the antioxidant activity of test samples. It is estimated as the antioxidant concentration is needed to minimize the initial DPPH concentration by 50% [28]. The order of antioxidant activity follows as Citrus grandis Osbeck red flesh, Citrus reticulata Blanco, Citrus sinensis Osbeck, and Citrus grandis Osbeck white flesh (C3 > C1 > C2 > C4), depicting Citrus grandis Osbeck red flesh as the highest antioxidant activity. The lowest IC50 value represents the highest scavenging activity of the essential oil as shown in Table 4. However, the Citrus grandis Osbeck white flesh (C4) sample demonstrated weaker antioxidant activities than that of positive control quercetin (5.60 ± 0.42 µL/mL).
Table 4.
Antioxidant activity of Citrus essential oil.
| Citrus Essential Oil | IC50 (µL/mL) |
|---|---|
| Mandarin Citrus reticulata Blanco (C1) |
2.30 ± 0.37 |
| Sweet Orange Citrus sinensis Osbeck (C2) |
3.33 ± 0.66 |
| Pummelo Citrus grandis Osbeck red flesh (C3) |
1.56 ± 0.08 |
| Pummelo Citrus grandis Osbeck white flesh (C4) |
52.34 ± 0.62 |
| Quercetin (Positive control) | 5.60 ± 0.42 |
The major component of essential oil, limonene and α-pinene, has shown bioactive properties such as antioxidant, antimicrobial, and antiulcer activities [29]. Limonene, due to the donation of hydrogen atoms, was converted into a stable form from free radical and the potential of antioxidant effect was determined which was directly proportional to the antioxidant activity that was reported previously [30]. However, in our study, Citrus grandis Osbeck white flesh (C4) has a greater percentage of limonene and monoterpene but has weaker antioxidant properties, which can be attributed that the overall performance as an antioxidant, which is the result of compounded interaction among components and oxidizable substance to be protected. Depending upon the experimental condition and essential oil composition, synergistic or antagonistic behavior is expected and exceptions may be obtained [31] and this could be an area of further exploration in CEO. Moreover, phenolic compounds such as polyphenols and phenylpropanoids, which are sometimes among the main components of essential oil, have been found to have higher antioxidant values [31,32]. Additionally, the major constituents of CEO, limonene, and β-pinene, have a grounded defensive activity against many kinds of malignant growth as reported in the literature [33].
2.5. Antibacterial Activity
CEOs have demonstrated a broad range of antibacterial activity against different classes of pathogens [34,35,36]. The antibacterial activity of the CEOs was tested against Staphylococcus aureus, Escherichia coli, Klebsiella pneumoniae, Shigella sonnei, and Salmonella typhi in this study. The effectiveness of antibacterial activities of various CEOs was evaluated by measuring the zone of inhibition (ZoI), summarized in Table 5. The result indicates that the CEO showed a moderate to poor antibacterial activity at 25% strength against test bacterial strain. Our study illustrated that Citrus grandis Osbeck white flesh (C4) has higher activity against Staphylococcus aureus and Escherichia coli whereas the Citrus reticulata Blanco (C1) sample showed noticeable activity against Salmonella typhi. The tested CEOs demonstrated weaker antibacterial activity as compared to neomycin. A previous study done on CEOs showed a higher ZoI against Gram-positive and Gram-negative bacteria than in our study, which might be due to the harvest of the sample at different ripening stages that significantly affects the antibacterial activity [20,21]. Additionally, limonene significantly inhibits antibacterial activity and can rupture the cell membrane of bacteria and lead to a change in morphology [37].
Table 5.
Antibacterial activity of citrus essential oil.
| Essential Oil | Bacterial Strains (ZoI) in mm | ||||
|---|---|---|---|---|---|
|
S. aureus ATCC 25923 |
E. coli ATCC 25922 |
K. pneumoniae ATCC 700603 |
S. sonnei ATCC 25931 |
S. typhi ATCC 14028 |
|
| Mandarin Citrus reticulata Blanco (C1) |
9 | 9 | 9 | 9 | 10 |
| Sweet Orange Citrus sinensis Osbeck(C2) |
8 | 7 | 10 | 8 | 9 |
| Pummelo Citrus grandis Osbeck (red flesh) (C3) |
8 | 8 | 8 | 10 | 9 |
| Pummelo Citrus grandis Osbeck (white flesh) (C4) |
11 | 11 | 9 | 10 | 8 |
| Positive control | 23 | 19 | 16 | 30 | 19 |
Note: Positive control: Neomycin 30 µg/disc and Negative control: DMSO.
