Abstract
Background
Information on tuberculosis (TB) and coronavirus disease 2019 (COVID-19) is still limited. The aim of this study was to describe the features of the TB/COVID-19 co-infected individuals from a prospective, anonymised, multicountry register-based cohort with special focus on the determinants of mortality and other outcomes.
Methods
We enrolled all patients of any age with either active TB or previous TB and COVID-19. 172 centres from 34 countries provided individual data on 767 TB-COVID-19 co-infected patients, (>50% population-based).
Results
Of 767 patients, 553 (74.0%) out of 747 had TB before COVID-19 (including 234 out of 747 with previous TB), 71 (9.5%) out of 747 had COVID-19 first and 123 (16.5%) out of 747 had both diseases diagnosed within the same week (n=35 (4.6%) on the same day). 85 (11.08%) out of 767 patients died (41 (14.2%) out of 289 in Europe and 44 (9.2%) out of 478 outside Europe; p=0.03): 42 (49.4%) from COVID-19, 31 (36.5%) from COVID-19 and TB, one (1.2%) from TB and 11 from other causes. In the univariate analysis on mortality the following variables reached statistical significance: age, male gender, having more than one comorbidity, diabetes mellitus, cardiovascular disease, chronic respiratory disease, chronic renal disease, presence of key symptoms, invasive ventilation and hospitalisation due to COVID-19. The final multivariable logistic regression model included age, male gender and invasive ventilation as independent contributors to mortality.
Conclusion
The data suggest that TB and COVID-19 are a “cursed duet” and need immediate attention. TB should be considered a risk factor for severe COVID disease and patients with TB should be prioritised for COVID-19 preventative efforts, including vaccination.
Short abstract
High mortality (11%) was observed with COVID-19/TB co-infection associated with older age, male gender and invasive ventilation. Efforts to avoid SARS-CoV-2 infection in TB patients are recommended to prevent excess morbidity and mortality. https://bit.ly/3mSylCK
Introduction
Tuberculosis (TB), with its estimated 10 million cases and 1.3 million deaths annually, continues to be a global health priority [1]. The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) coronavirus disease 2019 (COVID-19) pandemic has required concerted public health focus and action because of its rapid global spread, clinical severity, high mortality rate with 4 million deaths, and capacity to overwhelm healthcare systems [2–5]. The impact of COVID-19 on TB services has been well described, with a reduction of the number TB cases diagnosed and managed in most countries as a combined result of reduced access, delayed diagnosis with more advanced forms and overstretched health services among other reasons [6–11]. According to the World Health Organization (WHO) report, there was a 18% decrease of TB case notifications between 2019 and 2020 (from 7.1 to 5.8 million cases) [1]. Conservative models suggest that a 20% increase in TB deaths in the next 5 years is likely as a result of the pandemic [12, 13].
The clinical and immune-pathological interaction between the two diseases and the drivers of dual COVID-19/TB disease mortality are not yet fully understood [14–17]. A first pilot study of the Global Tuberculosis Network (GTN) on 49 TB/COVID-19 co-infected patients from eight countries was published in 2020 [18], suggesting that although signs and symptoms are largely the same, TB is frequently diagnosed concomitant with or after COVID-19 and that dual infection may be associated with an increased case-fatality rate. A second GTN study on 69 TB/COVID-19 patients [10] suggested an overall 12.6% case-fatality rate, higher than the 1–2% mortality rate reported for drug-susceptible TB [1] and for COVID-19 [4], identifying age and comorbidities as the main determinants for mortality. Subsequent studies from South Africa and the Philippines suggested that COVID-19 patients with TB have a 2.7 [19] and 2.17 [20], respectively, higher risk of mortality compared with COVID-19 patients without TB [20]. No large multicountry cohort of TB and COVID-19 patients has been reported to date.
In 2020 the GTN, in collaboration with several organisations (Groupe de Recherche et Enseignement en Pneumo-Infectiologie, a working group from the Société de Pneumologie de Langue Française; Sociedad Española de Neumología and Cirugía Torácica; Brazilian Society of Pulmonology and Tuberculosis; and the Moscow Society of Phthisiology, among others), national TB programmes (Chile, Colombia, Niger, Oman, Panama, Paraguay, Portugal, Serbia and Slovakia), partners and clinicians, developed a global repository of TB and COVID-19 patients. The repository was shared with the WHO to inform the development of global guidance [1, 21]. The aim of this study is to describe the features of the TB and COVID-19 co-infected individuals using this repository, with special focus on the determinants of mortality and other short-term outcomes.
Methods
Study design
The study is based on a prospective, anonymised, multicountry register-based cohort (annex 1). We worked with WHO and the GTN to identify respondents and send invitations to 175 centres in 37 countries [22]. The centres and countries providing data are listed in annex 2 and figure 1; we enrolled all patients (including children and adolescents) notified to these centres between March 2020 (first case reported on 12 March 2020) and June 2021. The questionnaire and process was piloted and has been described previously [10, 18, 23]. We enrolled all patients of any age from these centres with either active TB or previous TB and COVID-19 [18] simultaneously.
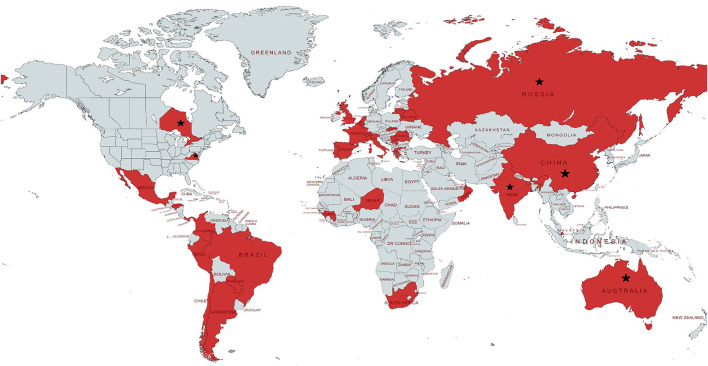
FIGURE 1.
Global distribution of the countries/states/regions participating in the study. The following states/territories are covered in the study (★): Australia (New South Wales); Canada (Ontario state): China (Wenzhou and Luzhou); India (New Delhi, Mumbai and Maharashtra states); the Russian Federation (Arkhangelsk, Moscow and Volvograd Oblasts); Switzerland (Vaud county); USA (Virginia state). 21 countries (Argentina, Belarus, Belgium, Brazil, Chile, China, France, Republic of Guinea, India, Italy, Mexico, Niger, Panama, Peru, Portugal, Romania, Russia, Singapore, Spain, Switzerland and UK) reported at least one tuberculosis/coronavirus disease 2019 case in 2020. Other countries (Australia, Canada, Colombia, Greece, Honduras, Lithuania, the Netherlands, Oman, Paraguay, Serbia, Slovakia, South Africa and USA) started reporting from 2021.
Variables and definitions
The data were obtained via an electronic collection form using variables standardised and harmonised with the WHO and piloted in our previous study [18, 21], including anonymised patients’ demographic data, laboratory, radiological and clinical status at diagnosis of TB and COVID-19, and details on follow-up.
Case definitions follow WHO classification [1]. We define previous TB patients as those who had TB and completed anti-TB treatment at any time in the past before diagnosis of COVID-19. The TB/COVID-19 cases collected in our study were compared with country/regional surveillance systems to estimate coverage in agreement with investigators (annex 2). All data were cleaned and harmonised throughout the dataset and investigators were contacted in at least two rounds of data cleaning to ensure quality of the dataset before final analysis. The cause of death was analysed as reported by each investigator.
Data analysis
A descriptive analysis was performed on all patients, presenting the details of TB and COVID-19 in the cohort. Considering the relevant proportion of patients from Europe and the number of European countries (15 out of 34) reporting data were also stratified by geographical origin.
We summarised variables using frequencies and percentages and calculated mean±sd for normally distributed data and medians with interquartile ranges (IQR) for non-normally distributed data. Unpaired t-tests were used to compare continuous variables with normal distributions and categorical variables were compared using Chi-squared or Fisher exact test. We used nonparametric tests (e.g. Mann–Whitney U-test) for data that could not be converted into a standard distribution.
