Abstract
A PCR method for rapid screening of Erysipelothrix spp. in the slaughterhouse was carried out by using four species-specific sets of oligonucleotide primers after initial amplification with the primer set MO101-MO102, which amplifies the 16S rRNA sequences of all four Erysipelothrix species. The DNA sequences coding for the rRNA gene cluster, including 16S rRNA, 23S rRNA, and the noncoding region downstream of 5S rRNA, were determined in order to design primers for the species-specific PCR detection system. The homology among the 4.5-kb DNA sequences of the rRNA genes of Erysipelothrix rhusiopathiae serovar 2 (DNA Data Bank of Japan accession no. AB019247), E. tonsillarum serovar 7 (accession no. AB019248), E. rhusiopathiae serovar 13 (accession no. AB019249), and E. rhusiopathiae serovar 18 (accession no. AB019250) ranged from 96.0 to 98.4%. The PCR amplifications were specific and were able to distinguish the DNAs from each of the four Erysipelothrix species. The results of PCR tests performed directly with tissue specimens from diseased animals were compared with the results of cultivation tests, and the PCR tests were completed within 5 h. The test with this species-specific system based on PCR amplification with the DNA sequences coding for the rRNA gene cluster was an accurate, easy-to-read screening method for rapid diagnosis of Erysipelothrix sp. infection in the slaughterhouse.
Erysipelothrix rhusiopathiae is a causative agent of swine erysipelas and human erysipeloid (14), a disease that occurs in acute and chronic forms and that causes development of arthritis and endocarditis (9, 10); this agent causes economic loss and remains an animal hygiene problem in swine production areas of the world. Pigs in which the pathogen is detected must be disused in Japan (13); therefore, methods of rapid diagnosis and appropriate treatment are needed.
Generally, routine bacteriological culture methods require a minimum of 3 days to detect Erysipelothrix cells and about 10 days to identify their serovars (3, 5).
Recently, several methods that can replace the time-consuming classical methods for detection of bacteria have been proposed, for example, DNA-DNA hybridization with bacterium-specific probes and PCR (8). Makino et al. (7) established a PCR system using highly specific primers, MO101 and MO102, for detection of Erysipelothrix species. On the basis of studies with DNA-DNA hybridization and with MO101 and MO102, the genus Erysipelothrix was reported to be divided into four species, E. rhusiopathiae (serovars 1a, 1b, 2, 4, 5, 6, 8, 9, 11, 12, 15, 16, 17, 19, 21, and N), E. tonsillarum (serovars 3, 7, 10, 14, 20, 22, and 23), E. rhusiopathiae serovar 13, and E. rhusiopathiae serovar 18; and those four species could not be distinguished from each other by PCR (4, 11, 12).
In this study, the DNA sequences of the rRNA gene clusters in each of the four Erysipelothrix species were determined, and consequently, we improved the PCR method so that it can be used to specifically identify each of the four Erysipelothrix species and applied the system to inspections in slaughterhouses.
MATERIALS AND METHODS
Bacterial strains and DNA preparation.
All standard bacterial strains used in this study are listed in Table 1. Lesions caused by Erysipelothrix infections were tested microbiologically immediately after the lesions were isolated by staff in the slaughterhouse. Total DNA from bacterial cells was prepared by using previously published methods (7). DNA from tissue samples was prepared for PCR as described previously (6) or by using a DNA preparation kit for gram-positive bacteria (GenTLE; TaKaRa Ltd., Kyoto, Japan).
TABLE 1.
