Abstract
Introduction and aim: As first receivers of suspected coronavirus disease 2019 (COVID-19) patients, clinicians of the Emergency Department (ED) have to rapidly perform the first clinical assessment evaluating the intensity of care needed. So far, clear management guidelines still lack. We identified variables associated with hospitalization in order to give a quick tool to assist clinicians in stratifying cases based on the severity at their arrival at the ED and in predicting the need for hospital care.
Methods: This is a monocentric observational prospective study enrolling COVID-19 patients. A score for hospitalization prediction (CovHos Score) was created using variables associated with hospitalization at multivariate analysis and then validated on an internal subsequent cohort.
Results: A total of 667 patients were included; 465 (69.7%) were hospitalized and 108 (16.2%) died at 30-days follow-up. In a multivariate analysis, male sex, age>65, alveolar-to-arterial oxygen gradient percentage increase compared to that expected for age, neutrophils/lymphocytes ratio and C-reactive protein levels were significantly associated with a higher rate of hospital admission. A CovHos score cut-off of 12 points predicted hospitalization with 85% sensitivity and 82.4 % specificity (area under a receiver operating characteristic curve [AUROC] = 0.909, 95% CI 0.884 - 0.935). Similar results were obtained in the validation court. A cut-off of 22 has 79% sensitivity and 77% specificity in predicting mortality (AUROC = 0.824; 95% CI 0.782-0.866); sensitivity and specificity were respectively 71.4% and 71.3% in the validation group.
Conclusions: Although medical judgment still remains crucial, the CovHos score is an effective tool to assist emergency clinicians in predicting the need for hospitalization or to optimize allocation in a shortage of hospital resources.
Keywords: covid-19, coronavirus, emergency department, score, discharge
Introduction
In December 2019, a new disease called ‘Coronavirus disease 2019 (COVID-19) appeared, generated by an unknown enveloped RNA beta-coronavirus named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1-3]. SARS-CoV-2 had a big impact, with an incidence of more than 34.8 million cases, causing more than 1 million deaths in the period going from the beginning of the epidemic to October 6th, 2020 [4] with a worldwide case fatality rate of about 12% [5]. The symptoms of COVID-19 in its clinical course are primarily respiratory, including cough, fever, and dyspnea; and in the most severe cases, it rapidly progresses to ARDS (Acute Respiratory Distress Syndrome) [6]. Arterial blood gas analysis (ABG) frequently highlights hypoxemic respiratory failure associated with respiratory alkalosis. Most patients typically reveal a discrepancy between clinical and ABG findings, presenting to the Emergency Department (ED) with extremely low partial pressure of arterial oxygen (PaO2) values not associated with dyspnea [7]. Moreover, a reduced partial pressure of arterial carbon dioxide (PaCO2) is often present, due to an increase in respiratory rate (RR) needed to maintain an adequate PaO2. Only a small proportion of patients will develop severe respiratory failure (SRF) but many of these require mechanical ventilation [8].
In Italy, from the beginning of the pandemic to October 5th, 2020, the number of people affected by COVID-19 was 327,586, with 36,002 deaths (0.11%) [9]. Italian hospitals were faced with a rising number of cases, with limited healthcare resources due to the sudden and ever-increasing pandemic. As first receivers of suspected COVID-19 patients, EDs have to rapidly perform the first clinical assessment and the identification of the most critical patients or those at worsening risk, evaluating the intensity of care needed. So far, ED physicians still lack clear management guidelines. According to literature, 14% of patients have severe disease and 5% a critical disease requiring intensive care while the majority of patients (81%) present with mild disease. Although emergency doctors are well trained to recognize and treat severe and critical COVID-19 patients, they are more likely to manage mild cases. Deciding, in an ED setting, whether patients with mild illness are suitable for home management or require in-hospital observation due to an increased worsening risk is challenging.
The aim of our study is to give a quick, reliable, and effective tool to assist clinicians in stratifying cases based on the severity at their arrival at the ED and in predicting the need for hospitalization, notably for unselected patients as in an emergency setting. We investigated COVID-19 features, as reported at the time of patient admission, in order to point out those variables associated with hospitalization. CovHos Score (COVID-19 score for hospitalization prediction) was thus created. CovHos score was then validated on a subsequent cohort.
To evaluate the severity of the respiratory failure and pulmonary impairment in patients affected by COVID-19, we used, among standard parameters, the Alveolar-to-arterial Oxygen Gradient (AaDO2). The AaDO2 measures the difference between the alveolar (pAO2) and arterial oxygen (PaO2) concentration, helping to determine the source of hypoxemia. In patients affected by pneumonia, where the physical barrier within the alveoli reduces the oxygen diffusion through the capillaries, AaDO2 is inappropriately high [10].
