Highlights
-
•
Survival analysis was conducted for 131 patients with coronavirus disease 2019 (COVID-19) in Somalia.
-
•
Interventions to improve outcomes in this low-resource and fragile setting were examined.
-
•
Risk factors for deaths included age ≥60 years, cardiovascular disease and use of non-invasive ventilation.
-
•
Patients who received oxygen alone were more likely to survive than patients who were ventilated.
-
•
Optimizing critical care for patients with COVID-19 in fragile states requires policy discourse.
Keywords: COVID-19, Non-invasive ventilation, Hospital mortality, Somalia, Survival analysis, Risk factors, Critical illness, Comorbidities, SARS-CoV-2, Fragile state, Mogadishu
Abstract
Objectives
To determine risk factors for death in patients with coronavirus disease 2019 (COVID-19) admitted to the main hospital in Somalia, and identify interventions contributing to improved clinical outcome in a low-resource and fragile setting.
Methods
A survival analysis was conducted of all patients with COVID-19 admitted to the main hospital in Somalia from 30 March to 12 June 2020.
Results
Of the 131 patients admitted to the hospital with COVID-19, 52 (40%) died and 79 (60%) survived. The main factors associated with the risk of in-hospital death were age ≥60 years {survival probability on day 21 was 0.789 [95% confidence interval (CI) 0.658–0.874] in patients aged <60 years vs 0.339 (95% CI 0.205–0.478) in patients aged ≥60 years}, cardiovascular disease [survival probability 0.478 (95% CI 0.332–0.610) in patients with cardiovascular disease vs 0.719 (95% CI 0.601–0.807) in patients without cardiovascular disease] and non-invasive ventilation on admission (patients who were not ventilated but received oxygen were significantly more likely to survive than patients who were ventilated; P<0.001).
Conclusion
Considering the risk factors (age ≥60 years, presence of cardiovascular disease and use of non-invasive ventilation) is critical when managing patients with severe COVID-19, especially in low-resource settings where availability of skilled healthcare workers for critical care units is limited. These findings also highlight the importance of use of medical oxygen for severely ill patients, and the critical aspect of deciding whether or not to ventilate critical patients with COVID-19 in order to improve clinical outcome.
Background
On 16 March 2020, the Federal Ministry of Health and Human Services of Somalia reported the country's first laboratory-confirmed case of coronavirus disease 2019 (COVID-19) in a Somali student arriving from China (WHO, 2020). By 31 October 2021, the country had officially reported 22,369 laboratory-confirmed cases of COVID-19, including 1238 associated deaths. Among the reported laboratory-confirmed COVID-19 cases, approximately 16% were admitted to isolation centres designated by the government (FMOH and WHO, 2021). Due to lack of consistent data, it is not clear how many of these admitted patients required critical care support. One study showed that, between 23 April and 28 June 2020, of the 443 patients with confirmed severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection admitted to the largest tertiary hospital in Mogadishu, the capital of Somalia, only 48 patients (10.8%) with severe symptoms were admitted to the intensive care unit (ICU) (Mohamud et al., 2021).
Like many other African countries, Somalia has not reported a very high number of cases and deaths. Given the fragility of the healthcare system in Somalia, it was anticipated that the system would be overwhelmed. The outbreak occurred at a time when the country had no ICU beds, no ventilators and no central supply of medical oxygen in the public sector. Ranked 193 out of 195 countries on the Global Health Security Index (Aitken et al., 2020, Index Project Team 2021), Somalia's health system, considered the second most fragile in the world (World Bank, 2021), has been debilitated by decades of civil war, insecurity and disease outbreaks, as well as natural disasters such as droughts and floods, leading to a deterioration in health outcomes. The universal health coverage index of Somalia, as a measure of effective health services coverage and its contribution to improved health outcome of all its people, is the lowest in the world: 25 out of 100 (GBD 2019 Universal Health Coverage Collaborators, 2020). The current health workforce density in Somalia (0.34 healthcare workers per 1000 population) is substantially lower than the density needed for universal health coverage: 4.45 healthcare workers per 1000 population by 2030 (WHO, 2015, 2016). When the COVID-19 pandemic reached Somalia, there were only 15 ICU beds (all in the private sector) for a population of more than 15 million. Broadly, the country's health system suffers from underperformance, fragmented and poor-quality service delivery, and limited and inequal access and inequitable distribution of the health workforce. The country's disease surveillance system to detect, monitor and facilitate a rapid response to any outbreak is rudimentary and dysfunctional.
To date, most of the laboratory-confirmed cases of COVID-19 in Somalia have not required hospitalization as >80% were either asymptomatic or had mild symptoms (FMOH and WHO, 2021) Between 30 March and 12 June 2020, 2922 COVID-19 laboratory-confirmed cases were reported in Mogadishu, of which 93 patients died and 131 patients with severe illness were admitted to the main hospital in Mogadishu.
Although data are available from developed, high-income and middle-income countries on the clinical characteristics of COVID-19, and the outcome and risk factors for clinical outcome (Bhatraju et al., 2020; Bialek et al., 2020; Salinas-Escudero et al., 2020; Thai et al., 2020; Yang et al., 2020), few studies investigating the links between interventions and clinical outcome have been published from less developed countries; as such, it is hoped that this contribution to the literature from a low-resource setting will be useful.
Documenting the length of hospitalization and survival of patients with COVID-19 and the risk factors associated with death in low-resource settings could provide a better understanding of the impact of the disease, the usefulness of medical interventions, and hospital capacity to cope with the surge in patients with COVID-19 in such settings. This information can also guide policy responses on the use of low-cost, high-impact interventions in such settings to save lives and manage a surge in cases.