3. Materials and Methods
3.1. Plant Material
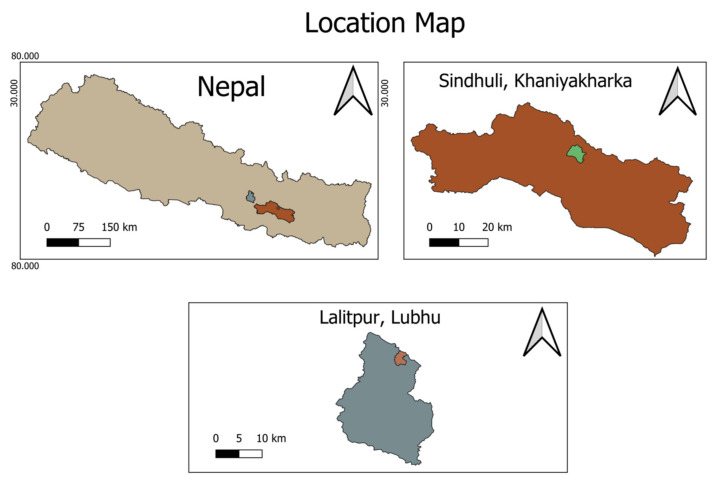
The fruit peel of Citrus species was collected in the area presented in Figure 2. It was collected at the beginning of the ripening period (November 2020), and fruit was identified by a horticulturist at the National Center for Fruit Development, Kirtipur, Kathmandu. Voucher specimens of the plant have been deposited at National Herbarium and Plant Laboratories (KATH), Government of Nepal, Lalitpur, Nepal.
Figure 2.
The geographical location of Citrus species collection site.
3.2. Isolation of Essential Oil
Fresh fruit peel of citrus was cut into small pieces and essential oil was extracted by hydrodistillation in Clevenger apparatus as previously described [38]. The essential oil was dried using anhydrous sodium sulfate and was stored in bottles at 4 °C until use in further studies. The percentage yield of essential oil of different samples is summarized in Table 1.
3.3. Chemical Composition Analysis by GC–MS
Analysis of the volatile chemical constituents in the CEOs was carried out using a GC–MS-QP2010 Ultra gas chromatograph-mass spectrometer (Shimadzu, Columbia, MD, USA) under the following conditions: mass selective detector (MSD), operated in the EI mode (electron energy = 70 eV), with scan range = 40–400 m/z, and scan rate = 3.99 scans/s. The GC column was a ZB-5 ms fused silica capillary with a (5% phenyl)-polydimethylsiloxane stationary phase, a film thickness of 0.25 μm, a length of 60 m, and an internal diameter of 0.25 mm. The carrier gas was helium (80 psi) with a column head pressure of 48.7 kPa and a flow rate of 1.0 mL/min. The injector temperature was 260 °C, and the detector temperature was 280 °C. The GC oven temperature was programmed as follows: hold a 40 °C initial temperature for 10 min; followed by an increase in temperature at 3 °C/min to 200 °C, and then increased at 2 °C/min to 260 °C. A 5% w/v solution of each CEO sample in dichloromethane was prepared, and 1 μL of the sample was injected using a 30:1 split ratio [38,39]. Identification of compounds was based on the retention indices determined by reference to a homologous series of n-alkanes and by comparison of the mass spectral fragmentation patterns. Relative percentages of the individual components of CEOs and different classes of compounds (%) are listed in Table 2.
