Abstract
Purpose
To describe the association between Sars-CoV-2 infection and small fiber neuropathy in the cornea identified by in vivo corneal confocal microscopy.
Methods
Twenty-three patients who had overcome COVID-19 were recruited to this observational retrospective study. Forty-six uninfected volunteers were also recruited and studied as a control group. All subjects were examined under in vivo confocal microscopy to obtain images of corneal subbasal nerve fibers in order to study the presence of neuroma-like structures, axonal beadings and dendritic cells. The Ocular Surface Disease Index (OSDI) questionnaire and Schirmer tear test were used as indicators of Dry Eye Disease (DED) and ocular surface pathology.
Results
Twenty-one patients (91.31%) presented alterations of the corneal subbasal plexus and corneal tissue consistent with small fiber neuropathy. Images from healthy subjects did not indicate significant nerve fiber or corneal tissue damage. Eight patients reported increased sensations of ocular dryness after COVID-19 infection and had positive DED indicators. Beaded axons were found in 82.60% of cases, mainly in patients reporting ocular irritation symptoms. Neuroma-like images were found in 65.22% patients, more frequently in those with OSDI scores >13. Dendritic cells were found in 69.56% of patients and were more frequent in younger asymptomatic patients. The presence of morphological alterations in patients up to 10 months after recovering from Sars-CoV-2 infection points to the chronic nature of the neuropathy.
Conclusions
Sars-CoV-2 infection may be inducing small fiber neuropathy in the ocular surface, sharing symptomatology and morphological landmarks with DED and diabetic neuropathy.
Keywords: COVID-19, Corneal neuropathy, In vivo confocal microscopy, Dry eye disease
1. Introduction
Infection with the Sars-CoV-2 virus is the cause of COVID-19 disease, which induces a wide variety of pathological signs in the body, even in patients with mild disease. A significant number of patients experience chronic pathological conditions after they overcome the infection, such as reduced respiratory capacity, vasculopathy or chronic fatigue [1]. Various neurological manifestations confirm the existence of the virus's impact on the central nervous system (CNS) [2,3]. Infection of the olfactory receptors in the nasal cavity is assumed to be related with the spread of viral infection to the CNS [4,5]. Olfactory nerve neuropathy produces anosmia and ageusia, neurological symptoms that have become relevant due to their uniqueness to and frequency in patients with mild COVID-19 [6].
In the eye, the conjunctival mucosa has also been studied as a route of entry of the virus into the body. Epithelium cells at the conjunctiva and the cornea express ACE2 and TMPRSS2 specific Sars-CoV-2 receptors [7]. However, conjunctivitis associated with viremia caused by Sars-Cov-2 is not severe and reverted within 10 days [8].
Another proposed route of entry for the virus into the body and into the CNS is through the sensory nerves [4,9]. Dorsal root ganglion from patients who have died from COVID-19 showed the expression of TMPRSS2 and ACE2 receptors in somata of sensory neurons, where viral RNA was also found [10]. Neuropilin receptors 1 and 2 (NRP1 and NRP2) have been also described as alternative receptors for Sars-Cov-2 [[10], [11], [12]]. NRP1 and NRP2 are important molecules for nerve growth and axon guidance and are expressed in peripheral nerves [13]. It is also believed that NRP1 and NRP2 receptors may be expressed in sensory nerve endings and that they may serve as a gateway for the virus.
The most densely innervated surface of the human body is the cornea of the eye [[14], [15], [16]]. Sensory axons from the ophthalmic branch of the trigeminal nerve account for more than 80% of the nerve fibers in the cornea [14] and are essential for the maintenance of the homeostasis of the cornea [[17], [18], [19]]. Corneal nerves show expression of NRP1, NRP2 and ACE2 receptors, making them suitable to Sars-CoV-2 infection [10]. Severe COVID-19 infection with hypoxemia has been associated with systemic neuropathic symptoms and widespread sensory dysfunction in patients with diabetes [20], including loss of sensitivity, alteration of tissue homeostasis and the generation of epithelial ulcers [21]. In the cornea, neuropathy of subbasal axons has been related with altered sensitivity, pain and the onset of dry eye disease (DED) [17,18,22,23].
Even in cases where COVID-19 related conjunctivitis was resolved in a few days, patients at the ophthalmology clinic reported increased ocular surface discomfort, irritation, and symptoms of DED between 2 and 10 months following Sars-CoV-2 infection. To date, there is no published evidence of sensory alterations in the cornea after Sars-CoV-2 virus infection. We used in vivo confocal microscopy (IVCM) to evaluate the morphological changes on the sensory subbasal plexus of the cornea after Sars-CoV-2 infection. To our knowledge, this is the first report of signs of corneal neuropathy in patients that have overcome COVID-19.
2. Methods
This observational retrospective study was conducted between January 1 and February 25, 2021. It follows the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline and the tenets of the Declaration of Helsinki. Data and images were obtained after informed consent explaining the objective of the study and the procedures was read and signed by the subjects.
The study involves 23 patients of both genders (18 women, 5 men) who had overcome Covid-19 between March and December 2020. Exclusion criteria included ocular surface surgery procedures; previous ocular infectious disease, such as bacterial keratitis, adenovirus, herpes virus, or acanthamoeba; and other ocular diseases that can affect corneal integrity indirectly (glaucoma, macular degeneration, etc.). Patients with systemic diseases that could cause alterations in corneal innervation, i.e., diabetes and fibromyalgia, were also excluded. A cohort of 46 uninfected gender and age-matched volunteers were recruited and studied as a control group (verified by an antigen test and/or antibody analysis, and a negative qPCR result).
2.1. In vivo confocal microscopy (IVCM)
Patients were examined using a Heidelberg® Retina Tomograph 3 confocal microscope equipped with the Rostock Cornea Module (Heidelberg Engineering, Heidelberg, Germany) with a 670 nm wavelength Helium–Neon diode laser, using a 63× objective (N.A. = 0.9). Topical anesthetic (Tetracaine 0.1% + Oxibuprocaine 0.4%; Alcon Cusí) and artificial tears (Lipolac, Carbomer 2 mg/g) were applied to the eye before testing. A sterile cap (TomoCap©, Heidelberg Engineering) was attached to the lens of the microscope and a drop of high viscosity gel (Recugel®, Bausch + Lomb) was used as a bonding agent between the cap and the lens.
Images of the corneal nerves of each eye were obtained, using the section mode, in the central and paracentral cornea in a total of 5 non-overlapping areas. Examination of the full thickness volume of the epithelium to stroma region was conducted to ascertain the complete morphology at the subbasal nerve fiber level. The size of the images obtained was 384 × 384 pixels, which corresponds to an area of 400 × 400 μm.