The main aim of this study was to assess the clinical characteristics of patients with COVID-19 admitted to the main public sector hospital in Somalia, estimate the length of hospital stay, and identify the risk factors for death in these patients. The study also assessed which interventions might help to improve clinical outcomes in patients with severe COVID-19 in low-resource and fragile settings.
Methods
Study design and patients
The current study took place at De Martino Hospital, the principal COVID-19 quarantine hospital in Mogadishu, during the early period of the pandemic from 30 March 2020 to 12 June 2020. The study cohort included all 131 patients admitted with laboratory-confirmed SARS-CoV-2 infection between 30 March and 12 June 2020.1 These dates represent the date of first hospital admission and the date when the entire cohort of 131 patients included in this study were either discharged alive or died. SARS-CoV-2 infection was diagnosed using reverse transcriptase polymerase chain reaction.
Data extraction and outcomes
Information was extracted from the clinical records of all 131 patients, including: place of residence, age, sex, date of admission, date of onset of symptoms, reported signs and symptoms (fever, shortness of breath, muscular pain, chest pain, abdominal pain, joint pain, general weakness, diarrhoea, cough, nausea or vomiting, sore throat, headache, runny nose, irritability and confusion), clinical characteristics (temperature, pharyngeal exudate, coma, abnormal lung X ray, conjunctival infection, dyspnoea or tachypnoea, seizure and abnormal lung auscultation), comorbidities as reported by the patient (cardiovascular disease including hypertension, immunodeficiency including human immunodeficiency virus, diabetes, renal disease and chronic lung disease), clinical interventions [delivery of ratio of the partial pressure of arterial oxygen over the fraction of inspired oxygen (PaO2/FiO2 ratio), ventilation support through a facemask under positive pressure without the need for endotracheal intubation, non-invasive ventilation, antibiotics, inotropes and vasopressors], and final outcome and date (survived or died). Inotropes and/or vasopressors were only used when needed (e.g. in the case of septic shock).
Individual patient data were transcribed into an Excel spreadsheet (Microsoft Corp., Redmond, WA, USA), and both the care providers and the information officers independently verified the data from the patient medical records for completeness and accuracy.
The primary outcome was survival status and time to outcome from date of hospitalization (in days).
Statistical analysis
Quantitative data are presented as mean and standard deviation (SD) (95% CI) and/or median and interquartile range (IQR). Age was dichotomized into <60 years and ≥60 years.
The distributions of cases, deaths, symptoms, clinical characteristics, comorbidities and type of treatment are reported by sex and age group.
Kaplan–Meier curves were performed to estimate survival probabilities, and the log-rank test was conducted to assess the significance of differences in survival curves between groups. The outcome of survival probabilities was reported at days 7, 14 and 21 after admission with 95% CI. As the final disposition of all patients was reported up to the last day of discharge or death (i.e. day 21), these points of analysis (7, 14 and 21 days) were selected for this study.
The survival analysis was extended using the Cox proportional hazard model to estimate the likelihood of death and simultaneously assess the effect of several risk factors on survival, adjusting for confounding or effect modification. In addition, the assumption of proportionality was examined. Age, diabetes and cardiovascular diseases were used as variables in the Cox proportional hazard model.
Stata Version 16.0 (StataCorp LLC, College Station, TX, USA) was used for data quality assessment and analysis. P<0.05 was considered to indicate statistical significance.
Results
Epidemiological characteristics
All 131 patients with COVID-19 admitted to the hospital were followed until the end of their stay. The first admission was on 30 March 2020 and the last date of discharge and/or death was on 12 June 2020. The length of hospital stay ranged between 1 day and 35 days (median 5.5 days, IQR 9.0 days).
Approximately two-thirds (68%) of the patients were male. The mean age was 58.5 (SD 16.1) years and the median age was 60.0 (IQR 18.0) years) for males vs 56.9 (SD 17.1) years and 55.0 (IQR 27) years, respectively, for females.
The total number of patients enrolled in this study is shown in Table 1. Patient data are stratified into age and sex groups. Of the 131 patients, 52 (40%) died and 79 (60%) survived (Table 1).
Table 1.
Number of patients with coronavirus disease 2019 and associated deaths, by age and sex, Somalia, 2020.
| Age group (years) | Males n (%) |
Females n (%) |
||
|---|---|---|---|---|
| Total no. of patients | No. died | Total no. of patients | No. died | |
| Mean (SD) | 58.5 (16.1) | 56.9 (17.1) | ||
| Median (IQR) | 60.0 (18.0) | 55.0 (27) | ||
| 10–19 | 2 | 0 | 0 | 0 |
| 20–29 | 3 | 0 | 4 | 2 |
| 30–39 | 9 | 0 | 6 | 0 |
| 40–49 | 5 | 2 | 4 | 0 |
| 50–59 | 15 | 4 | 11 | 4 |
| 60–69 | 32 | 19 | 9 | 4 |
| 70–79 | 17 | 8 | 3 | 1 |
| ≥80 | 6 | 5 | 5 | 3 |
| Total | 89 (68%) | 38 (73%) | 42 (32%) | 14 (27%) |
Clinical characteristics and comorbidities
Clinical features, comorbidities and interventions given, by age group and sex, are shown in Table 2.
Table 2.
