Abstract
Purpose:
Venetoclax-based therapy is a standard-of-care option in first-line and relapsed/refractory chronic lymphocytic leukemia (CLL). Patient management following venetoclax discontinuation remains nonstandard and poorly understood.
Experimental Design:
To address this, we conducted a large international study to identify a cohort of 326 patients who discontinued venetoclax and have been subsequently treated. Coprimary endpoints were overall response rate (ORR) and progression-free survival for the post-venetoclax treatments stratified by treatment type [Bruton’s tyrosine kinase inhibitor (BTKi), PI3K inhibitor (PI3Ki), and cellular therapies].
Results:
We identified patients with CLL who discontinued venetoclax in the first-line (4%) and relapsed/refractory settings (96%). Patients received a median of three therapies prior to venetoclax; 40% were BTKi naïve (n = 130), and 81% were idelalisib naïve (n = 263). ORR to BTKi was 84% (n = 44) in BTKi-naïve patients versus 54% (n = 30) in BTKi-exposed patients. We demonstrate therapy selection following venetoclax requires prior novel agent exposure consideration and discontinuation reasons.
Conclusions:
For BTKi-naïve patients, selection of covalently binding BTKis results in high ORR and durable remissions. For BTKi-exposed patients, covalent BTK inhibition is not effective in the setting of BTKi resistance. PI3Kis following venetoclax do not appear to result in durable remissions. We conclude that BTKi in naïve or previously responsive patients and cellular therapies following venetoclax may be the most effective strategies.
Introduction
Treatment paradigms for patients with chronic lymphocytic leukemia (CLL) have rapidly evolved over recent years. Novel kinase inhibitors targeting Bruton’s tyrosine kinase (BTKi) and PI3K (PI3Ki) that disrupt signaling downstream of the B-cell receptor (BCR) are now standard therapeutic options. Furthermore, the potent, selective, and orally bioavailable small-molecule BCL2 inhibitor (BCL2i), venetoclax, is licensed as both monotherapy and in combination with anti-CD20 mAbs (rituximab in relapsed/refractory CLL and obinutuzumab in first-line CLL) and now represents a key component of treatment pathways.
Pivotal randomized clinical trials established ibrutinib monotherapy and idelalisib-rituximab (1, 2) as standard treatment options in relapsed/refractory CLL prior to the pivotal venetoclax trial publications.(3, 4) As a result, outcomes with venetoclax following discontinuation of BCR inhibitors (BCRi) have been subsequently established both from prospective clinical trials (5, 6) and large retrospective cohort series (7, 8).
The success of the MURANO trial (9), the recent German CLL14 trial (10), and other pivotal venetoclax monotherapy phase I-II trials (3, 4, 6) encouraged prescribing physicians to utilize venetoclax (with or without anti-CD20 mAb), because of its favorable efficacy and safety profile (11, 12) earlier in treatment pathways. As a result, this change has identified a number of key issues regarding sequencing of therapy following venetoclax (13), in particular is the question of the activity of BTKi, PI3Ki, and cellular therapies in the setting of CLL progression following venetoclax discontinuation.
Four small series have reported on the response rate to ibrutinib following venetoclax in 6, 11, 8, and 27 BTKi-naïve patients, respectively (14–17). These very limited data suggest that a covalent BTKi may remain effective in venetoclax-exposed patients. Moreover, recent studies of venetoclax resistance mechanisms (18, 19) do not suggest overlapping BCL2i and BTKi resistance pathways. However, no prospective study has addressed the key question of BTKi efficacy post-venetoclax in BTKi-naïve patients, and large, robust case series are lacking. Similarly, no data exist regarding the efficacy and toxicity prolife of PI3Ki in venetoclax-exposed, PI3Ki-naïve patients with relapsed CLL.
Finally, patients who have discontinued both BCRi and BCL2i have poor outcomes, however these outcomes have typically been collected from series of heavily pretreated, poor risk, older patients (7, 8,20). At present, an understanding of the optimal therapeutic approaches in the dual pathway exposed setting remains limited. Cellular therapies including chimeric antigen receptor T cell (CAR-T; ref. 21) and allogeneic stem cell transplantation (alloSCT; ref. 22) have demonstrated efficacy in heavily pretreated CLL patients; however, data establishing the therapeutic value of these approaches in venetoclax-exposed patients is lacking.
To address these key unanswered questions, we conducted a large, international cohort study to establish the therapeutic efficacy of several treatment approaches for patients with relapsed/refractory CLL who discontinued venetoclax-based therapy for any reason and had received a subsequent therapy. To our knowledge, this is the largest series of patients with CLL, who have received and discontinued venetoclax-based therapy, for whom detailed outcome on post-venetoclax therapy is described.
Materials and Methods
We conducted an international, multicenter, retrospective cohort study to describe the characteristics and outcomes of patients with CLL who discontinued venetoclax-based therapy between 2014 and 2019. Thirty-one academic and community sites in the United States, European Union/United Kingdom (EU/UK), and South America participated in this study. The study was institutional review board approved by each participating institution in accordance with the Declaration of Helsinki and was conducted in partnership between U.S. centers, EU/UK centers, and CLL Collaborative Study of Real-World Evidence.