The images were analyzed and quantified automatically with ACCMetrics software (MA Dabbah, Imaging Science and Biomedical Engineering, Manchester, UK) [24,25]. The following seven parameters were calculated: 1) corneal nerve fiber density (CNFD), the total number of nerves/mm2; 2) corneal nerve branch density (CNBD), the number of second order branches emanating from primary axons/mm2; 3) corneal nerve fiber length (CNFL), the total length of all nerve fibers and branches (mm/mm2); 4) corneal nerve total branch density (CTBD), the total number of branches/mm2; 5) corneal nerve fiber area (CNFA), the total nerve fiber area (mm2/mm2); 6) corneal nerve fiber width (CNFW), the average nerve fiber width (mm/mm2); and 7) corneal nerve fractal dimension (CNFrD), which is an indicator of the structural complexity of the corneal nerve [26].
The images were then analyzed using the Cell Counter plugin of FIJI image analysis software (ImageJ 1.53c; NIH, USA) in order to quantify the incidence of neuromas (total number of neuromas/mm2), beaded axons (total number of beaded axons/mm2) and the density of dendritic cells (DC) in the center of the cornea (total number of DC/mm2). This analysis was performed in a semi-automatic fashion. The operator manually selected each parameter on the images and the Cell Counter plugin automatically calculated the total numbers. Three independent researchers carried out the analysis. The final value used for each parameter was the average of the three measurements.
In order to avoid any mistake in the classification of selected IVCM morphological alterations, once we located a possible pathological sign (neuroma, DC or axonal beads) we examined the candidate structure in detail in order to discount it being any other type of anatomical structure. We examined the whole volume of images of each neuroma to differentiate them from points where the stromal nerve penetrated the epithelium [22,27]. We also differentiated DC from small sections of axon collaterals on the basis of their dendritic morphology and enlarged cellular body. Finally, by following the entire thickness of beaded axons, we were able to identify and discard local refringent points that might interfere with the correct description of axonal beads [22,27].
The two types of analysis (using ACCMetrics and ImageJ softwares) were applied to each of the five selected images from each subject. The average of the five values obtained for each parameter was used for statistical analysis.
2.2. Ocular Surface Disease Index (OSDI)
The presence of ocular surface pathology symptoms, such as discomfort or pain, was evaluated using the Ocular Surface Disease Index (OSDI) test [28]. Patients were divided into 3 groups depending on their OSDI scores: scores >13; scores from 6 to 12; scores ≤5.
The Schirmer tear test (Katena) was conducted with all patients and healthy volunteers using paper strips placed in the lower lid of the eye. Strips with less than 5.5 mm of their length wet were considered as indicative of DED.
2.3. Statistical analysis
The SPSS statistical software for Mac, 16.0 (SPSS Inc., Chicago, Illinois, USA) was used for data analysis. Values were expressed as mean ± standard error of the mean (SEM). The Mann–Whitney U test was used to compare continuous variables between patients who had overcome COVID-19 and healthy control subjects.
3. Results
This study involved 23 patients diagnosed with COVID-19 by RT-PCR between March and December 2020 and 46 healthy age-matched subjects. Table 1 shows the age distribution of patients and healthy controls. All were subjected to IVCM examination and answered the OSDI questionnaire, as well as undergoing the Schirmer tear test for DED.
Table 1.
Number of patients and control subjects distributed by age intervals.
| <35 years | 36–55 years | 56–75 years | |
|---|---|---|---|
| COVID-19 | 7 | 10 | 6 |
| Healthy controls | 17 | 17 | 12 |
Only one patient, who was suffering from bilateral pneumonia (CURB-0.65 = mild grade) needed to be hospitalized. This was the only case in our cohort that could potentially be considered to be complicated COVID-19, although the patient's pneumonia was not considered severe and they fully recovered respiratory function and needed no further treatment. The rest of the patients showed mild symptoms or were asymptomatic and did not need hospitalization. All patients were asymptomatic or had mild symptoms such as headache, occasional fever or loss of olfactory or taste sensitivity. Two patients took only Paracetamol. One patient took Paracetamol, dexketoprofen and levofloxacin. The rest of the patients needed no treatment. Only 3 out of 23 post-COVID19 patients had comorbidities. One had hypertension and high cholesterol and the other two had high cholesterol. All 3 were aged 62–69 years. None of the patients suffered from long-COVID disease or post-COVID syndrome according to their Primary Health Care Team.
3.1. Signs of small fiber neuropathy in patients who had overcome COVID-19
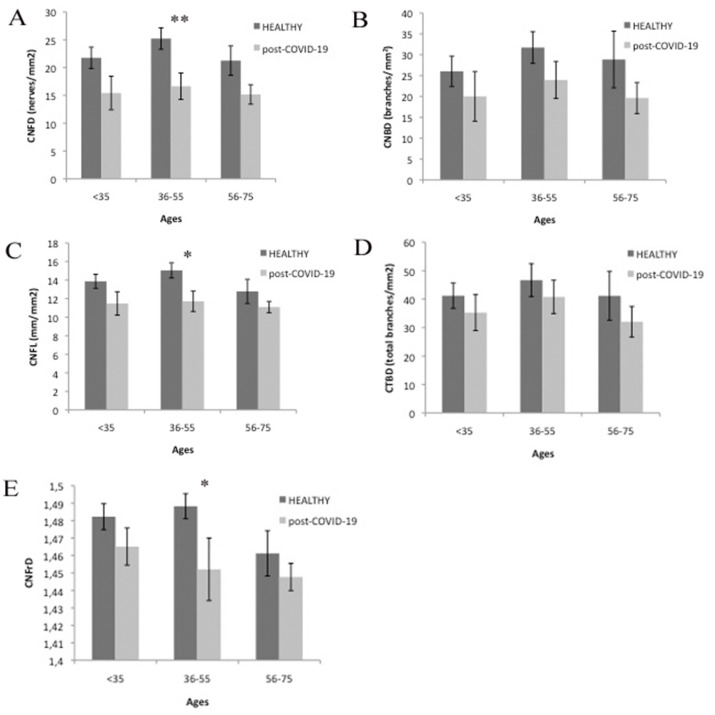
The automatic analysis of IVCM images using ACCMetrics software showed evident reductions in CNFD, CNBD, CNFL and CNBT in patients overcoming Sars-Cov-2 infection compared with healthy controls. These differences were significant for CNFD (p < 0.01) and CNFL (p < 0.05) parameters in patients in the 36 to 55 age range (Fig. 1 ). Also, CNFrD was significantly lower in post-COVID-19 patients compared to controls in the same age group (p < 0.05). The analysis of CNFA and CNFW showed no differences.
Fig. 1.