Results of PCR amplification with species-specific primer sets for serovars of Erysipelothrix strains
| Straina | Serovar | PCR result
|
|||
|---|---|---|---|---|---|
| ER1-F–ER1-R (399 bpb) | ER2-F–ER2-R (384 bp) | ER3-F–ER2-4 (289 bp) | ER4-F–ER4-R (387 bp) | ||
| E. rhusiopathiae | |||||
| ATCC 19414 | 2 | + | − | − | − |
| Fujisawa | 1 | + | − | − | − |
| Doggerscharbe | 4 | + | − | − | − |
| Pécs 67 | 5 | + | − | − | − |
| Dolphin E-1 | 6 | + | − | − | − |
| Gota | 8 | + | − | − | − |
| Kaparek | 9 | + | − | − | − |
| IV12/8 | 11 | + | − | − | − |
| Pécs 9 | 12 | + | − | − | − |
| Pécs 3597 | 15 | + | − | − | − |
| Tanzania | 16 | + | − | − | − |
| 545 | 17 | + | − | − | − |
| 2017 | 19 | + | − | − | − |
| Bãno 36 | 21 | + | − | − | − |
| MEW 22 | N | + | − | − | − |
| E. tonsillarum | |||||
| ATCC 43339 | 7 | − | + | − | − |
| Wittling | 3 | − | + | − | − |
| Lengy1-P | 10 | − | + | − | − |
| Iszap-4 | 14 | − | + | − | − |
| 2553 | 20 | − | + | − | − |
| Bãno 107 | 22 | − | + | − | − |
| KS 20A | 23 | − | + | − | − |
| Erysipelothrix sp. strain 1 | |||||
| Pécs 56 | 13 | − | − | + | − |
| Shiribeshi 17 | 13 | − | − | + | − |
| Shiribeshi 19 | 13 | − | − | + | − |
| Erysipelothrix sp. strain 2 715 | 18 | − | − | − | + |
ATCC 19414 and ATCC 43339 are type reference strains of E. rhusiopathiae and E. tonsillarum, respectively (3).
Product sizes are given in parentheses.
PCR primers and PCR.
The five universal primer sets used to amplify the 23S rRNA gene of Erysipelothrix were designed on the basis of the alignment of the DNA sequences of the 23S rRNA gene in 15 different bacteria (Fig. 1), whose species and GenBank accession numbers are as follows: Bacillus subtilis, D11460; Bacillus stearothermophilus, X01387; Clostridium botulinum, M94259; Listeria monocytogenes, X92951; Staphylococcus aureus, X68425; Staphylococcus carnosus, X68419; Streptococcus oralis, X68427; Streptococcus parauberis, S60368; Micrococcus luteus, X06484; Rhodopseudomonas capsulata; X06485; Pseudomonas marina, X07408; Escherichia coli, V00331; Pseudomonas aeruginosa, Y00432; Pseudomonas cepacia, X16368; and Leptospira interrogans, X14249. To amplify the remaining region of the 23S rRNA gene, four kinds of primer sets were designed (Fig. 1). PCR was performed in a 50-μl reaction mixture containing 10 ng of template DNA, each primer at a concentration of 25 mM, 50 mM KCl, 10 mM Tris-HCl (pH 8.3), 1.5 mM MgCl2, each deoxynucleoside triphosphate at a concentration of 200 μM, and 1.25 U of AmpliTaq DNA polymerase. The PCR was carried out for 35 cycles consisting of denaturation for 1 min at 94°C, annealing for 1 min at 58°C, and extension for 1 min at 72°C by using a Gene-Amp thermal cycler (model 2400; Perkin-Elmer Co., Foster City, Calif.). The PCR products were subjected to electrophoresis in 1.5% agarose gels for 30 min. After running for 30 min, the gels were stained with ethidium bromide solution and were then photographed under transillumination.
FIG. 1.
Designation of species-specific primer sets for detection of Erysipelothrix sp. DNA by PCR. (A) DNA sequences of 23S rRNA genes of gram-positive bacteria and positions of four primer sets for amplification of the remaining region of the 23S rRNA gene. (B) Region of DNA sequences determined after amplification by using the five universal primer sets and the remaining regions to be amplified by using the four kinds of primer sets. (C) In vitro cloning by combination of PCR with cassettes and cassette primers.
In vitro cloning by combination of PCR with cassettes and cassette primers.