Distribution of patients (number of cases) according to disease characteristics and treatment, by sex and age group, Somalia, 2020.
| Variable | Females (n) |
Males (n) |
||||
|---|---|---|---|---|---|---|
| Age (years) |
Age (years) |
|||||
| <40 | 40–59 | ≥60 | <40 | 40–59 | ≥60 | |
| Symptoms and signs | ||||||
| Fever | 9 | 13 | 17 | 13 | 19 | 54 |
| Shortness of breath | 8 | 15 | 16 | 14 | 20 | 52 |
| Muscular pain | 1 | 1 | 6 | 1 | 2 | 11 |
| Chest pain | 3 | 4 | 9 | 4 | 11 | 21 |
| Abdominal pain | 2 | 2 | 2 | 0 | 1 | 5 |
| Joint pain | 1 | 1 | 9 | 2 | 3 | 18 |
| General weakness | 3 | 4 | 10 | 3 | 6 | 24 |
| Diarrhoea | 1 | 1 | 1 | 0 | 2 | 5 |
| Cough | 9 | 15 | 16 | 14 | 19 | 52 |
| Nausea/vomiting | 2 | 3 | 0 | 0 | 1 | 6 |
| Sore throat | 4 | 6 | 8 | 5 | 9 | 26 |
| Headache | 4 | 5 | 12 | 7 | 12 | 30 |
| Runny nose | 0 | 0 | 0 | 0 | 0 | 4 |
| Irritability symptoms | 2 | 1 | 6 | 1 | 6 | 15 |
| Clinical characteristics | ||||||
| Abnormal lung X-ray findings | 7 | 13 | 17 | 14 | 20 | 54 |
| Dyspnoea/tachypnoea | 8 | 14 | 17 | 14 | 19 | 47 |
| Abnormal lung auscultation | 7 | 14 | 17 | 14 | 20 | 52 |
| Comorbidities | ||||||
| Cardiovascular disease | 0 | 6 | 8 | 0 | 10 | 29 |
| Immunodeficiency, including HIV | 0 | 0 | 0 | 1 | 0 | 1 |
| Diabetes | 1 | 9 | 10 | 2 | 9 | 21 |
| Renal disease | 1 | 1 | 1 | 0 | 1 | 2 |
| Chronic lung disease | 1 | 1 | 2 | 0 | 1 | 10 |
| Treatment | ||||||
| Oxygen therapy | 7 | 14 | 17 | 11 | 17 | 53 |
| Non-invasive ventilation | 1 | 1 | 5 | 0 | 3 | 20 |
| Inotropes and vasopressors | 0 | 1 | 3 | 0 | 4 | 20 |
| Antibiotics | 10 | 15 | 17 | 14 | 20 | 55 |
| Total | 10 | 15 | 17 | 14 | 20 | 55 |
HIV, human immunodeficiency virus.
Most patients reported fever, shortness of breath and cough, and a few patients had abdominal pain, diarrhoea or a runny nose. Muscular and joint pain, general weakness and headache increased with age for both sexes. None of the patients presented with pharyngeal exudate, coma, conjunctival infection or seizures. However, 95% of patients had abnormal lung findings on X ray and 95% had abnormal lung auscultation. Approximately 91% of patients presented with dyspnoea and/or tachypnoea.
Overall, males showed more acute clinical characteristics than females, but the frequency of symptoms often increased with age in females (Table 2).
The main comorbidities observed were cardiovascular disease (40%) and diabetes (40%). Cardiovascular disease was reported mainly by patients aged ≥40 years. Diabetes was more prevalent among females (48%) than males (36%). Only two male patients had immunodeficiency.
None of the female patients were pregnant or reported being pregnant in the 6 weeks preceding hospitalization.
All patients were given antibiotics according to the national protocol in Somalia. Oxygen therapy was given to 91% of the study patients, and the use of oxygen increased with patient age. Inotropes and vasopressors were given to 21% of patients, all of whom were aged ≥40 years. The criteria for an adult to receive oxygen therapy is that they present with acute respiratory distress syndrome with oxygen impairment of 200 mmHg < PaO2/FiO2 ≤ 300 mmHg (with positive end-expiratory pressure or continuous positive airway pressure ≥5 cmH2O, or non-ventilated) (FMOH, 2020).
Treatment outcome by epidemiological characteristics and comorbidities
Table 3 shows the survival probabilities with 95% CI at days 7, 14 and 21 after admission, by age group, sex, presence of cardiovascular disease and diabetes, and treatment with inotropes and vasopressors.
Table 3.
Survival probability according to patient characteristics and treatment on days 7, 14 and 21 after admission to hospital, Somalia, 2020.
| Variable | At day 7 | At day 14 | At day 21 |
|---|---|---|---|
| Survival probability (95% CI) | Survival probability (95% CI) | Survival probability (95% CI) | |
| Age group (years) | |||
| <60 | 0.789 (0.658–0.874) | 0.789 (0.658–0.874) | 0.789 (0.658–0.874) |
| ≥60 | 0.489 (0.365–0.602) | 0.440 (0.312–0.561) | 0.339 (0.205–0.478) |
| Sex | |||
| Female | 0.669 (0.497–0.794) | 0.669 (0.497–0.794) | 0.535 (0.254–0.752) |
| Male | 0.600 (0.487–0.696) | 0.557 (0.436–0.662) | 0.488 (0.348–0.613) |
| Cardiovascular disease | |||
| No | 0.719 (0.601–0.807) | 0.697 (0.574–0.790) | 0.558 (0.379–0.704) |
| Yes | 0.478 (0.332–0.610) | 0.425 (0.265–0.576) | 0.425 (0.265–0.576) |
| Diabetes | |||
| No | 0.704 (0.585–0.795) | 0.684 (0.561–0.779) | 0.547 (0.372–0.693) |
| Yes | 0.499 (0.353–0.629) | 0.449 (0.290–0.596) | 0.449 (0.290–0.596) |
| Inotropes or vasopressors | |||
| No | 0.769 (0.670–0.842) | 0.728 (0.614–0.813) | 0.655 (0.508–0.768) |
| Yes | 0.107 (0.027–0.251) | 0.107 (0.027–0.251) | 0.000 (0.000–0.000) |
CI, confidence interval.