Materials and methods
Study design and participants
From March 13th to April 4th, 2020, we conducted a monocentric observational prospective study enrolling consecutively all adult patients (≥ 18 years old), who came to the ED of Sant’Orsola-Malpighi Hospital (Bologna, Italy) showing suspicious signs and symptoms of COVID-19, like fever, cough, pharyngodynia, fatigue, anosmia, ageusia, conjunctivitis, headache, myalgias, diarrhoea, and syncope. All suspected cases of COVID-19 were admitted to a COVID area of our ED, where demographic, case history, and clinical data were collected, laboratory tests (ABG, general blood tests), and radiological exams (lung ultrasound, chest X-ray, and/or high resolution computed tomography [HRCT]) were performed. All patients underwent a nasal and pharyngeal swab for SARS-CoV-2. Out of all suspected patients admitted to ED, only those diagnosed with COVID-19 have been included in our analysis, both directly discharged and hospitalized patients. COVID-19 diagnoses have been established on a positive real-time reverse-transcription polymerase-chain-reaction (RT-PCR) assay for nasal and pharyngeal swab specimens and/or on typical imaging acquired by lung ultrasound, X-ray, or HRCT. Patients discharged or admitted to the hospital with an alternative diagnosis were excluded from the analysis. The study was approved by our local Ethics Committee (approval number: 551/2020/Oss/AOUBo). Verbal informed consent was obtained from all individual participants included in the study.
Epidemiological, demographic, clinical, laboratory, treatment, and outcome data were extracted from electronic medical records taken since the patient's arrival at the ED. Concerning the survival analysis, the 30-days all-cause mortality since the ED admission was considered. All patients have been contacted by telephone after 30 days in order to highlight a potential hospital re-admission other than Sant’Orsola-Malpighi Hospital.
Among the possible predictive variables for hospitalization, the following patient variables at hospital admission were included: demographic variables, medical history, clinical signs and symptoms, arterial blood gas, and laboratory findings. Besides, in order to evaluate the severity of the respiratory failure, we calculated AaDO2 and AaDO2 percentage increase compared to the expected value for a given age. AaDO2 was calculated using the formula:
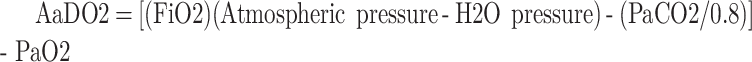
Normal gradient estimated = 
Statistical analysis and score creation
General characteristics were reported as mean ± standard deviation (SD) and frequencies (percentages) for continuous and categorical variables, respectively. Comparisons between groups were performed using the Chi-square test and the Student’s t-test or Mann-Whitney test for independent groups, for categorical and continuous variables, respectively, where appropriate.
Logistic binary regression analysis was run, taking hospitalization as the outcome. Statistically significant variables at univariate analysis were included in multivariate analysis. The strength of the association between variables and hospitalization was reported as odds ratios (ORs) with their 95% confidence intervals (95% CI). We built up a scoring system based on such findings to properly select COVID-19 patients requiring in-hospital treatment. CovHos score was constructed based on the coefficients from the logistic model, as the sum of each variable multiplied by its own OR. Receiver Operating Characteristic (ROC) analysis was used to determine the accuracy of the CovHos score in predicting hospitalization and to find the corresponding cut-off value. A ROC analysis was also performed to assess CovHos score accuracy in predicting mortality. P values <0.05 were considered significant for all the tests. SPSS statistics version 25 (IBM Corp., Armonk, NY) was used for statistical analyses.
Score validation
In order to validate the CovHos score, we tested it on a cohort of patients consecutively admitted to our ED from April 4th to April 30th that fulfilled the same inclusion and exclusion criteria. Exact Fisher’s test was used to assess the statistical significance of the CovHos score in the prediction of hospitalization and mortality. Specificity and sensitivity were calculated on 2x2 tables.
Results
Out of 1,366 patients admitted to the COVID area of our ED, 667 were included in the analysis. A total of 465 (69.7%) were hospitalized and 108 (16.2%) died at 30-days follow-up. Out of 202 discharged patients, 27 came back to the ED within 30 days; 15 of them were discharged and 12 hospitalized (6%).