The in vitro cloning (1, 2) and determination of the entire nucleotide sequence of the 23S rRNA genes of four strains, E. rhusiopathiae ATCC 19414, Pécs 56, and 715 and E. tonsillarum ATCC 43339, were performed with TaKaRa LA PCR in vitro cloning kits (TaKaRa Ltd.) according to the manufacturer's instructions. The purified genomic DNAs from each of the four strains were digested with EcoRI, the digested fragments were ligated to a synthetic EcoRI cassette; and then the first PCR was performed with the EcoRI cassette primer (primer C1; 5′-GTACATATTGTCGTTAGAACGCG-3′), known sequence-specific primers ER2 and ER9 (Table 2), and cassette-ligated DNA as a template. Nested PCR was then performed with nested primer sets ER-S1 and ER-S2 (Fig. 1) and with C2 (5′-TAATACGACTCACTATAGGGAGA-3′), and DNA was amplified with the product of the first PCR as the template.
TABLE 2.
Oligonucleotide primers synthesized in this study
| Primer purpose and name | Sequence | Nucleotide position |
|---|---|---|
| Universal primers for 23S rRNA gene | ||
| ER-1F | 5′-CACGGTGGATGCCTTGGC-3′ | 1186–1203 |
| ER-2R | 5′-CCTTTCCCTCACGGTACTG-3′ | 1654–1672 |
| ER-3F | 5′-GCGTGCCTTTTGTAGAATG-3′ | 1755–1773 |
| ER-4R | 5′-AGTGAGCTATTACGCACTCTTT-3′ | 2282–2303 |
| ER-5F | 5′-GCTCGTCCGCTCAGGGTTAG-3′ | 2571–2589 |
| ER-6R | 5′-CATTTTGCCGAGTTCCTTAA-3′ | 2861–2880 |
| ER-7F | 5′-GTTAAGGAACTCGGCAAAATG-3′ | 2860–2881 |
| ER-8R | 5′-GTTACGGCCGCCGTTTACTGG-3′ | 3062–3082 |
| ER-9F | 5′-AGTTCCGACCCGCACGAAAGG-3′ | 3172–3192 |
| ER-10R | 5′-AGGCGACCGCCCCAGTCAAAC-3′ | 3460–3480 |
| Primers for sequencing of 23S rRNA gene | ||
| ER1 · 2F | 5′-TCTAGTGGTATCCTGAGTACG-3′ | 1554–1574 |
| ER3 · 4R | 5′-TTCGGGTCTACCACAATGTAC-3′ | 1855–1875 |
| ER3 · 4F | 5′-CGTAGTCGAAAGGGAAACAGC-3′ | 2163–2183 |
| ER5 · 6R | 5′-TAAACCAGCACTTCCAGTCGC-3′ | 2644–2664 |
| ER5 · 6F | 5′-GATGCCAGCTCTCAAGAAAAG-3′ | 2752–2772 |
| ER7 · 8R | 5′-GTCTTGCGACTTTGCAGAGAG-3′ | 2959–2979 |
| ER7 · 8F | 5′-AGTCGCAAGACGAAGTATAGG-3′ | 2969–2989 |
| ER9 · 10R | 5′-CTAAGATTTCACCGAGTCTGC-3′ | 3169–3189 |
| Primers for LA PCR cloning | ||
| ER-S1 | 5′-TGCTTGTGTACGTACCACTCC-3′ | 1248–1265 |
| ER-S2 | 5′-GCCATTGGGATACCACTCTTG-3′ | 3330–3350 |
| Primers for sequencing of rRNA gene cluster | ||
| ER-S3 | 5′-CAAGGTATCCCTACCGGAAG-3′ | 817–836 |
| ER-S4 | 5′-GGAGCTGAATTAGGTTCCAAG-3′ | 3691–3711 |
| ER-S5 | 5′-CACCCTCTAATCGATATGCATC-3′ | 4567–4588 |
| ER-S6 | 5′-GTGTAGCGGTAAAATGCGTAG-3′ | 9–29 |
| ER-S7 | 5′-GAGACTGCCGGTGATAAACC-3′ | 467–486 |
| ER-S8 | 5′-CTCGCCCAAGAGTTCACATC-3′ | 3629–3648 |
| ER-S9 | 5′-GATATTGTCGCCGCTAAAGTG-3′ | 4200–4220 |
Direct sequencing of DNA.
