Abstract
An immunoglobulin G immunoblot was developed with antigenic extracts of Borrelia burgdorferi sensu stricto, B. garinii, B. afzelii, and B. valaisiana genospecies and was reacted with sera from patients with neuroborreliosis, acrodermatitis, and Lyme arthritis. A detailed analysis of the reactivities of the protein bands was performed, and a two-step scoring procedure was selected to determine the preferential reactivity of sera to one particular genospecies. The discriminative potential of 5 proteins (12-kDa, 16-kDa, 18-kDa, OspA, and 66-kDa proteins) was used as a rapid first-step scoring method, followed by scoring of 14 additional protein bands if necessary. The advantage of this procedure is the low percentage of serum samples with inconclusive results for one of the four species (10% for patients with neuroborreliosis, 6% for patients with acrodermatitis chronica atrophicans, and 6% for patients with Lyme arthritis). Among 31 serum samples from patients with neuroborreliosis, 16 were more reactive to B. garinii, 7 were more reactive to B. afzelii, 3 were more reactive to B. valaisiana, and 2 were more reactive to B. burgdorferi sensu stricto. Of 31 serum samples from patients with acrodermatitis, 26 showed a higher level of reactivity to B. afzelii. Of 34 serum samples from patients with Lyme arthritis, 21 were more reactive to B. burgdorferi sensu stricto, 10 were more reactive to B. afzelii, and 1 was more reactive to B. valaisiana. Our results suggest an organotropism of Borrelia species and provide some evidence of a pathogenic potential of B. valaisiana in humans.
Lyme borreliosis (LB) is a tick-borne multistage disease caused by the spirochetal bacterium Borrelia burgdorferi sensu lato. B. burgdorferi sensu lato is divided into several species on the basis of phenotypic and genotypic characteristics (35). In Europe, five species of B. burgdorferi sensu lato have been isolated from Ixodes ricinus: B. burgdorferi sensu stricto, B. garinii, B. afzelii (4, 9), group VS116, which has been classified as B. valaisiana novel species (44), and B. lusitaniae (24).
It appears that the geographic distributions of these species are not uniform, even within neighborhoods (34). However, in Western Europe and Switzerland, B. garinii is more frequently isolated, followed by B. afzelii, B. burgdorferi sensu stricto, and B. valaisiana, in that order (39). In Scandinavia and The Netherlands, B. afzelii is probably the most common Borrelia (36, 42), followed by B. valaisiana, B. burgdorferi sensu stricto, and B. garinii. In Ireland, B. valaisiana is described as the most prevalent genospecies, followed by B. garinii, B. burgdorferi sensu stricto, and B. afzelii (23). B. lusitaniae was first isolated from I. ricinus in Portugal and was subsequently isolated from ticks in other European countries (24).
After a person is bitten by an infected tick, the spirochetes undergo a hematogenous dissemination and can be found in many of the major organ systems. The first stage and hallmark of LB is a distinctive skin rash, erythema migrans (EM), that frequently appears at the site of the tick bite (40). Days to months after the tick bite, the disease may progress toward secondary and tertiary stages. In some patients, chronic diseases may develop. These may affect the skin, such as acrodermatitis chronica atrophicans (ACA), a clinical manifestation primarily observed in Europe, and possibly affect joints with arthritis, which is more common in the United States (40). In Europe, neurological symptoms appear in 30% of untreated patients and musculoskeletal symptoms appear in 20% of patients (1). These observations have suggested that clinical outcome could depend on infection with strains of different genospecies. Neurological symptoms are thought to be mainly caused by B. garinii, while B. afzelii is often associated with ACA and B. burgdorferi sensu stricto is often associated with Lyme arthritis (3, 13, 32, 42). However, controversial reports described a good match between the distribution of B. burgdorferi: sensu lato in ticks and in patients from the same area (14) or an organotropism linked to strain-specific characteristics, not to genotypes (45). Furthermore, the pathogenic potential of B. valaisiana was suggested among patients with EM by using PCR (37), but there is no previous indication of an association of B. valaisiana with chronic clinical symptoms.
Immunoblotting is unanimously reported to be a confirmatory test for LB. One of the factors affecting the performance of this assay is the polymorphism of Borrelia antigens which is evident among species and intraspecies. Norman et al. (30) found that the preferential reactivity to genospecies is not absolute and that regional variations in the reactivity to the genospecies strains may occur. The purpose of the current study was to compare the immunoglobulin G (IgG) immunoblots of four different Borrelia genospecies present in Switzerland (34). Therefore, we tested sera from patients living in Switzerland. In order to decrease the percentage of serum samples with an inconclusive predominance of one of the four species, we modified the scoring method described by Péter et al. (32). The preferential reactivity of sera led us to confirm the association between some manifestations of LB and the species of B. burgdorferi sensu lato.
(This research is part of the Ph.D dissertation of K. Ryffel.)
MATERIALS AND METHODS
Study samples.
Serum samples from Swiss patients with a clinical diagnosis of late LB and a positive screening test confirmed by immunoblotting were collected among sera referred for testing by a confirmatory diagnostic procedure. The sera were from 31 patients with neuroborreliosis, 31 patients with ACA, and 34 patients with Lyme arthritis. Sera from patients with several symptoms of LB were excluded. Among serum samples from patients with neuroborreliosis, all were confirmed to be positive by intrathecal antibody synthesis. The index of intrathecal antibody production was calculated as follows: IgG-specific titer in cerebrospinal fluid (CSF)/IgG-specific titer in serum divided by albumin concentration in CSF/albumin concentration in serum (negative result, index below 2.0).
In order to establish the persistence of the reactivity to the infecting species after antibiotic therapy, parallel serum samples from patients were tested. Patients with neuroborreliosis (n = 2), ACA (n = 6), and arthritis (n = 1) were selected. The first serum samples were taken during the first clinical visit, and the second ones were taken 6 months to 5 years after treatment.
Antigen preparation.