We were interested in determinants of mortality of COVID-19 and evaluated the effect of prognostic factors on these end-points by univariable and multivariable logistic regression models. Covariates that were significant prognostic factors at single variable analysis (p<0.05) were tested for inclusion in the multivariable model in a forward fashion using likelihood ratio tests at each step and used Akaike's information criterion to decide on the final model. For all variables, two-sided p-values ≤0.05 were considered statistically significant. All variables, when biologically plausible, were tested for interaction. Based on the results of the final multivariable model, we developed a nomogram for risk prediction (annex 3). The nomogram displays the predicted and confounding probabilities for each variable and overall as points on a scale from 0 to 100 in a user-friendly graphical interface and the overall scale corresponds to the predicted overall probability of the outcome for a patient.
Ethics
The ethics committee of the Maugeri Care and Research Institute, Tradate, Italy (the coordinating centre) approved the study on 26 May 2020 (CE 2020/May 26). Each participating centre or country signed a confidentiality and data-sharing agreement with the coordinating centre and obtained local ethics committee clearance or had a waiver indicating no requirement for ethical approval due to the local regulations [18, 23, 24].
Results
In total, 172 centres from 34 countries provided individual data on 767 TB-COVID-19 co-infected patients (annex 2). Ascertainment of COVID-19/TB was very high and in most of countries (or regions/states or metropolitan areas) (18 out of 34, 52.9%) >80% of these patients were notified to us.
Description of the TB/COVID-19 cohort
The demographic, epidemiological and clinical characteristics of the 767 TB/COVID-19 patients are summarised in table 1.
TABLE 1.
Demographic, epidemiological and clinical characteristics of 767 tuberculosis (TB)/coronavirus disease 2019 (COVID-19) cases
| Age, years | 44 (31–58) |
| Males | 540/767 (70.4) |
| Immigrated in the past 5 years | 80/717 (11.2) |
| Occupation | |
| Unemployed | 318/705 (45.1) |
| Employed | 254/705 (36.0) |
| Retired | 108/705 (15.3) |
| Student | 25/705 (3.6) |
| BCG vaccinated | 349/385 (90.7) |
| Pregnancy | 2/224 (0.9) |
| Alcohol abuse (≥14 drinks per week in men or ≥7 drinks per week in women) | 112/687 (16.3) |
| Smoking status | |
| Nonsmoker | 382/636 (60.1) |
| Current smoker | 184/636 (28.9) |
| Former smoker | 70/636 (11.0) |
| Vaping status | |
| No vape | 485/523 (92.7) |
| Current vape | 36/523 (6.9) |
| Former vape | 2/523 (0.4) |
| Intravenous drug user | |
| No drug user | 631/655 (96.3) |
| Current/regular | 9/655 (1.4) |
| Current/not regular | 4/655 (0.6) |
| Former drug user | 11/655 (1.7) |
| HIV positivity | 83/724 (11.5) |
| CD4 count pre-COVID-19 infection, cells·μL−1 (n=28) | 164.5 (46–344) |
| CD4 count during COVID-19 infection, cells·μL−1 (n=20) | 88 (41.3–247) |
| HIV treatment administered | 29/83 (34.9) |
| COPD | 59/751 (7.8) |
| Diabetes mellitus | 157/753 (20.8) |
| Uncontrolled diabetes mellitus (HbA1c ≥9%) | 40/136 (29.4) |
| Poorly controlled diabetes mellitus (HbA1c 7–9%) | 28/136 (20.6) |
| Well-controlled diabetes mellitus (HbA1c <7%) | 18/136 (13.2) |
| Unknown diabetes mellitus control | 50/136 (36.8) |
| Renal failure | 53/713 (7.4) |
| Dialysis | 17/43 (39.5) |
| Liver disease | 60/700 (8.6) |
| Timing of TB and COVID-19 diagnosis | |
| TB diagnosed before COVID-19# | 553/747 (74.0) |
| Days of TB diagnosis before COVID-19 diagnosis (n=318)¶ | 78 (38–145) |
| Years between TB end and COVID-19 diagnosis (n=229)+ | 2.3 (1.0–6.3) |
| COVID-19 diagnosed before TB | 71/747 (9.5) |
| Days of COVID-19 diagnosis before TB diagnosis (n=71) | 28 (15–42) |
| COVID-19 and TB diagnosed within the same week (including patients diagnosed on the same day) | 123/747 (16.5) |
| Days of TB and COVID-19 diagnosis within the same week (n=123) | 1 (0–4) |
| COVID-19 and TB diagnosed within the same day | 35/747 (4.7) |
Data are presented as median (interquartile range) or n/N (%). BCG: bacille Calmette–Guérin; HbA1c: glycated haemoglobin. #: patients with active TB and previous TB; ¶: patients with previous TB excluded; +: patients with previous TB.
Most patients were male (70.4%, 540 out of 767), with a median (IQR) age of 44 (31–58) years. The majority were vaccinated with bacillus Calmette–Guérin (90.7%, 349 out of 385). 11.1% (80 out of 717) had a history of migration in the past 5 years and 11.5% (83 out of 724) were HIV co-infected.
Of 767 patients, 553 (74.0%) out of 747 had TB before COVID-19 (including 234 out of 747 with previous TB), 71 (9.5%) out of 747 had COVID-19 first and 123 (16.5%) out of 747 had both diseases diagnosed within the same week (35 (4.6%) of them on the same day).
Characteristics of patients with TB in the TB/COVID-19 cohort
As shown in table 2, the majority of patients had newly diagnosed TB (618 out of 723, 85.5%) and bacteriologically confirmed disease (612 out of 732, 83.6%) with pulmonary localisation (648 out of 755, 85.8%); the majority (517 out of 607, 85.2%) had pan-susceptible TB.
TABLE 2.
Descriptive analysis of tuberculosis (TB) in the TB/coronavirus disease 2019 cohort
| TB form | |
| Failure | 17/723 (2.4) |
| Relapsed | 59/723 (8.2) |
| Loss to follow- up | 29/723 (4.0) |
| New case | 618/723 (85.5) |
| TB laboratory confirmation | 612/732 (83.6) |
| Site | |
| Pulmonary TB | 648/755 (85.8) |
| Extrapulmonary TB | 189/738 (25.6) |
| Pulmonary–extrapulmonary TB | 80/733 (10.9) |
| Extrapulmonary TB | |
| Pleural TB | 52/189 (27.5) |
| TB lymphadenitis | 42/189 (22.2) |
| Multiple locations | 31/189 (16.4) |
| Central nervous system | 17/189 (9.0) |
| Other | 15/189 (7.9) |
| Bone TB | 11/189 (5.8) |
| Gastrointestinal TB | 7/189 (3.7) |
| TB peritonitis | 5/189 (2.6) |
| Genitourinary TB | 4/189 (2.1) |
| TB pericarditis | 2/189 (1.0) |
| Unknown | 3/189 (1.6) |
| Radiology at TB diagnosis | |
| Bilateral pulmonary cavitary lesion | 118/633 (18.6) |
| Bilateral pulmonary cavitary lesion+other | 5/633 (0.8) |
| Unilateral pulmonary cavitary lesion | 121/633 (19.1) |
| Unilateral pulmonary cavitary lesion+other | 4/633 (0.6) |
| Bilateral pulmonary infiltrate (no cavities) | 108/633 (17.1) |
| Bilateral pulmonary infiltrate (no cavities)+other | 7/633 (1.1) |
| Unilateral pulmonary infiltrate (no cavities) | 94/633 (1.8) |
| Unilateral pulmonary infiltrate (no cavities)+other | 8/633 (1.3) |
| Other lesions | 143/633 (22.6) |
| Not done | 25/633 (3.9) |
| Lung function tests at TB diagnosis | |
| Lung function tests at TB diagnosis | 209/625 (33.4) |
| SO2, % (n=214) | 97 (94–98) |
| FiO2, % (n=112) | 21 (21–21) |
| PO2, mmHg (n=40) | 77.9 (65.7–93.8) |
| PCO2, mmHg (n=40) | 35.2±7.5 |
| pH (n=39) | 7.45 (7.40–7.47) |
| Microbiology | |
| TB microbiology (one or more tests) | 638/652 (97.8) |
| Solid culture | 441/638 (69.3) |
| Gene Xpert | 410/638 (64.5) |
| Liquid culture | 324/638 (50.9) |
| First-line LPA | 105/638 (16.5) |
| Second-line LPA | 28/638 (4.4) |
| Drug resistance pattern at TB diagnosis | |
| Pan-susceptible TB | 517/607 (85.2) |
| Drug-resistant TB | 90/607 (14.8) |
| Hospitalisation | |
| Hospitalisation during anti-TB treatment | 388/614 (63.2) |
| Duration of hospitalisation, days (n=342) | 31 (14–90) |
Data are presented as n/N (%), median (interquartile range) or mean±sd. SO2: oxygen saturation; FiO2: fraction of inspired oxygen; PO2: partial pressure of oxygen; PCO2: partial pressure of carbon dioxide; LPA: line probe assay.