To define the study cohort, medical chart review was performed to identify all patients with CLL at each institution who discontinued venetoclax-based therapies. Utilizing a standardized case report form, investigators collected data on patient’s pre-venetoclax demographics, disease characteristics, clinical and genetic characteristics, prior therapies, venetoclax dosing, response and survival outcomes of venetoclax, reasons for venetoclax discontinuation, and subsequent therapies. We collected data on whether venetoclax was administered in the context of a prospective clinical trial or in routine clinical practice. For patients who received venetoclax in combination with other therapies, we collected data on which agent(s) it was paired with, whether the combination was given on a fixed duration schedule, and number of cycles of therapy administered. Reasons for venetoclax discontinuation were characterized as adverse event (AE), progression of CLL, Richter transformation, alloSCT, CAR-T therapy, cost concerns, death not secondary to progression or toxicity, physician or patient preference, secondary malignancy, sudden death on therapy, or other reason not previously described or unknown. Patients who discontinued venetoclax due to Richter transformation were excluded from analyses related to the primary endpoint. Detailed information regarding available pre-venetoclax clinical, molecular, and genetic prognostic data were collected including Rai stage, del(17p), del(11q), karyotype complexity (defined in this study as >3 cytogenetic aberrations), IGHV mutation status, TP53 mutation, NOTCH1 mutation, BTK mutation, and PLCgamma2 mutation. For post-venetoclax therapies, we collected data on 19 unique regimens/therapies including overall response rate (ORR), complete remission (CR) rate, discontinuation rate, reason for discontinuation, and progression-free survival (PFS) and overall survival (OS). This report and all analyses focus on patients treated with a BTKi, PI3Ki, or cellular therapy. For patients who discontinued a post-venetoclax therapy due to an AE, we collected data on specific AEs leading to discontinuation. Toxicity assessment was defined according to the Common Terminology Criteria for AEs (version 4.0) where applicable.
Coprimary endpoints for this study were ORR and PFS for post-venetoclax treatments. We focused on outcomes of patients who were treated with a BTKi, PI3Ki, or cellular therapy (CAR-T or alloSCT) following venetoclax. Patients were further stratified on the basis of whether they were also treated with a BTKi or PI3Ki prior to venetoclax (BTKi/PI3Ki-exposed or BTKi/PI3Ki-naïve) and the reason for discontinuation of first kinase inhibitor (AE or progression of disease). Patients receiving a BTKi, PI3Ki, or cellular therapy as any line of therapy following venetoclax were included in assessment of study primary endpoints. Data regarding disease status at the time of alloSCT, conditioning regimen, donor source, degree of donor mismatch, and AEs (cytomegalovirus reactivation, graft-versus-host disease, and infections) were outside the scope of this study and were intentionally not collected. Of note, all patients who had received venetoclax in combination with a second novel agent (i.e., BTKi) were excluded from survival and sequencing analyses.
Investigators were requested to classify responses as defined by modified iwCLL criteria as CR, partial remission [PR; including PR with lymphocytosis (PR-L)], stable disease (SD), and progressive disease (PD; ref. 23). Because of study design, response assessments and follow-up intervals were not standardized, but time to best response was collected. PFS was defined as the time from the start of post-venetoclax therapy to last follow-up, progression of CLL, or death. OS was defined as the time from the start of post-venetoclax therapy to death from any cause. PFS and OS were both estimated by the Kaplan-Meier method (24). Patients with no progression or death were censored at last follow-up. Timing, frequency, causality, and duration of AEs for post-venetoclax therapies were not collected. All other comparisons were descriptive. Statistical analyses were performed using STATA 10.1 (Stata Statistical Software, Release 10, 2007; StataCorp LP). The database was locked on 8/1/2019 for analysis, last follow-up was defined as date of the most recent medical visit or death.
Results
Patient characteristics
We identified 326 patients with CLL who were treated with venetoclax and subsequently discontinued therapy; 4% (n = 13) as first-line and 96% (n = 313) for relapsed/refractory CLL. Of these, 67% received venetoclax as part of standard clinical practice (vs. clinical trial) and 73% received venetoclax as a monotherapy administered continuously (vs. combination or fixed duration schedule). Of those treated with a combination (n = 90), 78% were paired with anti-CD20 antibody (rituximab n = 41, obinutuzumab n = 13, and ofatumumab n = 8) and 16 patients were paired with BTKi with or without an anti-CD20 antibody. The median number of cycles of anti-CD20 mAb administered in combination with venetoclax was six (range 1–12). Baseline characteristics for the entire study cohort are included in Table 1. Baseline characteristics demonstrate this patient population to be a heavily pretreated (median three prior therapies) with several poor risk prognostic features including 47% del(17p) positive, 45% TP53 mutated, 39% complex karyotype, and 18% NOTCH1 mutated. The three most common reasons for discontinuing the regimen immediately preceding venetoclax were progression of CLL (59%), treatment-related AE (26%), and patient preference (6%).
Table 1.
Baseline characteristics.
| Characteristic | Result (range) | Number with available data |
|---|---|---|
| Median age at CLL diagnosis | 58 years (32–88) | 326 |
| Median age at venetoclax start | 66 years (38–91) | 324 |
| Median number therapies prior to venetoclax | 3 (0–11) | 326 |
| Male | 69% | 326 |
| White | 87% | 325 |
| Rai stage ≥ 3 | 64% | 318 |
| Del(17p) positive | 47% | 312 |
| TP53 mutation present | 45% | 221 |
| TP53 disruption (del17p or TP53 mutation) | 56% | 312 |
| Del(11q) positive | 27% | 311 |
| Complex karyotype present | 39% | 279 |
| NOTCH1 mutation present | 18% | 103 |
| IGHV unmutated | 82% | 171 |
| Ibrutinib prior to venetoclax | 60% | 324 |
| Any BTKi prior to venetoclax | 61% | 324 |
| Idelalisib prior to venetoclax | 19% | 324 |
Venetoclax response, dosing, and reasons for discontinuation
Following venetoclax dose escalation, 73% of patients achieved a stable venetoclax dose of 400 mg once daily (vs. 27% <400 mg daily). The ORR to venetoclax was 69% (28% CR). The median time to best response to venetoclax was 3 months (range 1–31 months). The median time to venetoclax discontinuation was 9 months (range 0.1–60 months). The most common reasons for discontinuation of venetoclax included progression of CLL (37%), AE (20%), Richter transformation (13%), physician or patient preference (8%), planned cellular therapy (7%), unrelated death (5%), secondary malignancy (3%), sudden death (3%), and other (4%). The median time to venetoclax discontinuation due to AE was 5.5 months, due to Richter transformation was 9 months, and due to CLL progression was 12 months. No patient discontinued venetoclax due to financial concerns (0%). Of the 326 patients who have discontinued venetoclax, 188 (58%) were treated with a subsequent line of therapy, 56 (17%) are alive untreated and 82 (25%) died prior to a subsequent line of therapy. Causes of death and why patients were not treated were not collected. With a median follow-up of 17 months (range 1–84 months), the median OS for the cohort from start of venetoclax to last follow-up or death was estimated to 28 months (Supplementary Fig. S1).