Each of the PCR products was extracted from the agarose gel and was purified with glass powder (TaKaRa EasyTrap; TaKaRa Ltd.) according to the manufacturer's instructions. The DNA sequences of both strands of the DNA fragments were then determined by use of a dye termination kit and a model 377 DNA sequencer (Perkin-Elmer Co.). The primers digested for PCR amplification were used for the cycle sequencing reaction (Table 2; Fig. 1). The Gene Works sequence analysis program from Teijin (Yokohama, Japan) was used to determine the complete sequence and to compare the sequences.
RESULTS
Nucleotide sequence of rRNA gene cluster.
The PCR was performed with the DNAs from four strains of Erysipelothrix by using five universal primer sets specific for the 23S rRNA gene (Fig. 1A; Table 2). The sizes of the PCR products, as determined by their mobilities on the agarose gel, were 500, 550, 350, 240, and 310 bp, which corresponded to the sizes of the products generated from the primer sets ER-1F–ER-2R, ER-3F–ER-4R, ER-5F–ER-6R, ER-7F–ER-8R, and ER-9F–ER-10R, respectively. Both DNA strands of the PCR products were then directly sequenced with the same 10 primers.
Further PCR was performed with the DNAs from the four strains by using four primer sets designed from the PCR products sequenced (Fig. 1B; Table 2), and then these PCR products were directly sequenced with the same eight primers, resulting in final nucleotide sequences of approximately 2,600 bp, corresponding to about 75% of the 23S rRNA gene sequence (Fig. 1B).
To clone the 5′ upstream and 3′ downstream regions of the 23S rRNA genes, cassette-mediated PCR cloning was adopted (Fig. 1C). The method consists of (i) digestion of genomic DNA with EcoRI, (ii) ligation of cleavage products to double-stranded DNA cassettes possessing an EcoRI site, and (iii) amplification of cassette-ligated restriction fragments containing known sequences by PCR with specific and cassette primers. The specific primers (primers ER2, ER9, ERS1, and ERS2) were designed to prime synthesis from the known sequences of the DNA, whereas the cassette primers (cassette primers C1 and C2) anneal to one strand of cassette DNA.
The amplified DNA fragments contained a 1,280-bp sequence upstream of the 23S rRNA genes of the four strains, a 1,400-bp sequence downstream of the 23S rRNA genes of E. rhusiopathiae ATCC 19414 and E. tonsillarum ATCC 43339, and a 1,200-bp sequence downstream of the 23S rRNA genes of the other two strains. Then, sequencing from the cassette primers and specific primer sets provided information for designing a new primer for the next “walking” step, which permitted direct sequencing without the need to synthesize internal sequencing primers. Finally, by using seven primers (primers ER-S3, ER-S4, ER-S5, ER-S6, ER-S7, ER-S8, and ER-S9), the entire sequences of approximately 4.5-kb genomic segments containing the rRNA genes of the four strains were determined.
The resulting nucleotide sequences of the four strains included the complete regions coding for 23S rRNA and 5S rRNA genes and major parts of the 16S rRNA gene and the 3′-end noncoding region (Fig. 2). The coding regions were deduced on the basis of alignments with other bacterial species whose sequences had high degrees of homology to those of the four strains examined. A comparative alignment of the sequence established in this study with the sequences of the four strains revealed that E. rhusiopathiae ATCC 19414 exhibited 98.2% (4,867 bp) identity with E. tonsillarum ATCC 43339, 96.0% (4,865 bp) identity with E. rhusiopathiae Pécs 56, and 98.4% (4,543 bp) identity with E. rhusiopathiae 715 (4,566 bp), respectively. It is apparent that the rRNA genes of the four strains exhibit a high degree of identity with each other.
FIG. 2.
Putative rRNA gene cluster localization and location of species-specific primers for each species. Primer sets specific for detection of E. rhusiopathiae, E. tonsillarum, and E. rhusiopathiae Pécs 56 were designed with a sequence from within the noncoding region near nucleotides 4110 and 4500; in contrast, the specific primer set for detection of strain 715 was designed with a sequence from within the 23S rRNA gene.
Design of primers specific for each species.