Features of the enrolled population
The mean ± SD age of our cohort was 61.9 ± 18.9 years and 355 (53.2%) were males. The most common comorbidities were hypertension (37.3%), diabetes (10.8%), and chronic obstructive pulmonary disease (9.9%). In 423 patients (63.4%), symptoms onset went back to less than six days before arriving at the ED and the most frequently observed symptoms were fever (81.7%), cough (55.6%), dyspnoea (37.3%), and fatigue (16.8%). Concerning the respiratory parameters, we have found that RR was 19.8 ± 5.4, SpO2 was 95.6 ± 4.4 and PaO2/FiO2 (P/F) was 347 ± 99.1.
Hospitalized and non-survivors were significantly older (68.5 ± 16.7; 81.2 ± 10.6) than discharged patients and survivors (46.7 ± 14.4; 58.1 ± 17.9) and had a higher prevalence of hypertension (48% and 65.7%, respectively). At the time of ED admission, they also complained of a higher rate of dyspnoea (40.6% vs 29.7%; 57.4% vs 33.5% respectively), showing a higher RR and a lower SpO2. The full demographic and clinical specifications are included in Table 1.
Table 1. Demographic and baseline clinical characteristics.
Data are mean ± SD or n (%). COPD: chronic obstructive pulmonary disease. SpO2: peripheral oxygen saturation. ED: emergency department. MBP: mean arterial blood pressure. HR: heart rate. RR: respiratory rate. BT: body temperature. NS: not significant
| Characteristics | All patients (n=667) | Discharged (n=202) | Hospitalized (n=465) | p-value | Survivors (n=559) | Non-survivors (n=108) | p-value | |
| Age, years | 61.9 ±18.9 | 46.7 ± 14.4 | 68.5 ± 16.7 | < .001 | 58.1 ± 17.9 | 81.2 ± 10.6 | < .001 | |
| Sex | ||||||||
| Male | 355 (53.2) | 86 (24.2) | 269 (75.8) | < .001 | 291 (82) | 64 (8) | < .001 | |
| Female | 312 (46.8) | 116 (37.2) | 196 (62.8) | < .001 | 268 (85.9) | 44 (14.1) | < .001 | |
| Comorbidities | ||||||||
| COPD | 66 (9.9) | 4 (2.0) | 62 (13.3) | < .001 | 47 (8.4) | 19 (17.6) | .007 | |
| Hypertension | 249 (37.3) | 26 (12.9) | 223 (48.0) | < .001 | 178 (31.8) | 71 (65.7) | < .001 | |
| Obesity | 41 (6.1) | 8 (4.0) | 33 (7.1) | NS | 30 (5.4) | 11 (10.2) | NS | |
| Diabetes | 72 (10.8) | 8 (4.0) | 64 (13.8) | < .001 | 48 (8.6) | 24 (22.2) | < .001 | |
| Chronic Kidney disease | 52 (7.8) | 1 (0.5) | 51 (11.0) | < .001 | 25 (4.5) | 27 (25.0) | < .001 | |
| Ischemic Heart Disease | 49 (7.3) | 2 (1.0) | 47 (10.1) | < .001 | 26 (4.7) | 23 (21.3) | < .001 | |
| Active cancer | 39 (5.8) | 5 (2.5) | 34 (7.3) | .012 | 25 (4.5) | 14 (13.0) | .002 | |
| Immunodeficiency | 8 (1.2) | 0 (0.0) | 8 (1.7) | NS | 7 (1.3) | 1 (0.9) | NS | |
| Symptoms onset > 6 days | 244 (36.6) | 74 (36.6) | 170 (36.6) | NS | 226 (40.4) | 18 (16.7) | < .001 | |
| Symptoms at ED admission | ||||||||
| Fever | 545 (81.7) | 154 (76.2) | 391 (84.1) | .016 | 460 (82.3) | 85 (78.7) | NS | |