Overall, 248 (39.2%) out of 633 patients presented unilateral or bilateral cavities. Approximtely one-third of the patients (209 out of 625, 33.4%) performed at least one lung function test; pulse oximetry being the most utilised.
The majority of patients with TB (388 of the 614 with information, 63.2%) were hospitalised during anti-TB treatment for a median (IQR) duration of 31 (14–90) days.
Characteristics of COVID-19 patients in the TB/COVID-19 cohort
SARS-CoV-2 laboratory confirmation was available for 723 (94.8%) out of 763 patients; the remaining patients’ diagnoses of COVID-19 were based on clinical and radiological criteria (table 3). The majority of COVID-19 patients reported signs and symptoms (538 out of 669, 80.4%); fever (386 out of 538, 71.7%) and dry cough (311 out of 538, 57.8%) being the most frequently reported. Other typical COVID-19 symptoms such as taste and olfactory disorders were reported by 56 (10.4%) and 48 (8.9%) out of 538 patients, respectively. Among the 266 patients who had a computed tomography (CT) scan, 228 (85.7%) had typical or atypical “ground-glass” opacities. 401 (64.8%) out of 619 patients with detailed information had at least one functional assessment of the respiratory system, most commonly pulse oximetry (397 out of 401, 99.0%).
TABLE 3.
Descriptive analysis of coronavirus disease 2019 (COVID-19) in the tuberculosis (TB)/COVID-19 cohort
| All patients | Alive | Deceased | |
| Patients | 682 | 85 | |
| SARS-CoV-2 laboratory confirmation | 723/763 (94.8) | ||
| Signs and symptoms | |||
| COVID-19 signs and symptoms (one or more symptoms) | 538/669 (80.4) | ||
| Fever | 386/538 (71.7) | ||
| Dry cough | 311/538 (57.8) | ||
| Shortness of breath | 192/538 (35.7) | ||
| Headache | 133/538 (24.7) | ||
| Tiredness | 114/538 (21.2) | ||
| Sore throat | 96/538 (17.8) | ||
| Malaise | 96/538 (17.8) | ||
| Chest pain | 88/538 (16.3) | ||
| Myalgia | 87/538 (16.2) | ||
| Nasal congestion | 73/538 (13.6) | ||
| Taste disorders | 56/538 (10.4) | ||
| Diarrhoea | 52/538 (9.7) | ||
| Olfactory disorders | 48/538 (8.9) | ||
| Vomiting/nausea | 38/538 (7.1) | ||
| Arthralgia | 36/538 (6.7) | ||
| Abdominal pain | 34/538 (6.3) | ||
| Irritability/confusion | 34/538 (6.3) | ||
| Other symptoms (loss of appetite, rhinorrhoea, difficulty of breathing, haemoptysis, conjunctivitis, among others) | 74/538 (13.7) | ||
| Diagnosis | |||
| COVID-19 laboratory confirmed (one or more tests) | 723/763 (94.7) | ||
| PCR diagnosis | 683/758 (90.1) | ||
| SARS-CoV-2 PCR diagnosis | 41/758 (5.4) | ||
| CT scan diagnosis | 54/758 (7.1) | ||
| Presumptive diagnosis | 61/758 (8.0) | ||
| Other diagnosis (chest radiography, rapid antigen test) | 17/758 (2.2) | ||
| Radiology at diagnosis | |||
| CT scan | 109/642 (17.0) | ||
| Chest radiography | 214/642 (33.3) | ||
| CT scan and chest radiography | 157/642 (24.5) | ||
| Radiology not done | 162/642 (25.2) | ||
| CT scan findings | |||
| Typical ground-glass opacity/opacities, bilateral | 126/266 (47.4) | ||
| Typical ground-glass opacity/opacities, unilateral | 40/266 (15.0) | ||
| Atypical ground-glass opacity/opacities | 56/266 (21.1) | ||
| Typical ground-glass opacity bilateral and atypical ones | 6/266 (2.2) | ||
| No COVID-19 lesion(s) (no opacity) | 38/266 (14.3) | ||
| Lung function tests at COVID-19 diagnosis | |||
| Lung function tests at COVID-19 diagnosis | 401/619 (64.8) | ||
| SO2, % (n=397) | 96 (94–98) | ||
| FiO2, % (n=269) | 21 (21–21) | ||
| PO2, mmHg (n=99) | 80 (63–95) | ||
| PCO2, mmHg (n=100) | 37 (33–41) | ||
| pH (n=100) | 7.4 (7.4–7.5) | ||
| Ventilation and oxygen therapy | |||
| No ventilation | 513/626 (81.9) | ||
| Noninvasive | 67/626 (10.7) | ||
| Invasive | 46/626 (7.4) | ||
| Supplemental oxygen during COVID-19 | 198/619 (32.0) | ||
| Hospitalisation | |||
| Hospitalisation due to COVID-19 | 452/732 (61.7) | ||
| Duration of hospitalisation, days (n=395) | 14 (8–22) | ||
| Concomitant hospitalisation due to TB/COVID-19 co-infection | 250/737 (33.9) | ||
| Duration of concomitant hospitalisation, days (n=223) | 16 (10–24) | ||
| PCR conversion rates | |||
| PCR conversion | 271/474 (57.2) | ||
| From start of treatment to PCR conversion, days (n=196) | 14.5 (11–22) | ||
| Treatment | |||
| Treatment for COVID-19 (one or more drugs) | 346/639 (54.1) | ||
| Antivirals | |||
| Lopinavir/ritonavir | 24/336 (7.1) | ||
| Darunavir/cobicistat or darunavir/ritonavir | 21/336 (6.2) | ||
| Favipiravir | 11/336 (3.3) | ||
| Remdesivir | 5/336 (1.5) | ||
| Other antivirals | 4/336 (1.2) | ||
| Immunomodulators | |||
| Glucocorticoids (methylprednisolone, betamethasone, ciclesonide, other glucocorticoids) | 115/336 (34.2) | ||
| Intravenous immunoglobulin | 3/336 (0.9) | ||
| IL-6 inhibitors | 2/336 (0.6) | ||
| Bevacizumab (antibody against VEGF-A) | 1/336 (0.3) | ||
| Anticoagulants | |||
| Enoxaparin | 72/336 (21.4) | ||
| Other therapeutic anticoagulants | 22/336 (6.5) | ||
| Miscellaneous | |||
| Azithromycin | 212/336 (63.1) | ||
| Hydroxychloroquine | 191/336 (56.8) | ||
| N-acetyl-cysteine | 22/336 (6.6) | ||
| Plasma from recovered patients | 3/336 (0.9) | ||
| Interferon | 3/336 (0.9) | ||
| Other nonsteroidal anti-inflammatory drugs | 2/336 (0.6) | ||
| Number of comorbidities | |||
| 0 | 339 (49.7) | 12 (14.1) | |
| 1 | 196 (28.7) | 24 (28.2) | |
| 2 | 97 (14.2) | 18 (21.2) | |
| 3 | 29 (4.3) | 9 (10.6) | |
| 4 | 15 (2.2) | 8 (9.4) | |
| 5 | 2 (0.3) | 4 (4.7) | |
| 6 | 2 (0.3) | 5 (5.9) | |
| 7 | 1 (0.1) | 3 (3.5) | |
| 8 | 1 (0.1) | 2 (2.4) |
Data are presented as n, n/N (%) or median (interquartile range). SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; CT: computed tomography; SO2: oxygen saturation; FiO2: fraction of inspired oxygen; PO2: partial pressure of oxygen; PCO2: partial pressure of carbon dioxide; IL: interleukin; VEGF-A: vascular endothelial growth factor.