3.4. Enantiomeric Analysis by Chiral GC–MS
Shimadzu GC–MS-QP2010S with EI mode (70 eV) and B-Dex 325 chiral capillary GC column was used to perform enantiomeric analysis of CEO samples. It scans in the 40–400 m/z range at a scan rate of 3.0 Scan/s. The column temperature was set at 50 °C, initially increased by a rate of 1.5 °C/min till 120 °C and then at 2 °C/min until 200 °C. The final temperature of the column was 200 °C and was kept constant. The carrier gas was helium with a constant flow rate of 1.8 mL/min. For each essential oil sample, 3% w/v solution in DCM was prepared, and 0.1 μL was injected using a split ratio of 1:45 [38,40,41]. The enantiomer percentages were determined from the peak area. Comparison of retention times and mass spectral fragmentation patterns with authentic samples obtained from Sigma-Aldrich (Milwaukee, WI, USA) was used to identify the enantiomers. Table 3 shows the enantiomeric distribution of components in the CEOs.
3.5. Antioxidant Activity of CEOs
The antioxidant activity of the CEOs was measured using the DPPH radical scavenging assay which was modified from the colorimetric method [42,43]. Briefly, 0.1 mM of DPPH solution was prepared in methanol and 100 µL of this solution was mixed with 100 µL of essential oil at different concentrations. The solution was then incubated at room temperature for 30 min. in dark and the absorbance was measured at 517 nm using a microplate reader (Epoch2, BioTek, Instruments, Inc., Winooski, VT, USA). Quercetin was used as a positive control and 30% DMSO was used as a negative control. The inhibitory concentration (IC50) was calculated from the plot of inhibition percentage, against sample concentration. Tests were carried out in triplicate. The calculated IC50 values are showcased in Table 4.
3.6. Antibacterial Activity of CEOs
The antimicrobial activity of the CEOs was done by the disc diffusion method as previously described by Puškárová et al. [44]. The bacterial inoculum was prepared in a Mueller Hinton broth by adding a single colony of bacteria from an overnight culture plate. Then it was incubated at 37 °C until the turbidity matched with 0.5 McFarland. The lawn culture was done in a pre-warmed sterile Mueller Hinton agar plate placed at the same temperature using sterile cotton swabs. The sterile filter paper discs (6 mm) prepared from Whatman No. 1 were pressed gently onto the surface of the agar plates and essential oil at 25% strength (diluted in DMSO) was pipetted onto the discs. The plates were then incubated at 37 °C for 24 h and the ZoI along with disc was measured in mm. Neomycin was used as positive control and DMSO was used as a negative control. The volume that comprised 10 mg pure essential oil was used to test antibacterial activity as each essential oil varied in density. The antibacterial activity of the CEOs is displayed in Table 5.
3.7. Data Analysis
The results were processed using BioTek Gen5 Microplate Data Collection & Analysis Software (BioTek, Instruments, Inc., Winooski, VT, USA) which was subsequently analyzed using Microsoft Excel. GraphPad Prism version 8 was used to calculate the IC50 value of essential oil. The results are presented as the mean ± standard deviation of the mean of triplicate data.
4. Conclusions
The essential oils from Citrus species of Nepalese origin have shown variation in chemical compositions, enantiomeric distributions, and antioxidant and antimicrobial activities. Limonene (63.76–89.15%) is a major component in all essential oils; it exists in dextrorotatory form, and its abundance is found to be in the order Citrus grandis Osbeck white flesh, Citrus grandis Osbeck red flesh, Citrus sinensis Osbeck, and Citrus reticulata Blanco (C4 > C3 > C2 > C1). Through this study, chiral terpenoids have been for the first time reported being found in essential oils produced from the peels of Citrus grandis Osbeck (C3 and C4). Furthermore, it is of interest that the peel of Citrus sinensis Osbeck has shown a higher extraction yield. The yield of extraction, its constituents, and ultimately the biological properties depends upon factors such as genetic variation and extraction procedure. The obtained results of the chiral and GC–MS analysis of these samples can be used as a fingerprint to detect adulteration and authentication issues. Furthermore, out of four CEOs: Citrus reticulata Blanco (C1), Citrus sinensis Osbeck (C2), and Citrus grandis Osbeck red flesh (C3) demonstrated strong antioxidant capacity, suggesting that they may be used as a natural antioxidant to prevent product oxidation. Despite showing promising antioxidant activities, the same show only marginal antimicrobial properties. Through this study, we have laid a path that leads to the commercial production of CEOs in Nepal, because of the observation of similar volatile chemotype and biological properties to other origins.