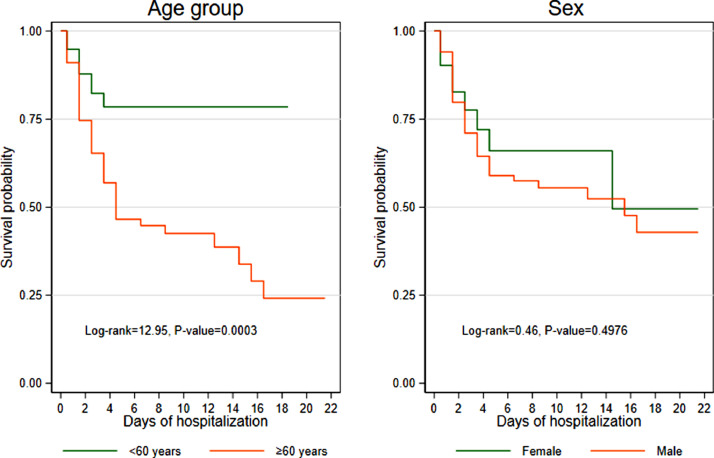
Table 3 shows that patients aged ≥60 years were significantly more likely to die than patients aged <60 years (P<0.003), and the risk of death was significantly different between the two age groups at days 7, 14 and 21 (Figure 1). The probability of surviving to day 21 in patients aged <60 years was 0.789 (95% CI 0.658–0.874), compared with 0.339 (95% CI 0.205–0.478) in patients aged ≥60 years. The probability of death was very similar for males and females (P=0.049) (Figure 1a).
Figure 1.
Kaplan–Meier survival estimates by age and sex.
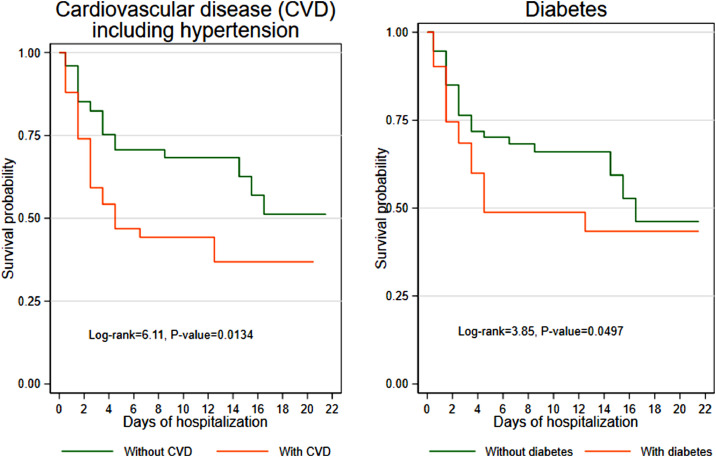
Patients without cardiovascular disease were significantly more likely to survive than patients with cardiovascular disease (P=0.0134) (Figure 2). Approximately half of patients with cardiovascular disease survived the first week of hospitalization (survival probability 0.478, 95% CI 0.332–0.610) compared with three-quarters of patients without cardiovascular disease (survival probability 0.719, 95% CI 0.601–0.807). However, the survival probabilities of patients without cardiovascular disease decreased by the third week and reached 0.558 (95% CI 0.379–0.704) compared with 0.425 (95% CI 0.265–0.576) (Table 3). A similar pattern was seen in patients with diabetes, although the difference was smaller (Figure 2).
Figure 2.
Kaplan–Meier survival estimates by comorbidity.
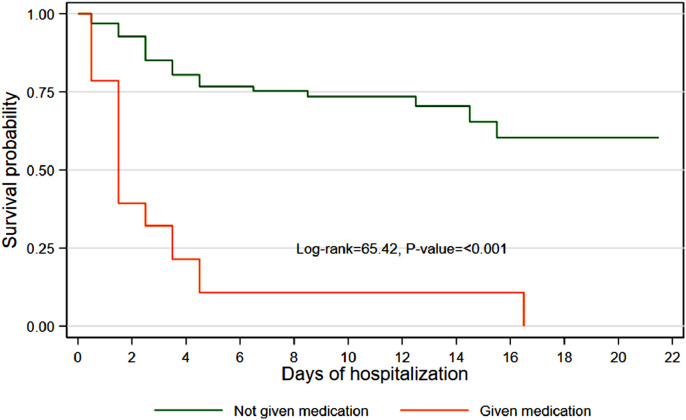
Of 29 patients given inotropes or vasopressors, 25 (86%) died in the first week of admission. The survival probability of those who did not receive inotropes or vasopressors was significantly higher at day 7 (survival probability 0.769, 95% CI 0.670–0.842), day 14 (survival probability 0.728, 95% CI 0.614–0.813) and day 21 (survival probability 0.655, 95% CI 0.508–0.768) after admission compared with those who received inotropes or vasopressors (Table 3). Overall, patients who did not receive inotropes or vasopressors were significantly more likely to survive than patients who did receive them (P<0.001) (Figure 3).
Figure 3.