Overall, 452 (61.7%) out of 732 patients were hospitalised for COVID-19 for a median (IQR) duration of 14 (8–22) days.
Mechanical ventilation was necessary for 113 patients; 46 (7.4%) out of 626 requiring intubation, while 67 (10.7%) out of 626 received noninvasive ventilation.
Azithromycin, hydroxychloroquine, antiretroviral drugs, corticosteroids and anticoagulants were the drugs most frequently prescribed during the first wave of the epidemic (table 3).
The number of comorbidities in the patients who survived and died are summarised in table 3 and annex 3. Cardiovascular and endocrine comorbidities were the most commonly observed; mostly hypertension and diabetes mellitus.
Age, gender and mortality
Out of 767 patients in the cohort, 85 (11.08%) died; 41 (14.2%) out of 289 in Europe and 44 (9.2%) out of 478 outside Europe (p=0.03) (table 4).
TABLE 4.
Characteristics of the patients alive or deceased in the coronavirus disease 2019 (COVID-19) cohort and stratification by geographical origin
| Vital status | Geographical location | |||||
| Alive # | Deceased ¶ | p-value | Europe | Not Europe | p-value | |
| Patients | 682 | 85 | 289 | 478 | ||
| Age, years | 41 (30–55) | 65 (48–77) | <0.0001 | 49 (36–63) | 39 (29–54) | <0.0001 |
| Age ≥65 years | 83 (12.2) | 44 (51.8) | <0.0001 | 68 (23.5) | 59 (12.3) | <0.0001 |
| Males | 470 (68.9) | 70 (82.4) | 0.01 | 209 (72.3) | 331 (69.3) | 0.37 |
| Non-European | 434 (63.6) | 44 (51.8) | 0.03 | |||
| ≥1 comorbidity | 343 (50.3) | 73 (85.9) | <0.0001 | 183 (63.3) | 233 (48.7) | <0.0001 |
| ≥2 comorbidities | 147 (21.6) | 49 (57.7) | <0.0001 | |||
| Number of comorbidities | 1 (0–1) | 2 (1–4) | <0.0001 | |||
| Diabetes mellitus | 125 (18.3) | 32 (37.7) | <0.0001 | 63 (21.8) | 94 (19.7) | 0.48 |
| Cardiovascular disease | 105 (15.4) | 41 (48.2) | <0.0001 | 79 (27.3) | 67 (14.0) | <0.0001 |
| Chronic respiratory disease | 71 (10.4) | 22 (25.9) | <0.0001 | 46 (15.9) | 47 (9.8) | 0.01 |
| HIV | 68 (10.0) | 12 (14.1) | 0.14 | 25 (8.7) | 55 (11.5) | 0.21 |
| Chronic liver disease | 50 (7.3) | 10 (11.8) | 0.15 | 47 (16.3) | 13 (2.7) | <0.0001 |
| Chronic renal disease | 38 (5.6) | 15 (17.7) | <0.0001 | 33 (11.4) | 20 (4.2) | <0.0001 |
| Invasive ventilation | 15 (2.7) | 31 (41.3) | <0.0001 | 14 (5.0) | 32 (9.3) | 0.04 |
| Previous TB | 200 (30.2) | 34 (40.0) | 0.07 | 79 (27.7) | 155 (33.6) | 0.09 |
| Hospitalisation due to COVID-19 | 381 (59.0) | 71 (83.5) | <0.0001 | 220 (76.4) | 232 (52.3) | <0.0001 |
| Duration of hospitalisation, days | 14 (8–22) | 10 (4–24) | 0.007 | 14 (9–22) | 13 (6–24) | 0.11 |
| Concomitant hospitalisation due to TB/COVID-19 co-infection | 216 (31.7) | 34 (40.0) | 0.19 | 136 (31.7) | 114(40.0) | 0.005 |
| Duration of concomitant hospitalisation | 16 (10–24) | 8 (4–20) | 0.11 | 16 (11–22) | 14 (6–26) | 0.36 |
| Death | 41 (14.2) | 44 (9.2) | 0.03 | |||
| Death at age ≥65 years | 26/41 (63.4) | 18/44 (40.9) | 0.04 | |||
| Age of patients who died, years | 70 (59–80.5) | 57.5 (44.3–71.8) | 0.004 | |||
| Dead with ≥1 comorbidity | 38/183 (20.8) | 35/233 (15.0) | 0.13 | |||
| Dead with diabetes mellitus | 19/63 (30.2) | 13/94 (13.8) | 0.01 | |||
| Dead with cardiovascular disease | 26/79 (32.9) | 15/67 (22.4) | 0.16 | |||
| Dead with chronic respiratory disease | 13/46 (28.3) | 9/47 (19.2) | 0.30 | |||
| Dead with HIV | 3 (12.0) | 9 (16.4) | 0.61 | |||
| Dead with chronic liver disease | 7/47 (14.9) | 3/13 (23.1) | 0.68 | |||
| Dead with chronic renal disease | 11/33 (33.3) | 4/20 (20.0) | 0.30 | |||
| Dead with active TB | 20/206 (9.7) | 31/307 (10.1) | 0.89 | |||
Data are presented as n, median (interquartile range) or n/N (%), unless otherwise stated. TB: tuberculosis. #: all patients in the cohort based on the latest information available (see text and figure 3 for details); ¶: all patients who died based on the latest information available. The detailed causes of death are reported in the text and in figure 3.
Overall, the median (IQR) age of the patients in Europe was higher than outside Europe: 49 (36–63) years versus 39 (29–54) years (p<0.0001). This is also true for the patients who died (70 years, 59–80.5 years versus 57.5 years, 44.3–71.8 years; p=0.004). In Europe, more patients aged >65 years died in comparison with the rest of the world (26 out of 41, 63.4% versus 18 out of 44, 40.9%; p=0.04).
More males were present among those who died versus those who survived (70 out of 85, 82.4% versus 470 out of 682, 68.9%; p=0.01) (table 4).
Comorbidities and their impact on COVID-19 mortality
The comorbidities per patient and geographical location, grouped into main categories, are summarised in tables 3 and 4, and annex 3.
Patients with more than one comorbidity were more frequently observed among those who died (73 out of 85, 85.9% versus 343 out of 682, 50.3%; p<0.0001) and in Europe (183 out of 289, 63.3% versus 233 out of 478, 48.7%; p<0.0001) (table 4)
In table 5, the results of the logistic regression analysis to assess the relationship between demographic, epidemiological, clinical variables and mortality are summarised.
TABLE 5.
Logistic regression analysis to assess the relationship between demographic, epidemiological, clinical variables and mortality
| Univariable analysis | Multivariable analysis | |||
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Age, years (10-year increase) | 1.82 (1.58–2.09) | <0.0001 | 1.93 (1.60–2.32) | <0.0001 |
| Male (yes versus no) | 2.08 (1.16–3.71) | 0.014 | 2.92 (1.38–6.16) | 0.005 |
| ≥1 comorbidity (yes versus no) | 6.01 (3.21–11.27) | <0.0001 | ||
| Diabetes mellitus (yes versus no) | 2.69 (1.67–4.35) | <0.0001 | ||
| Cardiovascular disease (yes versus no) | 5.12 (3.19–8.22) | <0.0001 | ||
| Chronic respiratory disease (yes versus no) | 3.00 (1.74–5.18) | <0.0001 | ||
| HIV (yes versus no) | 1.48 (0.77–2.87) | 0.241 | ||
| Chronic liver disease (yes versus no) | 1.69 (0.82–3.46) | 0.155 | ||
| Chronic renal disease (yes versus no) | 3.00 (1.74–5.18) | <0.0001 | ||
| Invasive ventilation (yes versus no) | 25.18 (12.64–50.13) | <0.0001 | 28.22 (1.37–64.39) | <0.0001 |
| Active TB (yes versus no) | 1.5 (1.0–2.5) | 0.069 | ||
| Presence of key symptoms (yes versus no) | 49.3 (19.7–123.9) | <0.0001 | ||
| Hospitalisation due to COVID-19 (yes versus no) | 3.54 (1.95–6.41) | <0.0001 | ||
| Duration of hospitalisation (1-day increase) | 0.98 (0.96–1.01) | 0.072 | ||
| Europe (yes versus no) | 1.63 (1.04–2.57) | 0.034 | ||
Multivariable model −2 log likelihood: 301.6, p<0.0001; percentage of cases correctly classified: 91%; area under the curve: 0.89 (0.86–0.91). TB: tuberculosis; COVID-19: coronavirus disease 2019.