Acknowledgments
The authors are thankful to the Aromatic Plant Research Center, Lehi, UT, USA for providing GC–MS and Chiral GC–MS data and other financial support to publish this article. The authors would like to express their gratitude towards Anil Rokaya, Prasamsa Panta, Seema Sapkota for their technical assistance.
Author Contributions
Conceptualization, N.P., D.P.B. and D.K.P.; methodology, D.P.B., K.K. and S.D.; validation, P.S.; formal analysis, D.P.B., D.K.P., D.A. and P.C.; investigation, D.P.B., D.K.P., D.A., P.C. and A.G.; data curation, N.P. and P.S.; writing—original draft preparation, D.P.B., K.K., and D.K.P.; writing—review and editing, D.P.B., D.K.P., P.S. and N.P.; supervision, N.P. and P.S.; antimicrobial activities, K.K. and S.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research is supported by University Grants Commission-Nepal to Devi Prasad Bhandari (UGC Award No: PhD-77/78-S&T-05).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are available in the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bora H., Kamle M., Mahato D.K., Tiwari P., Kumar P. Citrus Essential Oils (CEOs) and Their Applications in Food: An Overview. Plants. 2020;9:357. doi: 10.3390/plants9030357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caputo L., Cornara L., Bazzicalupo M., De Francesco C., De Feo V., Trombetta D., Smeriglio A. Chemical Composition and Biological Activities of Essential Oils from Peels of Three Citrus Species. Molecules. 2020;25:1890. doi: 10.3390/molecules25081890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li G., Xiang S., Pan Y., Long X., Cheng Y., Han L., Zhao X. Effects of Cold-Pressing and Hydrodistillation on the Active Non-Volatile Components in Lemon Essential Oil and the Effects of the Resulting Oils on Aging-Related Oxidative Stress in Mice. Front. Nutr. 2021;8:329. doi: 10.3389/fnut.2021.689094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Q.-W., Lin L.-G., Ye W.-C. Techniques for Extraction and Isolation of Natural Products: A Comprehensive Review. Chin. Med. 2018;13:1–26. doi: 10.1186/s13020-018-0177-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Masango P. Cleaner Production of Essential Oils by Steam Distillation. J. Clean. Prod. 2005;13:833–839. doi: 10.1016/j.jclepro.2004.02.039. [DOI] [Google Scholar]
- 6.Cozzi R., Ricordy R., Aglitti T., Gatta V., Perticone P., De Salvia R. Ascorbic Acid and Beta-Carotene as Modulators of Oxidative Damage. Carcinogenesis. 1997;18:223–228. doi: 10.1093/carcin/18.1.223. [DOI] [PubMed] [Google Scholar]
- 7.Kang H.J., Chawla S.P., Jo C., Kwon J.H., Byun M.W. Studies on the Development of Functional Powder from Citrus Peel. Bioresour. Technol. 2006;97:614–620. doi: 10.1016/j.biortech.2005.03.037. [DOI] [PubMed] [Google Scholar]
- 8.Mitropoulou G., Fitsiou E., Spyridopoulou K., Tiptiri-Kourpeti A., Bardouki H., Vamvakias M., Panas P., Chlichlia K., Pappa A., Kourkoutas Y. Citrus Medica Essential Oil Exhibits Significant Antimicrobial and Antiproliferative Activity. LWT. 2017;84:344–352. doi: 10.1016/j.lwt.2017.05.036. [DOI] [Google Scholar]
- 9.Wu Z., Li H., Yang Y., Zhan Y., Tu D. Variation in the Components and Antioxidant Activity of Citrus medica L. Var. sarcodactylis Essential Oils at Different Stages of Maturity. Ind. Crop. Prod. 2013;46:311–316. doi: 10.1016/j.indcrop.2013.02.015. [DOI] [Google Scholar]