Results of the ACCMetrics automatic quantification of subbasal plexus of patients that have overcome COVID-19 infection compared with healthy corneas. Significant differences are represented with asterisks (* = p < 0.05; ** = p < 0.01).
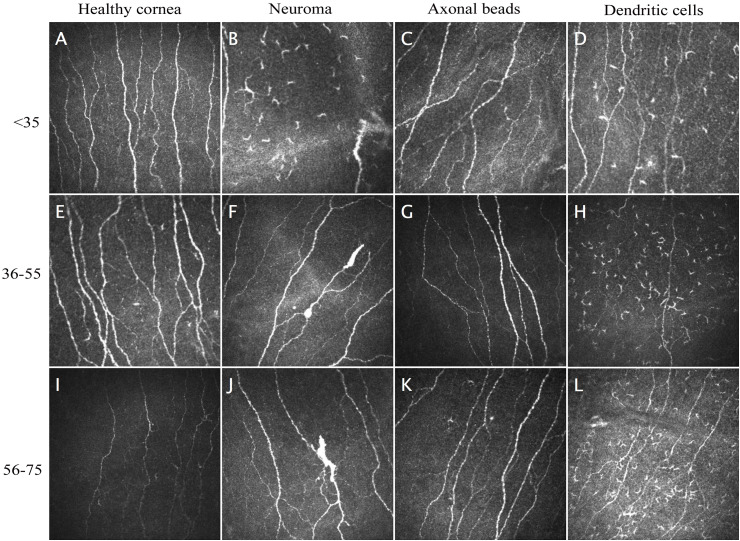
The semi-automatic analysis of IVCM images using FIJI software showed that 21 out of 23 patients (91.31%) presented morphological alterations of the corneal subbasal plexus (neuroma-like structures, beaded axons) and corneal cell infiltration (presence of abundant DC) consistent with small fiber neuropathy. On the other hand, images from healthy subjects did not show indications of nerve fiber or corneal tissue damage, irrespective of age or gender (Fig. 2 ).
Fig. 2.
IVCM captures from healthy subjects and patients that have overcome COVID19 infection, showing typical morphological signs of small fiber neuropathy.
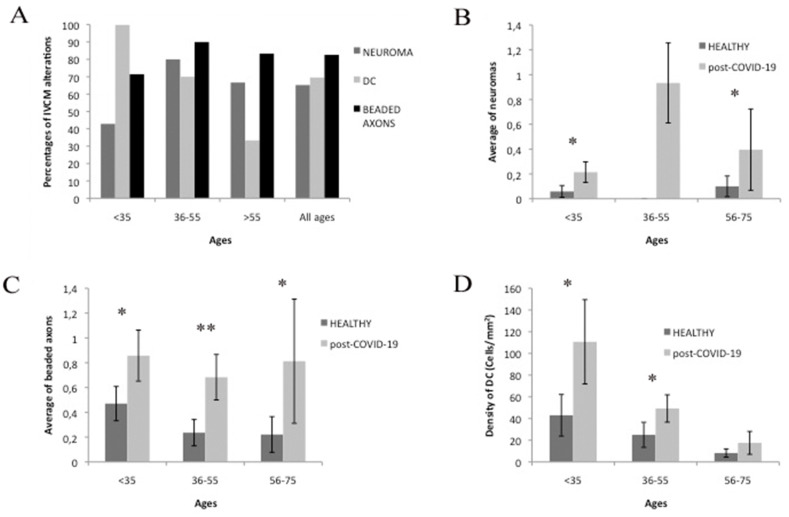
The quantification of neuromas in patients who had overcome COVID-19 infection and healthy subjects showed a significantly higher incidence in COVID-19 patients in all age groups. The occurrence of neuromas among healthy individuals was very rare. Indeed, we only detected 2 neuromas in the group of healthy subjects, one in the <35 group, the other in the 56–75 group, while the number of post-COVID-19 patients with identifiable neuromas was 15 (65.22% of patients across age groups). The proportion of neuromas was linked to age, reaching a maximum in the 36–55 group, where neuroma-like structures were identified in 80% (7 out of 10) of patients. The difference with respect to healthy subjects was significant for all age groups (p < 0.05). Beaded axons are characteristic signs of small fiber neuropathy and were frequently found in post-COVID-19 patients, affecting 82.60% of cases (19 out of 23) in the COVID-19 group and 26.01% of healthy individuals. These alterations were significantly more numerous in COVID-19 group than in healthy subjects (p < 0.05) for all age groups and were present in 90% of patients in the post-COVID-19 36–55 group and 71.43% of the <35 group. The frequency of beaded axons was also high in patients over 56 (83.33%). Finally, DC were found in 69.56% of COVID-19 patients and in 39.13% of healthy subjects. The density of DC in the central cornea of COVID-19 group was significantly higher (p < 0.05) in patients younger than 55 compared with age-matched controls.
3.2. COVID-19 infection increases severity of DED symptoms
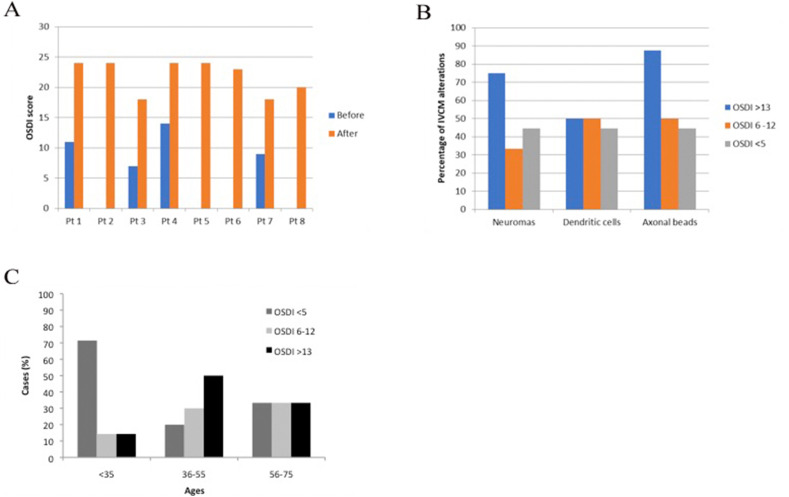
Eight COVID-19 patients scored OSDI >13 after COVID-19 infection. Their Schirmer test average was 3.00 ± 0.49 mm and they were thus considered to have acquired DED symptoms after COVID-19 infection (Fig. 3 ). Four of them had never experienced DED before their COVID-19 infection although post-infection they scored 20, 23, 24 and 24 on OSDI. The other four were patients with previous ophthalmological exams reflecting a non-pathological stage (OSDI scores 7, 9, 11 and 14; the latter presenting scores at the limit of mild DED and experiencing low grade symptoms but a normal Schirmer test before COVID-19 infection and so was not considered medically to have DED). These patients all experienced a great increase in symptoms, leading to a clear DED diagnosis (OSDI scores of 18, 18, 24 and 24 respectively). All eight subjects in this group (100%) showed IVCM signs of subbasal nerve lesion (neuromas, DC or beaded axons). A second group of six patients had OSDI values between 6 and 12. Such values are not usually considered as indicative of ocular dryness pathology, although they do represent the occurrence of some signs of ocular surface discomfort. The average score of these patients on the Schirmer test was 9.50 ± 1.26 mm, and 83% of these patients (n = 5) showed neuromas, DC or beaded axons. Finally, nine patients rated OSDI <5 and had no signs of discomfort. Their average Schirmer value was 11.25 ± 2.02 mm and the percentage of patients with neuromas, DC or beaded axons was 88.89% (n = 8).