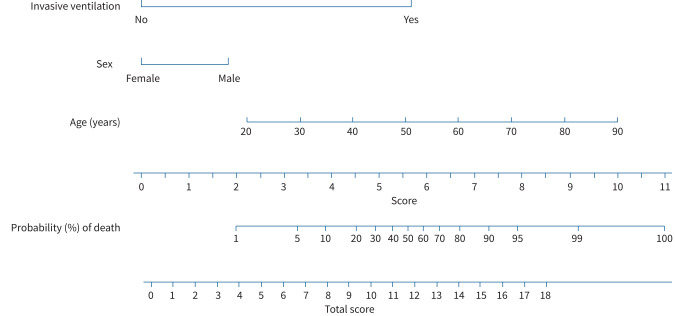
In the univariate analysis on mortality the following variables reached statistical significance: age, being male, having more than one comorbidity, type 2 diabetes mellitus, cardiovascular disease, chronic respiratory disease, chronic renal disease, presence of key symptoms, invasive ventilation and hospitalisation due to COVID-19 (table 5). The final multivariable logistic regression model included age (10-year increase), male gender and need for invasive ventilation as independent contributors to mortality (table 5). Adding other covariates did not significantly increase the performance of the model. A nomogram for the estimation of the risk of death was generated on the basis of the final multivariable model. As depicted in figure 2, each indicator is measured, and the corresponding points are assigned using the row “score”. Thus, the sum is reported on the row “total score”, and the corresponding probability of death is identified in the row “probability (%) of death”.
FIGURE 2.
Nomogram for the estimation of the risk of death, generated on the basis of the multivariable logistic regression analysis. As depicted, each indicator is measured, and the corresponding points are assigned using the row “score”. Thus, the sum is reported on the row “total score”, and the corresponding probability of the outcome is identified in the row “probability (%) of death”. As an example on how to use this nomogram, an 80-year-old woman not requiring invasive ventilation would have a probability of death <20%. In contrast, an 80-year-old woman requiring invasive ventilation during hospitalisation would have a probability of death >80%.
In the overall cohort, the presence of previous TB was higher among the patients who died than in those who survived (34 out of 85, 40.0% versus 200 out of 682, 30.2%), the difference not being statistically significant; no difference was found between European versus non-European patients (table 4).
Patients with active TB had higher probability of death (OR 1.5) compared with those with previous TB (table 5).
Clinical outcomes of COVID-19 patients
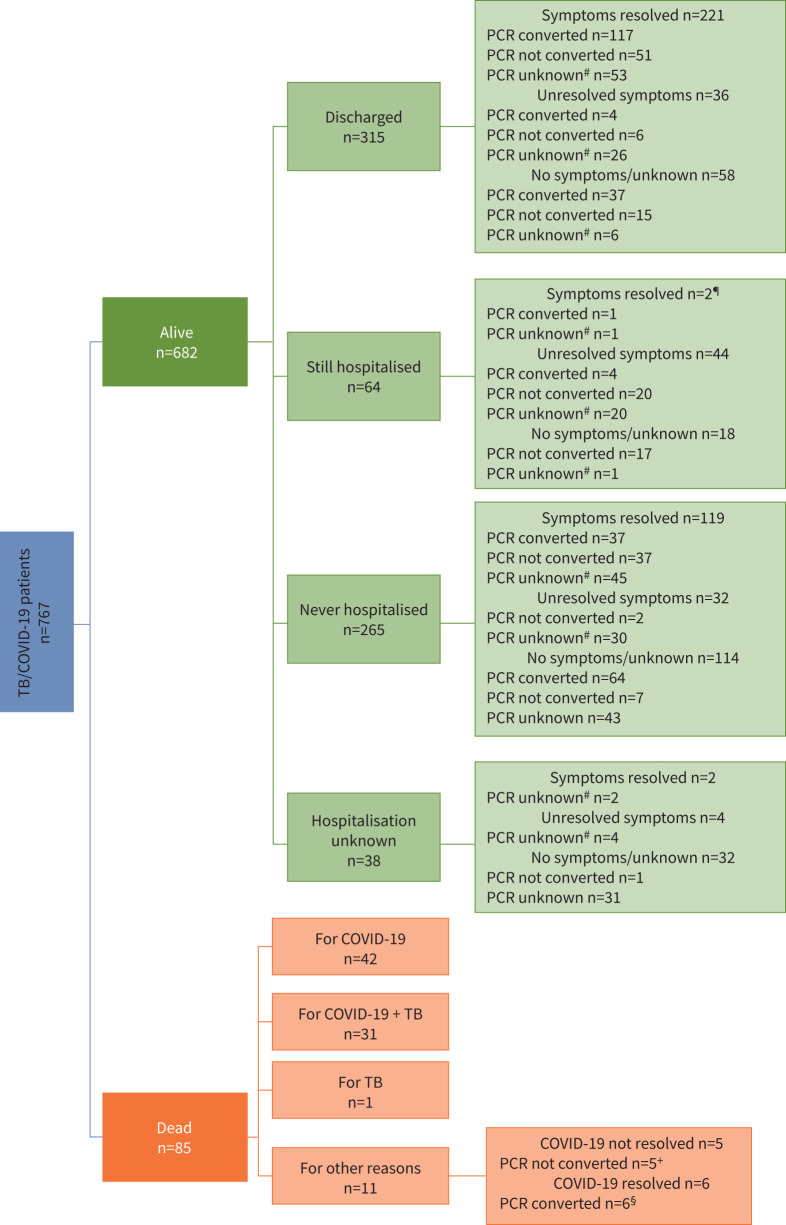
Out of 767 patients (figure 3), 682 (88.9%) survived and 85 (11.1%) died. Among 682 patients surviving, 379 (55.6%) were hospitalised, of whom 315 were discharged (221 with symptoms resolved, 36 not resolved and 58 with no or unknown symptoms) and 64 were still in hospital at the time of the analysis (two with symptoms resolved, 44 not resolved and 18 with no or unknown symptoms); 265 patients were never hospitalised (119 with symptoms resolved, 32 not resolved and 114 with no or unknown symptoms). No detailed information on hospitalisation was available for 38 patients (two with symptoms resolved, four not resolved and 32 with no or unknown symptoms).
FIGURE 3.
Clinical outcome of coronavirus disease 2019 (COVID-19) among tuberculosis (TB)/COVID-19 patients. #: including patients with PCR not done; ¶: n=2 with symptoms resolved remain hospitalised for TB; +: n=2 for multiple comorbidities, n=1 for suspected cancer, n=1 for sarcoidosis, n=1 for HIV; §: n=2 for sepsis, n=2 for multiple comorbidities, n=1 for bilateral Gram-negative nosocomial pneumonia, Morganella morganii, n=1 for pulmonary thromboembolism (with COVID-19 clinically diagnosed and PCR unknown).
Among the patients who died, 42 (49.4%) out of 85 died from COVID-19; 31 (36.5%) out of 85 from COVID-19 and TB and one (1.2%) out of 85 died from TB only. Among the patients who died for other reasons, five (5.9%) died with COVID-19 (n=2 multiple comorbidities, n=1 presumptive cancer, n=1 sarcoidosis, n=1 HIV); the remaining six (7.0%) died after resolution of COVID-19 (n=2 sepsis, n=2 multiple comorbidities, n=1 pneumonia, n=1 pulmonary thromboembolism).