Borrelia strains, B. burgdorferi sensu stricto VS215, B. garinii VS102, B. afzelii VS461, and B. valaisiana VS116 were used for antigen preparation (32, 44). All strains were isolated from ticks (I. ricinus) (33). Spirochetes were cultured in BSK II medium (Sigma, St. Louis, Mo.). During the late logarithmic phase of growth, the culture was centrifuged at 14,000 × g for 15 min and washed twice in phosphate-buffered saline (pH 7.2) to which MgCl2 (0.05 M) was added. The protein concentration of the suspension was determined by the biuret method and was adjusted to 1 mg/ml in distilled water. Washed Borrelia cells were stored at −20°C until use (34). The isolates used in this study were all low-passage strains. Prior to Western blotting, all antigen preparations were adjusted to contain equal amounts of p41, which is known to be present in rather constant amounts in all strains (16, 50) and which was quantified by serial dilution by Coomassie blue-stained immunoblotting and a densitometry analysis program.
Electrophoresis and immunoblotting.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting were performed by standard procedures (33). Briefly, samples were heated for 5 min at 95°C before undergoing electrophoresis on a polyacrylamide gel at 12.5% (constant voltage, 170 V). After electrophoresis the proteins were transferred to a polyvinylidene difluoride membrane and were cut into strips.
Before use, the strips were blocked for 1 h at 37°C with Tris-buffered saline (TBS; pH 7.2) with 5% gelatin and were washed three times for 5 min each time with washing buffer (W-buffer; TBS with 0.1% gelatin and 0.05% Tween 20) at room temperature. Each step of this procedure was performed at room temperature. The four antigen strips were incubated for 2 h with patient serum diluted 1/500 in D-buffer (TBS with 1% gelatin and 0.05% Tween 20). The strips were washed three times for 5 min each time with W-buffer. After the washing, rabbit anti-human IgG conjugated to alkaline phosphatase diluted 1/1,000 in D-buffer was added. At the end of the second 2-h incubation, two washes were done with W-buffer and one was done with TBS. The bound conjugate was visualized by addition of the chromogenic substrate 5-bromo-4-chloro-3-indolylphosphate–Nitro Blue Tetrazolium (Kirkegaard & Perry Laboratories). The reaction was stopped 10 to 15 min later by two rinses in distilled water. Incubations were always performed with all the strips of each antigen in the same well.
Characterization of protein bands.
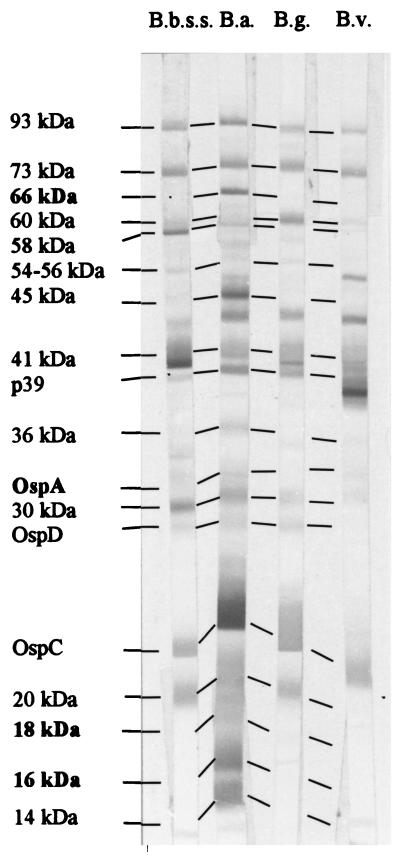
Reproducibility was confirmed by repeat probing of the immunoblot strips from different gels with four serum samples preferentially reactive with each species. In all strains the 39-kDa protein could be clearly differentiated from the flagellin (41 kDa), the 30-kDa protein could be differentiated from OspA, and the 58-kDa protein could be differentiated from the 60-kDa protein.
The blots were analyzed visually by one person, and the interpretation was confirmed independently by an other one. Since only minor divergences were observed for the typing of the serological reactions between the two readers, we used the classification of one reader only. For comparison between the four strips, scores (0 to 3 points) were allocated depending on the presence and intensity of the reaction to 19 proteins of Borrelia: the 93-kDa (p100), 75-kDa, 66-kDa, 60-kDa, 58-kDa, 54- to 56-kDa, 45-kDa, 41-kDa (flagellin), p39, 36-kDa, OspA, 30-kDa, OspD, OspC, 20-kDa, 18-kDa, 16-kDa, 14-kDa, and 12-kDa proteins. Some of these proteins were identified in the four Borrelia species with monoclonal antibodies: LA 114 Zs7 (93-kDa protein) sl60 (60-kDa protein), H9724 (flagellin), LA 112 Zs7 (39-kDa protein), and LA 22 2B8 (OspC) (kindly provided by A. G. Barbour, University of California, Irvine; R. Wallich, Ruprecht-Karls-Universität, Heidelberg, Germany; and B. Wilske, Max von Pettenkofer Institut, Munich, Germany). OspA was identified with two different monoclonal antibodies, H5332 (reactive with VS215, VS102, and VS461) and A116k (reactive with B. valaisiana). A report characterizing the A116k monoclonal antibody is in preparation. The other proteins were identified in the four Borrelia species by specific serum reactivities and Molecular Analysis software (Bio-Rad, Hercules, Calif.) (see Fig. 1 and Table 1).
FIG. 1.
Example of an immunoblot with a predominant score for B. afzelii. Immunoblots (IgG) were done with the serum from a patient with ACA. B.b.ss., B. burgdorferi sensu stricto VS215; B.a., B. afzelii VS461; B.g., B. garinii VS102; B.v., B. valaisiana VS116. Boldface indicates the five discriminative proteins used in method I.
TABLE 1.
Scores for reactivity of a serum sample to the four Borrelia species for 19 proteinsa
| Protein band | Scoreb
|
|||
|---|---|---|---|---|
| B.b.ss | B.a. | B.g. | B.v. | |
| 93 kDa | 2 | 2 | 1 | 1 |
| 73 kDa | 2 | 2 | 2 | 2 |
| 66 kDa | 0 | 3 | 0 | 0 |
| 60 kDa | 1 | 0 | 2 | 1 |
| 58 kDa | 2 | 1 | 0 | 1 |
| 54–56 kDa | 1 | 1 | 1 | 0 |
| 45 kDa | 1 | 3 | 0 | 0 |
| 41 kDa | 2 | 2 | 2 | 2 |
| p39 | 1 | 2 | 2 | 2 |
| 36 kDa | 0 | 1 | 0 | 1 |
| OspA | 1 | 1 | 0 | 1 |
| 30 kDa | 2 | 2 | 1 | 1 |
| OspD | 1 | 1 | 1 | 0 |
| OspC | 2 | 3 | 2 | 2 |
| 20 kDa | 2 | 2 | 2 | 0 |
| 18 kDa | 0 | 1 | 0 | 0 |
| 16 kDa | 0 | 2 | 0 | 0 |
| 14 kDa | 0 | 2 | 0 | 0 |
| 12 kDa | 0 | 0 | 0 | 0 |
| Score method Ic | 1 | 7 | 0 | 1 |
| Score method IId | 20 | 31 | 16 | 14 |
See Fig. 1. Boldface proteins and scores indicate the five discriminative proteins used in method I and the scores obtained with those proteins.