Fig. 3.
Results of the quantification of morphological signs of small fiber neuropathy (neuroma, beaded axons and dendritic cells) found in patients that have overcome COVID-19 infection and in healthy controls. In A we show the percentage of signs of lesions at the basal level of the corneal epithelium in groups of age. In B, C, and D, we show the comparison between the amount of neuroma, beaded axons or DC in post-COVID-19 patients and in healthy subjects. Significant differences are represented with asterisks (* = p < 0.05; ** = p < 0.01).
Beaded axons were strongly associated with patients reporting ocular surface irritation or discomfort, with OSDI values > 13 (87.5% of the patients with OSDI >13; Fig. 4 ). Patients with OSDI values under 12 showed percentages of beads occurrence between 44.44 and 50% (Fig. 4B). Neuroma-like images were more frequent (75%) in patients with OSDI values > 13, while patients with OSDI values under 12 showed percentages of neuroma occurrence between 33.33 and 55% (Fig. 4). The presence of DC seemed to be independent of OSDI values, the proportion of patients with DC being between 44 and 50% in all OSDI value groups (Fig. 4B). Nevertheless, DC were more frequent in younger patients (<35 years), most of them (71.43%) having OSDI values < 5 (Fig. 4C).
Fig. 4.
Summary of results of ocular surface IVCM biomarkers and DED symptoms developed after COVID-19 infection. In A, OSDI Scores before and after COVID19 infection (Pt = patient). In B, we show the distribution of IVCM biomarkers (neuroma, beaded axons or DC) attending to DED symptoms severity (OSDI scores). In C, we show the proportion of post-COVID-19 patients as a function of DED severity.
All healthy age-matched subjects obtained low values on the OSDI questionnaire (<12) and the average Schirmer stained length was 10.08 ± 1.31 mm.
In addition to DED symptoms, the 39.13% of COVID-19 patients reported past episodes of anosmia. The presence of anosmia was more numerous in young patients without DED symptoms (42.86%; <35 years; OSDI <5). The presence of neuromas in patients who had overcome COVID-19 was coincident with a 46.15% of anosmia. One 37.50% of patients showing DC had suffered also anosmia, while the proportion of patients with beaded axons and anosmia was 36.84%.
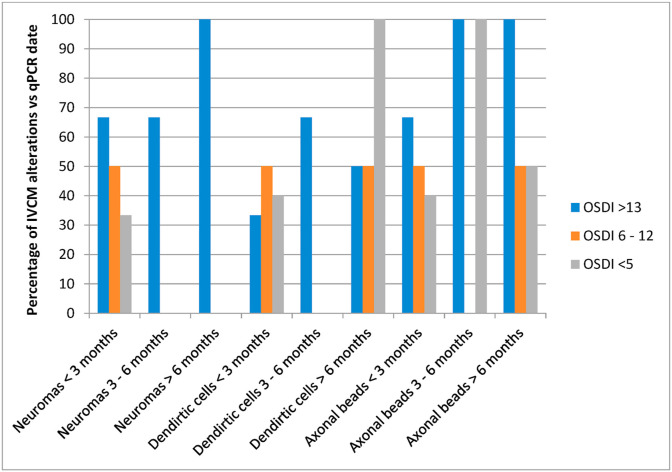
3.3. Temporal evolution of corneal COVID-19 symptoms
We later investigated whether corneal small fiber neuropathy as suggested by morphological alterations in patients overcoming COVID-19 infection was permanent or improved with time (Fig. 5 ). We grouped patients on the basis of the time elapsed from their RT-PCR COVID-19 positive diagnosis to the date of their ophthalmological examination, establishing 3 groups: 1) IVCM examination within 3 months of RT-PCR positive diagnosis (<3 months group); 2) Patients diagnosed with COVID-19 3–6 months before IVCM exam (3–6 months group); 3) Patients diagnosed 6–12 months before IVCM exam (>6 months group). The proportion of neuroma-like structures in patients with a recent COVID-19 diagnosis (<3 months) was significantly higher in patients with OSDI scores >13 compared to those with scores below 12. The percentage of neuromas seemed to increase in patients diagnosed longer ago (3–6 months and >6 months).
Fig. 5.
Proportion of IVCM morphological biomarkers related with the time elapsed from RT-PCR COVID-19 positive diagnosis and ophthalmological examination.
The percentage of patients with DC was higher in patients with longer periods from PCR + to eye exam. Interestingly DC were found in larger proportions in COVID-19 patients with lower OSDI scores (<5), normally considered non-pathological.
Subbasal nerve fibers showing beads were found in all temporal groups at high levels. The proportion of axonal beads was higher in patients with PCR diagnosis more than 3 months earlier and increased progressively with severity of OSDI symptoms among patients with more recent PCR+ (<3 months).
4. Discussion
To date, there is no report in the literature of serious ocular surface complications after Sars-CoV-2 infection, perhaps because the ocular surface expression level of ACE2 and TMPRSS2 receptors is generally low, as is the amount of Sars-CoV-2 RNA collected in ocular samples [29,30]. In addition, the ocular surface shows potent antiviral countermeasures that may explain the low prevalence of eye involvements [31].
Interestingly, our results demonstrate morphological changes in subbasal nerves of COVID-19 group associated with the generation of DED symptoms. The severity of morphological changes was related with worsening of DED symptoms: discomfort, irritation and mild pain. Gambini et al. [32] observed the prevalence of DED symptoms and indicators, such as OSDI questionnaire or Schirmer test, in a cohort of 64 COVID-19 patients, thus supporting thus our findings.
In this work we described lower CNFD and CNFL measurements in patients that have overcome COVID-19 compared with healthy corneas, as well as a reduced CNFRd. Also, high number of neuromas and beaded axons and high density of DC, all these morphological changes suggests clearly small fiber neuropathy [33]. The majority of the morphological changes were found in patients from 36 to 55 years of age. A recent study by Bitirgen et al. [34] reported also CNFD and CNFL reductions and an increase in DC density coincident with our results albeit these changes were observed only in relation with persisting systemic neurological symptoms in a subset of long COVID-19 patients.