Discussion
Our study described, for the first time, the features of the TB and COVID-19 co-infected individuals in a large cohort of 767 patients from 172 centres in 34 countries with specific focus on the risk factors for mortality and other outcomes.
The main characteristics of the cohort confirmed our previously described findings from the pilot study [18]: the patients are young (median age 44 years), and the majority are male, with drug-susceptible pulmonary TB. The commonest symptoms reported were fever, dry cough and dyspnoea, with approximately one out of 10 patients with typical symptoms for COVID-19 (olfactory and taste disorders). The majority of patients who underwent CT imaging presented typical or atypical ground-glass opacities, confirming the relevance of this radiological sign for the diagnosis of COVID-19 [25], which co-exist with the radiological features of TB (cavities and infiltrates).
Interestingly, 74% of the patients had TB diagnosed before COVID-19 (including 234 patients with previous TB, corresponding to 31.3% of the whole cohort); 16.5% were diagnosed within the same week (the presence of signs and symptoms prompted the clinicians to undertake imaging, which revealed potentially pre-existing TB on top of COVID-19) [18]; and 9.5% had COVID-19 diagnosed first.
A key question from our preliminary study [18] was on the role of SARS-CoV-2 in the progression of TB infection to disease as observed in other viral diseases (e.g. HIV) [5, 18]. While our study is not specifically designed to answer this question, we found 71 patients who had COVID-19 diagnosed before TB; of these, 35 were diagnosed >30 days prior (with sufficient time to develop TB disease) and 33 had pulmonary TB. Of 25 patients with complete radiological information, 12 (48%) had cavities, a condition which is likely to develop in >30 days. Therefore, this indirect evidence from our data suggests that COVID-19 may not have a major role in advancing TB infection to TB disease. Further longitudinal studies observing the patients with TB infection and COVID-19 over time and comparing the proportion of those who acquire TB disease with a control group without COVID-19 may offer better insight to an interaction.
The TB/COVID-19 patients with higher mortality were male, belonged to older age groups and underwent invasive ventilation, with more comorbidities than those with no need for (invasive) ventilation. These determinants of death are similar to those described for mono-disease COVID-19 or TB [4, 26].
Another important question arising from previous studies [10, 14, 18, 27] relates to the resources required for managing patients with TB and COVID-19. The study results indicate that an important proportion of patients needed ventilation (18%, of whom 7.4% required intubation) and 32% supplemental oxygen, the vast majority during hospitalisation (61.7% of the patients required a median of 14 days of admission because of COVID-19, in addition to those needed for TB). The need for competent staff to manage TB/COVID patients with respiratory failure has been a problem in several countries, where clinicians working within the TB programme were redeployed to work within the COVID-19 emergency [6–9, 14]. Evidence is continuing to emerge on the negative impact of COVID-19 on TB services [9, 28]. A recent global study indicates a significant decline in TB and TB infections diagnosed, with an increase of telemedicine use in 2020 in comparison with 2019 [9]. Reduction in the performance of global TB detection and care due to COVID-19 pandemic are expected to have devastating impact on TB mortality [29].
An issue that has recently gained increasing interest is that of post-TB lung disease (PTLD), as 13–68% of new TB cases and 75–96% of patients with multidrug-resistant TB completing anti-TB treatment suffer from TB sequelae [30, 31]. This condition [30–36] includes obstructive, restrictive or mixed-pattern lung function abnormalities, reduced exercise capacity and impaired quality of life. A summary of clinical standards to adequately manage PTLD, which includes post-treatment evaluation and identification of patients with sequelae likely to benefit from pulmonary rehabilitation, has been published recently [36].
Similarly, COVID-19 appears to commonly cause sequelae (the so-called “long-COVID” syndrome) [37–39], characterised by fatigue, sleeping difficulties, low grade fever, depression, anxiety, impacting cardiac, pulmonary and renal functions, and discussions are ongoing on the potential role of post-COVID-19 rehabilitation [14, 40–42].
A combination of post-COVID-19 and PTLD sequelae and the need for assessment and potential follow-up and rehabilitation can pose additional stress on health services in terms of human and economic resources.
Our study has several strengths, including a large sample size and the inclusion of countries from all continents. Furthermore, several variables collected in our study are not routinely collected in the surveillance systems at country level, making the study important to better understand the TB/COVID-19 interactions and to design ad hoc studies aimed at answering specific outstanding questions. Furthermore, approximately half of the countries/territories (18 out of 34) provided population-based data representative of their respective TB/COVID patients.
Among the main study limitations, Africa and Asia were under-represented; the number of paediatric patients was limited (six patients, two of them aged <1 year); some centres were unable to provide all the information requested on a few variables (particularly laboratory data); and ~10% of the patients had COVID-19 diagnosed based on the clinical and radiological findings, following the respective countries’ policy during the emergency phases of the pandemic. The timing of our study does not allow comment on the differential impact of emerging SARS-CoV-2 variants and TB, which will require ongoing monitoring and review.
In addition, as the cohort was composed of TB and COVID-19 patients, it was not possible undertake a comparative analysis against patients with TB or COVID-19 alone. In addition, it was not possible to draw conclusions on the effect of the different drugs prescribed, and we note that our cohort was prescribed a range of therapies by treating clinicians, including some now demonstrated to have no impact on COVID-19 outcomes. Future studies looking at the cohort will be able to examine the effect of steroids or monoclonal antibodies.
Furthermore, it was not possible to perform the analysis of TB-specific outcomes as an important proportion of patients are still undergoing anti-TB treatment.
The study will continue to evaluate early and final anti-TB treatment outcomes through periodic updates, as to make the “cohort” a “living” one.
Conclusions
This first description of a large global cohort provides important information for clinical and public health management of patients co-infected by TB and COVID-19. The similarity of signs and symptoms for the two diseases has been confirmed alongside the importance of the radiological presence of ground-glass opacities for the diagnosis of COVID-19. Preliminary information seems to suggest that COVID-19 is unlikely to represent a major determinant triggering TB infection to active TB.
The high (12%) mortality of co-infected patients may be explained by older age and male gender, with an important contribution also played by comorbidities (particularly cardiovascular disease and diabetes mellitus). The reason why males died more than females may be explained by the potential higher prevalence of comorbidities and risk factors.
Efforts to prevent SARS-CoV-2 infection in TB patients is warranted, including reinforcing of social distancing, mask wearing and other measures as appropriate to local epidemiology. Encouraging vaccination against SARS-CoV-2 for people with a current or past diagnosis of TB will also be valuable in preventing morbidity and mortality related to COVID-19 disease.
The combination of COVID-19 and TB adds to the clinical complexity in patient management (e.g. need for supplemental oxygen, invasive or noninvasive ventilation and specialised staff), significantly impacting health services. The impact of COVID-19 on long-term pulmonary sequelae in patients with TB and the need for pulmonary rehabilitation is yet to be determined.
As patients reported similar symptoms, it advisable for health services to screen patients for both diseases whenever possible, taking advantage of the possibility to obtain imaging rapidly, and stimulating adoption of rapid molecular testing for TB and COVID-19. Although our study does not provide specific data on this, it seems clinically advisable to treat both conditions as soon as possible following international recommendations.
Last, but not least, the experience gained during the COVID-19 pandemic will allow us to make better use of telemedicine interventions, thus reducing the burden of physical access to health services and transmission. Unnecessary hospitalisation should be actively discouraged [7, 9, 27].
Overall, the data suggest that TB and COVID-19 are a “cursed duet” and need immediate attention.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material: annexes 1 to 3 ERJ-02538-2021.Supplement (310.9KB, pdf)
Shareable PDF
Acknowledgement
The project is supported by the Global Tuberculosis Network (GTN). This article belongs to the scientific activities of the WHO Collaborating Centre for Tuberculosis and Lung Diseases, Tradate, ITA-80, 2020–2024- GBM/RC/LDA.
