Abstract
Group B Streptococcus (GBS) is an encapsulated Gram-positive pathogen that causes ascending infections of the reproductive tract during pregnancy. The capsule of this organism is a critical virulence factor that has been implicated in a variety of cellular processes to promote pathogenesis. Primarily comprised of carbohydrates, the GBS capsule, and its synthesis is driven by the capsule polysaccharide synthesis (cps) operon. The cpsE gene within this operon encodes a putative glycosyltransferase that is responsible for the transfer of a Glc-1-P from UDP-Glc to an undecaprenyl lipid molecule. We hypothesized that the cpsE gene product is important for GBS virulence and ascending infection during pregnancy. Our work demonstrates that a GBS cpsE mutant secretes less carbohydrates, has reduced capsule, and forms less biofilm than the wild-type parental strain. We show that compared to the parental strain, the ΔcpsE deletion mutant is more readily taken up by human placental macrophages and has significantly attenuated ability to invade and proliferate in the mouse reproductive tract. Taken together, these results demonstrate that the cpsE gene product is an important virulence factor that aids in GBS colonization and invasion of the gravid reproductive tract.
Keywords: Streptococcus, carbohydrate, capsule, pathogenesis, innate immunity
Graphical Abstract
Streptococcus agalactiae or Group B Streptococcus (GBS) is a gram positive, encapsulated bacterium that colonizes the gastrointestinal and reproductive tract of humans1,2. GBS colonization of the rectovaginal mucosa during pregnancy is a risk factor for invasive ascending infection of the gravid reproductive tract3. Upon invading these tissues, GBS causes inflammation of fetal membranes (chorioamnionitis) which can lead to preterm premature rupture of membranes (PPROM), preterm birth, neonatal sepsis, and maternal-fetal morbidity and mortality1. Intrapartum antibiotic use in developed countries has significantly reduced rates of Group B Streptococcus (GBS)-induced early onset sepsis in full term neonates4. However this treatment does not prevent GBS-induced preterm birth with associated long term neurodevelopmental deficits, nor have there been improvements in rates of late onset sepsis5,6. Additionally, emergence of antibiotic resistance in perinatal strains foreshadows the need for novel treatment approaches7. Understanding mechanisms of GBS virulence is critical to develop effective preventative and therapeutic treatments for pregnant people.
The polysaccharide capsule is a major virulence factor for pathogens in the Streptococcus genus. The streptococcal polysaccharide capsule facilitates evasion of the innate immune response by protecting the bacterial cell from deposition of complement, opsonization, and phagocytosis8-10. Additionally, the capsule mediates interaction with viral pathogens that result in superinfection11. Capsular synthesis occurs via Wzy- and synthase-dependent mechanisms in gram positive bacteria, including streptococci12. Wzy-dependent mechanisms involve cytoplasmic synthesis of polysaccharides anchored to undecaprenyl-phosphate lipid carrier. Subsequent transmembrane transport to the extracellular peptidoglycan environment occurs via the Wzx flippase12. On the cytoplasmic face of the bacterial cell wall, Wzy polymerase further elaborates the lipid-polysaccharide capsular component13.
The GBS capsular polysaccharide (CPS) forms the outermost layer made of repeating structures of the monosaccharides glucose, galactose, N-acetylneuraminic acid and N-acetylglucosamine that define serotypes14. There are 10 GBS CPS serotypes (Ia, Ib, II – IX) that demonstrate varying involvement in specific human disease conditions. For example, CPSIII strains are associated with higher rates of invasive neonatal disease15. Despite variable disease outcomes, all of the CPS structures across serotype share terminal sialic acid (Sia) residues that allow molecular mimicry of human cell surface sialic acids. This allows interaction with Sia-receptors, Siglecs, on innate immune cells that serve to dampen inflammatory responses16. GBS Sia binding to Siglec-9 on platelets inhibits platelet killing of GBS17.
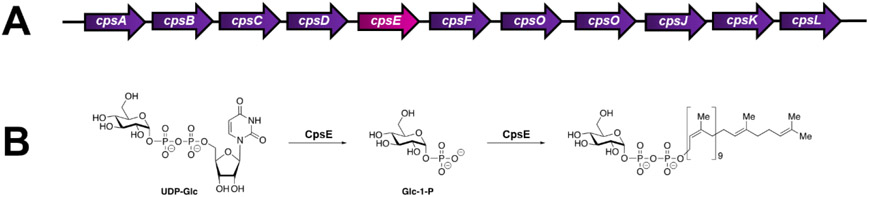
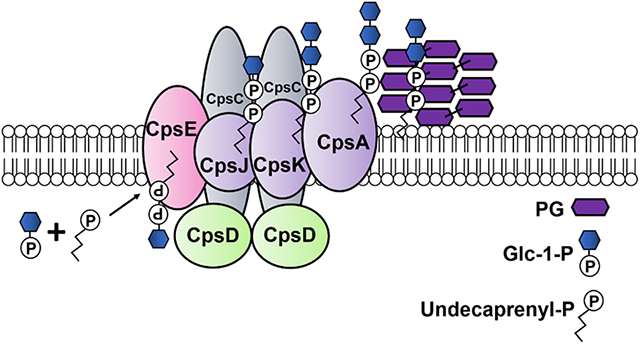
The initiation of GBS CPS synthesis occurs in the bacterial cytoplasm with protein products of the cps operon (Figure 1A), however details of this process are not fully elucidated. The GBS cps operon consists of genes that encode proteins involved in bacterial regulation and transport, capsular assembly and polymerization, and sialic acid synthesis and transport18. The cpsE gene encodes a putative galactosyltransferase involved in capsular assembly and polymerization which likely occurs at the cytoplasmic face of the bacterial cell wall. cpsE adds a monosaccharide to an undecaprenyl phosphate acceptor to initiate assembly of the oligosaccharide repeating unit as shown in Figure 1B. Deletion of the cpsE gene from the type Ia GBS strain 515 (cpsIaE) causes complete loss of reactivity with antiserum to the type Ia GBS polysaccharide19. Despite genetic deletion, the rest of the cps operon is still transcribed indirectly indicating that CpsIaE function is necessary for capsule formation19. Interestingly the role of S. pneumoniae CpsE in capsule formation is serotype specific12.
Figure 1. Genetic architecture and putative activity of CpsE.
A) Genetic arrangement of the cps operon in S. agalactiae GB37. B) Putative activity of CpsE (as determined in S. pneumoniae). CpsE cleaves a glucose-1-phosphate from uridine diphosphate glucose and transfers this to a polyprenyl phosphate acceptor (such as undecaprenyl phosphate).
Biofilm formation is critical for colonization of the host and an important primary step in streptococcal pathogenesis. GBS strains vary in their capacity to form biofilms, and these differences are associated with phylogenetic lineage, isolation source, and capsular serotype20. Previous work has shown that carbohydrate metabolism influences GBS biofilm formation21. Specifically, GBS serotype V strains form strong biofilms in the presence of glucose22. In this work, we sought to determine the role of capsular synthesis in a hypervirulent GBS serotype V in biofilm formation, immune evasion, and ascending infection of a pregnant host.
Results
CpsE is implicated in production of serotype V capsule
To evaluate the production of capsule, wild-type GB37 and the isogenic ΔcpsE mutant cells were evaluated by negative stain transmission electron microscopy (TEM) analyses. TEM of negatively stained whole bacterial cells revealed the production of capsule by GB37 cells (Figure 2A and 2C). Conversely, capsule production was severely attenuated in the isogenic ΔcpsE mutant cells (Figure 2B and 2D), indicating that the cpsE locus is critical for the production of capsule in GBS strain GB37. Prior studies have demonstrated that CpsE function in serotype Ia is upstream of other tested gene products of the cps operon19. Our data demonstrate similar significant loss of capsule and support the role of CpsE early in the production of the capsule in serotype V as well.
Figure 2. Transmission electron microscopy analysis of GBS capsule biosynthesis.