Kaplan–Meier survival estimates by use of inotropes and vasopressors.
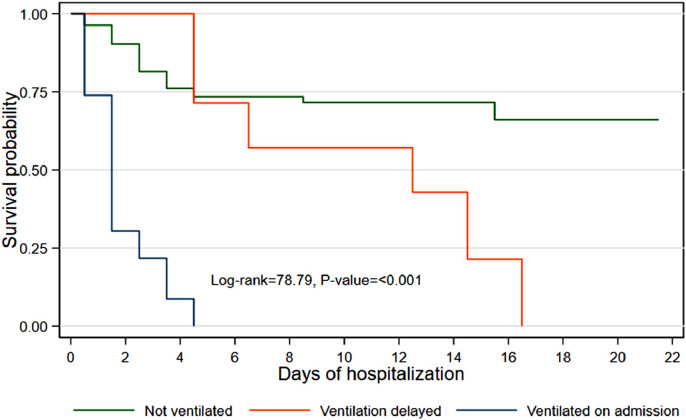
Of the 131 patients, 12 did not receive medical oxygen as their condition was considered mild; all of these patients survived. The remaining 119 patients who were given oxygen on admission were categorized into three groups – did not receive non-invasive ventilation, received delayed non-invasive ventilation, and non-invasive ventilation given on admission – and their survival probabilities were calculated (Figure 4 and Table 4). Patients who were not ventilated or whose ventilation was delayed had a higher probability of survival than patients who were ventilated on admission. Patients who were ventilated on admission had a 22% probability of survival on day 3 compared with 83% for those who were not ventilated at all. None of the patients ventilated on admission survived after day 4 (Table 4).
Figure 4.
Kaplan–Meier survival estimates by ventilation use.
Table 4.
Survival probability according to length of time after admission, by administration of non-invasive ventilation, Somalia, 2020.
| Length of time after | No ventilation | Delayed non-invasive ventilation | Non-invasive ventilation on admission |
|---|---|---|---|
| admission (days) | Survival probability (95% CI) | Survival probability (95% CI) | Survival probability (95% CI) |
| 1 | 0.966 (0.899–0.989) | 1.000 (1.000–1.000) | 0.739 (0.509–0.873) |
| 2 | 0.910 (0.827–0.954) | 1.000 (1.000–1.000) | 0.304 (0.135–0.493) |
| 3 | 0.828 (0.731–0.893) | 1.000 (1.000–1.000) | 0.217 (0.079–0.399) |
| 4 | 0.778 (0.675–0.853) | 1.000 (1.000–1.000) | 0.087 (0.015–0.242) |
| 5 | 0.753 (0.647–0.832) | 0.714 (0.258–0.920) | |
| 7 | 0.753 (0.647–0.832) | 0.571 (0.172–0.837) | |
| 9 | 0.737 (0.627–0.819) | 0.571 (0.172–0.837) | |
| 14 | 0.737 (0.627–0.819) | 0.429 (0.098–0.734) | |
| 15 | 0.737 (0.627–0.819) | 0.214 (0.012–0.586) | |
| 16 | 0.698 (0.564–0.798) | 0.214 (0.012–0.586) | |
| 21 | 0.698 (0.564–0.798) |
CI, confidence interval.
All patients who received delayed non-invasive ventilation as a response to worsening symptoms did not survive beyond day 16. Overall, patients who were not ventilated had a significantly better probability of survival than patients who were ventilated on admission (P<0.001) (Figure 4).
The likelihood of death for females and males by ventilation status was estimated using a Cox proportional hazard model, adjusting for age and two comorbidities. Table 5 shows the adjusted hazard ratios. The likelihood of death in female patients who were ventilated on admission (hazard ratio 33.79, 95% CI 4.09–279.07) was higher compared with male patients who were ventilated on admission (hazard ratio 8.65, 95% CI 3.93–19.06).
Table 5.
Adjusted hazard ratios for death in hospitalized patients with coronavirus disease 2019, by sex, Somalia, 2020.
| Variable | Adjusted hazard ratios (95% CI) |
|
|---|---|---|
| Females | Males | |
| Age (years) | ||
| <60 | 1.00 | |
| ≥60 | 1.08 (0.34–3.40) | 1.84 (0.74–4.57) |
| Cardiovascular disease | ||
| No | 1.00 | 1.00 |
| Yes | 0.72 (0.16–3.28) | 1.67 (0.86–3.26) |
| Diabetes | ||
| No | 1.00 | 1.00 |
| Yes | 2.14 (0.65–7.08) | 1.33 (0.69–2.56) |
| Non-invasive ventilation | ||
| No | 1.00 | 1.00 |
| Delayed | 2.20 (0.42–11.40) | 2.26 (0.71–7.18) |
| On admission | 33.79 (4.09–279.07) | 8.65 (3.93–19.06) |
CI, confidence interval.
Discussion
This study included a large cohort of patients in the analysis with the aim of identifying risk factors for in-hospital deaths, and identifying which interventions had the highest probability of improved clinical outcome in Somalia. The analysis included all 131 patients who were hospitalized in the main public sector hospital of Somalia with laboratory-confirmed SARS-CoV-2 infection during the first 3 months of the COVID-19 pandemic in the country from 30 March to 12 June 2020. This analysis only included this cohort of 131 patients as no patients were admitted to the study hospital with laboratory-confirmed SARS-CoV-2 infection after 12 June 2020, and all the patients included in the cohort were either discharged alive or died between 30 March and 12 June 2020. The demographic and clinical characteristics, outcomes and risk factors for death were assessed for this cohort of 131 patients. Before the COVID-19 outbreak, the main hospital in Somalia, where the study took place, had no ICU beds and was not designed to care for severely ill patients. When COVID-19 reached Somalia, the government refurbished the De Martino Hospital and fitted it with 20 ICU beds. The decision was made to transfer all severely ill patients diagnosed with COVID-19 to this hospital. This article also reports the findings of a survival analysis that assessed which interventions, given to this cohort of 131 patients in accordance with the national treatment guidelines of Somalia for SARS-CoV-2 infection, improved the probability of survival.