Specific primer sets for detection of each species of Erysipelothrix were designed on the basis of the rRNA sequences determined, as shown in Table 2 and Fig. 2. Specific primer sets for detection of E. rhusiopathiae, E. tonsillarum, and E. rhusiopathiae Pécs 56 were designed from sequences within the noncoding region near nucleotides 4110 and 4500 (Table 2), because the nucleotide sequences of those strains exhibited weak homology with each other. In contrast, a specific primer set for detection of E. rhusiopathiae 715 was designed from a sequence within the 23S rRNA gene (Fig. 2).
PCR was performed with the DNAs from different strains by using the four primer sets. PCR products were obtained only when the DNAs extracted from the isolates and the matching specific primer sets were used, as shown in Fig. 3. The sizes of the amplified products, based on the mobilities in agarose gels of E. rhusiopathiae ATCC 19414, E. tonsillarum ATCC 43339, E. rhusiopathiae Pécs 56, and E. rhusiopathiae 715, were 399, 384, 288, and 387 bp, respectively, which correspond to the sizes predicted from the nucleotide sequences.
FIG. 3.
Amplification of rRNA gene fragments by PCR. PCR was carried out with DNAs extracted from four species and corresponding species-specific primer sets. Lane M, DNA size marker (100-bp DNA ladder); lane 1, strain ATCC 19414 DNA amplified with ER1-F–ER1-R (399 bp) but negative for amplification with ER1-F–ER1-R from ATCC 13339 (lane 2), Pécs 56 (lane 3), and 715 (lane 4) DNAs; lane 6, strain ATCC 43339 DNA amplified with ER2-F–ER2-R (384 bp) but negative for amplification with ER2-F–ER2-R from ATCC 19414 (lane 5), Pécs 56 (lane 7), and 715 (lane 8) DNAs; lane 11, strain Pécs 56 DNA amplified with ER3-F–ER3-R (289 bp) but negative for amplification with ER3-F–ER3-R from ATCC 19414 (lane 9), ATCC 43339 (lane 10), and 715 (lane 12) DNAs; lane 16, strain 715 DNA amplified with ER4-F–ERF-R (387 bp) but negative for amplification with ER4-F–ERF-R from ATCC 19414 (lane 13), ATCC 43339 (lane 14), and Pécs 56 (lane 15) DNAs.
PCR was then performed with Erysipelothrix strains, including strains of 26 serotypes which were classified into one of the four species. PCR products were obtained when DNA sequences from the particular strains matched those of the species-specific primer sets, as indicated in Table 3. The results demonstrated that the four species of Erysipelothrix could be distinguished from each other by PCR with the species-specific primer sets with specificity (Table 1).
TABLE 3.
Sequences of species-specific primer sets
| Species | Primer | Sequence | Position | Size of product (bp) |
|---|---|---|---|---|
| E. rhusiopathiae | ER1F | 5′-GTTCATCTCTCTAATGCACTAC-3′ | 4119–4140 | 399 |
| ER1R | 5′-TGTTGGACTACTAATCGTTTCG-3′ | 4497–4515 | ||
| E. tonsillarum | ER2F | 5′-ATGTAATATGATCTGGTGATTTG-3′ | 4132–4154 | 384 |
| ER2R | 5′-AGGACTGCTGATTGTCTCATG-3′ | 4495–4515 | ||
| Erysipelothrix sp. strain 1 | ER3F | 5′-TGGAGGACCGAACCGACTG-3′ | 1897–1915 | 289 |
| ER3R | 5′-AATTTTGGGACCTTAACTGGC-3′ | 2165–2185 | ||
| Erysipelothrix sp. strain 2 | ER4F | 5′-TAAAGCACTAAGATCTGGTGG-3′ | 4130–4150 | 387 |
| ER4R | 5′-TCGGACTACTAATTGTCTCAG-3′ | 4496–4516 |
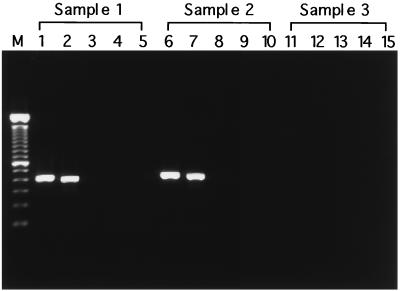
Furthermore, PCR was performed with 17 swine specimens obtained at the slaughterhouse from animals with endocarditis, arthritis, and dermatitis (Fig. 4). Amplified DNA products were obtained with the E. rhusiopathiae-specific primer set from nine of the specimens, which were confirmed to be infected with E. rhusiopathiae by the cultivation tests. No PCR product was obtained with any of the four kinds of primer sets from eight of the specimens from which Actinomyces pyogenes, Staphylococcus aureus, and group C Streptococcus were isolated in cultivation tests. The sequences of the 399-bp products amplified from E. rhusiopathiae isolates from slaughterhouses were determined to be identical to the sequences from E. rhusiopathiae ATCC 19414 determined in this study (data not shown).