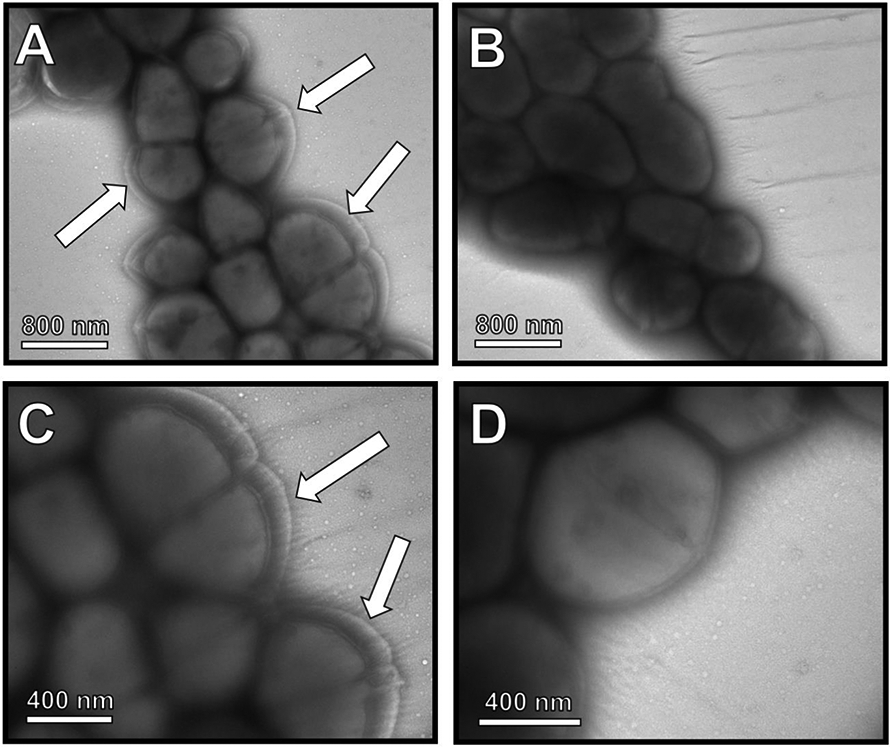
Ammonium molybdate staining and TEM of whole bacterial cells reveals A & C) wild-type GB37 produces a thick capsule visualized by negative stain as a white region surrounding the cell (arrows). B & D) An isogenic ΔcpsE mutant exhibits diminished capsule associated with the cell surface. Magnification is 6500X (A & B) and 11,000X (C & D). Micrographs are representative of three biological replicates.
CpsE aids in GBS biofilm formation
Extracellular surface features like capsule have been implicated in cellular processes, such as biofilm formation, which are critical for colonization and pathogenesis in the vertebrate host. We hypothesized that the isogenic ΔcpsE mutant cells, which form less capsule, would have perturbed biofilm formation compared to the parental strain. To test this, bacteria were grown under static conditions to facilitate biofilm formation. The following day, biofilms were analyzed by standard crystal violet spectrophotometric biofilm quantification (Figure 3). This revealed that the isogenic ΔcpsE mutant was 60% attenuated in its ability to form biofilms compared to the parental strain (P=0.0039, paired Student’s t test, P=0.0019, unpaired Student’s t test with Welch’s correction). Interestingly, the ΔcpsE and the WT parental strain have no significant difference in growth rate in vitro (Figure S1, panel A). High resolution scanning electron microscopy also showed that the isogenic ΔcpsE mutant had sparse cells adhering to the abiotic substrate (glass coverslip) compared to the parental strain which formed robust biofilms. Together, these results indicate that GBS biofilm formation is aided by the cpsE locus.
Figure 3. Analysis of the role of cpsE in GBS biofilm formation.
A) Bacterial biofilm was visualized with crystal violet stain (purple) which revealed that GB37 forms robust biofilm when cultured in THB supplemented with glucose. The isogenic ΔcpsE mutant has diminished capacity to form biofilms on polystyrene. B) Quantification of biofilm by spectrophotometric measurement, (bars indicate mean optical density of 560 nm (OD560) of solubilized crystal violet in ratio to cellular density (OD600) prior to staining) indicates wild-type GB37 forms significantly more quantifiable biofilm than the isogenic ΔcpsE mutant. High-resolution scanning electron microscopy imaging reveals wild-type GB37 forms larger, tertiary architectural structures of cells with well-formed channels between cellular clusters (C & D). Conversely, the isogenic ΔcpsE mutant adheres sparsely to the abiotic surface and rarely forms tertiary cellular structures. C & E) 5,000X magnification. D & F) 10,000X magnification. Bars indicate mean +/− SEM. N=3-6 biological replicates. **P<0.01, Student’s t test with Welch’s correction.
Inactivation of the cpsE locus results in attenuated carbohydrate secretion into biofilms
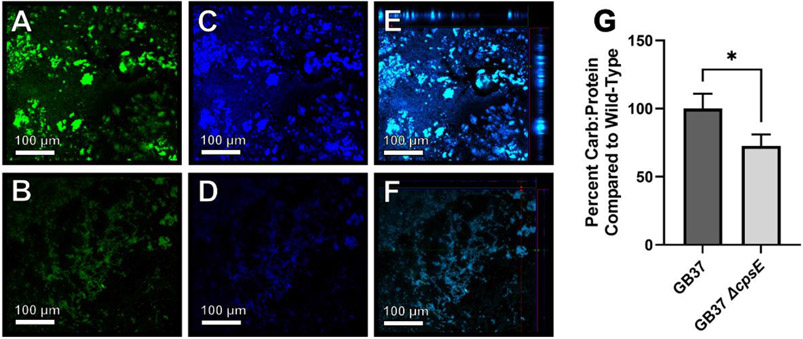
The cpsE gene is predicted to encode a galactosyltransferase that is critical for cell surface polysaccharide transport. Therefore, we hypothesized that cell-associated surface carbohydrates within the biofilm would be attenuated in the isogenic ΔcpsE mutant cells compared to the parental strain. To test this, we employed confocal laser scanning microscopy of biofilm samples stained with Syto9 (green fluorescent signal) to visualize cells and calcofluor white stain (blue fluorescent signal) to visualize carbohydrate matrices within the biofilms (Figure 4A-F). Microscopical analyses of the wild-type GB37 strain revealed robust carbohydrate production within biofilms (Figure 4A-C), which was attenuated in the isogenic ΔcpsE mutant (Figure 4D-F). Confirmation of changes in biofilm-associated carbohydrates was done by employing the L-cysteine sulfuric acid monomeric carbohydrate assay to quantify carbohydrates (normalized to total cellular protein content) of bacterial cells adhering to a polystyrene substrate. Quantitation of total carbohydrate to protein ratio (Figure 4G) revealed that the isogenic ΔcpsE mutant cells had diminished carbohydrates associated with adherent cells compared to the parental strain.
Figure 4. Analyses of carbohydrates within GBS biofilm.
Confocal laser scanning microscopy of GBS (stained green with Syto9, panels A and B) and cell-associated carbohydrates (stained blue with calcofluor white stain, panels C and D) reveals GB37 (A, C, E) secretes more carbohydrate matrix in its biofilm than a ΔcpsE mutant (B, D, F). Merged images (Panels E and F) include ortho stack analyses which confirm that GB37 (Panel E) forms thicker biofilms than the ΔcpsE mutant (Panel F). G) Monomeric carbohydrate assay of adherent bacterial cells indicates the ΔcpsE mutant secretes less carbohydrates compared to the wild-type strain. *P<0.05, Student’s t test.
Inactivation of the cpsE locus results in enhanced phagocytosis of GBS by placental macrophages
Capsule is an important virulence factor which aids in bacterial evasion of the innate immune cell phagocytosis. We hypothesized that, due to its defect in capsule production, a ΔcpsE mutant would exhibit enhanced phagocytosis by innate immune cells in the gravid reproductive tract. To test this, we utilized human placental macrophages in co-culture with either a wild-type parental strain of GBS (GB37) or the cpsE isogenic mutant strain. Bacterial cells were labeled with a fluorescence marker and evaluation of phagocytosis was measured by determining the fluorescence of bacteria associated with macrophages. There were no discernible differences in FITC labeling between the ΔcpsE and the WT parental strain as determined by mean fluorescence of serial dilutions of known cellular densities (Figure S1, panel B). Our results indicate that the ΔcpsE mutant had a 42% enhancement of bacteria associated with placental macrophages compared to the parental strain (Figure 5). This indicates that GBS CPS V capsule can aid in evasion of phagocytosis of GBS by human placental macrophages.