B.b.ss., B. burgdorferi sensu stricto; B.a., B. afzelii; B.g., B. garinii; B.v., B. valaisiana.
Score with the five discriminative proteins (the score was discriminative if the score for reactivity to one species was two points greater than the scores for reactivity to the other species).
Total score with 19 proteins (the score was discriminative if the score for reactivity to one species was three points greater than the scores for reactivity to the other species).
Determination of predominant reactivity for each species.
To determine the preferential reactivities of the sera, a detailed analysis was performed. Five of 13 proteins with a discriminative potential (the 12-kDa, 16-kDa, 18-kDa, OspA and 66-kDa proteins) were selected and were used in a rapid first-step scoring procedure (method I). A total score for these five proteins that was greater by two points for one individual species compared with the scores for the other species was considered as preferential reactivity. If preferential reactivity could not be found, the scores for all 19 protein bands were taken into account (method II). A total score that was three points greater for one individual species compared with the scores for the other species was considered a preferential reactivity. The differential number of scored points necessary to define this preferential reactivity to one particular species was established on the basis of a previous statistical study (32).
Comparison of immunoreactivity with genotypic detection.
Oligonucleotide primers that recognize the OspA sequences of most B. burgdorferi strains (11) were used to amplify DNA by PCR for patients for whom there was a clinical suspicion of Lyme arthritis. The presence of B. burgdorferi sensu lato DNA in synovial fluid (n = 15) and urine (n = 1) samples was shown by agarose gel analysis. The specificities of the PCR amplicons were controlled by colorimetric solid-phase capture hybridization assay (28). Stored aliquots of each PCR-positive synovial fluid or urine sample were retrospectively analyzed with species-specific primers to type the infecting strains by PCR. (11). Without knowing the results of the PCR, sera from these patients were analyzed and the results were interpreted independently.
RESULTS
Characterization of serological responses.
In order to determine the preferential reactivities of sera from patients with neuroborreliosis (n = 31), ACA (n = 31), and Lyme arthritis (n = 34), the bands for following proteins were scored and analyzed on four species-specific immunoblots: the 93-kDa (p100), 75-kDa, 66-kDa, 60-kDa, 58-kDa, 54- to 56-kDa, 45-kDa, 41-kDa (flagellin), p39, 36-kDa, OspA, 30-kDa, OspD, OspC, 20-kDa, 18-kDa, 16-kDa, 14-kDa, and 12-kDa proteins (Fig. 1; Table 1).
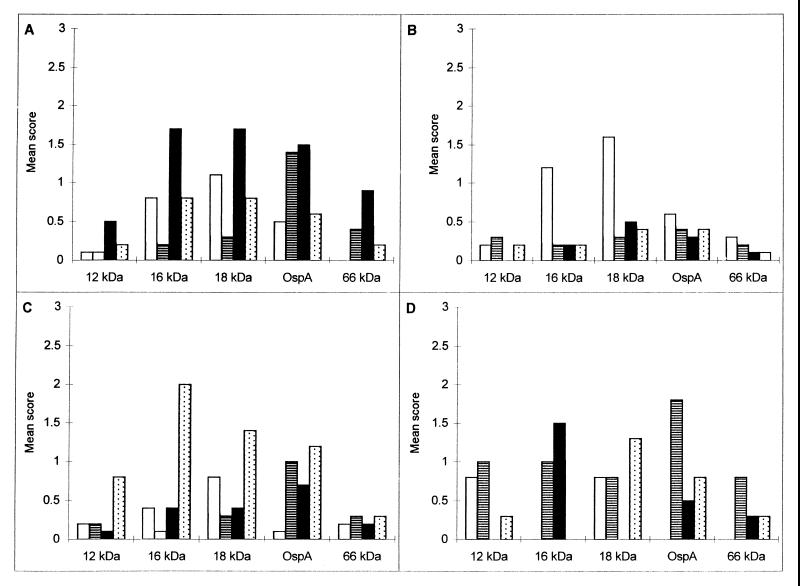
When the specimens were grouped by their preferential reactivity to each of the four species, only a few bands appeared to have discriminative potential. Among the sera with a preferential reactivity to B. burgdorferi sensu stricto (n = 23), the 12-kDa, 16-kDa, OspC, 58-kDa, and 66-kDa antigen bands were found to have a higher discriminative power with B. burgdorferi sensu stricto than with the other species. Sera with a preferential reactivity to B. garinii (n = 17) had greater reactivities to the 16-kDa, 18-kDa, 20-kDa, OspC, OspD, 30-kDa, OspA, 45-kDa, and 60-kDa antigen bands. Sera with a preferential reactivity to B. afzelii (n = 43) had greater reactivities to the 12-kDa, 14-kDa, 16-kDa, 18-kDa, OspC, and 45-kDa antigen bands. Among sera with a preferential reactivity to B. valaisiana (n = 4), the reactivities to the OspA, 45-kDa, and 66-kDa antigen bands were greater. The 12-kDa, 14-kDa, 16-kDa, 18-kDa, 20-kDa, OspC, OspD, 30-kDa, OspA, 45-kDa, 58-kDa, 60-kDa, and 66-kDa proteins had discriminative potential for the four species. On the basis of these results, the five proteins with the best discriminative potential (the 12-kDa, 16-kDa, 18-kDa, OspA, and 66-kDa proteins) were used in a rapid first-step procedure to determine the preferential reactivities of sera to the four species (Fig. 2A to D). If this procedure was not discriminative, the second step was then used and the results were determined on the basis of the scores for all 19 proteins.