Sequencing of therapies following venetoclax
For patients who were treated with BTKi, PI3Ki, CAR-T, and anti-CD20 antibody monotherapy following venetoclax discontinuation, response data, median PFS, and reasons for discontinuation for post-venetoclax therapy are summarized in Table 2. The most common class of therapy administered in the post-venetoclax setting was BTKi. We identified 74 patients treated with a BTKi, of which 44 were BTKi naïve and 30 were previously BTKi exposed (33% BTKi intolerant and 66% BTKi refractory of whom 6 had confirmed BTK or PLCgamma2 mutations in a limited number of patients analyzed). BTKi-naïve patients received ibrutinib (n = 43) or acalabrutinib monotherapy (n = 1). BTKi-exposed patients received ibrutinib (n = 4), acalabrutinib (n = 20), or a noncovalently binding BTKi monotherapy within a clinical trial (n = 6). No patient received BTKi in combination with either an anti-CD20 mAb or venetoclax. The ORR to BTKi in BTKi-naïve patients was 84% (CR 9%, n = 44). This was significantly higher than the ORR to BTKi in patients who were previously BTKi-exposed pre-venetoclax (ORR = 54%, n = 30; P < 0.001). In the BTKi-exposed patients, the ORR to BTKi was 50% in prior BTKi-resistant patients and 70% in prior BTKi-intolerant patients.
Table 2.
Response to subsequent therapy in patients with CLL previously treated with venetoclax.
| Subsequent therapy | BTKi | BTKi | PI3Ki | CAR-T | Anti-CD20 absent |
|---|---|---|---|---|---|
| Ibrutinib | Ibrutinib Acalabrutinib |
Idelalisib | Rituximab Obinutuzumab |
||
| Agents | Acalabrutinib | Noncovalent BTKi BTKi exposed |
Duvelisib | Anti-CD19 | Ofatumumab |
| Pre-ven exposure | BTKi-naïve | 33% BTKi intolerant 66% BTKi resistant |
PI3Ki naïve BTKi exposed |
BTKi exposed |
|
| Patient number | 44 | 30 | 17 | 18 | 19 |
| Lines of therapy pre-ven, median (range) | 2(0–8) | 4 (1–11) | 4 (1–6) | 4 (1–10) | 3 (1–9) |
| ORR | 83.9% | 53.4% | 46.9% | 66.6% | 32% |
| CR | 9.0% | 10.0% | 5.9% | 33.3% | 16% |
| PR | 56.8% | 26.7% | 35.2% | 33.3% | 16% |
| PR-L | 18.1% | 16.7% | 5.8% | 0% | 0% |
| SD | 11.6% | 23.3% | 23.7% | 5.7% | 32% |
| PD | 4.5% | 23.3% | 29.4% | 27.7% | 37% |
| Median PFS (months) | 32 | 12 | 5 | 9 | 2 |
| Median follow-up (months) | 10.5 | 3.5 | 5.0 | 2.0 | 2.0 |
| DC rate | 38% | 38% | 78% | NA | 72% |
| Reasons for DC (% discontinuations) | |||||
| CLL progression | 21.4% | 66.6% | 58.3% | — | 62% |
| Adverse event | 14.3% | 8.3% | 25% | — | 15.4% |
| Transformation | 14.3% | — | 16.7 | — | 7.6% |
| Planned cellular Tx | 14.2% | — | — | — | — |
| Unrelated death | 7.1% | 8.3% | — | — | — |
| Sudden death on Tx | 7.1% | 16.6% | — | — | — |
| Patient preference | 7.1% | — | — | — | — |
| Other | 14.2% | — | — | — | 15.3% |
Abbreviations: abs, antibody; DC, discontinuation; Tx, therapy; Ven, venetoclax.
With a median follow-up of 7.7 months (1–48 months) for patients treated with BTKi post-venetoclax, the estimated median PFS to post-venetoclax BTKi was 32 months in BTKi-naïve patients, not reached in BTKi-intolerant patients but was only 4 months in patients who were known to be BTKi resistant (Fig. 1A and B, BTKi naïve vs. BTKi resistant). Of note, median follow-up for the group of BTKi-naïve patients treated with BTKi post-venetoclax was 10.5 months (1–48 months). In the subset of patients who discontinued venetoclax due to progression, the estimated median PFS to post-venetoclax BTKi was not reached (n = 29) in BTKi-naïve patients (Supplementary Fig. S2). While the overall discontinuation rates were comparable, the reasons for discontinuation of a BTKi varied depending on whether patients were exposed to a BTKi pre-venetoclax (Table 2) with a higher proportion of BTKi-exposed patients discontinuing due to CLL progression (66.6% discontinuations vs. 21.4% discontinuations; P = 0.0001). While testing for ibrutinib resistance in BTKi-exposed patients was limited to 61 patients, BTKCys481 mutations were documented in 18 patients and PLCgamma2 in 4 patients. Because of small numbers, outcomes were not analyzed separately in patients with ibrutinib-associated resistance mutations.