Figure 5. Evaluation of placental macrophage phagocytosis of wild-type vs. cpsE mutant.
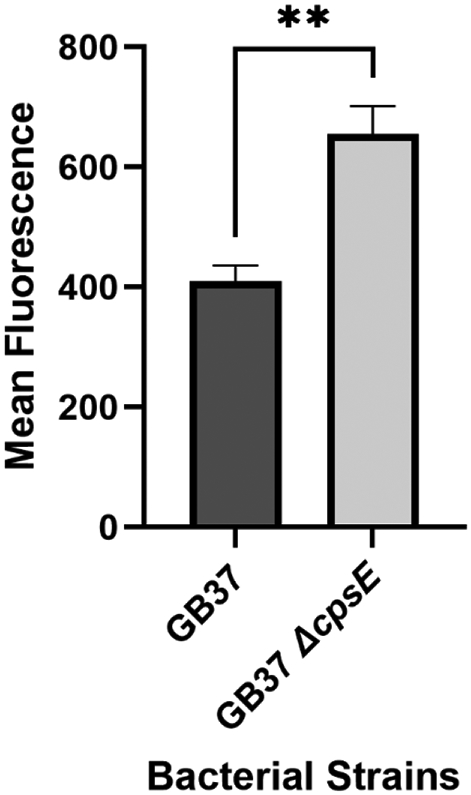
Bacterial cells were labeled with fluorescent dye (FITC) and co-cultured with primary human placental macrophages for 3 hours before extracellular bacteria were washed away or quenched with trypan blue stain. Mean fluorescence was measured in placental macrophages co-cultured with fluorescently labeled GB37 or isogenic ΔcpsE mutant (+/− SEM). An isogenic isogenic ΔcpsE mutant is more readily taken up by placental macrophages than the parental strain, indicating cpsE aids in evasion of phagocytosis. **P<0.01, Student’s t test with Welch’s correction (N=4).
A cpsE mutant has diminished capacity to invade the gravid reproductive tract of a mouse
Because the ΔcpsE mutant was attenuated in its ability to evade innate immune cells, we hypothesized that this isogenic strain might also be attenuated in virulence in a vertebrate host model of infection and disease. To test this, we employed the mouse model of ascending GBS vaginal infection during pregnancy pioneered by the Randis and Ratner laboratories, which our lab has refined23. Pregnant mice were challenged on embryonic day 13.5 with a vaginal inocula of either wild-type GB37 or ΔcpsE mutant, and uninfected controls were also maintained. Two days post-infection, mice were sacrificed and reproductive tissues were collected for analysis of bacterial burden by quantitative culture techniques (Figure 6). A 3-log decrease in bacterial burden was observed in the vaginal and uterine tissues derived from animals infected with a ΔcpsE mutant compared to animals infected with the parental strain, a result that was statistically significant (P<0.0001 and P=0.0019, respectively, Student’s t test with Welch’s correction). A 4-log decrease in bacterial burden was observed in decidua, placenta, fetal membrane, and fetal tissues from animals infected with a ΔcpsE mutant compared to animals infected with the parental strain, a result that was statistically significant (P=0.0002, P<0.0001, P=0.0004, P=0.0006, respectively by Student’s t test with Welch’s correction).
Figure 6. Analysis of bacterial burden within reproductive tissues from the mouse model of ascending GBS infection of the vagina during pregnancy.
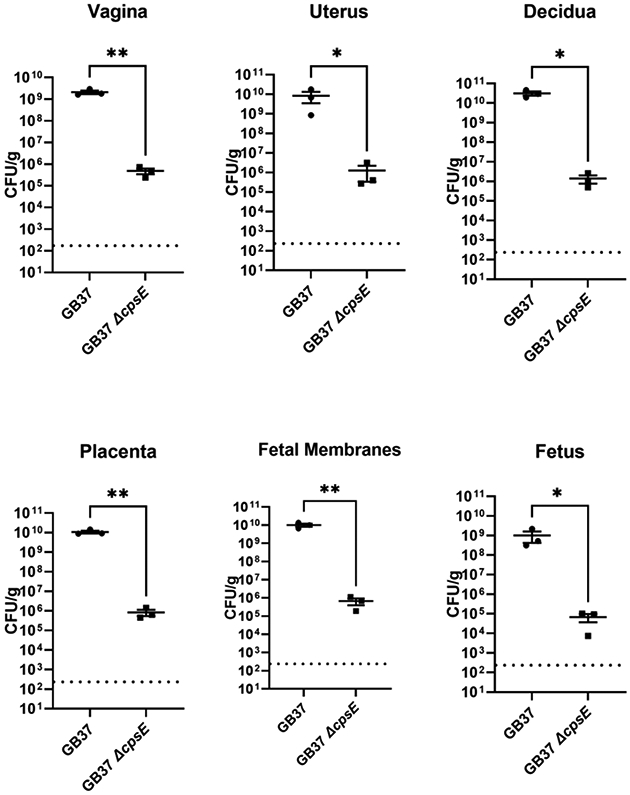
Pregnant mice received a vaginal inoculation of bacterial cells (either GB37 or isogenic ΔcpsE mutant) at embryonic day 13.5. Two days post-infection, mice were sacrificed and reproductive tissues were collected, homogenized, and analyzed by quantitative culture to determine bacterial burden (colony forming units per gram of tissue; CFU/g). GB37 was able to ascend and invade the reproductive tract more effectively and had enhanced burden in the vagina, uterus, decidua, placenta, fetal membranes, and fetus compared to the isogenic ΔcpsE mutant. N=3, *P<0.05, **P<0.01, Student’s t test with Welch’s correction. Dotted line indicates the limit of detection.
A ΔcpsE mutant has attenuated ability to cause tissue destruction and inflammation of the decidua and placenta
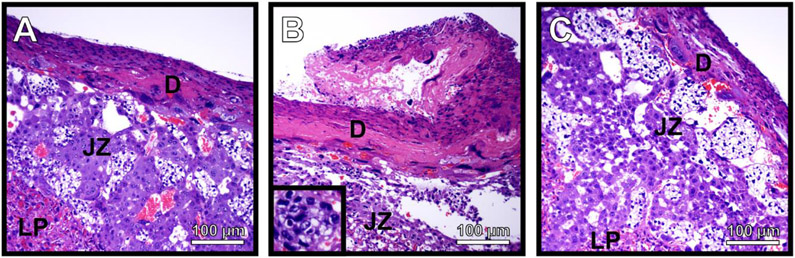
Our previously published work has shown that GBS can invade and cross the placenta to traverse the maternal-fetal interface and cause infection of the fetus24. This work also showed that in response to GBS infection, polymorphonuclear immune cells (neutrophils) were recruited to the decidua and placenta to exert antimicrobial activity against the bacterial invasion. These neutrophils are a hallmark of chorioamnionitis in human patient samples and are often correlated with active infection and cognate inflammation which leads to tissue injury and disease progression. We hypothesized that decreased bacterial burden in these tissues could be correlated with decreased changes in immunopathology associated with ΔcpsE mutant infection compared to animals infected with the parental strain. To test this, we collected fetal-placental units from uninfected, wild-type-infected, and ΔcpsE mutant-infected animals and performed histopathological examination under light microscopy (Figure 7). Results indicate that uninfected animals have preserved architecture of the decidua (maternal-facing tissues), and placenta with limited presence of polymorphonuclear cells. Conversely, infection of the host with wild-type GBS results in a disruption of tissue architecture consistent with loss of tissue integrity and enhanced presence of polymorphonuclear cells indicative of acute inflammation. Interestingly, infection of the host with a ΔcpsE mutant resulted in diminished disruption of tissue architecture and presence of polymorphonuclear cells compared to animals infected with the parental strain, a result which was similar to uninfected controls.