To the authors’ knowledge, this is the first evidence from a fragile and vulnerable health setting on risk factors for in-hospital deaths, and which interventions have proven successful in an environment with limited resources and capacities. The study sample had a high number of deaths. Of the patient cohort, 60% recovered and were discharged, and the remaining 40% died in the hospital between 30 March and 12 June 2020.
The median length of hospital stay for the study sample was 5.5 days (IQR 9 days), with a range of 1–35 days and a mean of 7.7 days (SD 6.9). In a similar study in Vietnam, the median length of hospital stay was 21 (range 16–34) days (Thai et al., 2020), while in the USA and the UK, the length of stay was shorter with an average of 7–8 days (Bhatraju et al., 2020; Bialek et al., 2020; Docherty et al., 2020).
Two-thirds of patients were male (69%). The mean age of male patients was 58.5 years vs 56.9 years for females. These findings are consistent with studies in Vietnam (Thai et al., 2020) and China (Yang et al., 2020) which suggest a gender difference in hospitalized patients with COVID-19. The signs and symptoms by age group and sex in this study do not differ from those reported in studies outside of Africa (Bhatraju et al., 2020; Bialek et al., 2020; Docherty et al., 2020; Salinas-Escudero et al., 2020; Thai et al., 2020; Wu and McGoogan, 2020; Wu et al., 2020); however, there are no comparable data from African settings, especially sub-Saharan African countries.
The main comorbidities found in the 131 patients with COVID-19 in this study are similar to the findings of other studies performed in Africa (Dalal et al., 2011). However, this study is limited by the lack of population-based data on the prevalence of diabetes and cardiovascular diseases in males and females in Somalia. Also, the proportion of female COVID-19 patients with diabetes in this study (48%) was higher than males (36%), and this is consistent with a study from Somaliland (Ahmed et al., 2019). This study found that the prevalence of diabetes was higher in females than males, and cardiovascular disease was more prevalent among males; in both sexes, the risk factors for diseases increased with age (Ahmed et al., 2019). These findings are consistent with a study that examined the burden of non-communicable diseases in sub-Saharan Africa (Dalal et al., 2011), and another study which examined comorbidities in a sample of patients hospitalized with COVID-19 in New York City and surrounding areas (Richardson et al., 2020).
This survival analysis and estimation of the hazard ratio of death showed that age ≥60 years and having cardiovascular disease as a co-morbidity significantly increased the likelihood of death due to COVID-19. However, the patient's sex did not increase the likelihood of death. In a study in China (Meng et al., 2020), men were more than twice as likely to die from COVID-19 than women, which differs from the present study, and age >50 years increased the risk of death from COVID-19, which concurs with the present study. The use of vasopressors and inotropes did not reduce the risk of death, as patients who received such treatment had very low probability of survival, and the adjusted hazard ratio of death was 6.24 (95% CI 3.55–10.99) compared with patients who did not receive vasopressors and inotropes (calculation not shown). Another study from China reported similar low survival rates among patients who received treatments such as vasoconstrictive agents and oxygen therapy, as these measures were administered to patients with higher severity of disease (Yang et al., 2020).
Similar to a study from Italy (Grasselli et al., 2020), the present study found that the use of non-invasive ventilation was an indicator of severe disease and increased the risk of death. An important finding of the present study was the comparative benefit to clinical outcome of non-invasive ventilation and the use of medical oxygen. The survival probability of patients who received medical oxygen alone was higher (75%) at day 7, and consistently remained >70% even at day 14 after admission, compared with patients treated with both oxygen and non-invasive ventilation (10%). The risk of death for patients who received non-invasive ventilation with medical oxygen was 5.43 times higher compared with patients who received oxygen alone (calculation not shown). The lack of clinical benefit from the use of ventilation has been reported in other settings (Bhatraju et al., 2020; Zareifopoulos et al., 2020). In addition, sophisticated hospital equipment, such as ventilators, is often operated by healthcare workers with little training on such specialized equipment, as has been the case in Somalia during the COVID-19 pandemic where short supply of equipment and trained staff has been a concern. Using hastily supplied ventilators with limited training can lead to improper use, discomfort and even death (Branson, 2018; Kohbodi et al., 2020; Zareifopoulos et al., 2020).
As the COVID-19 pandemic spread throughout the world, there was concern that the infection would have a substantial impact on African countries because they were unprepared to deal with such a crisis (El-Sadr and Justman, 2020; Nkengasong and Mankoula, 2020). While this is true, the level of preparedness was judged by the number and availability of ICU beds and ventilators per millions of people's use. However, our experience has shown that ventilators, although useful for patients suffering from severe symptoms, are not a feasible treatment in countries where skilled and trained staff, such as specialized nurses and intensivists to manage ICUs and ventilators, are not available, as was the case in the study hospital where this study was conducted. Although a causal link between the skill level and training of Somali healthcare workers and the relatively high number of deaths observed among the admitted cohort of 133 patients with SARS-CoV-2 infection could not be established, it seems likely that the relative lack of experience of healthcare workers on the use of ventilators and managing a high-dependency critical care unit contributed to the high mortality rate (40%) among the admitted patients.