| Dyspnea | 249 (37.3) | 60 (29.7) | 189 (40.6) | .007 | 187 (33.5) | 62 (57.4) | < .001 | |
| Cough | 371 (55.6) | 130(64.4) | 241 (51.8) | .003 | 336 (60.1) | 35 (32.4) | < .001 | |
| Conjunctivitis | 10 (1.5) | 8 (4.0) | 2 (0.4) | .002 | 10 (1.8) | 0 (0.0) | NS | |
| Pharingodynia | 46 (6.9) | 31 (15.3) | 15 (3.2) | < .001 | 45 (8.1) | 1 (0.9) | .006 | |
| Headache | 58 (8.7) | 37 (18.3) | 21 (4.5) | < .001 | 57 (10.2) | 1 (0.9) | .001 | |
| Fatigue | 112 (16.8) | 39 (19.3) | 73 (15.7) | NS | 102 (18.2) | 10 (9.3) | .024 | |
| Myalgia | 77 (11.5) | 46 (22.8) | 31 (6.7) | < .001 | 74 (13.2) | 3 (2.8) | .001 | |
| Diarrhoea | 96 (14.4) | 41 (20.3) | 55 (11.8) | .006 | 90 (16.1) | 6 (5.6) | .004 | |
| Anosmia | 34 (5.1) | 25 (12.4) | 9 (1.9) | < .001 | 34 (6.1) | 1 (0.9) | .003 | |
| Ageusia/Disgeusia | 58 (8.7) | 37 (18.3) | 21 (4.5) | < .001 | 58 (10.4) | 0 (0.0) | < .001 | |
| Syncope | 10 (1.5) | 2 (1.0) | 8 (1.7) | NS | 6 (1.1) | 4 (3.7) | NS | |
| Vital Signs | ||||||||
| MBP, mmHg | 92.4 ± 14.5 | 95.7 ± 12.6 | 91.6 ±14.8 | .029 | 93.3 ± 13.3 | 89.4 ± 17.6 | .044 | |
| HR, beats per min | 90.3 ±17.3 | 88.4 ±15.4 | 91.2 ± 18.1 | NS | 89.8 ± 16.6 | 93.1 ± 20.7 | NS | |
| RR, breaths per min | 19.8 ± 5.4 | 17.3 ±2.7 | 20.9 ±5.9 | < .001 | 19.1 ± 4.6 | 24,2 ± 7.5 | < .001 | |
| SpO2, % | 95.6 ± 4.4 | 97.9 ±1.5 | 94.6 ± 4.8 | < .001 | 96.1 ± 3.8 | 92.2 ± 5.7 | < .001 | |
| BT, °C | 37.2 ± 0.9 | 36.8 ±0.6 | 37.3 ± 0.9 | < .001 | 37.1 ± 0.9 | 37.3 ± 0.9 | .036 | |
| Glasgow Coma Scale | ||||||||
| 15 | 621 (93.1) | 202 (32.5) | 419 (67.5) | < .001 | 540 (87) | 81 (13) | < .001 | |
| ≤14 | 46 (6.9) | 0 (0.0) | 46 (100) | < .001 | 19 (41.3) | 27 (58.7) | < .001 | |
| Kelly Scale | ||||||||
| grade 1-2 | 644 (96.6) | 202 (31.4) | 442 (68.6) | < .001 | 552 (85.7) | 92 (14.3) | < .001 | |
| grade 3-4 | 18 (2.7) | 0 (0.0) | 18 (100) | < .001 | 6 (33.3) | 12 (66.6) | < .001 | |
| grade 5-6 | 5 (0.7) | 0 (0.0) | 5 (100) | < .001 | 1 (20) | 4 (80) | < .001 | |
On ABG we have found a standard pH value in all examined cohorts, while hypocapnia occurred in all patient categories with the exception of the discharged ones, who showed normal pCO2 values. As to the laboratory tests performed on admission, patients typically showed normal white blood cells (WBC), elevated C-reactive protein (CRP) (6.2 ± 7.4 mg/dL) and, lactate dehydrogenase (LDH) (289.6 ± 183.2 U/L). Lymphocytopenia was found in 40.3% of cases. Patients who required hospitalization or died, featured more significant laboratory abnormalities, including lymphocytopenia, neutrophils/lymphocytes ratio (N/L), D-dimer, LDH and, CRP, than those who got discharged. ABG and laboratory findings are listed in Table 2.
Table 2. Arterial blood gas and laboratory findings.