FIG. 2.
Mean score of specific reactivity to different Borrelia species. (A) Specific reactivity to B. burgdorferi sensu stricto (n = 23). (B) Specific reactivity to B. garinii (n = 17). (C) Specific reactivity to B. afzelii (n = 43). (D) Specific reactivity to B. valaisiana (n = 4). ■, B. burgdorferi sensu stricto; □, B. garinii; , B. afzelii; ▤, B. valaisiana.
Among the sera from patients with neuroborreliosis, 18 serum samples had a preferential reactivity to one species after the first step; the results for 16 of them were confirmed by the second step. For two samples the total score was not greater by three points. Among the sera from patients with ACA and Lyme arthritis, 21 and 19 serum samples, respectively, had a preferential reactivity to one species after the first step. The results for 19 and 17 of the samples respectively, were confirmed by the second step. For the four remaining serum samples the total score was not greater by three points for one species than for the others. The percentages of serum samples with preferential reactivity to a particular species are shown in Table 2. The percentages of serum samples with a preferential reactivity by the combined method were 90% for patients with neuroborreliosis (28 of 31), 94% for patients with ACA (29 of 31), and 94% for patients with Lyme arthritis (32 of 34). Of the three serum samples from patients with neuroborreliosis with undetermined reactivities, one was from a patient with an early infection and had a weak reaction for IgG. We suspect a mixed infection for another sample, as the total scores for reactivity to the 19 bands were clearly higher with the B. garinii and B. afzelii species-specific immunoblots than those with the B. valaisiana and B. burgdorferi sensu stricto species-specific immunoblots. Of the two serum samples from patients with Lyme arthritis and inconclusive reactivities to a particular genospecies, one seemed to be from a patient with a mixed infection. The total scores for the five specific bands were much higher with the B. burgdorferi sensu stricto and B. afzelii species-specific immunoblots than with the B. garinii and B. valaisiana species-specific immunoblots.
TABLE 2.
Percentage of preferential reactivity to particular Borrelia species by scoring method
| Clinical manifestation | % Reactivity
|
||
|---|---|---|---|
| Method I | Method II | Methods I and II | |
| Neuroborreliosis (n = 31) | 58 | 84 | 90 |
| ACA (n = 31) | 68 | 87 | 94 |
| Arthritis (n = 34) | 56 | 88 | 94 |
Two protein bands presented a preferential reactivity to one specific species, regardless of the clinical symptoms. The 14-kDa protein of B. afzelii and the 58-kDa protein of B. burgdorferi sensu stricto had higher reactivities.
Preferential serological reactivities of sera from patients with different clinical symptoms.
The percentages of serum samples from each patient group showing a preferential reactivity to each species are presented in Table 3. Reactivities of sera from patients with neuroborreliosis were higher with the B. garinii-specific immunoblot (52%) than with those specific for other species (6 to 22%). The reactivities of sera from patients with ACA were predominant with the B. afzelii-specific immunoblot (84%). The reactivities of sera from patients with Lyme arthritis were higher with the B. burgdorferi sensu stricto-specific immunoblot (62%) and with the B. afzelii-specific immunoblot (29%). Three serum samples from patients with neuroborreliosis and one serum sample from a patient with Lyme arthritis had preferential reactivities to B. valaisiana-specific immunoblot.
TABLE 3.
Percentage of serum samples per disease group showing preferential reactivity for individual species by methods I and II
| Clinical manifestation | %
Reactivitya
|
|||
|---|---|---|---|---|
| B.b.ss | B.g. | B.a. | B.v. | |
| Neuroborreliosis (n = 31) | 6 | 52 | 22 | 10 |
| ACA (n = 31) | 7 | 3 | 84 | 0 |
| Arthritis (n = 34) | 62 | 0 | 29 | 3 |
B.b.ss. B. burgdorferi sensu stricto; B.g., B. garinii; B.a., B. afzelii; B.v., B. valaisiana.
Persistence of reactivity to species-specific immunoblot.
The persistence of the predominant reactivity after treatment with antibiotics was evaluated by comparing sequential serum samples from nine patients. Two paired serum samples from patients with neuroborreliosis were analyzed. Both serum samples from one of them had a preferential reactivity to B. valaisiana-specific immunoblot. For the other patient, the reactivity of the first serum sample was inconclusive and the second serum sample had a preferential reactivity to B. afzelii-specific immunoblot. A decreasing IgG antibody response was observed for both of these patients. Six pairs of serum samples from patients with ACA were analyzed. For both serum samples from five of the patients, the sera had a preferential reactivity to B. afzelii-specific immunoblot and the second serum sample had a decreased immune response. The second serum sample from one of the patients had a preferential reactivity only to B. burgdorferi sensu stricto-specific immunoblot, although a decrease in the immune response was observed. One pair of serum samples from a patient with Lyme arthritis was analyzed. Both serum samples had preferential reactivity to B. burgdorferi sensu stricto-specific immunoblot, with an increased immune response for the second serum sample (obtained 3.5 years later).
Immunoblot reactivity compared with genomic detection.
PCR with synovial and urine samples detected five B. burgdorferi sensu stricto isolates one B. garinii isolate, nine B. afzelii isolates, and one untypeable Borrelia isolate. Preferential reactivities were observed for serum samples from these patients by immunoblotting (to B. burgdorferi sensu stricto for 3 serum samples and to B. afzelii for 13 serum samples). The results of these two methods, performed independently, agreed with each other for samples from 11 patients (69%) (two samples had preferential reactivity to B. burgdorferi sensu stricto and nine samples had preferential reactivity to B. afzelii). One sample had preferential reactivity to B. burgdorferi sensu stricto by immunoblotting but could not be amplified with any species-specific primers. One serum sample had a preferential reactivity to B. afzelii by immunoblotting although the results of PCR with urine from this patient indicated an infection with B. garinii. Only three discrepancies were found. For two samples the strain detected in the synovial fluid was B. afzelii, whereas the IgG immunoblots presented a preferential reactivity to B. burgdorferi sensu stricto, and for the third sample, B. burgdorferi sensu stricto was detected in the synovial fluid by PCR, but the IgG immunoblot suggested a B. afzelii infection.