As reported in most sub-Saharan African countries (Mangipudi et al., 2020), medical oxygen in secondary and tertiary healthcare settings is not always available, even though it is well established that this therapy is fundamental for the treatment of acutely ill patients, especially for pneumonia, which is a leading cause of death in elderly people and children aged <5 years. Its usefulness in the treatment of patients with COVID-19 is further evidence of the urgent need to ensure that medical oxygen is always available in these settings. However, for myriad reasons, including a lack of data on the availability of medical oxygen, supplying and delivering oxygen to sub-Saharan African countries has not been a priority despite the fact that it is recognized as a fundamental hospital component.
This study has a number of limitations and the results should be interpreted in light of these limitations. First, the study examined a small cohort of patients who were admitted to one public sector hospital in Somalia during the first 3 months of the pandemic. The dataset does not include all clinical variables related to the evolution of COVID-19, and data were collected manually, retrospectively, although the data were checked by two people to minimize error. Nonetheless, human error and many other missing pieces of information (e.g. obesity, smoking habits, etc.) should be taken into consideration when analysing and interpreting the data. Therefore, the results may not be generalizable for the rest of the country or other population groups, and may not be representative of the whole outbreak trajectory in Somalia. Second, the study data include information on deaths from laboratory-confirmed cases and do not include other patients who could not be tested. This may lead to underestimation of the findings (of the benefit of oxygen over non-invasive ventilation) and limit their generalizability. Third, the absence of important data on oxygen saturation levels and respiratory rates upon admission and upon discharge or death represent another limitation of this study. All of these limiting factors may confound the observations to be inferred from the study findings.
Despite these limitations, this study contributes to an understanding of the risk factors for in-hospital death from COVID-19 in Somalia. Risk factors for in-hospital death from COVID-19 have not been studied or published from sub-Saharan countries previously. In addition, the study has used findings from survival analyses to assess which interventions have a higher probability of improved clinical outcome of patients with severe COVID-19 in resource-poor settings by early use of medical oxygen.
Conclusion
Considering the risk factors mentioned in this study – age ≥60 years, presence of cardiovascular disease and use of non-invasive ventilation – is critical when dealing with patients with severe COVID-19 in an environment where a trained and skilled health workforce to manage patients in high-dependency units is limited and critical care services are rudimentary. This study confirms the importance of high levels of hospital emergency preparedness including decision-making in managing critical care. The findings highlight the importance of the critical aspect of deciding whether or not to ventilate critical patients in a low-resource setting when advanced levels of critical care are not available, and instead optimizing the use of available healthcare resources in these settings, such as the use of medical oxygen to improve the probability of survival. Thus, the study results have important policy implications by highlighting the value of available, accessible and affordable low-cost interventions in a fragile and resource-poor setting to inform case management for severe acute respiratory diseases. The scaling up of availability of medical oxygen in such a setting will also promote improved access to care for childhood pneumonia and other respiratory diseases, and result in improved outcome in terms of lives saved and deaths averted as lower respiratory infections are the third leading cause of death and second leading cause of disability-adjusted life-years for both sexes in Somalia (GBD 2019 Universal Health Coverage Collaborators, 2020).
Acknowledgements
The authors wish to thank Jonathan Aaron Polonsky for his review and Ms Fiona Curlet for her review and editing. This work was supported by the Alliance for Health Policy and Systems Research, World Health Organization. The Alliance is supported through core funding and project-specific designated funds. The full list of Alliance donors is available at: https://www.who.int/alliance-hpsr/partners/en/
Conflict of interest statement
None declared.
Funding
None.
Ethical approval
Not applicable.
Footnotes
Regarding admission criteria for hospitalization of patients with COVID-19 in Somalia, and according to the National Standard Operating Procedure for Case Management of COVID-19 published by the Federal Ministry of Health of Somalia, patients who fall into the high-risk category as per the World Health Organization's classification such as age >60 years, heavy smoker and chronic medical conditions (diabetes, hypertension, chronic kidney disease) and immunodeficiency should be admitted to an isolation centre designated by the government. Regarding ICU admission criteria for COVID-19, a patient must meet one or more of the following criteria: acute and potentially reversible organ dysfunction responding poorly to initial resuscitation; severe respiratory failure or intubated (SpO2/FiO2 ratio <200); circulatory shock (systolic blood pressure <90 mmHg, lactate >4); and more than single organ failure. Please refer to the National Standard Operating Procedures (SOP): case management COVID-19 handbook for further information. https://www.humanitarianresponse.info/ru/operations/somalia/document/somalia-final-sop-case-management-covid-19-v1-26042020 (accessed 1 November 2021).