Data are mean ± SD, median (min-max) or n (%). PaCO2, PaO2: arterial carbon dioxide and oxygen tensions. P/F: arterial oxygen partial pressure/fractional inspired oxygen ratio. AaDO2: alveolar-to-arterial oxygen gradient. HCO3-: bicarbonate. FiO2: fractional inspired oxygen. WBC: white blood cells. N/L ratio: neutrophils/lymphocytes ratio. LDH: lactate dehydrogenase. CPK: creatine phosphokinase. CRP: C-reactive protein. PCT: procalcitonin. NS: not significant. ROX index: the ratio of oxygen saturation as measured by pulse oximetry/FiO2 to respiratory rate. FEU: fibrinogen equivalent units
| All patients (n=667) | Discharged (n=202) | Hospitalized (n=465) | p-value | Survivors (n=559) | Non-survivors (n=108) | p-value | |
| Arterial Blood Gas Analysis | |||||||
| pH | 7.4 ± 0.6 | 7.4 ± 0.0 | 7.4 ± 0.7 | < .001 | 7.4 ± 0.6 | 7.4 ± 0.1 | NS |
| pO2, mmHg | 75.9 ± 22.1 | 90.1 ± 13.6 | 69.7 ± 22.3 | < .001 | 77.8 ± 19.9 | 65.9 ± 29.5 | < .001 |
| pCO2, mmHg | 33.7 ± 6.7 | 35.5 ± 4.6 | 32.9 ± 7.3 | < .001 | 33.6 ± 6.1 | 34.1 ± 9.3 | NS |
| HCO3- mmol/L | 23.8 ± 3.4 | 24.1 ± 2.3 | 23.6 ± 3.8 | .016 | 23.9 ± 2.8 | 23.1 ± 5.4 | .007 |
| Lactate, mmol/L | 1.2 ± 1.1 | 1 ± 0.6 | 1.4 ± 1.3 | < .001 | 1.1 ± 0.7 | 2.1 ± 2.2 | < .001 |
| FiO2 | 23.1 ± 9.9 | 21 ± 0.0 | 24.0 ± 11.8 | < .001 | 21.8 ± 6.0 | 30.0 ± 19.4 | < .001 |
| P/F | 347.0 ± 99.1 | 426.8 ± 63.5 | 312.4 ± 91.5 | < .001 | 362.8 ± 90.5 | 261.7 ± 100.4 | < .001 |
| AaDO2 | 34.8 ± 17.7 | 18 ± 9.9 | 42.4 ± 14.9 | < .001 | 32.8 ± 17.1 | 47.8 ± 15.6 | < .001 |
| AaDO2 expected for age | 19.4 ± 4.7 | 15.6 ± 3.6 | 21 ± 4.2 | < .001 | 18.5 ± 4.4 | 24.2 ± 2.8 | < .001 |
| Rox Index | 24.3 ± 6.5 | 27.7 ± 4.2 | 22.8 ± 6.7 | < .001 | 25.3 ± 5.9 | 18.6 ± 7.1 | < .001 |
| Laboratory tests | |||||||
| WBC, 109/L | 7.9 ± 5.5 | 6.8 ± 2.9 | 8.3 ± 6.2 | .028 | 7.4 ± 5.2 | 10.0 ± 6.5 | < .001 |
| Neutrophils, 109/L | 6.1 ± 6.4 | 4.4 ± 2.2 | 6.8 ± 7.3 | < .001 | 5.7 ± 6.4 | 8.2 ± 6.0 | < .001 |
| Lymphocytes, 109/L | 1.6 ± 2.7 | 1.8 ± 1.1 | 1.6 ± 3.1 | < .001 | 1.7 ± 2.9 | 1.3 ± 1.3 | < .001 |
| Eosinophils, 109/L | 0.1 ± 0.1 | 0.1 ± 0.2 | 0.0 ± 0.1 | < .001 | 0.1 ± 0.1 | 0.0 ± 0.1 | < .001 |
| N/L ratio | 5.8 ± 6.9 | 2.9 ± 1.9 | 6.9 ± 7.8 | < .001 | 5.0 ± 5.9 | 9.8 ± 9.9 | < .001 |
| Platelets, 109/L | 220.4 ± 21.3 | 230.6 ± 71.5 | 216.4 ± 97.7 | .001 | 221.2 ± 83.5 | 215.9 ± 123.5 | .047 |
| D-dimer, mg/L FEU | 1.6 ± 4.0 | 0.4 ± 0.4 | 2.0 ± 4.6 | < .001 | 1.1 ± 2.8 | 3.9 ± 7.3 | < .001 |
| Creatinine, mg/dL | 1.1 ± 1.0 | 0.8 ± 0.6 | 1.2 ± 1.0 | < .001 | 1.0 ± 0.8 | 1.6 ± 1.2 | < .001 |
| LDH, U/L | 289.6 ± 183.2 | 206.1 ± 70.5 | 322.6 ± 202.5 | < .001 | 262.4 ± 104.0 | 437.4 ± 363.5 | < .001 |
| CPK, U/L | 83.0 (11-26758) | 68.0 (30-276) | 83.0 (11-26758) | NS | 77.5(11-26758) | 148.0 (16-953) | .012 |
| Bilirubin, mg/dL | 0.8 ± 1.9 | 0.6 ± 0.5 | 0.8 ± 2.0 | < .001 | 0.6 ± 0.3 | 1.5 ± 4.2 | < .001 |
| CRP, mg/dL | 6.2 ± 7.4 | 1.3 ± 2.3 | 8.1 ± 7.8 | < .001 | 4.9 ± 6.0 | 13.1 ± 10.0 | < .001 |
| PCT, ng/mL | 1.7 ± 11.3 | 0.1 ± 0.0 | 1.8 ± 11.6 | .017 | 0.7 ± 3.5 | 5.8 ± 23.8 | < .001 |
| Lymphopenia, | 269 (40.3) | 35 (17.3) | 234 (50.3) | < .001 | 203 (36.3) | 66 (61.1) | < .001 |
Overall, AaDO2 values were significantly higher in the hospitalized versus discharged patients (21 ± 4.2 vs 15.6 ± 3.6; p< .001) and in non-survivor versus survivor patients (24.2 ± 2.8 vs 18.5 ± 4.4; p< .001).