DISCUSSION
Our four species-specific immunoblots allowed us to observe predominant serum antibody reactivity to one Borrelia species in the majority of serum samples from patients with chronic LB (90% for patients with neuroborreliosis [28 of 31], 94% for patients with ACA [29 of 31], and 94% for patients with Lyme arthritis [32 of 34]). The association of a given clinical manifestation with a defined Borrelia species was confirmed by these results. The predominant reactivity persisted long after treatment of the disease. Indirect evidence suggested the involvement of B. valaisiana in some chronic clinical manifestations. Results of serological tests for LB may depend on the different isolates causing infection and on the antigenic source, because isolates from different species are rather heterogeneous (47, 48) and lead to differences in reactivities on Western blots (3, 21, 26, 27, 30, 46, 49, 50). Moreover, as the infecting species is geographic area specific (8), we studied the four observed species in Switzerland and tested patients with LB from the same area. Norman et al. (30) suggested that development, optimization, and validation of immunoblotting criteria must be done for each specific strain as different levels of reactivity to each strain may occur. Therefore, we selected the best way to determine the preferential reactivity to each Borrelia species. Species-specific immunoblot analysis achieved by scoring individual discriminative bands allowed determination of predominant reactivity for a large percentage of serum samples. The preferential reactivity of sera to one species was determined by a first-step analysis based on the scores for five protein bands (the 12-kDa, 16-kDa, 18-kDa, OspA, and 66-kDa proteins) with a high discriminative potential. Two of these five proteins (the 18- and 66-kDa proteins) are recommended by the Centers for Disease Control and Prevention for use in the confirmation of positive serological tests. First, the 18-kDa protein has already been described by other investigators and is now considered a good marker for LB (12, 22, 32, 38). Second, the 66-kDa protein has been described as a heat shock protein with a high degree of specificity for LB (7, 12, 25, 26). Analysis of isolates of B. burgdorferi sensu lato obtained in North America and Europe has demonstrated that OspA has antigenic variability and that several distinct groups can be serologically defined (45, 47). However, there are controversial reports about its specificity in immunoblots (6, 10, 12, 20). The functions of the 12- and 16-kDa antigens are not yet clear. When the rapid first-step scoring method was inconclusive, it was followed by a second-step scoring method based on the total score for the 19 selected proteins. In most instances, the analysis with 19 protein bands allowed the confirmation and extension of the initial typing, leaving only a few specimens with inconclusive reactivities. This two-step procedure presented the advantage of a first rapid scoring method, with about two-thirds of the serum samples having preferential reactivity at the end of this step and 93% having preferential reactivity at the end of the second-step. No divergence in preferential reactivities was observed between the first-step approach and the second-step approach.
A preliminary blinded study comparing typing by immunoblotting with genome detection in synovial fluids and urine from selected patients showed agreement for 69% (11 of 16) of the samples. Only 4 of 16 samples showed discrepancies, and for the remaining sample no comparison was possible. Among the four patients who provided the four samples for which discrepancies were found, one had had frequent tick bites over several years and so could have been in contact with several B. burgdorferi sensu lato species. Only urine was available from a second patient, and tests with the urine sample revealed the presence of B. garinii, whereas the serological interpretation was in favor of the presence of B. afzelii. A mixed infection in this patient could also be envisaged. Serum from one patient showed by IgG immunoblotting a preferential reactivity to B. burgdorferi sensu stricto, but no amplification with species-specific primers was possible.
The reproducibility of typing by immunoblotting was high with the sera from a given patient. Among paired serum samples from seven patients, the seven first serum samples with preferential reactivity to one of the four species were shown to have the same reactivity as the second serum samples in the pairs. Excluding the possibility of a risk of a reinfection, the preferential reactivity of a serum sample may be observed for several years. Although the possibility of reinfection caused by the bites of separate ticks cannot be excluded, the presence of several species in the lesions of EM and ACA patients may reflect the occurrence of mixed infections in the local tick populations (37). It was also shown that infection with multiple species can persist in patients for a prolonged period (11). In our study, the proportion of serum samples with undetermined reactivity was 10% for patients with neuroborreliosis, 6% for patients with ACA, and 6% for patients with Lyme arthritis. However, significantly higher scores for reactivity to two of the four Borrelia species were observed for two of the seven serum samples with inconclusive reactivities, suggesting mixed infections. Sera from patients with ACA clearly showed greater reactivity to B. afzelii, which confirmed the results described by other investigators (2, 3, 13, 42). The reactivities of sera from patients with Lyme arthritis were predominantly specific to B. burgdorferi sensu stricto (62%) and B. afzelii (29%), which has also been confirmed by other serological analyses (2, 3). However, a PCR analysis performed with small numbers of synovial fluid samples in Germany revealed no association between Lyme arthritis and B. burgdorferi sensu stricto (15, 43). In our study, we also observed discrepant results between the serological study (62% association with B. burgdorferi sensu stricto) and the PCR analysis with a small number of samples (31% B. burgdorferi sensu stricto). We think that the selection of patients with Lyme arthritis on the basis of their PCR results is highly dependent on the test characteristics and could have led to a biased prevalence of Borrelia species. In addition, in the two studies (15, 43), large proportions of samples from patients with Lyme arthritis (4 of 11 [36%] and 7 of 20 [35%], respectively) were negative by PCR.
Our results lead us to confirm an association between B. garinii (52% of preferential reactivity) and neuroborreliosis, although some investigators are opposed to such an association. The good match described by Eiffert et al. (14) between the distribution of B. burgdorferi sensu lato in ticks and CSF from patients in the same area was interpreted to occur as a result of the random selection of organisms detected in the examined ticks, but it does not take into account the predominant occurrence of nymph bites on humans (17). In Switzerland nymphs are mainly infected with B. afzelii (16a). The association between neuroborreliosis and B. garinii in Europe has already been described in other reports (2, 10, 11, 32, 42). The percentage of association in this study was nevertheless lower than that described by others. This may be explained by a falsely attributed preferential reactivity to B. garinii in the absence of B. valaisiana antigen. In this study B. valaisiana was tested by comparative immunoblotting for the first time, and a pathogenic potential similar to that of B. garinii was suggested. Moreover B. garinii and B. valaisiana have both been demonstrated to have enzootic cycles in avian hosts (18, 19, 29, 31). Furthermore, the phylogenetic position of VS116 (B. valaisiana) has been described as being closely related to that of B. garinii (5, 41). A preferential reactivity to B. valaisiana was observed among three serum samples from patients with neuroborreliosis and one patient with Lyme arthritis. These preliminary results provided some additional clues as to the potential pathogenicity of B. valaisiana in humans. As recently reported (37), B. valaisiana has been detected by PCR and restriction fragment length polymorphism analysis in skin biopsy specimens from two EM patients, and mixed infections that included B. valaisiana were identified in both EM and ACA patients. The clinical histories of the three patients who had neuroborreliosis and who were potentially infected with B. valaisiana were obtained. The three patients had partial facial palsy. One of them presented with EM 14 days after a tick bite.