References
- Ahmed SH, Meyer HE, Kjøllesdal MK, Marjerrison N, Mdala I, Htet AS, et al. The prevalence of selected risk factors for non-communicable diseases in Hargeisa, Somaliland: a cross-sectional study. BMC Public Health. 2019;19:878. doi: 10.1186/s12889-019-7101-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aitken T, Lee Chin K, Liew D, Ofori-Asenso R. Rethinking pandemic preparation: Global Health Security Index (GHSI) is predictive of COVID-19 burden, but in the opposite direction. J Infect. 2020;81:318–356. doi: 10.1016/j.jinf.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatraju PK, Ghassemieh BJ, Nichols M, Kim R, Jerome KR, Nalla AK, et al. COVID-19 in critically ill patients in the Seattle region – case series. N Engl J Med. 2020;382:2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialek S, Boundy E, Bowen V, Chow N, Cohn A, Dowling N, et al. Severe outcomes among patients with coronavirus disease 2019 (COVID-19) – United States, February 12–March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:343–346. doi: 10.15585/mmwr.mm6912e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branson RD. Automation of mechanical ventilation. Crit Care Clin. 2018;34:383–394. doi: 10.1016/j.ccc.2018.03.012. [DOI] [PubMed] [Google Scholar]
- Dalal S, Beunza JJ, Volmink J, Adebamowo C, Bajunirwe F, Njelekela M, et al. Non-communicable diseases in sub-Saharan Africa: what we know now. Int J Epidemiol. 2011;40:885–901. doi: 10.1093/ije/dyr050. [DOI] [PubMed] [Google Scholar]
- Docherty AB, Harrison EM, Green CA, Hardwick HE, Pius R, Norman L, et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:1–12. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sadr WM, Justman J. Africa in the path of Covid-19. N Engl J Med. 2020;383:e11. doi: 10.1056/NEJMp2008193. [DOI] [PubMed] [Google Scholar]
- FMOH, WHO. COVID-19 Situation Report – Somalia. Issue 85. 2021. Available at: https://mcusercontent.com/cd9d6d0a38b5bbbd90d426e7e/files/005b0414-8c0b-b636-d620-401d4bba40ce/Somalia_COVID_19_Situation_Report_17_23_Oct_2021.pdf (accessed 1 November 2021).
- FMOH. National Standard Operating Procedures (SOP): case management COVID-19. 2020. Available at: https://www.humanitarianresponse.info/ru/operations/somalia/document/somalia-final-sop-case-management-covid-19-v1-26042020 (accessed 1 November 2021).
- GBD 2019 Universal Health Coverage Collaborators Measuring universal health coverage based on an index of effective coverage of health services in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1250–1284. doi: 10.1016/S0140-6736(20)30750-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Index Project Team. GHS index. 2021. Available at: https://www.ghsindex.org/ (accessed 15 September 2021).
- Kohbodi GNA, Rajasurya V, Noor A. StatPearls Publishing; Treasure Island, FL: 2020. Ventilator-associated pneumonia. [PubMed] [Google Scholar]
- Mangipudi S, Leather A, Seedat A, Davies J. Oxygen availability in sub-Saharan African countries: a call for data to inform service delivery. Lancet Glob Health. 2020;8:e1123–e1124. doi: 10.1016/S2214-109X(20)30298-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Y, Wu P, Lu W, Liu K, Ma K, Huang L, et al. Sex-specific clinical characteristics and prognosis of coronavirus disease-19 infection in Wuhan, China: a retrospective study of 168 severe patients. PLoS Pathog. 2020;16 doi: 10.1371/journal.ppat.1008520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamud MFY, Hashi AS, Mohamed AH, Yusuf AM, Ali IH, Ahmed MA. Clinical characteristics, comorbidities, initial management and outcome of COVID-19 infected patients admitted to intensive care unit in Somalia: a national retrospective study. Research Square. 2021 doi: 10.21203/rs.3.rs-66767/v1. preprint. [DOI] [Google Scholar]
- Nkengasong JN, Mankoula W. Looming threat of COVID-19 infection in Africa: act collectively, and fast. Lancet. 2020;395:841–842. doi: 10.1016/S0140-6736(20)30464-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas-Escudero G, Carrillo-Vega MF, Granados-García V, Martínez-Valverde S, Toledano-Toledano F, Garduño-Espinosa J. A survival analysis of COVID-19 in the Mexican population 2020 Research Square 2020 preprint. https://doi.org/10.21203/rs.3.rs-39083/v1. [DOI] [PMC free article] [PubMed]
- Thai PQ, Toan DTT, Dinh TS, Van Hoang TH, Luu NM, Xuan Hung L, et al. Factors associated with the duration of hospitalization among COVID-19 patients in Vietnam: a survival analysis. Epidemiol Infect. 2020;145:e114. doi: 10.1017/S0950268820001259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . World Health Organization Regional Office for the Eastern Mediterranean; Cairo: 2015. Somalia. Health profile.https://rho.emro.who.int/sites/default/files/Profiles-briefs-files/EMROPUB_EN_19617-SOM.pdf Available at: (accessed 15 September 2021) [Google Scholar]
- WHO . World Health Organization; Geneva: 2016. Health workforce requirements for universal health coverage and the Sustainable Development Goals.https://apps.who.int/iris/bitstream/handle/10665/250330/9789241511407-eng.pdf;sequence=1 Available at: (accessed 15 October 2021) [Google Scholar]
- WHO . World Health Organization Regional Office for the Eastern Mediterranean; Cairo: 2020. COVID-19 situation report-21.http://www.emro.who.int/images/stories/coronavirus/documents/covid-19_sitrep_21.pdf?ua=1 Available at: (accessed 15 October 2021) [Google Scholar]
- World Bank. Somalia economic update: investing in health to anchor growth. 2021. Available at: https://www.worldbank.org/en/country/somalia/publication/somalia-economic-update-investing-in-health-to-anchor-growth (accessed 1 November 2021).
- Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, McGoogan J. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zareifopoulos N, Lagadinou M, Karela A, Platanaki C, Karantzogiannis G, Velissaris D. Management of COVID-19: the risks associated with treatment are clear, but the benefits remain uncertain. Monaldi Arch Chest Dis. 2020:90. doi: 10.4081/monaldi.2020.1342. [DOI] [PubMed] [Google Scholar]