- 10.Juráň S., Pallozzi E., Guidolotti G., Fares S., Šigut L., Calfapietra C., Alivernini A., Savi F., Večeřová K., Křůmal K., et al. Fluxes of Biogenic Volatile Organic Compounds above Temperate Norway Spruce Forest of the Czech Republic. Agric. For. Meteorol. 2017;232:500–513. doi: 10.1016/j.agrformet.2016.10.005. [DOI] [Google Scholar]
- 11.Juráň S., Šigut L., Holub P., Fares S., Klem K., Grace J., Urban O. Ozone Flux and Ozone Deposition in a Mountain Spruce Forest Are Modulated by Sky Conditions. Sci. Total Environ. 2019;672:296–304. doi: 10.1016/j.scitotenv.2019.03.491. [DOI] [PubMed] [Google Scholar]
- 12.Bustamante J., van Stempvoort S., García-Gallarreta M., Houghton J.A., Briers H.K., Budarin V.L., Matharu A.S., Clark J.H. Microwave Assisted Hydro-Distillation of Essential Oils from Wet Citrus Peel Waste. J. Clean. Prod. 2016;137:598–605. doi: 10.1016/j.jclepro.2016.07.108. [DOI] [Google Scholar]
- 13.Hosni K., Zahed N., Chrif R., Abid I., Medfei W., Kallel M., Brahim N.B., Sebei H. Composition of Peel Essential Oils from Four Selected Tunisian Citrus Species: Evidence for the Genotypic Influence. Food Chem. 2010;123:1098–1104. doi: 10.1016/j.foodchem.2010.05.068. [DOI] [Google Scholar]
- 14.González-Mas M.C., Rambla J.L., López-Gresa M.P., Blázquez M.A., Granell A. Volatile Compounds in Citrus Essential Oils: A Comprehensive Review. Front. Plant Sci. 2019;10:12. doi: 10.3389/fpls.2019.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khadayat K., Sherpa D.D., Malla K.P., Shrestha S., Rana N., Marasini B.P., Khanal S., Rayamajhee B., Bhattarai B.R., Parajuli N. Molecular Identification and Antimicrobial Potential of Streptomyces Species from Nepalese Soil. Int. J. Microbiol. 2020;2020:1–8. doi: 10.1155/2020/8817467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Puvača N., de Llanos Frutos R. Antimicrobial Resistance in Escherichia coli Strains Isolated from Humans and Pet Animals. Antibiotics. 2021;10:69. doi: 10.3390/antibiotics10010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friedly E.C., Crandall P.G., Ricke S.C., Roman M., O’Bryan C., Chalova V.I. In Vitro Antilisterial Effects of Citrus Oil Fractions in Combination with Organic Acids. J. Food Sci. 2009;74:M67–M72. doi: 10.1111/j.1750-3841.2009.01056.x. [DOI] [PubMed] [Google Scholar]
- 18.O’Bryan C.A., Crandall P.G., Chalova V.I., Ricke S.C. Orange Essential Oils Antimicrobial Activities against Salmonella Spp. J. Food Sci. 2008;73:M264–M267. doi: 10.1111/j.1750-3841.2008.00790.x. [DOI] [PubMed] [Google Scholar]
- 19.Yi F., Jin R., Sun J., Ma B., Bao X. Evaluation of Mechanical-Pressed Essential Oil from Nanfeng Mandarin (Citrus reticulata Blanco Cv. Kinokuni) as a Food Preservative Based on Antimicrobial and Antioxidant Activities. LWT. 2018;95:346–353. doi: 10.1016/j.lwt.2018.05.011. [DOI] [Google Scholar]
- 20.Bourgou S., Rahali F.Z., Ourghemmi I., Saïdani Tounsi M. Changes of Peel Essential Oil Composition of Four Tunisian Citrus during Fruit Maturation. Sci. World J. 2012;2012:1–10. doi: 10.1100/2012/528593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Değirmenci H., Erkurt H. Relationship between Volatile Components, Antimicrobial and Antioxidant Properties of the Essential Oil, Hydrosol and Extracts of Citrus aurantium L. Flowers. J. Infect. Public Health. 2020;13:58–67. doi: 10.1016/j.jiph.2019.06.017. [DOI] [PubMed] [Google Scholar]