Figure 7. Histopathological evaluation of gravid reproductive tissues in response to GBS infection.
Microscopical analysis of hematoxylin and eosin-stained placental tissue sections shows that, A) uninfected tissues have intact tissue architecture with few detectable polymorphonuclear cells. B) Infection with wild-type GB37 results in a disruption of tissue integrity and polymorphonuclear cell infiltrates (inset panel) indicative of inflammation. C) Infection with the isogenic ΔcpsE mutant results in less disruption of tissue integrity, and fewer polymorphonuclear cell infiltrates, indicating less tissue damage in these animals. Micrographs were collected at 400X magnification and are representative of three separate experiments. Tissue compartments are labeled as follows: decidua (D), junctional zone (JZ), labyrinthine placenta (LP).
Discussion
Encapsulated and nonencapsulated strains of Streptococcus species are frequently isolated from clinical samples25-27. The polysaccharide capsules in S. pneumoniae and S. agalactiae (GBS) are synthesized by the gene products of the cps operon15,28,29. Within this operon, the cpsE locus encodes an enzyme responsible for the addition of activated sugars to a lipid carrier in the bacterial membrane29. Previous work in the human pathogen S. pneumoniae has shown that a single nucleotide base substitution (C to G at the 1135 nucleotide position) in the cpsE locus results in an amino acid change from arginine to glycine at residue 379 in the CpsE protein, rendering the CpsE protein enzymatically inactive. This single nucleotide polymorphism (SNP) is responsible for lack of capsule production30. Similarly, our work has demonstrated that loss of the cpsE locus results in a significant defect related to serotype V capsule production, indicating that the cpsE locus is required for capsule biogenesis in S. agalactiae.
Variations in capsule serotype have been associated with variation in bacterial biofilm formation in Streptococcus species; highlighting the important intersection of capsule, biofilm, and pathogenesis31. Acidic conditions that mimic the vaginal environment enhance biofilm formation in GBS serotypes III and V22, 32. In our study, the cpsE mutant exhibited attenuated capacity to form biofilms in vitro in THB supplemented with glucose. This result demonstrates that proper capsule production is important for biofilm formation and that CpsE plays an essential role in GBS serotype V capsule biosynthesis. Similarly, previous work by Xia et al. has shown that capsule is critical for GBS serotype III biofilm formation in cell culture medium supplemented with human plasma33. Interestingly, several groups have reported that acapsular mutants of S. pneumoniae have enhanced ability to adhere and form biofilms on abiotic and biotic surfaces29; this indicates the molecular mechanisms which underpin these processes could vary across strains or species, or that other environmental factors could influence Streptococcus biofilm formation independent of bacterial capsule biogenesis.
In S. pneumoniae, inactivation of galU, a gene involved in UDP-glucose metabolism which influences capsule biosynthesis, results in enhanced adherence to epithelial cells and phagocytosis by macrophages34. Similarly, capsule has been implicated in resistance to complement activity as well as neutrophil phagocytosis in S. pneumoniae35 and CPS III S. agalactiae36. Bacterial capsule has been shown to confer resistance to dendritic cell phagocytosis in both S. agalactiae and S. suis37,38. Results from these studies demonstrate that GBS CPS V which lack cpsE and consequently experience defects in capsule biosynthesis, are unable to evade phagocytosis by placental macrophages to the same extent as GBS with a functional cpsE gene. Consistent with our results is a similar uptake defect by mouse alveolar macrophages of a type III GBS cpsE mutant39. Interestingly, the absence of capsule has also been associated with worse clinical outcomes. For example, non-encapsulated S. pneumoniae display multi-drug resistance35 and lack of capsule in GBS CPS III was isolated from a case of endocarditis26. In a GBS CPS III hyperhemolytic strain (A909), a cpsE deletion mutant caused increased phagocytosis in mouse bone marrow derived macrophages40. This is consistent with what we see in ex vivo infected human placental macrophages. However the double mutant in that study, ΔcpsEΔcovr, caused significantly increased mortality in a mouse model after intravenous injection of a large CFU burden (1x108)40. Important differences in these two mouse models are the routes of infection (intravenous vs. intravaginal), the strain capsular serotypes (III vs. V), and the dose of infection. Our mouse model, with an infectious vaginal dose of 1x104 CFU, phenotypically mimics human GBS intrauterine infection in that there is very little maternal mortality, the primary sites of infection are the gestational tissues, the strain was isolated from human neonatal infection. It is clear that capsule dependent virulence varies among and within bacterial species. Additionally, the pregnant reproductive tract is an immunologically unique environment with specific susceptibility to certain pathogens, including GBS. Our work is the first to implicate cpsE-associated capsule biogenesis as an important immune evasion strategy that GBS CPS V uses to circumnavigate phagocytosis by primary human placental macrophages. This has major implications for understanding the pathogenesis of a devastating infectious disease during pregnancy.
Variations in capsule-associated serotypes have been associated with streptococcal disease outcomes31. S. pneumoniae rapidly shed their capsule upon interaction with antimicrobial peptides which allows enhanced invasion of host epithelial cells41. Additional results demonstrate that CPS type and amount affect transmission dynamics and may contribute to the marked differences in prevalence and shedding phenotypes among streptococcal strains42. In S. pneumoniae, cpsE expression is linked to capsule level in serotype 4 strain TIGR4. Mild reductions in cpsE transcript levels result in reduction in capsule and avirulence in murine models of lung and blood infection43. Similarly, we show that the cpsE mutant bacterial ascension in pregnant mice is significantly lower compared to infection with the parental wild-type strain. Thus, our study implicates cpsE and cognate capsule biosynthesis as an important virulence factor in ascending GBS serotype V infections of the reproductive tract during pregnancy. Additionally, infection with the isogenic cpsE mutant yields reduced decidual and placental architectural disruption and inflammation compared to the wild-type strain. This, in combination with decreased bacterial burden, suggests that the cpsE mutant does not ascend the reproductive tract as readily as the wild-type strain. It is also possible that the ascension kinetics of the mutant and wild type strain are similar, but the differences noted in burden and tissue destruction are secondary to increased killing of the cpsE mutant. In either case, this likely results in a diminished capacity to cause tissue damage.
Notably, regional shifts in the relative abundance of circulating GBS7, potential capsular switching44, and the presence of nontypeable strains45 highlight the need for vaccine strategies that are independent of capsular structure. Recently, a live attenuated vaccine strain of S. pneumoniae was generated by Amonov and colleagues29. This strain features gene deletion of cpsE and endA (encoding an endonuclease required for neutrophil immune evasion). This cpsE-endA double mutant strain has 23-fold attenuation of virulence in a mouse model of nasopharyngeal infection. Furthermore, the murine immune response to the double mutant vaccine strain results in protection from high dose mucosal challenge of the D39 wild-type strain of S. pneumoniae29. This study highlights the importance of understanding the biology of serotype specific capsular streptococcal mutants for vaccine generation. Our work demonstrates that cpsE is a critical virulence factor in serotype V strains of GBS. We have shown that cpsE is vital for GBS capsule biosynthesis, biofilm formation, immune evasion, and pathogenicity. Future studies are needed to explore the use of the GBS serotype V cpsE mutant as a vaccine candidate to prevent GBS-associated diseases, especially in pregnancy.