Conclusion.
Our scoring method with discriminative proteins on immunoblots allows attribution of the serum reactivity of patients with chronic Lyme borreliosis to one Borrelia species for most samples. Our results suggest an organotropism of Borrelia species and provide some evidence of the pathogenic potential of B. valaisiana in humans.
ACKNOWLEDGMENTS
This work was supported by the Foundation for Research and Development from the Institut Central des Hôpitaux Valaisans and the Swiss Foundation for Scientific Research (grant 32.52739.97).
We thank A. F. Rodriguez and S. Bochuz for excellent technical assistance and A. G. Barbour, B. Wilske, and R. Wallich for providing monoclonal antibodies.
REFERENCES
- 1.Altpeter E S, Meier C. Epidemiologische Aspekte der neurologischen Komplikationen der Lyme-Borreliose in der Schweiz. Schweiz Med Wochenschr. 1992;122:22–26. [PubMed] [Google Scholar]
- 2.Anthonissen F M, De Kesel M, Hoet P P, Bigaignon G H. Evidence for the involvement of different genospecies of Borreliain the clinical outcome of Lyme disease in Belgium. Res Microbiol. 1994;145:327–331. doi: 10.1016/0923-2508(94)90187-2. [DOI] [PubMed] [Google Scholar]
- 3.Assous M V, Postic D, Paul G, Nevot P, Baranton G. Western blot analysis of sera from Lyme borreliosis patients according to the genomic species of the Borreliastrains used as antigens. Eur J Clin Microbiol Infect Dis. 1993;12:261–268. doi: 10.1007/BF01967256. [DOI] [PubMed] [Google Scholar]
- 4.Baranton G, Postic D, Saint-Girons I, Boerlin P, Piffaretti J C, Assous M, Grimont P A D. Delineation of Borrelia burgdorferi sensu stricto, Borrelia gariniisp. nov., and group VS461 associated with Lyme borreliosis. Int J Syst Bacteriol. 1992;42:378–383. doi: 10.1099/00207713-42-3-378. [DOI] [PubMed] [Google Scholar]
- 5.Bernasconi M V, Valsangiacomo C, Balmelli T, Péter O, Piffaretti J C. Tick zoonoses in the southern part of Switzerland (Canton Ticino): occurrence of Borrelia burgdorferi sensu lato and Rickettsiasp. Eur J Epidemiol. 1997;13:209–215. doi: 10.1023/a:1007394901846. [DOI] [PubMed] [Google Scholar]
- 6.Bruckbauer H R, Preac-Mursic V, Fuchs R, Wilske B. Cross-reactive proteins of Borrelia burgdorferi. Eur J Clin Microbiol Infect Dis. 1992;11:224–232. doi: 10.1007/BF02098084. [DOI] [PubMed] [Google Scholar]
- 7.Bunikis J, Luke C J, Bunikiene E, Bergstrom S, Barbour A G. A surface-exposed region of a novel outer membrane protein (P66) of Borreliaspp. is variable in size and sequence. J Bacteriol. 1998;180:1618–1623. doi: 10.1128/jb.180.7.1618-1623.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bunikis J, Olsen B, Westman G, Bergstrom S. Variable serum immunoglobulin responses against different Borrelia burgdorferisensu lato species in a population at risk for and patients with Lyme disease. J Clin Microbiol. 1995;33:1473–1478. doi: 10.1128/jcm.33.6.1473-1478.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Canica M M, Nato F, du Merle L, Mazie J C, Baranton G, Postic D. Monoclonal antibodies for identification of Borrelia afzeliisp. nov. associated with late cutaneous manifestations of Lyme borreliosis. Scand J Infect Dis. 1993;25:441–448. doi: 10.3109/00365549309008525. [DOI] [PubMed] [Google Scholar]
- 10.Cinco M, Murgia R, Ruscio M, Andriolo B. IgM and IgG significant reactivity to Borrelia burgdorferi sensu stricto, Borrelia garinii and Borrelia afzeliiamong Italian patients affected by Lyme arthritis or neuroborreliosis. FEMS Immunol Med Microbiol. 1996;14:159–166. doi: 10.1111/j.1574-695X.1996.tb00283.x. [DOI] [PubMed] [Google Scholar]
- 11.Demaerschalck I, Messaoud A B, de Kesel M, Hoyois B, Lobet Y, Hoet P, Bigaignon G, Bollen A, Godfroid E. Simultaneous presence of different Borrelia burgdorferigenospecies in biological fluids of Lyme disease patients. J Clin Microbiol. 1995;33:602–608. doi: 10.1128/jcm.33.3.602-608.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dressler F, Whalen J A, Reinhardt B N, Steere A C. Western blotting in the serodiagnosis of Lyme disease. J Infect Dis. 1993;167:392–400. doi: 10.1093/infdis/167.2.392. [DOI] [PubMed] [Google Scholar]
- 13.Dunand V, Bretz A G, Suard A, Praz G, Dayer E, Péter O. Acrodermatitis chronica atrophicans and serologic confirmation of infection due to Borrelia afzelii and/or Borrelia gariniiby immunoblot. Clin Microbiol Infect. 1998;4:159–163. doi: 10.1111/j.1469-0691.1998.tb00381.x. [DOI] [PubMed] [Google Scholar]
- 14.Eiffert H, Ohlenbusch A, Christen H-J, Thompsen R, Spielman A, Matuschka F-R. Nondifferentiation between Lyme disease spirochetes from vector ticks and human cerebrospinal fluid. J Infect Dis. 1995;171:476–479. doi: 10.1093/infdis/171.2.476. [DOI] [PubMed] [Google Scholar]
- 15.Eiffert H, Karsten A, Thompsen R, Christen H-J. Characterization of Borrelia burgdorferistrains in Lyme arthritis. Scand J Infect Dis. 1998;30:265–268. doi: 10.1080/00365549850160918. [DOI] [PubMed] [Google Scholar]
- 16.Engstrom S M, Shoop E, Johnson R C. Immunoblot interpretation criteria for serodiagnosis of early Lyme disease. J Clin Microbiol. 1995;33:419–427. doi: 10.1128/jcm.33.2.419-427.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16a.Gern, L. Personal communication.