Figure 1.
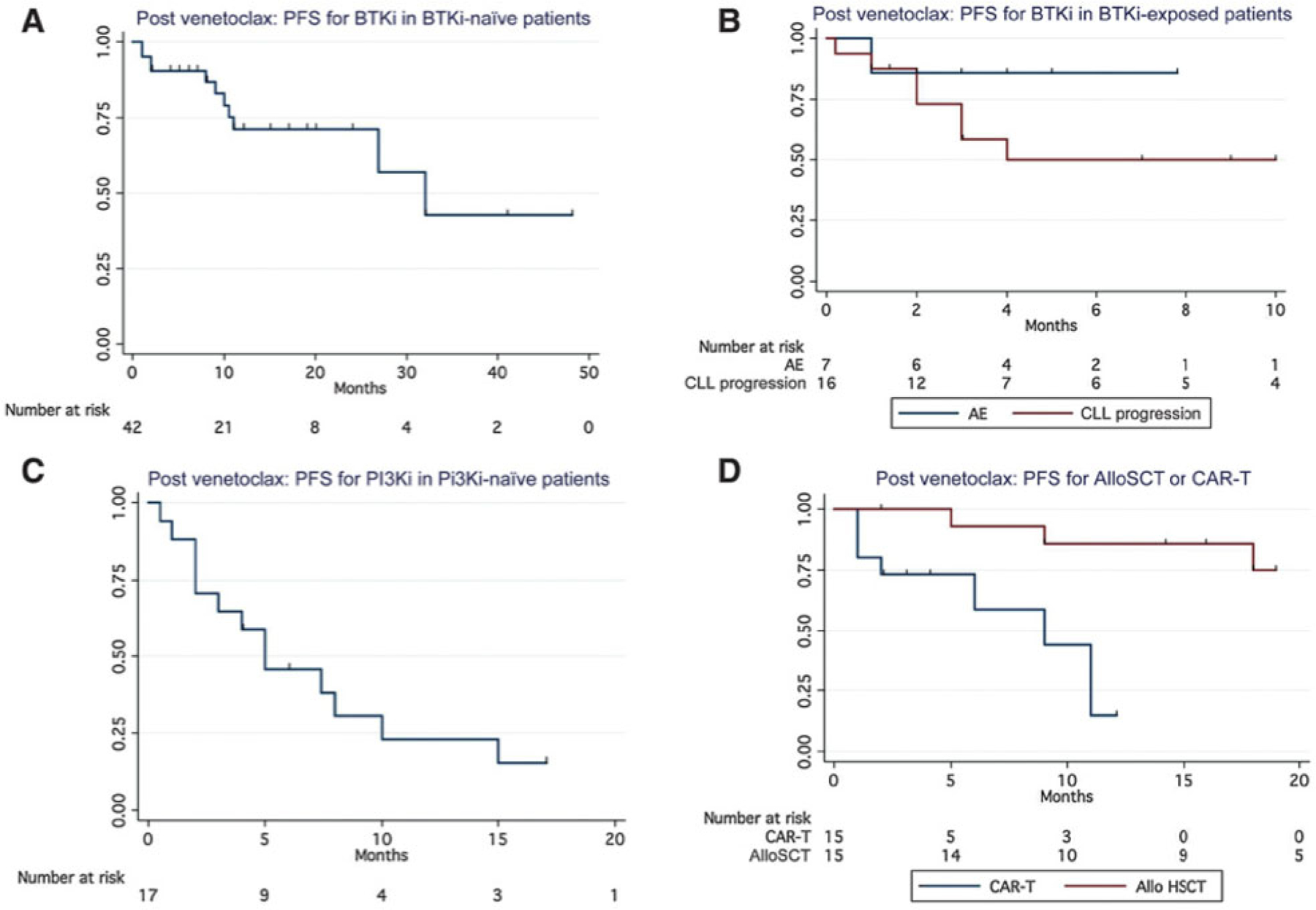
A, PFS in for BTKi in BTK-naïve patients. B, PFS for BTKi in BTK-exposed patients. C, PFS for PI3Ki in PI3Ki-naïve patients. D, PFS for alloSCT and CAR-T following venetoclax in BTKi-exposed patients.
In this series, patients receiving a PI3Ki were all PI3Ki-naïve and BTKi-exposed prior to venetoclax (n = 17). While the ORR to PI3Ki was 46.9%, responses were not durable with a median PFS of only 5 months (Fig. 1C) and an overall discontinuation rate of 78% (most commonly due to progression of CLL). The ORR to CD19-directed CAR-T therapy (various products on clinical studies) was 66% in 18 patients all of whom were both BTKi- and venetoclax-exposed; the median PFS was 9 months (Fig. 1D). The median PFS was not reached for 19 patients who underwent alloSCT post venetoclax (Fig. 1D). The use of anti-CD20 mAb monotherapy did not result in durable remissions following venetoclax with an ORR of 32% and a median PFS of only 2 months.
Discussion
In this largest experience to date of therapies following venetoclax discontinuation, we demonstrated that the therapeutic selection following venetoclax requires careful consideration of prior novel agent exposure and reasons for discontinuation. By virtue of the period in which the population studied received venetoclax and the study design in which patients were only included if they had discontinued venetoclax, this is a predominantly relapsed/refractory, heavily pretreated, and genetically poor risk CLL patient population with a short median time on venetoclax of only 9 months.