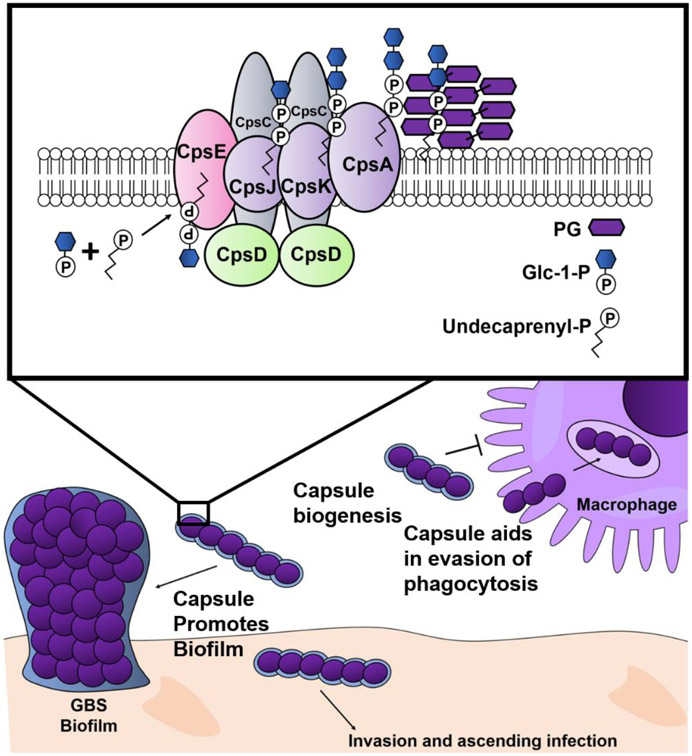
Conclusions: A conceptual model of the action of cpsE in GBS capsule synthesis, biofilm formation, immune evasion, and ascending infection during pregnancy
In summary, this study revealed that the cpsE gene is required for secretion of polysaccharides and capsule biogenesis by GBS. The acapsular cpsE mutant is attenuated in its ability to form biofilms, evade phagocytosis by placental macrophages, and cause ascending infection of the reproductive tract in a pregnant host (Figure 8).
Figure 8. Conceptual model of the role of cpsE during GBS infection of the reproductive tract.
GBS CpsE catalyzes the transfer of glucose-1-phosphate (Glc-1-P) to an undecaprenyl phosphate acceptor, initiating capsule synthesis. CpsJ is a predicted glycosyltransferase which transports the molecule to the opposite side of the cellular membrane where CpsK facilitates sialylation of the capsular polysaccharide. CpsA is a LytR-domain protein that acts as a transcriptional regulator and assists in insertion of the capsular polysaccharide into the peptidoglycan (PG) cell wall. Capsular biogenesis promotes biofilm formation, evasion of phagocytosis by placental macrophages, invasion and ascending infection during pregnancy.
Methods
Bacterial strains and culture conditions
S. agalactiae strain GB37 (capsular type V), a highly virulent clinical strain derived from a human case of neonatal sepsis, was used for these studies46. This strain is amongst the most common serotypes found in invasive neonatal disease, is genetically tractable, and is an established strain to study GBS pathogenesis in a pregnant mouse model of ascending vaginal infection. The GB37 cpsE isogenic mutant was generated as previously described40. Briefly, the cpsE mutation was amplified from A909 ΔDcpsE mutant chromosomal DNA with primers designed with flanking BamH1 and KpnI ends, which were subsequently digested with BamH1 and Kpn1 and ligated into BamHI/KpnI-digested pJR233, a temperature sensitive mutagenic plasmid, to create pJR22ΔcpsE. The resulting plasmid was introduced into GB37 by electroporation and allelic exchange was achieved by a two-step process. First, selection for isogenic derivatives carrying the plasmid was performed on agar plates supplemented with 3 μg/mL erythromycin at 30°C. Subsequently, cultures were shifted to 37-42°C to promote integration into the chromosome by homologous recombination. Integrant strains were serially passaged on solid medium at 37°C and erythromycin-sensitive strains were screened for the expected deletion mutant by PCR and sequencing. All bacterial strains were cultured from freezer stocks onto tryptic soy agar plates supplemented with 5% sheep blood (blood agar plates) at 37°C in ambient air overnight. Bacteria were sub-cultured from blood agar plates into Todd-Hewitt broth (THB) or THB supplemented with 1% glucose (THB +1% glucose) and incubated (aerobically, shaking at 200 RPM) at 37°C in ambient air overnight. To analyze bacterial growth, bacterial density was measured spectrophotometrically at an optical density of 600 nm (OD600), and bacterial numbers were determined with a coefficient of 1 OD600 = 109 colony forming units (CFU) per mL.
Transmission Electron Microscopy analyses
Bacterial strains were grown in THB +1% glucose overnight. The following day, bacteria were prepared for whole cell negative stain and transmission electron microscopy analyses as previously described47. Briefly, 10 μL of bacterial culture were spotted onto formvar-coated copper 100 mesh grids (Electron Microscopy Sciences) and allowed to settle onto the grid for 5-10 min before supernatants were removed. Cells were stained with 1% ammonium molybdate negative stain and imaged with a Philips/FEI T12 transmission electron microscope to visualize cellular features.
Monomeric carbohydrate assay
Total monomeric carbohydrates within bacterial biofilms were quantified using the L-cysteine monomeric carbohydrate assay as previously described48. Briefly, bacteria were grown in THB overnight and sub-cultured at a 1:100 dilution into fresh THB +1% glucose in 3 mL polystyrene tubes. Cultures were incubated statically at 37°C in ambient air overnight. The following day, cultures were decanted and adherent bacteria cells were scraped from the sides of the tubes into 0.5 mL of sterile PBS. 0.2 mL of culture was removed and protein concentration was determined using a standard Bradford reagent assay in conjunction with a known standard curve. 0.2 mL of culture was removed and subjected to the colorimetric monomeric carbohydrate assay. 0.8 mL of L-cysteine in sulfuric acid (0.07 g L-cysteine in 86% sulfuric acid solution) was added and each sample was incubated on ice for 10 minutes. Samples were gently mixed and heated to 100°C for 3 minutes before being plunged into ice again for 5 minutes. Spectrophotometric readings were taken at OD415 nm of both experimental samples, negative control samples and a standard curve. A ratio of total carbohydrates to total protein was calculated to evaluate total carbohydrates normalized to cellular density.
Quantitative analysis of biofilms
Biofilm formation was determined by crystal violet staining of overnight static cultures as described before49, 50. Briefly, bacterial cultures were grown overnight in THB and sub-cultured at a 1:100 dilution into fresh THB +1% glucose in culture plates (12-well). Cultures were incubated statically at 37°C in ambient air overnight. The following day, OD600 was measured for each well to ascertain cell density, and cultures were decanted and washed three times before staining with 0.1% crystal violet. Wells were washed three times with water and allowed to dry before macroscopic imaging. Crystal violet was re-solubilized in 80% ethanol: 20% acetone solution and the total biofilm quantification was measured at OD560. Total biofilm to biomass was calculated by the ratio of OD560 of re-solubilized crystal violet to the OD600 measurement of total cell density.
Confocal laser scanning microscopy analyses
Bacterial biofilms were analyzed by confocal laser scanning microscopy analyses (CLSM) and fluorescent staining. Bacterial cells were stained with 10 μg/mL Syto 9 (ThermoFisher; green fluorescence; excitation wavelength of 486 nm and emission at 501 nm) and extracellular carbohydrates were stained with 10 μg/mL calcofluor white (Sigma Aldrich; blue fluorescence; excitation wavelength at 380 nm and emission at 475 nm). Samples were mounted with ProLong Gold antifade reagent and imaged with a Zeiss LSM 710 CLSM. Samples were analyzed in both widefield and confocal modalities at 630X magnification and micrographs were collected with Zen 2010 software. Micrographs shown are representative of three biological replicates.
Scanning Electron Microscopy analyses
Samples were prepared for scanning electron microscopy analyses as previously described49-51. Briefly, samples were subjected to primary fixation with 2.5% glutaraldehyde, 2.0% paraformaldehyde, in 0.05 M sodium cacodylate buffer at room temperature for 24 hours. Subsequently, samples were washed three times with 0.05 M sodium cacodylate buffer and subjected to a secondary fixation step with 0.1% osmium tetroxide for 15 minutes. Samples were washed three times with 0.05 M sodium cacodylate buffer before being sequentially dehydrated with increasing concentrations of ethanol. After dehydration, samples were dried with a Tousimis CO2 critical point dryer, mounted onto aluminum SEM stubs, and painted with a thin stripe of colloidal silver to dissipate excess charging. Samples were imaged with an FEI Quanta 250 field emission gun scanning electron microscope at an accelerating voltage of 5.0 KeV.