- 22.Hou H.-S., Bonku E.M., Zhai R., Zeng R., Hou Y.-L., Yang Z.-H., Quan C. Extraction of Essential Oil from Citrus reticulate Blanco Peel and Its Antibacterial Activity against Cutibacterium acnes (Formerly Propionibacterium acnes) Heliyon. 2019;5:e02947. doi: 10.1016/j.heliyon.2019.e02947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tran T.H., Quyen N.T., Linh H.T.K., Ngoc T.T.L., Quan P.M., Toan T.Q. Essential Oil from Vietnamese Mandarin (Citrus reticulata Blanco) Using Hydrodistillation Extraction Process and Identification of It’s Components. Solid State Phenom. 2019;298:100–105. doi: 10.4028/www.scientific.net/SSP.298.100. [DOI] [Google Scholar]
- 24.Zhang L.-L., Yang Z.-Y., Fan G., Ren J.-N., Yin K.-J., Pan S.-Y. Antidepressant-like Effect of Citrus sinensis (L.) Osbeck Essential Oil and Its Main Component Limonene on Mice. J. Agric. Food Chem. 2019;67:13817–13828. doi: 10.1021/acs.jafc.9b00650. [DOI] [PubMed] [Google Scholar]
- 25.Chen G.-W., Lin Y.-H., Lin C.-H., Jen H.-C. Antibacterial Activity of Emulsified Pomelo (Citrus grandis Osbeck) Peel Oil and Water-Soluble Chitosan on Staphylococcus aureus and Escherichia coli. Molecules. 2018;23:840. doi: 10.3390/molecules23040840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ou M.-C., Liu Y.-H., Sun Y.-W., Chan C.-F. The Composition, Antioxidant and Antibacterial Activities of Cold-Pressed and Distilled Essential Oils of Citrus paradisi and Citrus grandis (L.) Osbeck. Evid.-Based Complement. Altern. Med. 2015;2015:1–9. doi: 10.1155/2015/804091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uysal B., Sozmen F., Aktas O., Oksal B.S., Kose E.O. Essential Oil Composition and Antibacterial Activity of the Grapefruit (Citrus paradisi L.) Peel Essential Oils Obtained by Solvent-Free Microwave Extraction: Comparison with Hydrodistillation: Essential Oil Composition and Antibacterial Activity of the Grapefruit. Int. J. Food Sci. Technol. 2011;46:1455–1461. doi: 10.1111/j.1365-2621.2011.02640.x. [DOI] [Google Scholar]
- 28.Rivero-Cruz J.F., Granados-Pineda J., Pedraza-Chaverri J., Pérez-Rojas J.M., Kumar-Passari A., Diaz-Ruiz G., Rivero-Cruz B.E. Phytochemical Constituents, Antioxidant, Cytotoxic, and Antimicrobial Activities of the Ethanolic Extract of Mexican Brown Propolis. Antioxidants. 2020;9:70. doi: 10.3390/antiox9010070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Demir N., Yildiz O., Alpaslan M., Hayaloglu A.A. Evaluation of Volatiles, Phenolic Compounds and Antioxidant Activities of Rose Hip (Rosa L.) Fruits in Turkey. LWT—Food Sci. Technol. 2014;57:126–133. doi: 10.1016/j.lwt.2013.12.038. [DOI] [Google Scholar]
- 30.Shah B.B., Mehta A.A. In Vitro Evaluation of Antioxidant Activity of d-Limonene. Asian J. Pharm. Pharmacol. 2018;4:883–887. doi: 10.31024/ajpp.2018.4.6.25. [DOI] [Google Scholar]
- 31.Amorati R., Foti M.C., Valgimigli L. Antioxidant Activity of Essential Oils. J. Agric. Food Chem. 2013;61:10835–10847. doi: 10.1021/jf403496k. [DOI] [PubMed] [Google Scholar]