The neuropathy of cornea nerve endings has been established as a determinant for the appearance of pathologies such as DED in mice [17,35,36] and in humans [22,23,37]. The degeneration affects the distal end of the neuron, altering not only the fiber morphology but also modifying the neuron function [38].
In healthy corneas, sensitive nerve ending remodeling is a normal process in the corneal epithelium during epithelial cell turnover. Insults and diseases may exceed its capacity for regenerative response and the damaged corneal nerve endings degenerate causing the neuron loss of function [17,19,39,40]. In this case, our results suggest that Sars-Cov-2 infection is related with corneal nerve degeneration. The presence of NRP1 and NRP2 receptors in corneal nerves may explain the generation of small fiber neuropathy and the recruitment of DC to central cornea of patients that have overcome COVID-19 described in our results. Unlike the Sars-CoV virus, which can only infect through interaction with ACE2, Sars-Cov-2 also uses NRP1 and NRP2 as alternative receptors in tissues with very low or absent ACE2 expression [11,12]. Other factor affecting nerve degeneration could be the loss of the neuroprotective role of ACE2 on the neurons expressing it [41,42]. The occupation of the receptor ACE2 by the spike protein of Sars-CoV-2 virus would contribute to the reduction of the survival and regeneration capacity of the axonal endings of the sensory neurons of the cornea.
In our study beaded axons were the most frequent sign of neuropathy in post-COVID-19 patients and also indicative of severity of DED symptoms, as they were predominantly found in OSDI >13 group. Beaded axons were also found in asymptomatic OSDI (<5) even after more than 6 months after overcoming the infection, indicating the possible chronicity of the neuropathy, as is common in other ocular surface viral infections such as HSV, where morphological and functional alterations of corneal nerves are still present months after overcoming HSV keratitis [43]. One cause of the generation of beadings along corneal axons is oxidative stress, as reported in skin sensory nerve endings in diabetic patients induced by high glucose concentrations [[44], [45], [46]]. The lesion is a distal axonopathy that interrupts axonal vesicular traffic and, above all, it is an underlying mitochondrial dysfunction. In the cornea, this may be represented by the increased beading of subbasal nerves observed in COVID-19 patients, it having been suggested that beads observed in peripheral neuropathies, including diabetic neuropathy, are the result of an accumulation of vesicles and mitochondria traveling towards the periphery in regenerating axons, resulting in axon swelling [[44], [45], [46]]. Also, the degeneration and loss of distal nerve endings in the innervated tissue causes not only a loss of sensitivity, but also the alteration of tissue homeostasis and the generation of epithelial ulcers [21].
Our results show that neuroma were preferentially found in patients reporting severe symptoms of DED (OSDI >13 and Schirmer 3.00 ± 0.49 mm), although they were also present in patients with a recent COVID19 diagnosis (less than 3 months after PCR+) and low OSDI (scoring <12). Neuromas are presumed to be disorganized neural and/or glial tissue caused by unsuccessful attempt to regenerate at the stump of the injured nerve [47]. In our work, the identification of neuromas occurred as early as 3 months after patients tested PCR+. This is consistent, for example, with the period of development of neuromas in postsurgical procedures [48]. The lack of neuroma structures in asymptomatic patients and patients with only slight DED symptoms diagnosed with COVID-19 3–10 months before their ophthalmological examination might suggest the worsening of the neuropathy over time. Nerve activity is altered in neuromas, mostly due to the accumulation of Na + channels, whose expression is upregulated in regenerating neurons. As a result of the altered expression of ion channels of neuroma nerve fibers, they lose the ability to detect the natural stimuli they use to be sensitive, which manifests as reduced sensitivity to stimulation, that is, sensitivity loss [18]. In addition, neuroma nerves become hyperexcitable and fire action potentials even in the absence of any stimulation, causing continuous pain and discomfort [18,22,27]. As such, patients with corneal neuroma-like structures will experience ocular discomfort and pain [22] and, at the same time, will have reduced sensitivity to corneal stimulation.
The altered sensitivity of neuropathic fibers we observed in the cornea may be equivalent to the loss of chemical sensitivity described in patients with anosmia/hyposmia or ageusia/dysgeusia due to the altered function of sensory receptors of the olfactory and trigeminal nerves in the olfactory and nasal mucosae and in the taste buds [6,49,50]. Our study showed high coincidence between the presence of neuromas at IVCM examination and loss of smell during Sars-Cov-2 infection. While all patients reporting anosmia recovered total olfactory functionality, corneal morphological changes remained at least the time elapsed between infection and ophthalmological exam.
Accompanying axon degeneration, we observed significantly high density of DC in the cornea of patients who have overcome COVID-19 disease. They were found in higher proportion in young (<35 years) and asymptomatic (low OSDI scores) patients. DC are involved in corneal immunoregulation [51] and inflammatory [52] processes. Attending to morphological parameters such as dendritic processes extension [53], the majority of DC observed was immature DC. Although immature DC did not work as antigen presenting cells, they may be involved in phagocytic activity in the diseased cornea [53]. In healthy corneas, DC were located mostly at the periphery in the corneal epithelium, with a decrease in numbers at the center, which accounted for less than 20% of the total DC [53]. The presence of DC, regardless of whether the patient has symptoms or not, could be explained by the fact that several molecules such as CD209, CD26, CD30 and CD66 present in DC have been found to be receptors for receptors for SARS-CoV-2 [54,55].
Our results add new evidence for the use of IVCM technology in the diagnosis and follow up of post-COVID-19 syndromes or complications, as well as to the study of small fiber neuropathies. Many authors and clinicians are promoting the study of the subbasal nerve plexus alterations as indicators of peripheral neuropathies associated with systemic or neurodegenerative diseases [19,[56], [57], [58]], DED [22,23,33,52,59], or associated with an inflammatory reaction [60,61]. The use of IVCM for the evaluation of corneal nerves is gaining interest and it is now used as a diagnostic marker of diabetic neuropathy [33,56,62,63] and other peripheral neuropathies [[64], [65], [66]], as well as in DED [23,67]. Our work, as well as other recent publications [34], show that IVCM can be used to study of COVID-19 disease.
Taken together, our results indicate the induction of a subclinical immunopathological scenario upon Sars-CoV-2 infection of the cornea. DC were also observed also in patients that had overcome Sars-CoV-2 infection up to 10 months earlier, suggesting that the immunocompromised environment is persistent for a long periods of time, and supporting also the hypothesis of the generation of a chronic DED-like state on the ocular surface of post-COVID19 patients.