Isolation of placental macrophages
Placental macrophages were isolated from de-identified placenta samples from term, non-laboring caesarean section deliveries as previously described30 and in accordance with a protocol approved by the Vanderbilt University Medical Center Institutional Review Board #IRB #181998 and #00005756. Villous core tissue was macerated and enzymatically digested with hyaluronidase, collagenase, and DNAse before being strained through a stainless-steel filter and suspended in RPMI with HEPES, L-glutamine, and fetal bovine serum supplemented with antibiotic and antifungal factors. Sample was centrifuged at 1,500 RPM at room temperature for 10 minutes and supernatant decanted leaving a cell pellet. Pellet was strained through increasingly fine screens and enriched via Percoll gradient. Red blood cells (RBC) were lysed with RBC lysis buffer and macrophages were positively selected with CD14+ beads and magnet selection. After purification, placental macrophages were cultured in RPMI overnight at 37°C in ambient air supplemented with 5% CO2.
Evaluation of placental macrophage phagocytosis of GBS
Evaluation of placental macrophage phagocytosis of GBS was performed as previously described53. Briefly, placental macrophages were cultured in fresh medium alone or with a 10:1 inocula of bacterial cells that were pre-treated with FITC stain. Bacteria were serially diluted and mean fluorescence was measured to ensure FITC labeling was consistent across strains. Co-cultures were incubated for 3-4 hours before being washed three times with sterile PBS. Extracellular bacterial fluorescence was quenched with trypan blue stain, and intracellular fluorescence was measured at an excitation wavelength of 495 nm and emission at 519 nm to ascertain the fluorescence intensity as a proxy for intracellular bacterial presence within placental macrophages.
Ascending vaginal infection of pregnant mice
GBS infection of pregnant mice and subsequent analyses were performed as previously described 23, 24, 54. Briefly, C57BL6/J mice were purchased from Jackson laboratories and mated in harem breeding strategies overnight. The following day, pregnancy was confirmed by the presence of a vaginal mucus plug, establishing the embryonic date (E0.5). On embryonic day 13.5 (E13.5) pregnant dams were anesthetized via inhalation of isoflurane and vaginally infected with 102-103 colony forming units (CFU) in 0.05 mL of THB plus 10% gelatin. Uninfected controls were also maintained. On embryonic day 15.5 (E15.5) animals were euthanized and necropsy was performed to harvest reproductive tissues including vagina, uterus, placenta, decidua, fetal membranes, and fetus.
Ethics Statement
All animal experiments were performed in accordance with the Animal Welfare Act, U.S. federal law, and NIH guidelines. All experiments were carried out under a protocol approved by Vanderbilt University Institutional Animal Care and Use Committee (IACUC: M/14/034 and M/17/012), a body that has been accredited by the Association of Assessment and Accreditation of Laboratory Animal Care Act (AAALAC).
Quantitative culture to determine bacterial burden in tissues
To determine bacterial burden in reproductive tissues quantitative culture methods were employed as previously described24. Briefly, reproductive tissues were weighed and homogenized in sterile THB. Homogenates were subjected to serial dilution and plated onto blood agar to determine the CFU per mg of host tissue.
Histopathological examination of reproductive tissues
Reproductive tissues were subjected to a primary fixation in 10% formalin (neutral buffered) overnight. The following day, tissues were embedded in paraffin and sectioned into 5 m thick sections for staining and microscopical analyses. Sections were stained with hematoxylin and eosin for histopathological examination and imaged with an OMAX M83ES compound light microscope.
Statistical Analyses
Statistical analysis of biofilm, carbohydrate, and fluorescence quantifications was performed using Student’s t-test. Bacterial burden assays were analyzed by log transformation of CFU and Student’s t-test with Welch’s correction. P ≤ 0.05 were considered significant. All data analyzed in this work were derived from at least three separate biological replicates. Statistical analyses were performed using GraphPad Prism Software (Version 9.0, GraphPad Software Inc., La Jolla CA) and Microsoft Excel (Version 14.6.3, Microsoft Corporation, Redmond WA).
Supplementary Material
Acknowledgments
This work has been funded by the National Institutes of Health grant R01 HD090061 (to J.A.G.), by the Department of Veterans Affairs Office of Research BX005352 (to J.A.G.), and by NIH 7K12HD000850-37 (supporting K.N.N.), T32 HL007411-36S1 (supporting J.L.) 2T32AI112541-06 (supporting J.F.), K08AAI151100 (supporting R.S.D.), R35GM133602 (to S.D.T.), and F32HD100087 (to A.J.E.). Additional funding from the National Science Foundation Award Numbers 1547757 and 1400969, and NIH grant GM05551 (to S.M.D.) supported this work. Additional support was provided by NIH U01TR002398, NIH R01AI134036, and the March of Dimes (to D.M.A.), and from the Vanderbilt Institute for Clinical and Translational Research program supported by the National Center for Research Resources, Grant UL1 RR024975-01, and the National Center for Advancing Translational Sciences, Grant 2 UL1 TR000445-06. Microscopy experiments were performed in part through the use of the Vanderbilt Cell Imaging Shared Resource (supported by NIH grants CA68485, DK20593, DK58404, DK59637 and EY08126) as well as S10 RR026373 and S10 RR027396.
Footnotes
Supporting Information Available
Figure S1. Bacterial growth and mean fluorescence assays are available as supporting files in “Supporting information”. This information is available free of charge on the ACS Publications website.
References
- (1).Patras KA; Nizet V Group B Streptococcal Maternal Colonization and Neonatal Disease: Molecular Mechanisms and Preventative Approaches. Front. Pediatr 2018, 6, 27. 10.3389/fped.2018.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Shabayek S; Spellerberg B Group B Streptococcal Colonization, Molecular Characteristics, and Epidemiology. Front. Microbiol 2018, 9, 437. 10.3389/fmicb.2018.00437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Benitz WE; Gould JB; Druzin ML Risk Factors for Early-Onset Group B Streptococcal Sepsis: Estimation of Odds Ratios by Critical Literature Review. Pediatrics 1999, 103 (6), e77. 10.1542/peds.103.6.e77. [DOI] [PubMed] [Google Scholar]
- (4).Schrag SJ; Verani JR Intrapartum Antibiotic Prophylaxis for the Prevention of Perinatal Group B Streptococcal Disease: Experience in the United States and Implications for a Potential Group B Streptococcal Vaccine. Vaccine 2013, 31 Suppl 4, D20–6. 10.1016/j.vaccine.2012.11.056. [DOI] [PubMed] [Google Scholar]