- 32.Tabaszewska M., Antoniewska A., Rutkowska J., Skoczylas Ł., Słupski J., Skoczeń-Słupska R. Bioactive Components, Volatile Profile and In Vitro Antioxidative Properties of Taxus baccata L. Red Arils. Molecules. 2021;26:4474. doi: 10.3390/molecules26154474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jabri karoui I., Marzouk B. Characterization of Bioactive Compounds in Tunisian Bitter Orange (Citrus aurantium L.) Peel and Juice and Determination of Their Antioxidant Activities. BioMed Res. Int. 2013;2013:1–12. doi: 10.1155/2013/345415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ambrosio C.M.S., Ikeda N.Y., Miano A.C., Saldaña E., Moreno A.M., Stashenko E., Contreras-Castillo C.J., Da Gloria E.M. Unraveling the Selective Antibacterial Activity and Chemical Composition of Citrus Essential Oils. Sci. Rep. 2019;9:17719. doi: 10.1038/s41598-019-54084-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mancuso M., Catalfamo M., Laganà P., Rappazzo A.C., Raymo V., Zampino D., Zaccone R. Screening of Antimicrobial Activity of Citrus Essential Oils against Pathogenic Bacteria and Candida Strains. Flavour Fragr. J. 2019;34:187–200. doi: 10.1002/ffj.3491. [DOI] [Google Scholar]
- 36.Raspo M.A., Vignola M.B., Andreatta A.E., Juliani H.R. Antioxidant and Antimicrobial Activities of Citrus Essential Oils from Argentina and the United States. Food Biosci. 2020;36:100651. doi: 10.1016/j.fbio.2020.100651. [DOI] [Google Scholar]
- 37.Han Y., Sun Z., Chen W. Antimicrobial Susceptibility and Antibacterial Mechanism of Limonene against Listeria monocytogenes. Molecules. 2019;25:33. doi: 10.3390/molecules25010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poudel D.K., Rokaya A., Ojha P.K., Timsina S., Satyal R., Dosoky N.S., Satyal P., Setzer W.N. The Chemical Profiling of Essential Oils from Different Tissues of Cinnamomum camphora L. and Their Antimicrobial Activities. Molecules. 2021;26:5132. doi: 10.3390/molecules26175132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Satyal P., Jones T.H., Lopez E.M., McFeeters R.L., Ali N.A.A., Mansi I., Al-kaf A.G., Setzer W.N. Chemotypic Characterization and Biological Activity of Rosmarinus officinalis. Foods. 2017;6:20. doi: 10.3390/foods6030020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.DeCarlo A., Zeng T., Dosoky N.S., Satyal P., Setzer W.N. The Essential Oil Composition and Antimicrobial Activity of Liquidambar Formosana Oleoresin. Plants. 2020;9:822. doi: 10.3390/plants9070822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Satyal P., Powers C., Parducci V.R., McFeeters R., Setzer W. Chemical Composition, Enantiomeric Distribution, and Antifungal Activity of the Oleoresin Essential Oil of Protium amazonicum from Ecuador. Medicines. 2017;4:70. doi: 10.3390/medicines4040070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Subedi A., Amatya M.P., Shrestha T.M., Mishra S.K., Pokhrel B.M. Antioxidant And Antibacterial Activity of Methanolic Extract of Machilus odoratissima. Kathmandu Univ. J. Sci. Eng. Technol. 2012;8:73–80. doi: 10.3126/kuset.v8i1.6045. [DOI] [Google Scholar]
- 43.Khadayat K., Marasini B.P., Gautam H., Ghaju S., Parajuli N. Evaluation of the alpha-amylase inhibitory activity of Nepalese medicinal plants used in the treatment of diabetes mellitus. Clin. Phytosci. 2020;6:1–8. doi: 10.1186/s40816-020-00179-8. [DOI] [Google Scholar]
- 44.Puškárová A., Bučková M., Kraková L., Pangallo D., Kozics K. The Antibacterial and Antifungal Activity of Six Essential Oils and Their Cyto/Genotoxicity to Human HEL 12469 Cells. Sci. Rep. 2017;7:8211. doi: 10.1038/s41598-017-08673-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are available in the manuscript.