5. Conclusions
To our knowledge, this is the first report of Sars-CoV-2 induced neuropathy on the ocular surface. Viral infection causes sensory fiber axonopathy that became chronic after the patients’ recovery. Morphological alterations found in corneas of COVID-19 patients are similar to those found in diabetic corneas and DED, and are accompanied by functional loss and alteration in sensitivity. COVID-19 patients suffer pain and discomfort consistent with DED symptoms.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
None.
Acknowledgements
The authors want to especially acknowledge the devoted work of medical and technical staff at the Instituto Oftalmológico Fernández-Vega and for valuable comments and guidance of the research team at the Fundación de Investigación Oftalmológica.
References
- 1.Reinhold A., Tzankov A., Matter M., Mihic-Probst D., Scholl H.P.N., Meyer P. Ocular pathology and occasionally detectable intraocular SARS-CoV-2 RNA in five fatal COVID-19 cases. Ophthalmic Res. 2021 doi: 10.1016/j.exer.2021.10845510.1159/000514573. [DOI] [PubMed] [Google Scholar]
- 2.Payus A.O., Liew Sat Lin C., Mohd Noh M., Jeffree M.S., Ali R.A. SARS-CoV-2 infection of the nervous system: a review of the literature on neurological involvement in novel coronavirus disease-(COVID-19) Bosn J Basic Med Sci. 2020;20:283–292. doi: 10.17305/bjbms.2020.4860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berger J.R. COVID-19 and the nervous system. J Neurovirol. 2020;26:143–148. doi: 10.1007/s13365-020-00840-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Satarker S., Nampoothiri M. Involvement of the nervous system in COVID-19: the bell should toll in the brain. Life Sci. 2020;262:118568. doi: 10.1016/j.lfs.2020.118568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi R., Goldstein B.J. Olfactory epithelium: cells, clinical disorders, and insights from an adult stem cell niche. Laryngoscope Investig. Otolaryngol. 2018;3:35–42. doi: 10.1002/lio2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barón-Sánchez J., Santiago C., Goizueta-San Martín G., Arca R., Fernández R. Smell and taste disorders in Spanish patients with mild COVID-19. Neurologia. 2020;35:633–638. doi: 10.1016/j.nrl.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sungnak W., Huang N., Bécavin C., Berg M., Queen R., Litvinukova M., et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. 2020;26:681–687. doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Douglas K.A.A., Douglas V.P., Moschos M.M. Ocular manifestations of COVID-19 (SARS-CoV-2): a critical review of current literature. In Vivo (Brooklyn) 2020;34:1619–1628. doi: 10.21873/invivo.11952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meinhardt J., Radke J., Dittmayer C., Franz J., Thomas C., Mothes R., et al. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat Neurosci. 2021;24:168–175. doi: 10.1038/s41593-020-00758-5. [DOI] [PubMed] [Google Scholar]
- 10.McFarland A.J., Yousuf M.S., Shiers S., Price T.J. Neurobiology of SARS-CoV-2 interactions with the peripheral nervous system: implications for COVID-19 and pain. Pain Rep. 2021;6:e885. doi: 10.11604/pamj.supp.2020.35.2.2500310.1097/pr9.0000000000000885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daly J.L., Simonetti B., Klein K., Chen K.-E., Williamson M.K., Antón-Plágaro C., et al. Neuropilin-1 is a host factor for SARS-CoV-2 infection. Science. 2020;370:861–865. doi: 10.1126/science.abd3072. 80- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cantuti-Castelvetri L., Ojha R., Pedro L.D., Djannatian M., Franz J., Kuivanen S., et al. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science. 2020;370:856–860. doi: 10.1126/science.abd2985. 80- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee P.S., Gao N., Dike M., Shkilnyy O., Me R., Zhang Y., et al. Opposing effects of neuropilin-1 and -2 on sensory nerve regeneration in wounded corneas: role of Sema3C in ameliorating diabetic neurotrophic keratopathy. Diabetes. 2019;68:807–818. doi: 10.2337/db18-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marfurt C.F., Cox J., Deek S., Dvorscak L. Anatomy of the human corneal innervation. Exp Eye Res. 2010;90:478–492. doi: 10.1016/j.exer.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 15.Oliveira-Soto L., Efron N. Morphology of corneal nerves using confocal microscopy. Cornea. 2001;20:374–384. doi: 10.1097/00003226-200105000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Al-Aqaba M.A., Fares U., Suleman H., Lowe J., Dua H.S. Architecture and distribution of human corneal nerves. Br J Ophthalmol. 2010;94:784–789. doi: 10.1136/bjo.2009.173799. [DOI] [PubMed] [Google Scholar]
- 17.Alcalde I., Íñigo-Portugués A., González-González O., Almaraz L., Artime E., Morenilla-Palao C., et al. Morphological and functional changes in TRPM8-expressing corneal cold thermoreceptor neurons during aging and their impact on tearing in mice. J Comp Neurol. 2018 doi: 10.1002/cne.24454. [DOI] [PubMed] [Google Scholar]
- 18.Belmonte C., Acosta M.C., Merayo-Lloves J., Gallar J. What causes eye pain? Curr Ophthalmol Rep. 2015;3:111–121. doi: 10.1007/s40135-015-0073-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Badian R.A., Andréasson M., Svenningsson P., Utheim T.P., Lagali N. The pattern of the inferocentral whorl region of the corneal subbasal nerve plexus is altered with age. Ocul Surf. 2021;22:204–212. doi: 10.1016/j.jtos.2021.08.015. [DOI] [PubMed] [Google Scholar]