- 17.Gray J S, Kahl O, Robertson J N, Daniel M, Estrada-Pena A, Gettinby G, Jaenson T G, Jensen P, Jongejan F, Korenberg F, Kurtenbach K, Zeman P. Lyme borreliosis habitat assessment. Zentralbl Bakteriol. 1998;287:211–228. doi: 10.1016/s0934-8840(98)80123-0. [DOI] [PubMed] [Google Scholar]
- 18.Humair P F, Postic D, Wallich R, Gern L. An avian reservoir (Turdus merula) of the Lyme borreliosis spirochetes. Zentbl Bakteriol Parasitenkd Infektkrankh Hyg Abt 1 Orig. 1998;287:521–538. [PubMed] [Google Scholar]
- 19.Isogai E, Tanaka S, Braga III I S, Itakura C, Isogai H, Kimura K, Fujii N. Experimental Borrelia gariniiinfection of Japanese quail. Infect Immun. 1994;62:3580–3582. doi: 10.1128/iai.62.8.3580-3582.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalish R A, Leong J M, Steere A C. Association of treatment-resistant chronic Lyme arthritis with HLA-DR4 and antibody reactivity to OspA and OspB of Borrelia burgdorferi. Infect Immun. 1993;61:2774–2779. doi: 10.1128/iai.61.7.2774-2779.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karlsson M. Antibody response against autologous and heterologous isolates of Borrelia burgdorferiin four patients with Lyme neuroborreliosis. Eur J Clin Microbiol Infect Dis. 1991;10:742–745. doi: 10.1007/BF01972499. [DOI] [PubMed] [Google Scholar]
- 22.Karlsson M, Mollegard I, Stiernstedt G, Wretlind B. Comparison of Western blot and enzyme-linked immunosorbent assay for diagnosis of Lyme borreliosis. Eur J Clin Microbiol Infect Dis. 1989;8:871–877. doi: 10.1007/BF01963773. [DOI] [PubMed] [Google Scholar]
- 23.Kirstein F, Rijpkema S, Molkenboer M, Gray J S. The distribution and prevalence of B. burgdorferi genomospecies in Ixodes ricinusticks in Ireland. Eur J Epidemiol. 1997;13:67–72. doi: 10.1023/a:1007360422975. [DOI] [PubMed] [Google Scholar]
- 24.Le Fleche A, Postic D, Girardet K, Péter O, Baranton G. Characterization of Borrelia lusitaniaesp. nov. by 16S ribosomal DNA. Int J Syst Bacteriol. 1997;47:921–925. doi: 10.1099/00207713-47-4-921. [DOI] [PubMed] [Google Scholar]
- 25.Luft B J, Gorevic P D, Jiang W, Munoz P, Dattwyler R J. Immunologic and structural characterization of the dominant 66- to 73-kDa antigens of Borrelia burgdorferi. J Immunol. 1991;146:2776–2782. [PubMed] [Google Scholar]
- 26.Ma B, Christen B, Leung D, Vigo-Pelfrey C. Serodiagnosis of Lyme borreliosis by Western immunoblot: reactivity of various significant antibodies against Borrelia burgdorferi. J Clin Microbiol. 1992;30:370–376. doi: 10.1128/jcm.30.2.370-376.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Magnarelli L A, Anderson J F, Johnson R C, Nadelman R B, Wormser G P. Comparison of different strains of Borrelia burgdorferisensu lato used as antigens in enzyme-linked immunosorbent assays. J Clin Microbiol. 1994;32:1154–1158. doi: 10.1128/jcm.32.5.1154-1158.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mansy F, Hoyois B, de Vos M J, van Elsen A, Bollen A, Godfroid E. Colorimetric solid-phase capture hybridization assay for detection of amplified Borrelia burgdorferiDNA. BioTechniques. 1996;21:122–125. doi: 10.2144/96211rr03. [DOI] [PubMed] [Google Scholar]
- 29.Nakao M, Miyamoto K, Fukunaga M. Lyme disease spirochetes in Japan: enzootic transmission cycles in birds, rodents, and Ixodes persulcatusticks. J Infect Dis. 1994;170:878–882. doi: 10.1093/infdis/170.4.878. [DOI] [PubMed] [Google Scholar]
- 30.Norman G L, Antig J M, Bigaignon G, Hogrefe W R. Serodiagnosis of Lyme borreliosis by Borrelia burgdorferi sensu stricto, B. garinii, and B. afzeliiWestern blots (immunoblots) J Clin Microbiol. 1996;34:1732–1738. doi: 10.1128/jcm.34.7.1732-1738.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olsen B, Jaenson T G, Noppa L, Bunikis J, Bergstrom S. A Lyme borreliosis cycle in seabirds and Ixodes uriaeticks. Nature. 1993;362:340–342. doi: 10.1038/362340a0. [DOI] [PubMed] [Google Scholar]
- 32.Péter O, Bretz A-G, Postic D, Dayer E. Association of distinct species of Borrelia burgdorferisensu lato with neuroborreliosis in Switzerland. Clin Microbiol Infect. 1997;3:423–431. doi: 10.1111/j.1469-0691.1997.tb00278.x. [DOI] [PubMed] [Google Scholar]
- 33.Péter O, Bretz A G. Polymorphism of outer surface proteins of Borrelia burgdorferias a tool for classification. Zentbl Bakteriol Parasitenkd Infektkrankh Hyg Abt 1 Orig. 1992;277:28–33. doi: 10.1016/s0934-8840(11)80867-4. [DOI] [PubMed] [Google Scholar]
- 34.Péter O, Bretz A G, Bee D. Occurrence of different genospecies of Borrelia burgdorferisensu lato in Ixodid ticks of Valais, Switzerland. Eur J Epidemiol. 1995;11:463–467. doi: 10.1007/BF01721234. [DOI] [PubMed] [Google Scholar]