Score creation and validation
Statistically significant variables at the univariate analysis were included in a multivariate regression. The results showed that male sex (OR, 1.856; IC 1.093-3.149 p <.022), age > 65 years (OR, 6.796; IC 3.622-12.753, p <.001), AaDO2 % (OR, 5.212; IC 3.373-8.054, p < .001), N/L (OR, 1.099; IC 1.039-1.235, p = .05) and increased levels of CRP (OR, 1.247; IC 1.130-1.376, p <.001) were associated with a higher rate of hospital admission (Table 3).
Table 3. Multivariate regression model for predicting hospitalization.
AaDO2: alveolar to arterial oxygen gradient. N/L: neutrophils/lymphocytes ratio. CRP: C-reactive protein.
| Variables | Odds Ratio (95% CI) | p-value |
| Age > 65 years | 6.796 (3.622 - 12.753) | < .001 |
| Male sex | 1.856 (1.093 - 3.149) | .022 |
| AaDO2 percentage increase compared to expected for age | 5.212 (3.373 - 8.054) | < .001 |
| N/L | 1.099 (1.039 - 1.235) | .05 |
| CRP, mg/dL | 1.247 (1.130 - 1.376) | < .001 |
| Constant | 1.115 |
CovHos score was thus created using the formula:
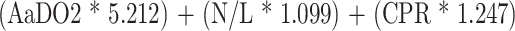 |
adding 1.856 in case of male sex and 6.796 in case of age >65 years.
CovHos score, based on the multivariable regression, identified a cut-off of 12 points in predicting hospitalization with 85 % sensitivity and 82.4 % specificity (area under a receiver operating characteristic curve [AUROC] = 0.909, 95% CI 0.884 - 0.935, p < .001). Figure 1 shows ROC curve of CovHos score.
Figure 1. Receiver operating characteristic (ROC) analysis of CovHos Score in predicting hospitalization in COVID-19 patients .
The ROC analysis allowed to define 22 points as the most accurate cut-off in predicting mortality: indeed, it showed a sensitivity of 79% and a specificity of 77% (AUROC 0.824; 95% CI 0.782-0.866).
We validated the score on 309 patients who fulfilled the same inclusion and exclusion criteria. Among these, 228 were hospitalized and 81 were discharged. The results showed that a CovHos score of 12 points has a sensitivity of 82% and a specificity of 74% in predicting the need for in-hospital treatment (p<0.001, AUROC 0.905, 95% CI 0.880-0.930). Figure 2 shows the ROC curve of the CovHos score in the validation group.
Figure 2. Receiver operating characteristic (ROC) analysis of CovHos Score in the validation group.
Moreover, the CovHos score of 22 points demonstrated a sensitivity of 71.4% and a specificity of 71.3% in mortality prediction (p<0.001, AUROC 0.820, 95%CI 0.778-0.863).
Examples of applicability of the CovHos score are presented in Figure 3.
Figure 3. Examples of CovHos score application.
AaDO2 = Alveolar-to-arterial Oxygen Gradient; y = years
Patient A was a 41-years-old man that came to ED with a fever for three days without dyspnea. The score was automatically calculated by filling values in a spreadsheet previously prepared. Despite good ABG values (pO2=75 mmHg, pCO2=29 mmHg), CovHos was 14.8 points. HRCT scan demonstrated the presence of spared ground-glass areas. The patient was admitted to the hospital and, during the hospital stay (six days) O2 was administered for the occurrence of dyspnea and worsening of ABG values. Patient B was an 87-years-old female patient admitted to ED for fever. Her CovHos score was 21.8 points. During her hospital stay (seven days) the patient remained eupnoeic and became afebrile. She was then discharged without complications. Patient C was a 59-years-old male deceased six days after intensive care admission. His CovHos score was 28.3 points.