- (5).Nanduri SA; Petit S; Smelser C; Apostol M; Alden NB; Harrison LH; Lynfield R; Vagnone PS; Burzlaff K; Spina NL; Dufort EM; Schaffner W; Thomas AR; Farley MM; Jain JH; Pondo T; McGee L; Beall BW; Schrag SJ Epidemiology of Invasive Early-Onset and Late-Onset Group B Streptococcal Disease in the United States, 2006 to 2015: Multistate Laboratory and Population-Based Surveillance. JAMA Pediatr. 2019, 173 (3), 224–233. 10.1001/jamapediatrics.2018.4826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Stoll BJ; Hansen NI; Sánchez PJ; Faix RG; Poindexter BB; Van Meurs KP; Bizzarro MJ; Goldberg RN; Frantz ID 3rd; Hale EC; Shankaran S; Kennedy K; Carlo WA; Watterberg KL; Bell EF; Walsh MC; Schibler K; Laptook AR; Shane AL; Schrag SJ; Das A; Higgins RD; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Early Onset Neonatal Sepsis: The Burden of Group B Streptococcal and E. coli Disease Continues. Pediatrics 2011, 127 (5), 817–826. 10.1542/peds.2010-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Shipitsyna E; Shalepo K; Zatsiorskaya S; Krysanova A; Razinkova M; Grigoriev A; Savicheva A Significant Shifts in the Distribution of Vaccine Capsular Polysaccharide Types and Rates of Antimicrobial Resistance of Perinatal Group B Streptococci within the Last Decade in St. Petersburg, Russia. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol 2020, 39 (8), 1487–1493. 10.1007/s10096-020-03864-1. [DOI] [PubMed] [Google Scholar]
- (8).Winkelstein JA; Abramovitz AS; Tomasz A Activation of C3 via the Alternative Complement Pathway Results in Fixation of C3b to the Pneumococcal Cell Wall. J. Immunol 1980, 124 (5), 2502–2506. [PubMed] [Google Scholar]
- (9).Brown EJ; Joiner KA; Cole RM; Berger M Localization of Complement Component 3 on Streptococcus pneumoniae: Anti-Capsular Antibody Causes Complement Deposition on the Pneumococcal Capsule. Infect. Immun 1983, 39 (1), 403–409. 10.1128/IAI.39.1.403-409.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Abeyta M; Hardy GG; Yother J Genetic Alteration of Capsule Type but Not PspA Type Affects Accessibility of Surface-Bound Complement and Surface Antigens of Streptococcus pneumoniae. Infect. Immun 2003, 71 (1), 218–225. 10.1128/iai.71.1.218-225.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Okamoto S; Kawabata S; Terao Y; Fujitaka H; Okuno Y; Hamada S The Streptococcus pyogenes Capsule Is Required for Adhesion of Bacteria to Virus-Infected Alveolar Epithelial Cells and Lethal Bacterial-Viral Superinfection. Infect. Immun 2004, 72 (10), 6068–6075. 10.1128/IAI.72.10.6068-6075.2004.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Yother J Capsules of Streptococcus Pneumoniae and Other Bacteria: Paradigms for Polysaccharide Biosynthesis and Regulation. Annu. Rev. Microbiol 2011, 65, 563–581. 10.1146/annurev.micro.62.081307.162944. [DOI] [PubMed] [Google Scholar]
- (13).Islam ST; Lam JS Synthesis of Bacterial Polysaccharides via the Wzx/Wzy-Dependent Pathway. Can. J. Microbiol 2014, 60 (11), 697–716. 10.1139/cjm-2014-0595. [DOI] [PubMed] [Google Scholar]
- (14).Cieslewicz MJ; Chaffin D; Glusman G; Kasper D; Madan A; Rodrigues S; Fahey J; Wessels MR; Rubens CE Structural and Genetic Diversity of Group B Streptococcus Capsular Polysaccharides. Infect. Immun 2005, 73 (5), 3096–3103. 10.1128/IAI.73.5.3096-3103.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Alhhazmi A; Pandey A; Tyrrell GJ Identification of Group B Streptococcus Capsule Type by Use of a Dual Phenotypic/Genotypic Assay. J. Clin. Microbiol 2017, 55 (9), 2637–2650. 10.1128/JCM.00300-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Carlin AF; Lewis AL; Varki A; Nizet V Group B Streptococcal Capsular Sialic Acids Interact with Siglecs (Immunoglobulin-like Lectins) on Human Leukocytes. J. Bacteriol 2007, 189 (4), 1231–1237. 10.1128/JB.01155-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Uchiyama S; Sun J; Fukahori K; Ando N; Wu M; Schwarz F; Siddiqui SS; Varki A; Marth JD; Nizet V Dual Actions of Group B Streptococcus Capsular Sialic Acid Provide Resistance to Platelet-Mediated Antimicrobial Killing. Proc. Natl. Acad. Sci. U. S. A 2019, 116 (15), 7465–7470. 10.1073/pnas.1815572116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Chaffin DO; Beres SB; Yim HH; Rubens CE The Serotype of Type Ia and III Group B Streptococci Is Determined by the Polymerase Gene within the Polycistronic Capsule Operon. J. Bacteriol 2000, 182 (16), 4466–4477. 10.1128/jb.182.16.4466-4477.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Cieslewicz MJ; Kasper DL; Wang Y; Wessels MR Functional Analysis in Type Ia Group B Streptococcus of a Cluster of Genes Involved in Extracellular Polysaccharide Production by Diverse Species of Streptococci. J. Biol. Chem 2001, 276 (1), 139–146. 10.1074/jbc.M005702200. [DOI] [PubMed] [Google Scholar]
- (20).Parker RE; Laut C; Gaddy JA; Zadoks RN; Davies HD; Manning SD Association between Genotypic Diversity and Biofilm Production in Group B Streptococcus. BMC Microbiol. 2016, 16, 86. 10.1186/s12866-016-0704-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Rinaudo CD; Rosini R; Galeotti CL; Berti F; Necchi F; Reguzzi V; Ghezzo C; Telford JL; Grandi G; Maione D Specific Involvement of Pilus Type 2a in Biofilm Formation in Group B Streptococcus. PLoS One 2010, 5 (2), e9216. 10.1371/journal.pone.0009216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).D’Urzo N; Martinelli M; Pezzicoli A; De Cesare V; Pinto V; Margarit I; Telford JL; Maione D Acidic PH Strongly Enhances in vitro Biofilm Formation by a Subset of Hypervirulent ST-17 Streptococcus agalactiae Strains. Appl. Environ. Microbiol 2014, 80 (7), 2176–2185. 10.1128/AEM.03627-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Randis TM; Gelber SE; Hooven TA; Abellar RG; Akabas LH; Lewis EL; Walker LB; Byland LM; Nizet V; Ratner AJ Group B Streptococcus β-Hemolysin/Cytolysin Breaches Maternal-Fetal Barriers to Cause Preterm Birth and Intrauterine Fetal Demise in vivo. J. Infect. Dis 2014, 210 (2), 265–273. 10.1093/infdis/jiu067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Kothary V; Doster RS; Rogers LM; Kirk LA; Boyd KL; Romano-Keeler J; Haley KP; Manning SD; Aronoff DM; Gaddy JA Group B Streptococcus Induces Neutrophil Recruitment to Gestational Tissues and Elaboration of Extracellular Traps and Nutritional Immunity. Front. Cell. Infect. Microbiol 2017, 7, 19. 10.3389/fcimb.2017.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Takeuchi N; Ohkusu M; Hishiki H; Fujii K; Hotta M; Murata S; Ishiwada N First Report on Multidrug-Resistant Non-Encapsulated Streptococcus pneumoniae Isolated from a Patient with Pneumonia. Journal of infection and chemotherapy : official journal of the Japan Society of Chemotherapy. Netherlands: July 2020, pp 749–751. 10.1016/j.jiac.2020.02.009. [DOI] [PubMed] [Google Scholar]
- (26).Sellin M; Linderholm M; Norgren M; Håkansson S Endocarditis Caused by a Group B Streptococcus Strain, Type III, in a Nonencapsulated Phase. J. Clin. Microbiol 1992, 30 (9), 2471–2473. 10.1128/JCM.30.9.2471-2473.1992.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Philips JB 3rd; Li JX; Gray BM; Pritchard DG; Oliver JR Role of Capsule in Pulmonary Hypertension Induced by Group B Streptococcus. Pediatr. Res 1992, 31 (4 Pt 1), 386–390. 10.1203/00006450-199204000-00016. [DOI] [PubMed] [Google Scholar]