Until now, patient series of BTKi outcomes following venetoclax exposure were very limited in terms of patient number and follow-up. Anderson and colleagues (15) described 6 patients receiving ibrutinib as their first therapy following progression of CLL on venetoclax. Five partial responses were noted, and two patient deaths were considered ibrutinib related. Brown and colleagues described 10 partial responses in 11 patients and have subsequently updated this series to include 27 patients with an ORR of 56% (16). Recently, Greil and colleagues described eight partial responses to ibrutinib in all 8 patients discontinuing venetoclax following treatment, typically at first relapse, on the MURANO trial (17). These series did not provide information regarding durability of responses and were limited to the study of ibrutinib following venetoclax.
To the best of the authors’ knowledge, we provide the largest series outlining outcomes for patients with CLL receiving BTKi therapy after venetoclax. For BTKi-naïve patients, selection of a covalently binding BTKi results in high response rates (ORR 84%) and durable remissions (median PFS 32 months). PFS in this patient population did not appear to be negatively impacted by the reason for discontinuation of venetoclax (resistance vs. intolerance), suggesting nonoverlapping mechanisms of resistance. Although indirect comparisons across patient populations are challenging, the median PFS for BTKi following venetoclax from this dataset (32 months) appears comparable with the median PFS of venetoclax monotherapy following ibrutinib (24.7 months; ref. 6). It is likely that the second novel agent chosen in a sequence, whether it is venetoclax or a BTKi, will result in a shorter PFS than the first agent regardless of the order. In addition, while it may be tempting to compare these PFS data with PFS data for from recent relapsed/refractory clinical trials such as RESONATE or MURANO, it is important to note that the patient populations enrolled in these landmark studies have essentially not been exposed to novel agents and are not comparable (9, 25).
In the MURANO trial (9), patients in the experimental arm were treated with fixed duration venetoclax-rituximab. Most of these patients had received one prior immunochemotherapy and virtually no patients enrolled had received prior BCR inhibitors. Fixed duration, nontoxic, and highly effective therapy such as venetoclax-rituximab is an attractive option. Now our data enhance the credibility of this approach as the first novel agent in the relapsed/refractory setting by providing more robust and definitive evidence of efficacy and durable responses of BTK inhibition following BCL2 inhibition. Mechanisms of resistance to venetoclax are becoming increasingly well understood (17, 18), and no mechanism to date~has described enhanced interference with BCR signaling as a contributing event. Our data, coupled with this evolving literature, are grounds for reassurance, particularly in a field where sequencing randomized trials [venetoclax +/− anti-CD20 antibodies → ibrutinib vs. ibrutinib → venetoclax (+/− anti-CD20 antibodies)] are highly unlikely to be performed.
We also examined the influence of the reason for prior BTKi discontinuation on retreatment with a BTKi post-venetoclax discontinuation. We found that the reason for prior BTKi discontinuation strongly influences subsequent responses. For previously BTKi-exposed patients, BTK inhibition with a covalently binding BTKi is, unsurprisingly, ineffective in the setting of BTKi failure but can be considered if BTKi was discontinued for intolerance. This strategy is particularly relevant as access to next-generation covalently binding BTKis such as acalabrutinib are now approved in the first-line and relapsed/refractory settings (26, 27). While the discontinuation rates observed for BTK-exposed and BTK-naïve populations were equivalent, importantly more discontinuations in the BTK-exposed group were due to CLL progression. Observed similarity in discontinuation rates are likely attributable to small number of patients studied and the limited follow-up. Furthermore, we highlight that a subset of the BTKi-exposed patients received noncovalent BTKis on a clinical trial, which may account for the relatively low discontinuation rate observed. Sequencing recommendations may need to be further modified as more data become available from the early clinical studies of noncovalently binding BTKi in BTKi-resistant patients (28–30).
We also examined outcomes in a small subset of patients treated with PI3Ki following venetoclax. Durable remissions were not seen in this small subpopulation, all of whom were PI3K-naïve and both BTKi- and venetoclax-exposed. This finding suggests that there may be possible overlap in resistance mechanisms to either agent, although this needs further exploration in larger numbers of patients with pharmacodynamic studies before conclusions can be definitive. It is important to note that currently approved and commercially available PI3Kis were studied in essentially BTKi-naïve and venetoclax-naïve patient populations and reported results may not translate to a patient population previously treated with novel agents. While the PI3Ki duvelisib is approved in patients who have failed at least two prior therapies, no patient treated on that study had been previously exposed to a BTKi or venetoclax (31).
We also examined the role of CAR-T and alloSCT following venetoclax discontinuation. Small patient numbers in a heterogeneous population with various stem cells products and techniques preclude definitive conclusions, however, both approaches seem to demonstrate therapeutic efficacy and can be considered in appropriate high-risk patients for whom limited other options exist. These findings also support current guidance from The European Society for Blood and Marrow Transplantation regarding the utility and timing of alloSCT following the introduction of novel agents (particularly in fit patients with an available donor who are being treated with their second novel agent; ref. 32). Outcomes in the post-venetoclax alloSCT setting are being explored in considerably more detail in a separate case series (33).