Footnotes
The contributors of the TB/COVID-19 Global Study Group are: Nicolas Casco, Alberto Levi Jorge, Domingo Juan Palmero (Argentina); Jan-Willem Alffenaar, Justin Denholm, Greg J. Fox, Wafaa Ezz, Jin-Gun Cho (Australia); Alena Skrahina, Varvara Solodovnikova (Belarus); Pierre Bachez, Alberto Piubello (Belgium); Marcos Abdo Arbex, Tatiana Alves, Marcelo Fouad Rabahi, Giovana Rodrigues Pereira, Roberta Sales, Denise Rossato Silva (Brazil); Muntasir M. Saffie (Canada); Ruth Caamaño Miranda, Viviana Cancino, Monica Carbonell, Catalina Cisterna, Clorinda Concha, Arturo Cruz, Nadia Escobar Salinas, Macarena Espinoza Revillot, Joaquín Farías Valdés, Israel Fernandez, Ximena Flores, Patricia Gallegos Tapia, Ana Garavagno, Carolina Guajardo Vera, Martina Hartwig Bahamondes, Luis Moyano Merino, Eduardo Muñoz, Camila Muñoz, Indira Navarro, Jorge Navarro Subiabre, Carlos Ortega, Sofia Palma, Ana María Pradenas, Gloria Pereira, Patricia Perez Castillo, Mónica Pinto, Rolando Pizarro, Francisco Rivas Bidegain, Patricia Rodriguez, Cristina Sánchez, Angeles Serrano Salinas, Aline Soto, Carolina Taiba, Margarita Venegas, Maria Soledad Vergara Riquelme, Evelyn Vilca, Claudia Villalón, Edith Yucra (Chile); Yang Li (China); Andres Cruz, Beatriz Guelvez, Regina Victoria Plaza, Kelly Yoana Tello Hoyos (Colombia); Claire Andréjak, François-Xavier Blanc, Samir Dourmane, Antoine Froissart, Armine Izadifar, Frédéric Rivière, Frédéric Schlemmer (France); Katerina Manika (Greece); Boubacar Djelo Diallo, Souleymane Hassane-Harouna (Republic of Guinea); Norma Artiles, Licenciada Andrea Mejia (Honduras); Nitesh Gupta, Pranav Ish, Gyanshankar Mishra, Samridhi Sharma, Rupak Singla, Zarir F. Udwadia (India); Francesca Alladio, Fabio Angeli, Andrea Calcagno, Rosella Centis, Luigi Ruffo Codecasa, Lia D'Ambrosio, Angelo De Lauretis, Susanna Esposito, Beatrice Formenti, Alberto Gaviraghi, Vania Giacomet, Delia Goletti, Gina Gualano, Alberto Matteelli, Giovanni Battista Migliori, Ilaria Motta, Fabrizio Palmieri, Emanuele Pontali, Tullio Prestileo, Niccolò Riccardi, Laura Saderi, Matteo Saporiti, Giovanni Sotgiu, Claudia Stochino, Marina Tadolini, Alessandro Torre, Simone Villa, Dina Visca (Italy); Edvardas Danila, Saulius Diktanas (Lithuania); Ruy López Ridaura, Fátima Leticia Luna López, Marcela Muñoz Torrico, Adrian Rendon (Mexico); Onno W. Akkerman (the Netherlands); Mahamadou Bassirou Souleymane (Niger); Seif Al-Abri, Fatma Alyaquobi, Khalsa Althohli (Oman); Edwin Aizpurua, Rolando Gonzales, Julio Jurado, Alejandra Loban (Panama); Sarita Aguirre, Rosarito Coronel Teixeira, Viviana De Egea, Sandra Irala, Angélica Medina, Guillermo Sequera, Natalia Sosa, Fátima Vázquez (Paraguay); Félix K. Llanos-Tejada, Selene Manga, Renzo Villanueva-Villegas (Peru); David Araujo, Raquel Duarte, Tânia Sales Marques (Portugal); Victor Ionel Grecu, Adriana Socaci (Romania); Olga Barkanova, Maria Bogorodskaya, Sergey Borisov, Andrei Mariandyshev, Anna Kaluzhenina (Russian Federation); Tatjana Adzic Vukicevic, Maja Stosic (Serbia); Darius Beh, Deborah Ng, Catherine W.M. Ong (Singapore); Ivan Solovic (Slovakia); Keertan Dheda, Phindile Gina (South Africa); José A Caminero, José Cardoso-Landivar, Maria Luiza De Souza Galvão, Angel Dominguez-Castellano, José-María García-García, Israel Molina Pinargote, Sarai Quirós Fernandez, Adrián Sánchez-Montalvá, Eva Tabernero Huguet, Miguel Zabaleta Murguiondo (Spain); Pierre-Alexandre Bart, Jesica Mazza-Stalder (Switzerland); Freya Bakko, James Barnacle, Annabel Brown, Shruthi Chandran, Kieran Killington, Kathy Man, Padmasayee Papineni, Simon Tiberi, Natasa Utjesanovic, Dominik Zenner (UK); Jasie L. Hearn, Scott Heysell, Laura Young (Virginia, USA).
This article has supplementary material available from erj.ersjournals.com
This article has an editorial commentary: https://doi.org/10.1183/13993003.00149-2022
Conflict of interest: None declared.
References
- 1.World Health Organization (WHO) . Global Tuberculosis Report 2021. Geneva, WHO, 2021. apps.who.int/iris/bitstream/handle/10665/336069/9789240013131-eng.pdf/ Date last accessed: 19 September 2021. [Google Scholar]
- 2.Guan WJ, Ni ZY, Hu Y, et al. . Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382: 1708–1720. doi: 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu XW, Wu XX, Jiang XG, et al. . Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ 2020; 368: m606. doi: 10.1136/bmj.m606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou F, Yu T, Du R, et al. . Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395: 1054–1062. doi: 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ong CWM, Migliori GB, Raviglione M, et al. . Epidemic and pandemic viral infections: impact on tuberculosis and the lung. A consensus by the World Association for Infectious Diseases and Immunological Disorders (WAidid), Global Tuberculosis Network (GTN) and members of ESCMID Study Group for Mycobacterial Infections (ESGMYC). Eur Respir J 2020; 56: 2001727. doi: 10.1183/13993003.01727-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Migliori GB, Thong PM, Akkerman O, et al. . Worldwide effects of coronavirus disease pandemic on tuberculosis services, January–April 2020. Emerg Infect Dis 2020; 26: 2709–2712. doi: 10.3201/eid2611.203163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McQuaid CF, Vassall A, Cohen T, et al. . The impact of COVID-19 on TB: a review of the data. Int J Tuberc Lung Dis 2021; 25: 436–446. doi: 10.5588/ijtld.21.0148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McQuaid CF, McCreesh N, Read JM, et al. . The potential impact of COVID-19-related disruption on tuberculosis burden. Eur Respir J 2020; 56: 2001718. doi: 10.1183/13993003.01718-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Migliori GB, Thong PM, Alffenaar JW, et al. . Gauging the impact of the COVID-19 pandemic on tuberculosis services: a global study. Eur Respir J 2021: 2101786. doi: 10.1183/13993003.01786-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Motta I, Centis R, D'Ambrosio L, et al. . Tuberculosis, COVID-19 and migrants: preliminary analysis of deaths occurring in 69 patients from two cohorts. Pulmonology 2020; 26: 233–240. doi: 10.1016/j.pulmoe.2020.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zenner D. Time to regain lost ground: tuberculosis in the COVID-19 era. Euro Surveill 2021; 26: 2100564. doi: 10.2807/1560-7917.ES.2021.26.24.2100564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hogan AB, Jewell BL, Sherrard-Smith E, et al. . Potential impact of the COVID-19 pandemic on HIV, tuberculosis, and malaria in low-income and middle-income countries: a modelling study. Lancet Glob Health 2020; 8: e1132–e1141. doi: 10.1016/S2214-109X(20)30288-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cilloni L, Fu H, Vesga JF, et al. . The potential impact of the COVID-19 pandemic on the tuberculosis epidemic a modelling analysis. EClinicalMedicine 2020; 28: 100603. doi: 10.1016/j.eclinm.2020.100603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Visca D, Ong CWM, Tiberi S, et al. . Tuberculosis and COVID-19 interaction: a review of biological, clinical and public health effects. Pulmonology 2021; 27: 151–165. doi: 10.1016/j.pulmoe.2020.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stochino C, Villa S, Zucchi P, et al. . Clinical characteristics of COVID-19 and active tuberculosis co-infection in an Italian reference hospital. Eur Respir J 2020; 56: 2001708. doi: 10.1183/13993003.01708-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petrone L, Petruccioli E, Vanini V, et al. . Coinfection of tuberculosis and COVID-19 limits the ability to in vitro respond to SARS-CoV-2. Int J Infect Dis 2021; in press [https://doi.org/S1201-9712(21)00176-4]. doi:10.1016/j.ijid.2021.02.090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riou C, du Bruyn E, Stek C. Relationship of SARS-CoV-2-specific CD4 response to COVID-19 severity and impact of HIV-1 and tuberculosis coinfection. J Clin Invest 2021; 131: e149125. doi: 10.1172/JCI149125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tadolini M, Codecasa LR, García-García J-M, et al. . Active tuberculosis, sequelae and COVID-19 co-infection: first cohort of 49 cases. Eur Respir J 2020; 56: 2001398. doi: 10.1183/13993003.01398-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boulle A, Davies MA, Hussey H, et al. . Risk factors for coronavirus disease 2019 (COVID-19) death in a population cohort study from the Western Cape Province, South Africa. Clin Infect Dis 2020; 73: e2005–e2015. doi: 10.1093/cid/ciaa1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sy KTL, Haw NJL, Uy J. Previous and active tuberculosis increases risk of death and prolongs recovery in patients with COVID-19. Infect Dis 2020; 52: 902–907. doi: 10.1080/23744235.2020.1806353 [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization (WHO) . The WHO Global Clinical Platform for COVID-19. www.who.int/teams/health-care-readiness-clinical-unit/covid-19/data-platform/ Date last accessed: 19 September 2021.