The use of the CovHos score allowed to suggest the hospitalization of a young man who got worse in the following days. Moreover, by evaluating the score of patient B, indicative of hospitalization with a good prognosis, ED clinicians could foresee a relatively rapid discharge without complications. Lastly, the high CovHos score of patient C reflected the bad prognosis of a middle-aged man.
CovHos score in the study group
Out of 465 hospitalized patients, only one had a one-day observation for cardiological reasons, 72 patients (15.5%) had a length of hospital stay between two and five days, and 392 patients (84.3%) between six and 45 days. CovHos score was 18.6 ± 13 in the short hospital stay group and 24 ± 14 in the long hospital stay group (p < 0.05). Patients readmitted to hospital after a prior discharge (12 out of 202 discharged) showed a higher CovHos score (9.8 vs 3.8; p=0.006), higher AaDO2 (29 vs 16; p=0.001) and higher AaDO2 percentage increase (0.5 vs 0.03; p=0.007).
Discussion
COVID-19 is a relatively unknown and challenging disease, featuring a broad spectrum of appearances and severity degrees. Based on our experience, and considering the sudden and sometimes unexpected evolution of this condition, it is crucial to early identify and stratify patients needing hospitalization or those who are more susceptible to adverse events, especially in a heterogeneous population setting like the ED. This is even more important in case of a pandemic, where hospital resources are limited. So far, scientific literature available on this subject has been mainly addressing severe cases involving acute respiratory failure until the development of ARDS. Although critical patients are more challenging from a clinical perspective, our data showed, like those from the general literature [11,12] that at the time of ED admission the majority of COVID-19 cases was mild. Therefore, the aim of this study is to identify risk factors for hospitalization in adults affected by COVID-19 presenting at the ED, in order to build a score as a quick and effective aid to the physician in determining the need for hospitalization, and potentially, the correct intensity of care. A similar approach was used by Bartoletti et al. [13] who demonstrated that age, obesity, body temperature, RR, lymphocytes, CRP, creatinine, and LDH>350 IU/L, considered together in the so-called PREDI-CO score, have good accuracy in predicting severe respiratory failure (SRF) (AUROC 0.89, sensitivity 80% and specificity 76% at a score>3 points). However, the only parameter influenced by respiratory dynamics in such a score is RR, without any attention to ABG parameters. We have already demonstrated the utility of the ROX index, which is defined as the ratio of peripheral oxygen saturation and the fraction of inspired oxygen to respiratory rate, in predicting hospitalization and mortality in patients with a diagnosis of COVID-19 in the ED [14]. However, even such an approach does not take into consideration ABG parameters.
The analysis of ABG findings showed that, although a large number of patients had hypocapnia, pH values were regular. Hypoxemia was more frequent among severe patients and since their first medical contact, they generally underwent oxygen therapy. Considering the well-known importance of ABG in determining the level of respiratory impairment, we analyzed P/F values, which were significantly lower in hospitalized patients and non-survivors. However, in order to have a more accurate identification of an incipient respiratory failure, we considered AaDO2, which seemed to play a relevant clinical role. It is well known that due to the sigmoid shape of the oxygen dissociation curve, the accuracy of SpO2 readings above 90% becomes uncertain. Given the flatness of the upper oxygen-dissociation curve, a pulse oximetry reading of 95% may denote a PaO2 anywhere between 60 and 200 mmHg. In this connection, AaDO2 allows for a more precise evaluation of the pathophysiological basis of hypoxemia than the more widely used PaO2/FiO2 [15], giving support to the differential diagnosis with other respiratory diseases.
Multivariate regression showed that age>65, male sex, AaDO2%, N/L, and CRP values were the main statistically significant variables able to identify COVID-19 patients needing an in-hospital observation period.
In order to help emergency physicians who first evaluate patients suspected of COVID-19 at the time of ED admission, we built a score based on the five above-mentioned variables. CovHos score could be a useful tool to identify patients needing admittance to a hospital ward. In our context, a CovHos score higher than 12 points had 85 % sensitivity and 82.4 % specificity in the identification of hospital admission. A different cut-off may be considered in other health care systems, depending on local resources of a pandemic that devastated global healthcare systems. Indeed, emergency physicians should decide every day to hospitalize or to discharge patients on a probabilistic basis in many clinical situations, considering together demographic, clinical, laboratory, and radiological variables. In some contexts, precautionary hospitalization may be unrealistic due to limited offers or disproportionate demand. For these reasons, we suggest a cut-off that appears a good compromise between patient safety and resource sparing; however, the score increase in severe conditions does not impede small variations of proposed cut-off values in different situations. Moreover, the CovHos score may be used to allocate patients in the appropriate setting of care even in wards other than intensive care units. Worth to remark that the higher CovHos score is associated with a longer hospital stay and with mortality.