Our study has several limitations. It is retrospective and hypothesis generating only. However, in the absence of a planned clinical trial to address sequencing questions, our data may help to guide practice and may also aid in the design of future prospective clinical trials. We recognize that clinical and prognostic data were not uniformly collected, and responses are not confirmed centrally. Information regarding treatment schedule, dose, interruptions, adherence, and AEs to post-venetoclax therapies were not collected and follow-up is limited and may be different among patients, centers, and treatment regimens. To try to address these limitations, we partnered with investigators who are both well versed in the use of venetoclax and other novel agents, routinely perform response assessments on prospective clinical studies, and who also have considerable experience in conducting real world evidence studies in CLL. In addition, the large number of centers who participated makes it unlikely that any one anomaly in practice variation or data collection would significantly impact results. The protocol and case report form requested investigators to follow iwCLL criteria to define response and progression of disease and was standardized with drop down menus to allow for consistency of data collection. Given limited follow-up and relatively small numbers in each cohort, PFS estimates must be interpreted with caution. These are hypothesis generating only, however, they do suggest potential differences in durability of response to agents based on prior treatment history and reasons for discontinuation. In addition, not all patients for whom we have response data have available data for follow-up in survival analyses, which may affect PFS data. In addition, we highlight that these data do not provide a definitive comparison of outcomes between alloSCT andCAR-T products in this setting and should not be used to guide the selection of cellular therapy modality over another. We recognize that we do not have data regarding the remission status at time of cellular therapy and additional important prognostic data, which may impact outcomes. Finally, the patient population included here is considerably older, more heavily pretreated, and with a higher proportion of poor risk genetic features as compared with patients currently treated in clinical practice and those treated on recent venetoclax studies such as MURANO and CLL14 (9, 10).
Our cohort represents the first wave of patients treated at our centers who are discontinuing venetoclax and, like patients who discontinued ibrutinib in early series, are likely to be heavily pretreated and high risk (34–36). Despite this, we believe these data are still informative in guiding the development of sequencing algorithms.
In conclusion we provide the first series describing efficacy and survival of a large population of patients who had discontinued venetoclax-based therapy. Critically, the well-known efficacy of BTKi monotherapy does not seem to be considerably compromised by prior venetoclax treatment, providing reassurance and flexibility when sequencing therapeutic novel agent options. BTKi in patients who are BTKi-naïve or responding at time of BTKi discontinuation and alloSCT following venetoclax appear to be the most effective strategies with durable responses. Overall, our data provide further support for the use of venetoclax-based therapy earlier in the treatment course of CLL.
Supplementary Material
Translational Relevance.
BTK (BTKi) and BCL2 inhibitors (BCL2i) are now commonly utilized, highly effective agents for treatment of patients with chronic lymphocytic leukemia in the first-line (CLL14) and relapsed/refractory settings. While efficacy data for the use of venetoclax post-BTKi has been well described, data for the opposite sequence is limited. We present the largest series of patients receiving BTKi post-venetoclax and demonstrate efficacy and durability of response. Outcomes in patients with prior BTKi exposure were dependent on the discontinuation reason for BTKi pre-venetoclax. These data support the utility of venetoclax-based therapy early in treatment pathways before BTKi. From a translational perspective, these observations suggest minimal overlap between BCL2i (BCL2 Gly101Val mutations) and BTKi (Cys481/PLCgamma2 mutations) mechanisms of resistance. While less is known about PI3K inhibitor (PI3Ki) resistance, PI3Kis were not effective in double novel agent-exposed patients, suggesting potential Pi3Ki resistance in BTKi- and/or venetoclax-resistant cells.
Acknowledgments
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnotes
Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Prior presentation: Presented at The 2019 American Society of Hematology Annual Meeting, oral presentation.
Disclosure of Potential Conflicts of Interest
A.R. Mato is an employee/paid consultant for TG Therapeutics, Celgene, Genentech, AbbVie, Johnson & Johnson, Pharmacyclics, Verastem, Sunesis, Loxo, Pfizer, and AstraZeneca, and is an advisory board member/unpaid consultant for NCCN, AbbVie, and Verastem. L.E. Roeker is an employee/paid consultant for Curio Science, holds ownership interest (including patents) in AbbVie and Abbott Laboratories, and is an advisory board member/unpaid consultant for Verastem and AbbVie. R. Jacobs is an employee/paid consultant for AbbVie, AstraZeneca, Pharmacyclics, and Verastem, reports receiving commercial research grants from Pharmacyclics and TG Therapeutics, and speakers bureau honoraria from Pharmacyclics, Janssen, Genentech, AstraZeneca, AbbVie, and Sanofi. B.T. Hill is an employee/paid consultant for Pharmacyclics, Genentech, AbbVie, and AstraZeneca, and reports receiving commercial research grants from Genentech, Pharmacyclics, and AbbVie. N. Lamanna is an advisory board member/unpaid consultant for AbbVie, AstraZeneca, BeiGene, Genentech, Janssen, Pharmacyclics, Celgene, and Gilead. D. Brander is an employee/paid consultant for AbbVie, ArQule, AstraZeneca, Genentech, Pfizer, Pharmacyclics, TG Therapeutics, Verastem, and Novartis, reports receiving commercial research grants from AbbVie, ArQule, Ascentage, BeiGene, DTRM, Genentech, Juno, MEI, Pharmacyclics, TG Therapeutics, and Tolero, and is an advisory board member/unpaid consultant for NCCN. M. Shadman is an employee/paid consultant for AbbVie, Genentech, AstraZeneca, Sound Biologics, Pharmacyclics, Verastem, ADC therapeutics, Cellectar, Bristol-Myers Squibb, and Atara Biotherapeutics. C.S. Ujjani is an employee/paid consultant for AbbVie. M.S. Yazdy is an employee/paid consultant for Octapharma, Janssen, and AbbVie, reports receiving commercial research grants from Genentech, and speakers bureau honoraria from Bayer. G.F. Perini reports receiving speakers bureau honoraria from and is an advisory board member/unpaid consultant for Janssen and AbbVie. J.A. Pinilla-Ibarz reports receiving speakers bureau honoraria from AbbVie and Janssen. J. Barrientos is an employee/paid consultant for Genentech, AstraZeneca, Gilead, and Celgene. A.P. Skarbnik is an employee/paid consultant for AbbVie, Pharmacyclics, Janssen, and AstraZeneca, reports receiving commercial research grants from AstraZeneca, Pharmacyclics, and Bristol-Myers Squibb, speakers bureau honoraria from AbbVie, Pharmacyclics, Janssen, Genentech, Jazz Pharmaceuticals, Gilead Sciences, Celgene, Kite Pharma, BeiGene, Verastem, and Seattle Genetics, and holds ownership interest (including patents) in COTA Healthcare. J.J. Pu reports receiving speakers bureau honoraria from Roche. S. Gohil reports receiving other remuneration from AbbVie. M. Choi reports receiving speakers bureau honoraria from AbbVie, PCYC, and Genentech. C.C. Coombs is an employee/paid consultant for Covance (via AbbVie) and AbbVie, and is an advisory board member/unpaid consultant for Octapharma, Loxo, MEI Pharma, and AstraZeneca. J. Rhodes is an employee/paid consultant for AstraZeneca and Verastem. No potential conflicts of interest were disclosed by the other authors.