- 22.Casco N, Jorge AL, Palmero D, et al. . TB and COVID-19 co-infection: rationale and aims of a global study. Int J Tuberc Lung Dis 2021; 25: 78–80. doi: 10.5588/ijtld.20.0786 [DOI] [PubMed] [Google Scholar]
- 23.Borisov S, Danila E, Maryandyshev A, et al. . Surveillance of adverse events in the treatment of drug-resistant tuberculosis: first global report. Eur Respir J 2019; 54: 1901522. doi: 10.1183/13993003.01522-2019 [DOI] [PubMed] [Google Scholar]
- 24.Borisov SE, Dheda K, Enwerem M, et al. . Effectiveness and safety of bedaquiline-containing regimens in the treatment of MDR- and XDR-TB: a multicentre study. Eur Respir J 2017; 49: 1700387. doi: 10.1183/13993003.00387-2017 [DOI] [PubMed] [Google Scholar]
- 25.Wen Z, Chi Y, Zhang L, et al. . Coronavirus disease 2019: initial detection on chest CT in a retrospective multicenter study of 103 Chinese patients. Radiol Cardiothorac Imaging 2020; 2: e200092. doi: 10.1148/ryct.2020200092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duarte R, Aguiar A, Pinto M, et al. . Different disease, same challenges: social determinants of tuberculosis and COVID-19. Pulmonology 2021; 27: 338–344. doi: 10.1016/j.pulmoe.2021.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Migliori GB, Visca D, van den Boom M, et al. . Tuberculosis, COVID-19 and hospital admission: consensus on pros and cons based on a review of the evidence. Pulmonology 2021; 27: 248–256. doi: 10.1016/j.pulmoe.2020.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alves A, Aguiar A, Migliori GB, et al. . COVID -19 related hospital re-organization and trends in tuberculosis diagnosis and admissions: reflections from Portugal. Arch Bronconeumol 2021; in press [ 10.1016/j.arbres.2021.09.005]. doi: [DOI] [PubMed] [Google Scholar]
- 29.Glaziou P. Predicted impact of the COVID-19 pandemic on global tuberculosis deaths in 2020. medRxiv 2021; preprint [ 10.1101/2020.04.28.20079582]. doi: 10.1101/2020.04.28.20079582 [DOI] [Google Scholar]
- 30.Visca D, Centis R, D'Ambrosio L, et al. . The need for pulmonary rehabilitation following tuberculosis treatment. Int J Tuberc Lung Dis 2020; 24: 720–722. doi: 10.5588/ijtld.20.0030 [DOI] [PubMed] [Google Scholar]
- 31.Visca D, Zampogna E, Sotgiu G, et al. . Pulmonary rehabilitation is effective in patients with tuberculosis pulmonary sequelae. Eur Respir J 2019; 53: 1802184. doi: 10.1183/13993003.02184-2018 [DOI] [PubMed] [Google Scholar]
- 32.Allwood BW, Stolbrink M, Baines N, et al. . Persistent chronic respiratory symptoms despite TB cure is poorly correlated with lung function. Int J Tuberc Lung Dis 2021; 25: 262–270. doi: 10.5588/ijtld.20.0906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Allwood BW, van der Zalm MM, Amaral AFS, et al. . Post-tuberculosis lung health: perspectives from the First International Symposium. Int J Tuberc Lung Dis 2020; 24: 820–828. doi: 10.5588/ijtld.20.0067 [DOI] [PubMed] [Google Scholar]
- 34.Muñoz-Torrico M, Cid-Juárez S, Gochicoa-Rangel L, et al. . Functional impact of sequelae in drug-susceptible and multidrug-resistant tuberculosis. Int J Tuberc Lung Dis 2020; 24: 700–705. doi: 10.5588/ijtld.19.0809 [DOI] [PubMed] [Google Scholar]
- 35.Muñoz-Torrico M, Rendon A, Centis R, et al. . Is there a rationale for pulmonary rehabilitation following successful chemotherapy for tuberculosis? J Bras Pneumol 2016; 42: 374–385. doi: 10.1590/S1806-37562016000000226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Migliori GB, Marx FM, Ambrosino N, et al. . Clinical standards for the assessment, management, and rehabilitation of post-TB lung disease. Int J Tuberc Lung Dis 2021; 25: 797–813. doi: 10.5588/ijtld.21.0425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zampogna E, Paneroni M, Belli S, et al. . Pulmonary rehabilitation in patients recovering from COVID-19. Respiration 2021; 100: 416–422. doi: 10.1159/000514387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zampogna E, Migliori GB, Centis R, et al. . Functional impairment during post-acute COVID-19 phase: preliminary finding in 56 patients. Pulmonology 2021; 27: 452–455. doi: 10.1016/j.pulmoe.2020.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Visca D, Migliori GB, Dinh-Xuan AT, et al. . The role of blood gas analysis in the post-acute phase of COVID-19 pneumonia. Arch Bronconeumol 2021; in press [ 10.1016/j.arbres.2021.06.003].doi: 10.1016/j.arbres.2021.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zampogna E, Ambrosino N, Migliori GB, et al. . Time course of exercise capacity in patients recovering from COVID-19-associated pneumonia. Authors’ reply. J Bras Pneumol 2021; 47: e20210328. doi: 10.36416/1806-3756/e20210328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singh S. Post-COVID rehabilitation. In: Fabre A, Hurst J, Ramjug S, eds. COVID -19 (ERS Monograph). Sheffield, European Respiratory Society, 2021; pp. 197–213.doi:10.6084/m9.FigShare.14096275.v11. [Google Scholar]
- 42.Gramegna A, Mantero M, Amati F, et al. . Post-COVID sequelae. In: Fabre A, Hurst J, Ramjug S, eds. COVID-19 (ERS Monograph). Sheffield, European Respiratory Society, 2021; pp. 180–196. doi:10.6084/m9.figshare.14096263.v1 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material: annexes 1 to 3 ERJ-02538-2021.Supplement (310.9KB, pdf)
This one-page PDF can be shared freely online.
Shareable PDF ERJ-02538-2021.Shareable (230.4KB, pdf)