- (28).Kapatai G; Sheppard CL; Al-Shahib A; Litt DJ; Underwood AP; Harrison TG; Fry NK Whole Genome Sequencing of Streptococcus pneumoniae: Development, Evaluation and Verification of Targets for Serogroup and Serotype Prediction Using an Automated Pipeline. PeerJ 2016, 4, e2477. 10.7717/peerj.2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Amonov M; Simbak N; Wan Hassan WMR; Ismail S; A Rahman NI; Clarke SC; Yeo CC Disruption of the CpsE and EndA Genes Attenuates Streptococcus pneumoniae Virulence: Towards the Development of a Live Attenuated Vaccine Candidate. Vaccines 2020, 8 (2). 10.3390/vaccines8020187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Schaffner TO; Hinds J; Gould KA; Wüthrich D; Bruggmann R; Küffer M; Mühlemann K; Hilty M; Hathaway LJ A Point Mutation in CpsE Renders Streptococcus pneumoniae Nonencapsulated and Enhances Its Growth, Adherence and Competence. BMC Microbiol. 2014, 14, 210. 10.1186/s12866-014-0210-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Sempere J; de Miguel S; González-Camacho F; Yuste J; Domenech M Clinical Relevance and Molecular Pathogenesis of the Emerging Serotypes 22F and 33F of Streptococcus pneumoniae in Spain. Front. Microbiol 2020, 11, 309. 10.3389/fmicb.2020.00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Yadav P; Verma S; Bauer R; Kumari M; Dua M; Johri AK; Yadav V; Spellerberg B Deciphering Streptococcal Biofilms. Microorganisms 2020, 8 (11). 10.3390/microorganisms8111835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Xia F. Di; Mallet A; Caliot E; Gao C; Trieu-Cuot P; Dramsi S Capsular Polysaccharide of Group B Streptococcus Mediates Biofilm Formation in the Presence of Human Plasma. Microbes Infect. 2015, 17 (1), 71–76. 10.1016/j.micinf.2014.10.007. [DOI] [PubMed] [Google Scholar]
- (34).Cools F; Torfs E; Vanhoutte B; de Macedo MB; Bonofiglio L; Mollerach M; Maes L; Caljon G; Delputte P; Cappoen D; Cos P Streptococcus pneumoniae galU Gene Mutation Has a Direct Effect on Biofilm Growth, Adherence and Phagocytosis in vitro and Pathogenicity in vivo. Pathog. Dis 2018, 76 (7). 10.1093/femspd/fty069. [DOI] [PubMed] [Google Scholar]
- (35).Hyams C; Camberlein E; Cohen JM; Bax K; Brown JS The Streptococcus pneumoniae Capsule Inhibits Complement Activity and Neutrophil Phagocytosis by Multiple Mechanisms. Infect. Immun 2010, 78 (2), 704–715. 10.1128/IAI.00881-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Takahashi S; Aoyagi Y; Adderson EE; Okuwaki Y; Bohnsack JF Capsular Sialic Acid Limits C5a Production on Type III Group B Streptococci. Infect. Immun 1999, 67 (4), 1866–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Lemire P; Houde M; Lecours M-P; Fittipaldi N; Segura M Role of Capsular Polysaccharide in Group B Streptococccus Interactions with Dendritic Cells. Microbes Infect. 2012, 14 (12), 1064–1076. 10.1016/j.micinf.2012.05.015. [DOI] [PubMed] [Google Scholar]
- (38).Meijerink M; Ferrando ML; Lammers G; Taverne N; Smith HE; Wells JM Immunomodulatory Effects of Streptococcus suis Capsule Type on Human Dendritic Cell Responses, Phagocytosis and Intracellular Survival. PLoS One 2012, 7 (4), e35849. 10.1371/journal.pone.0035849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Jones AL; Mertz RH; Carl DJ; Rubens CE A Streptococcal Penicillin-Binding Protein Is Critical for Resisting Innate Airway Defenses in the Neonatal Lung. J. Immunol 2007, 179 (5), 3196–3202. 10.4049/jimmunol.179.5.3196. [DOI] [PubMed] [Google Scholar]
- (40).Gendrin C; Merillat S; Vornhagen J; Coleman M; Armistead B; Ngo L; Aggarwal A; Quach P; Berrigan J; Rajagopal L Diminished Capsule Exacerbates Virulence, Blood-Brain Barrier Penetration, Intracellular Persistence, and Antibiotic Evasion of Hyperhemolytic Group B Streptococci. J. Infect. Dis 2018, 217 (7), 1128–1138. 10.1093/infdis/jix684.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Kietzman CC; Gao G; Mann B; Myers L; Tuomanen EI Dynamic Capsule Restructuring by the Main Pneumococcal Autolysin LytA in Response to the Epithelium. Nat. Commun 2016, 7, 10859. 10.1038/ncomms10859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Zafar MA; Hamaguchi S; Zangari T; Cammer M; Weiser JN Capsule Type and Amount Affect Shedding and Transmission of Streptococcus pneumoniae. MBio 2017, 8 (4). 10.1128/mBio.00989-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Shainheit MG; Mulé M; Camilli A The Core Promoter of the Capsule Operon of Streptococcus pneumoniae Is Necessary for Colonization and Invasive Disease. Infect. Immun 2014, 82 (2), 694–705. 10.1128/IAI.01289-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Wang R; Li L; Huang T; Huang W; Lei A; Chen M Capsular Switching and ICE Transformation Occurred in Human Streptococcus agalactiae ST19 With High Pathogenicity to Fish. Front. Vet. Sci 2018, 5, 281. 10.3389/fvets.2018.00281.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Rosini R; Campisi E; De Chiara M; Tettelin H; Rinaudo D; Toniolo C; Metruccio M; Guidotti S; Sørensen UBS; Kilian M; DEVANI Consortium; Ramirez M; Janulczyk R; Donati C; Grandi G; Margarit I Genomic Analysis Reveals the Molecular Basis for Capsule Loss in the Group B Streptococcus Population. PLoS One 2015, 10 (5), e0125985. 10.1371/journal.pone.0125985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Singh P; Aronoff DM; Davies HD; Manning SD Draft Genome Sequence of an Invasive Streptococcus agalactiae Isolate Lacking Pigmentation. Genome Announc. 2016, 4 (1). 10.1128/genomeA.00015-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Radin JN; Gaddy JA; González-Rivera C; Loh JT; Algood HMS; Cover TL Flagellar Localization of a Helicobacter pylori Autotransporter Protein. MBio 2013, 4 (2), e00613–12. 10.1128/mBio.00613-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Nwugo CC; Gaddy JA; Zimbler DL; Actis LA Deciphering the Iron Response in Acinetobacter baumannii: A Proteomics Approach. J. Proteomics 2011, 74 (1), 44–58. 10.1016/j.jprot.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Gaddy JA; Tomaras AP; Actis LA The Acinetobacter baumannii 19606 OmpA Protein Plays a Role in Biofilm Formation on Abiotic Surfaces and in the Interaction of This Pathogen with Eukaryotic Cells. Infect. Immun 2009, 77 (8), 3150–3160. 10.1128/IAI.00096-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Lu J; Francis JD; Guevara MA; Moore RE; Chambers SA; Doster RS; Eastman AJ; Rogers LM; Noble KN; Manning SD; Damo SM; Aronoff DM; Townsend SD; Gaddy JA Antibacterial and Anti-Biofilm Activity of the Human Breast Milk Glycoprotein Lactoferrin against Group B Streptococcus. Chembiochem 2021. 10.1002/cbic.202100016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Ackerman DL; Doster RS; Weitkamp J-H; Aronoff DM; Gaddy JA; Townsend SD Human Milk Oligosaccharides Exhibit Antimicrobial and Antibiofilm Properties against Group B Streptococcus. ACS Infect. Dis 2017, 3 (8), 595–605. 10.1021/acsinfecdis.7b00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Sutton JA; Rogers LM; Dixon BREA; Kirk L; Doster R; Algood HM; Gaddy JA; Flaherty R; Manning SD; Aronoff DM Protein Kinase D Mediates Inflammatory Responses of Human Placental Macrophages to Group B Streptococcus. Am. J. Reprod. Immunol 2019, 81 (3), e13075. 10.1111/aji.13075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Rogers LM; Anders AP; Doster RS; Gill EA; Gnecco JS; Holley JM; Randis TM; Ratner AJ; Gaddy JA; Osteen K; Aronoff DM Decidual Stromal Cell-Derived PGE(2) Regulates Macrophage Responses to Microbial Threat. Am. J. Reprod. Immunol 2018, 80 (4), e13032. 10.1111/aji.13032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Doster RS; Sutton JA; Rogers LM; Aronoff DM; Gaddy JA Streptococcus agalactiae Induces Placental Macrophages To Release Extracellular Traps Loaded with Tissue Remodeling Enzymes via an Oxidative Burst-Dependent Mechanism. MBio 2018, 9 (6). 10.1128/mBio.02084-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.