References
- 1.Furman RR, Sharman JP, Coutre SE, Cheson BD, Pagel JM, Hillmen P, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med 2014;370:997–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones JA, Wach M, Robak T, Brown JR, Menter AR, Vandenberghe E, et al. Results of a phase III randomized, controlled study evaluating the efficacy and safety of idelalisib (IDELA) in combination with ofatumumab (OFA) for previously treated chronic lymphocytic leukemia (CLL). J Clin Oncol 33:15s, 2015. (suppl; abstr 7023). [Google Scholar]
- 3.Stilgenbauer S, Eichhorst B, Schetelig J, Coutre S, Seymour JF, Munir T, et al. Venetoclax in relapsed or refractory chronic lymphocytic leukaemia with 17p deletion: a multicentre, open-label, phase 2 study. Lancet Oncol 2016;17:768–78. [DOI] [PubMed] [Google Scholar]
- 4.Roberts AW, Davids MS, Pagel JM, Kahl BS, Puvvada SD, Gerecitano JF, et al. Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. N Engl J Med 2016;374:311–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coutre S, Choi M, Furman RR, Eradat H, Heffner L, Jones JA, et al. Venetoclax for patients with chronic lymphocytic leukemia who progressed during or after idelalisib therapy. Blood 2018;131:1704–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones JA, Mato AR, Wierda WG, Davids MS, Choi M, Cheson BD, et al. Venetoclax for chronic lymphocytic leukaemia progressing after ibrutinib: an interim analysis of a multicentre, open-label, phase 2 trial. Lancet Oncol 2018;19: 65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mato AR, Thompson M, Allan JN, Brander DM, Pagel JM, Ujjani CS, et al. Real-world outcomes and management strategies for venetoclax-treated chronic lymphocytic leukemia patients in the United States. Haematologica 2018;103: 1511–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eyre TA, Kirkwood AA, Gohill S, Follows G, Walewska R, Walter H, et al. Efficacy of venetoclax monotherapy in patients with relapsed chronic lymphocytic leukaemia in the post-BCR inhibitor setting: a UK wide analysis. Br J Haematol 2019;185:656–69. [DOI] [PubMed] [Google Scholar]
- 9.Seymour JF, Kipps TJ, Eichhorst B, Hillmen P, D’Rozario J, Assouline S, et al. Venetoclax-rituximab in relapsed or refractory chronic lymphocytic leukemia. N Engl J Med 2018;378:1107–20. [DOI] [PubMed] [Google Scholar]
- 10.Fischer K, Al-Sawaf O, Bahlo J, Fink AM, Tandon M, Dixon M, et al. Venetoclax and obinutuzumab in patients with CLL and coexisting conditions. N Engl J Med 2019;380:2225–36. [DOI] [PubMed] [Google Scholar]
- 11.Davids MS, Hallek M, Wierda W, Roberts AW, Stilgenbauer S, Jones JA, et al. Comprehensive safety analysis of venetoclax monotherapy for patients with relapsed/refractory chronic lymphocytic leukemia. Clin Cancer Res 2018;24: 4371–9. [DOI] [PubMed] [Google Scholar]
- 12.Roeker LE, Fox CP, Eyre TA, Brander DM, Allan JN, Schuster SJ, et al. Tumor lysis, adverse events, and dose adjustments in 297 venetoclax-treated CLL patients in routine clinical practice. Clin Cancer Res 2019;25:4264–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sarraf Yazdy M, Mato AR, Cheson BD. Combinations or sequences of targeted agents in CLL: is the whole greater than the sum of its parts (Aristotle, 360 BC)? Blood 2019;133:121–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown JR, Davids MS, Chang JE, Ma S, Biondo JML, Mun Y, et al. Outcomes of ibrutinib (Ibr) therapy in Ibr-naïve patients (pts) with chronic lymphocytic leukemia (CLL) progressing after venetoclax (Ven). Blood 2019;134:4320. [Google Scholar]
- 15.Anderson MA, Tam C, Lew TE, Juneja S, Juneja M, Westerman D, et al. Clinicopathological features and outcomes of progression of CLL on the BCL2 inhibitor venetoclax. Blood 2017;129:3362–70. [DOI] [PubMed] [Google Scholar]
- 16.Brown JR, Davids MS, Chang J, Ma S, Biondo J, Mobasher M, et al. Outcomes of ibrutinib therapy given after prior venetoclax therapy in ibrutinib-naïve patients with relapsed/refractory (R/R) chronic lymphocytic leukemia (CLL). Blood 2018;132:5556. [Google Scholar]
- 17.Greil R, Fraser G, Leber B, Marks R, Quaresmini G, Middeke JM, et al. PS1161 efficacy and safety of ibrutinib in relapsed/refractory chronic lymphocytic leukemia patients previously treated with venetoclax in the Murano study. HemaSphere 2019;3:527. [Google Scholar]
- 18.Blombery P, Anderson MA, Gong JN, Thijssen R, Birkinshaw RW, Thompson ER, et al. Acquisition of the recurrent Gly101Val mutation in BCL2 confers resistance to venetoclax in patients with progressive chronic lymphocytic leukemia. Cancer Discov 2019;9:342–53. [DOI] [PubMed] [Google Scholar]