Some limitations should be noted, such as the monocentric nature of our study. We are also aware that we have not defined beforehand the reference standard to determine the hospitalization need, which was decided by the association of clinical judgment, patients’ characteristics, and medical reports. However, the low number of patients re-admitted to the hospital after a prior discharge and the extremely low prevalence of one-day hospital stay corroborate our appropriateness of hospitalization evaluation.
Conclusions
The CovHos score could be a useful tool to help physicians select patients who might be suitable for home management, especially in a shortage of hospital beds. Although we believe medical judgment still remains crucial in the final clinical decision, our aim was to create a useful score for ED management of COVID-19 patients. A high CovHos score should increase clinicians' attention in discharging patients. The relation between the CovHos score and patient worsening is also evident with respect to mortality and length of hospital stay. Further studies especially on a larger scale from different places would help in understanding and appreciating the validity of the score even better.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study. Comitato Etico Indipendente di Area Vasta Emilia Centro (CE-AVEC) issued approval 551/2020/Oss/AOUBo. The study was approved by our local Ethics Committee (approval number: 551/2020/Oss/AOUBo). Verbal informed consent was obtained from all individual participants included in the study
Animal Ethics
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
References
- 1.Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Zhou F, Yu T, Du R, et al. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lu R, Zhao X, Li J, et al. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clinical characteristics and outcomes are similar in ARDS diagnosed by oxygen saturation/Fio2 ratio compared with Pao2/Fio2 ratio. Chen W, Janz DR, Shaver CM, Bernard GR, Bastarache JA, Ware LB. Chest. 2015;148:1477–1483. doi: 10.1378/chest.15-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO epidemiological update (2019). [ Nov; 2020 ];https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports
- 5.A comprehensive literature review on the clinical presentation, and management of the pandemic coronavirus disease 2019 (COVID-19) Kakodkar P, Kaka N, Baig MN. Cureus. 2020;12:0. doi: 10.7759/cureus.7560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Severe Covid-19. Berlin DA, Gulick RM, Martinez FJ. N Engl J Med. 2020;383:2451–2460. doi: 10.1056/NEJMcp2009575. [DOI] [PubMed] [Google Scholar]
- 7.Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Huang C, Wang Y, Li X, et al. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Distinct phenotypes require distinct respiratory management strategies in severe COVID-19. Robba C, Battaglini D, Ball L, et al. Respir Physiol Neurobiol. 2020;279:103455. doi: 10.1016/j.resp.2020.103455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Covid-19 - Situazione in Italia. [ Oct; 2020 ];Ministero della Salute. http://www.salute.gov.it/portale/nuovocoronavirus/dettaglioContenutiNuovoCoronavirus.jsp?area=nuovoCoronavirus&id=5351&lingua=italiano&menu=vuoto 2019
- 10.Hantzidiamantis PJ, Amaro E. Treasure Island, FL: StatPearls Publishing; [ Mar; 2020 ]. 2021. Physiology, alveolar to arterial oxygen gradient. [PubMed] [Google Scholar]
- 11.Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. Wu Z, McGoogan JM. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 12.Characteristics of COVID-19 patients dying in Italy. [ Nov; 2020 ];Istituto Superiore di Sanità. https://www.epicentro.iss.it/en/coronavirus/sars-cov-2-analysis-of-deaths 2020
- 13.Development and validation of a prediction model for severe respiratory failure in hospitalized patients with SARS-CoV-2 infection: a multicentre cohort study (PREDI-CO study) Bartoletti M, Giannella M, Scudeller L, et al. Clin Microbiol Infect. 2020;26:1545–1553. doi: 10.1016/j.cmi.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Role of ROX index in the first assessment of COVID-19 patients in the emergency department. Gianstefani A, Farina G, Salvatore V, et al. Intern Emerg Med. 2021 doi: 10.1007/s11739-021-02675-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Basing respiratory management of COVID-19 on physiological principles. Tobin MJ. Am J Respir Crit Care Med. 2020;201:1319–1320. doi: 10.1164/rccm.202004-1076ED. [DOI] [PMC free article] [PubMed] [Google Scholar]