- 35.Postic D, Assous M V, Grimont P A, Baranton G. Diversity of Borrelia burgdorferi sensu lato evidenced by restriction fragment length polymorphism of rrf (5S)-rrl(23S) intergenic spacer amplicons. Int J Syst Bacteriol. 1994;44:743–752. doi: 10.1099/00207713-44-4-743. [DOI] [PubMed] [Google Scholar]
- 36.Rijpkema S G, Herbes R G, Verbeek-De Kruif N, Schellekens J F. Detection of four species of Borrelia burgdorferi sensu lato in Ixodes ricinus ticks collected from roe deer (Capreolus capreolus) in The Netherlands. Epidemiol Infect. 1996;117:563–566. doi: 10.1017/s0950268800059252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rijpkema S G T, Tazelaar D, Molkenboer M, Noordhoek G, Plantinga G, Schouls L, Schellekens J. Detection of Borrelia afzelii, Borrelia burgdorferi sensu stricto, Borrelia gariniiand group VS116 by PCR in skin biopsies of patients with erythema migrans and acrodermatitis chronica atrophicans. Clin Microbiol Infect. 1997;3:109–116. doi: 10.1111/j.1469-0691.1997.tb00259.x. [DOI] [PubMed] [Google Scholar]
- 38.Ryffel K, Péter O, Binet L, Dayer E. Interpretation of immunoblots for Lyme borreliosis using a semiquantitative approach. Clin Microbiol Infect. 1998;4:205–212. doi: 10.1111/j.1469-0691.1998.tb00670.x. [DOI] [PubMed] [Google Scholar]
- 39.Saint Girons I, Gern L, Gray J S, Guy E C, Korenberg E, Nuttall P A, Rijpkema S G T, Schönberg A, Stanek G, Postic D. Identification of Borrelia burgdorferisensu lato species in Europe. Zentbl Bakteriol Parasitenkd Infektkrankh Hyg Abt 1 Orig. 1998;287:190–195. doi: 10.1016/s0934-8840(98)80120-5. [DOI] [PubMed] [Google Scholar]
- 40.Steere A C. Lyme disease. N Engl J Med. 1989;321:586–596. doi: 10.1056/NEJM198908313210906. [DOI] [PubMed] [Google Scholar]
- 41.Valsangiacomo C, Balmelli T, Piffaretti J C. A phylogenetic analysis of Borrelia burgdorferi sensu lato based on sequence information from the hbbgene, coding for a histone-like protein. Int J Syst Bacteriol. 1997;47:1–10. doi: 10.1099/00207713-47-1-1. [DOI] [PubMed] [Google Scholar]
- 42.van Dam A P, Kuiper H, Vos K, Widdjojokusumo A, de Jongh B M, Spanjaard S, Ramselaar A C T, Kramer M D, Dankert J. Different genospecies of Borrelia burgdorferiare associated with distinct clinical manifestations of Lyme borreliosis. Clin Infect Dis. 1993;17:708–717. doi: 10.1093/clinids/17.4.708. [DOI] [PubMed] [Google Scholar]
- 43.Vasiliu V, Herzer P, Rossler D, Lehnert G, Wilske B. Heterogeneity of Borrelia burgdorferisensu lato demonstrated by an ospA-type-specific PCR in synovial fluid from patients with Lyme arthritis. Med Microbiol Immunol (Berlin) 1998;187:97–102. doi: 10.1007/s004300050079. [DOI] [PubMed] [Google Scholar]
- 44.Wang G, van Dam A P, Le Flèche A, Postic D, Péter O, Baranton G, de Boer R, Spanjaard L, Dankert J. Genetic and phenotypic analysis of Borrelia valaisiana sp. nov. (Borreliagenomic groups VS116 and M19) Int J Syst Bacteriol. 1997;47:926–932. doi: 10.1099/00207713-47-4-926. [DOI] [PubMed] [Google Scholar]
- 45.Wilske B, Busch U, Eiffert H, Fingerle V, Pfister H W, Rössler D, Preac-Mursic V. Diversity of OspA and OspC among cerebrospinal fluid isolates of Borrelia burgdorferisensu lato from patients with neuroborreliosis in Germany. Med Microbiol Immunol (Berlin) 1996;184:195–201. doi: 10.1007/BF02456135. [DOI] [PubMed] [Google Scholar]
- 46.Wilske B, Fingerle V, Preac-Mursic V, Jauris-Heipke S, Hofmann A, Loy H, Pfister H W, Rössler D, Soutschek E. Immunoblot using recombinant antigens derived from different genospecies of Borrelia burgdorferisensu lato. Med Microbiol Immunol (Berlin) 1994;183:43–59. doi: 10.1007/BF00193630. [DOI] [PubMed] [Google Scholar]
- 47.Wilske B, Preac-Mursic V, Göbel U B, Graf B, Jauris S, Soutschek E, Schwab E, Zumstein G. An OspA serotyping system for Borrelia burgdorferibased on reactivity with monoclonal antibodies and OspA sequence analysis. J Clin Microbiol. 1993;31:340–350. doi: 10.1128/jcm.31.2.340-350.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wilske B, Preac-Mursic V, Jauris S, Hofmann A, Pradel I, Soutschek E, Schwab E, Will G, Wanner G. Immunological and molecular polymorphisms of OspC, an immunodominant major outer surface protein of Borrelia burgdorferi. Infect Immun. 1993;61:2182–2191. doi: 10.1128/iai.61.5.2182-2191.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wilske B, Preac-Mursic V, Schierz G, Busch K V. Immunochemical and immunological analysis of European Borrelia burgdorferistrains. Zentbl Bakteriol Mikrobiol Hyg Reihe A. 1986;263:92–102. doi: 10.1016/s0176-6724(86)80108-0. [DOI] [PubMed] [Google Scholar]
- 50.Zoller L, Burkard S, Schafer H. Validity of Western immunoblot band patterns in the serodiagnosis of Lyme borreliosis. J Clin Microbiol. 1991;29:174–182. doi: 10.1128/jcm.29.1.174-182.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]