- 20.Odriozola A., Ortega L., Martinez L., Odriozola S., Torrens A., Corroleu D., et al. Widespread sensory neuropathy in diabetic patients hospitalized with severe COVID-19 infection. Diabetes Res Clin Pract. 2020;172:108631. doi: 10.1097/pr9.000000000000088510.1016/j.diabres.2020.108631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zherebitskaya E., Akude E., Smith D.R., Fernyhough P. Development of selective axonopathy in adult sensory neurons isolated from diabetic rats: role of glucose-induced oxidative stress. Diabetes. 2009;58:1356–1364. doi: 10.2337/db09-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moein H.R., Akhlaq A., Dieckmann G., Abbouda A., Pondelis N., Salem Z., et al. Visualization of microneuromas by using in vivo confocal microscopy: an objective biomarker for the diagnosis of neuropathic corneal pain? Ocul Surf. 2020;18(4):651–656. doi: 10.1016/j.jtos.2020.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cox S.M., Kheirkhah A., Aggarwal S., Abedi F., Cavalcanti B.M., Cruzat A., et al. Alterations in corneal nerves in different subtypes of dry eye disease: an in vivo confocal microscopy study. Ocul Surf. 2021;22:135–142. doi: 10.1016/j.jtos.2021.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dabbah M.A., Graham J., Petropoulos I.N., Tavakoli M., Malik R.A. Automatic analysis of diabetic peripheral neuropathy using multi-scale quantitative morphology of nerve fibres in corneal confocal microscopy imaging. Med Image Anal. 2011;15:738–747. doi: 10.1016/j.media.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 25.Chen X., Graham J., Dabbah M.A., Petropoulos I.N., Tavakoli M., Malik R.A. An automatic tool for quantification of nerve fibers in corneal confocal microscopy images. IEEE Trans Biomed Eng. 2017;64:786–794. doi: 10.1109/TBME.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen X., Graham J., Petropoulos I.N., Ponirakis G., Asghar O., Alam U., et al. Corneal nerve fractal dimension: a novel corneal nerve metric for the diagnosis of diabetic sensorimo corneal nerve fractal dimension: a novel corneal nerve metric for the diagnosis of diabetic sensorimotor polyneuropathy. Invest Ophthalmol Vis Sci. 2018;59:1113–1118. doi: 10.1167/iovs.17-23342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stepp M.A., Pal-Ghosh S., Downie L.E., Zhang A.C., Chinnery H.R., Machet J., et al. Corneal epithelial “neuromas”: a case of mistaken identity? Cornea. 2020;39:930–934. doi: 10.1097/ICO.0000000000002294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wolffsohn J.S., Arita R., Chalmers R., Djalilian A., Dogru M., Dumbleton K., et al. TFOS DEWS II diagnostic methodology report. Ocul Surf. 2017;15:539–574. doi: 10.1016/j.jtos.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 29.Azzolini C., Donati S., Premi E., Baj A., Siracusa C., Genoni A., et al. SARS-CoV-2 on ocular surfaces in a cohort of patients with COVID-19 from the lombardy region, Italy. JAMA Ophthalmol. 2021 doi: 10.1001/jamaophthalmol.2020.5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sawant O.B., Singh S., Wright R.E., Jones K.M., Titus M.S., Dennis E., et al. Prevalence of SARS-CoV-2 in human post-mortem ocular tissues. Ocul Surf. 2021;19:322–329. doi: 10.1016/j.jtos.2020.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leonardi A., Rosani U., Brun P. Ocular surface expression of SARS-CoV-2 receptors. Ocul Immunol Inflamm. 2020;28:735–738. doi: 10.1080/09273948.2020.1772314. [DOI] [PubMed] [Google Scholar]
- 32.Gambini G., Savastano M.C., Savastano A., De Vico U., Crincoli E., Cozzupoli M.G., et al. Ocular surface impairment after COVID-19: a cohort study. Cornea. 2020 doi: 10.1097/ico.0000000000002643. [DOI] [PubMed] [Google Scholar]
- 33.Tummanapalli S.S., Issar T., Yan A., Kwai N., Poynten A.M., Krishnan A.V., et al. Corneal nerve fiber loss in diabetes with chronic kidney disease. Ocul Surf. 2020;18(1):178–185. doi: 10.1016/j.jtos.2019.11.010. [DOI] [PubMed] [Google Scholar]
- 34.Bitirgen G., Korkmaz C., Zamani A., Ozkagnici A., Zengin N., Ponirakis G., et al. Corneal confocal microscopy identifies corneal nerve fibre loss and increased dendritic cells in patients with long COVID. Br J Ophthalmol. 2021 doi: 10.1136/bjophthalmol-2021-319450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parra A., Madrid R., Echevarria D., del Olmo S., Morenilla-Palao C., Acosta M.C., et al. Ocular surface wetness is regulated by TRPM8-dependent cold thermoreceptors of the cornea. Nat Med. 2010;16:1396–1399. doi: 10.1038/nm.2264. [DOI] [PubMed] [Google Scholar]
- 36.Alcalde I., Íñigo-Portugués A., Carreño N., Riestra A.C., Merayo-Lloves J.M. Effects of new biomimetic regenerating agents on corneal wound healing in an experimental model of post-surgical corneal ulcers. Arch Soc Esp Oftalmol. 2015;90 doi: 10.1016/j.oftal.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 37.Kovács I., Luna C., Quirce S., Mizerska K., Callejo G., Riestra A., et al. Abnormal activity of corneal cold thermoreceptors underlies the unpleasant sensations in dry eye disease. Pain. 2016;157:399–417. doi: 10.1097/j.pain.0000000000000455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malmberg A.B., Mizisin A.P., Calcutt N.A., von Stein T., Robbins W.R., Bley K.R. Reduced heat sensitivity and epidermal nerve fiber immunostaining following single applications of a high-concentration capsaicin patch. Pain. 2004;111:360–367. doi: 10.1016/j.pain.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 39.Wang S., Davis B.M., Zwick M., Waxman S.G., Albers K.M. Reduced thermal sensitivity and Nav1.8 and TRPV1 channel expression in sensory neurons of aged mice. Neurobiol Aging. 2006;27:895–903. doi: 10.1016/j.neurobiolaging.2005.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ma F., Zhang L., Westlund K.N. Trigeminal nerve injury ErbB3/ErbB2 promotes mechanical hypersensitivity. Anesthesiology. 2012;117:381–388. doi: 10.1097/ALN.0b013e3182604b2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dolatshahi M., Sabahi M., Aarabi M.H. Pathophysiological clues to how the emergent SARS-CoV-2 can potentially increase the susceptibility to neurodegeneration. Mol Neurobiol. 2021:1–16. doi: 10.1002/mus.2708310.1007/s12035-020-02236-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bennion D.M., Haltigan E., Regenhardt R.W., Steckelings U.M., Sumners C. Neuroprotective mechanisms of the ACE2-angiotensin-(1-7)-Mas axis in stroke. Curr Hypertens Rep. 2015;17:3. doi: 10.1007/s11906-014-0512-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gallar J., Tervo T.M.T., Neira W., Holopainen J.M., Lamberg M.E., Miñana F., et al. Selective changes in human corneal sensation associated with herpes simplex virus keratitis. Invest Ophthalmol Vis Sci. 2010;51:4516–4522. doi: 10.1167/iovs.10-5225. [DOI] [PubMed] [Google Scholar]
- 44.Müller L.J., Pels L., Vrensen G.F. Ultrastructural organization of human corneal nerves. Invest Ophthalmol Vis Sci. 1996 Mar;37(4):476–488. PMID: 8595948. [PubMed] [Google Scholar]