- 19.Guieze R, Liu VM, Rosebrock D, Jourdain AA, Hernandez-Sanchez M, Martinez Zurita A, et al. Mitochondrial reprogramming underlies resistance to BCL-2 inhibition in lymphoid malignancies. Cancer Cell 2019;36:369–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eyre TA, Roeker LE, Fox CP, Gohill SH, Walewska R, Walter HS, et al. The efficacy and safety of venetoclax therapy in elderly patients with relapsed, refractory chronic lymphocytic leukaemia. Br J Haematol 2020; 188:918–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turtle CJ, Hay KA, Hanafi LA, Li D, Cherian S, Chen X, et al. Durable molecular remissions in chronic lymphocytic leukemia treated with CD19-specific chimeric antigen receptor-modified T cells after failure of ibrutinib. J Clin Oncol 2017; 35:3010–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dreger P, Michallet M, Bosman P, Dietrich S, Sobh M, Boumendil A, et al. Ibrutinib for bridging to allogeneic hematopoietic cell transplantation in patients with chronic lymphocytic leukemia or mantle cell lymphoma: a study by the EBMT chronic malignancies and lymphoma working parties. Bone Marrow Transplant 2019;54:44–52. [DOI] [PubMed] [Google Scholar]
- 23.Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Dohner H, et al. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood 2018;131:2745–60. [DOI] [PubMed] [Google Scholar]
- 24.Bland JM, Altman DG. Survival probabilities (the Kaplan-Meier method). BMJ 1998;317:1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Byrd JC, Brown JR, O’Brien S, Barrientos JC, Kay NE, Reddy NM, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med 2014;371:213–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharman JP, Banerji V, Fogliatto LM, Herishanu Y, Munir T, Walewska R, et al. ELEVATE TN: Phase 3 study of acalabrutinib combined with obinutuzumab (O) or alone vs. O plus chlorambucil (Clb) in patients (Pts) with treatment-naive chronic lymphocytic leukemia (CLL). Blood 2019;134:31. [Google Scholar]
- 27.Ghia P, Pluta A, Wach MJEL. ASCEND phase 3 study of acalabrutinib vs. investigator’s choice of rituximab plus idelalisib (IdR) or bendamustine (BR) in patients with relapsed/refractory (R/R) chronic lymphocytic leukemia (CLL). EHA Library 273259 (2019).
- 28.Allan JN, Patel K, Mato AR, Wierda WG, Pinilla-Ibarz J, Choi MY, et al. PS1148 preliminary results of a phase 1B/2 dose-escalation and cohort-expansion study of the noncovalent, reversible Bruton’s tyrosine kinase inhibitor (BTKI), vecabrutinib, in B-cell malignancies. HemaSphere 2019; 3:520. [Google Scholar]
- 29.Brandhuber B, Gomez E, Smith S, Eary T, Spencer S, Rothenberg SM, et al. LOXO-305, a next generation reversible BTK inhibitor, for overcoming acquired resistance to irreversible BTK inhibitors. Clin Lymphoma Myeloma Leuk 2018; 18:S216. [Google Scholar]
- 30.Woyach J, Stephens D, Flinn I, Bhat S, Savage RE, Chai F, et al. PS1150 a phase 1 dose escalation study of ARQ 531 in patients with relapsed or refractory B-cell lymphoid malignancies. HemaSphere 2019;3:521–2. [Google Scholar]
- 31.Flinn IW, Hillmen P, Montillo M, Nagy Z, Illes A, Etienne G, et al. The phase 3 DUO trial: duvelisib vs. ofatumumab in relapsed and refractory CLL/SLL. Blood 2018;132:2446–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dreger P, Schetelig J, Andersen N, Corradini P, van Gelder M, Gribben J, et al. Managing high-risk CLL during transition to a new treatment era: stem cell transplantation or novel agents? Blood 2014;124:3841–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roeker LE, Brown JR, Dreger P, Lahoud OB, Eyre TA, Brander DM, et al. Allogeneic stem cell transplantation (alloHSCT) for chronic lymphocytic leukemia (CLL) in the era of novel agents. Blood 2019;134:3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maddocks KJ, Ruppert AS, Lozanski G, Heerema NA, Zhao W, Abruzzo L, et al. Etiology of ibrutinib therapy discontinuation and outcomes in patients with chronic lymphocytic leukemia. JAMA Oncol 2015;1:80–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jain P, Keating M, Wierda W, Estrov Z, Ferrajoli A, Jain N, et al. Outcomes of patients with chronic lymphocytic leukemia after discontinuing ibrutinib. Blood 2015;125:2062–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.UL CLL Forum. Ibrutinib for relapsed/refractory chronic lymphocytic leukemia: a UK and Ireland analysis of outcomes in315 patients. Haematologica 2016;101: 1563–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