- 45.Ishibashi F., Kojima R., Taniguchi M., Kosaka A., Uetake H., Tavakoli M. The expanded bead size of corneal C-nerve fibers visualized by corneal confocal microscopy is associated with slow conduction velocity of the peripheral nerves in patients with type 2 diabetes mellitus. J Diabetes Res. 2016:3653459. doi: 10.1155/2016/3653459. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ishibashi F., Kojima R., Taniguchi M., Kosaka A., Uetake H., Tavakoli M. The expanded bead size of corneal C-nerve fibers visualized by corneal confocal microscopy is associated with slow conduction velocity of the peripheral nerves in patients with type 2 diabetes mellitus. J Diabetes Res. 2016:3653459. doi: 10.1155/2016/3653459. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lu C., Sun X., Wang C., Wang Y., Peng J. Mechanisms and treatment of painful neuromas. Rev Neurosci. 2018;29:557–566. doi: 10.1515/revneuro-2017-0077. [DOI] [PubMed] [Google Scholar]
- 48.Henrot P., Stines J., Walter F., Martinet N., Paysant J., Blum A. Radiogr a Rev Publ Radiol Soc North Am Inc; 2000. Imaging of the painful lower limb stump. 20 Spec No:S219-35. [DOI] [PubMed] [Google Scholar]
- 49.Fotuhi M., Mian A., Meysami S., Raji C.A. Neurobiology of COVID-19. J Alzheimers Dis. 2020;76:3–19. doi: 10.3233/jad-200581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meunier N., Briand L., Jacquin-Piques A., Brondel L., Pénicaud L. COVID 19-induced smell and taste impairments: putative impact on physiology. Front Physiol. 2021;11 doi: 10.3389/fphys.2020.625110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schaumburg C.S., Siemasko K.F., De Paiva C.S., Wheeler L.A., Niederkorn J.Y., Pflugfelder S.C., et al. Ocular surface APCs are necessary for autoreactive T cell-mediated experimental autoimmune lacrimal keratoconjunctivitis. J Immunol. 2011;187:3653–3662. doi: 10.4049/jimmunol.1101442. [DOI] [PubMed] [Google Scholar]
- 52.Marsovszky L., Németh J., Resch M.D., Toldi G., Legány N., Kovács L., et al. Corneal Langerhans cell and dry eye examinations in ankylosing spondylitis. Innate Immun. 2014;20:471–477. doi: 10.1177/1753425913498912. [DOI] [PubMed] [Google Scholar]
- 53.Mastropasqua L., Nubile M., Lanzini M., Carpineto P., Ciancaglini M., Pannellini T., et al. Epithelial dendritic cell distribution in normal and inflamed human cornea: in vivo confocal microscopy study. Am J Ophthalmol. 2006 Nov;142(5):736–744. doi: 10.1016/j.ajo.2006.06.057. 10.1016/j.ajo.2006.06.057. PMID: 17056357. [DOI] [PubMed] [Google Scholar]
- 54.Amraei R., Rahimi N. COVID-19, renin-angiotensin system and endothelial dysfunction. Cells. 2020;9 doi: 10.3390/cells9071652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Willcox M.D., Walsh K., Nichols J.J., Morgan P.B., Jones L.W. The ocular surface, coronaviruses and COVID-19. Clin Exp Optom. 2020;103:418–424. doi: 10.1111/cxo.13088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cruzat A., Qazi Y., Hamrah P. In vivo confocal microscopy of corneal nerves in Health and disease. Ocul Surf. 2017;15:15–47. doi: 10.1016/j.jtos.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roszkowska A.M., Licitra C., Tumminello G., Postorino E.I., Colonna M.R., Aragona P. Corneal nerves in diabetes-The role of the in vivo corneal confocal microscopy of the subbasal nerve plexus in the assessment of peripheral small fiber neuropathy. Surv Ophthalmol. 2020 doi: 10.1016/j.survophthal.2020.09.003. [DOI] [PubMed] [Google Scholar]
- 58.Arrigo A., Rania L., Calamuneri A., Postorino E.I., Mormina E., Gaeta M., et al. Early corneal innervation and trigeminal alterations in Parkinson disease: a pilot study. Cornea. 2018;37:448–454. doi: 10.1097/ICO.0000000000001517. [DOI] [PubMed] [Google Scholar]
- 59.Labbé A., Liang Q., Wang Z., Zhang Y., Xu L., Baudouin C., et al. Corneal nerve structure and function in patients with non-sjogren dry eye: clinical correlations. Invest Ophthalmol Vis Sci. 2013;54:5144–5150. doi: 10.1167/iovs.13-12370. [DOI] [PubMed] [Google Scholar]
- 60.Alzahrani Y., Pritchard N., Efron N. Changes in corneal Langerhans cell density during the first few hours of contact lens wear. Contact Lens Anterior Eye. 2016;39:307–310. doi: 10.1016/j.clae.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 61.Ferdousi M., Romanchuk K., Mah J.K., Virtanen H., Millar C., Malik R.A., et al. Early corneal nerve fibre damage and increased Langerhans cell density in children with type 1 diabetes mellitus. Sci Rep. 2019;9:8758. doi: 10.1038/s41598-019-45116-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Petropoulos I.N., Manzoor T., Morgan P., Fadavi H., Asghar O., Alam U., et al. Repeatability of in vivo corneal confocal microscopy to quantify corneal nerve morphology. Cornea. 2013;32:e83–e89. doi: 10.1097/ICO.0b013e3182749419. [DOI] [PubMed] [Google Scholar]
- 63.Binotti W.W., Bayraktutar B., Ozmen M.C., Cox S.M., Hamrah P. A review of imaging biomarkers of the ocular surface. Eye Contact Lens. 2020;46(Suppl 2):S84–S105. doi: 10.1097/ICL.0000000000000684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Oudejans L., He X., Niesters M., Dahan A., Brines M., van Velzen M. Cornea nerve fiber quantification and construction of phenotypes in patients with fibromyalgia. Sci Rep. 2016;6:23573. doi: 10.1038/srep23573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jeziorska M., Atkinson A., Kass-Iliyya L., Kobylecki C., Gosal D., Marshall A., et al. Small fibre neuropathy in Parkinson's disease: comparison of skin biopsies from the more affected and less affected sides. J Parkinsons Dis. 2019;9:761–765. doi: 10.3233/JPD-191697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Petropoulos I.N., Ponirakis G., Khan A., Gad H., Almuhannadi H., Brines M., et al. Corneal confocal microscopy: ready for prime time. Clin Exp Optom. 2020;103:265–277. doi: 10.1111/cxo.12887. [DOI] [PubMed] [Google Scholar]
- 67.Giannaccare G., Pellegrini M., Sebastiani S., Moscardelli F., Versura P., Campos E.C. In vivo confocal microscopy morphometric analysis of corneal subbasal nerve plexus in dry eye disease using newly developed fully automated system. Graefes Arch Clin Exp Ophthalmol. 2019;257:583–589. doi: 10.1007/s00417-018-04225-7. [DOI] [PubMed] [Google Scholar]