FIG. 4.
Specific amplification of E. rhusiopathiae DNA by PCR from clinical isolates. Template DNAs were prepared from the diseased tissues as described in the text, and then PCR was carried out with one genus-specific primer set and four species-specific primer sets. Lane M, DNA size marker (100-bp DNA ladder); lanes 1 and 2, 407- and 399-bp DNA fragments amplified from sample 1 (from an animal with dermatitis), respectively; lanes 3 to 5, negative for amplification with ER2-F–ER2-R (lane 3), ER3-F–ER3-R (lane 4), and ER4-F–ER4-R (lane 5); lanes 6 and 7, 407- and 399-bp DNA fragments amplified from sample 2 (from an animal with endocarditis), respectively; lanes 8 to 10, negative for amplification with ER2-F–ER2-R (lane 8), ER3-F–ER3-R (lane 9), and ER4-F–ER4-R (lane 10); lanes 11 to 15, negative for amplification with one genus-specific and four species-specific primer sets and DNA from sample 3 (from an animal with endocarditis), which was negative for Erysipelothrix spp. in cultivation tests.
DISCUSSION
It has been thought that the genus Erysipelothrix consists of a single species, E. rhusiopathiae. However, Takahashi et al. (12) reported that the genus Erysipelothrix contains two main species, E. rhusiopathiae and E. tonsillarum, and two other species represented by E. rhusiopathiae serovars 13 and 18, respectively, on the basis of DNA-DNA hybridization experiments. As to the detection of Erysipelothrix species, Makino et al. (7) reported that the PCR system with primer set MO101-MO102 corresponding to E. rhusiopathiae DNA coding for 16S rRNA could produce amplified products from the DNA samples from 35 Erysipelothrix strains, including those of the 4 species used in this study. This is compatible with the fact that the homologies of the DNA sequences of the 16S rRNAs of the four species ranged from 98.5 to 98.8%. Accordingly, the four species of Erysipelothrix are indistinguishable from each other with the PCR system based on the MO101-MO102 primer set. Generally, the results from pathogenicity tests (5) have confirmed that E. rhusiopathiae strains are pathogenic for swine but E. tonsillarum strains are not. When Erysipelothrix is suspected in joints and/or other organs of swine on the basis of a clinical diagnosis, it is important for detection purposes to distinguish among the four species of Erysipelothrix as well as other causative bacterial pathogens.
In this study, we have established a method for the direct detection of Erysipelothrix sp. DNA from tissue samples from diseased animals without cultivation by PCR using oligonucleotide primers complementary to the DNA sequences of the rRNA gene cluster (including 16S, 23S, and 5S rRNAs and the noncoding region) of the four species by cassette cloning and primer-walking methods. This PCR system is able to distinguish the four species of Erysipelothrix from each other, giving different sizes of PCR products corresponding to each species.
The usefulness of this PCR method of diagnosis was confirmed with tissue specimens derived from animals with endocarditis, arthritis, and dermatitis which were suspected to be caused by Erysipelothrix. The rRNA gene fragments were amplified by PCR from diseased tissue specimens, and the species of Erysipelothrix identified on the basis of the fragments amplified with the species-specific primer sets was identical to that identified by the cultivation tests. From these data, it is concluded that the PCR system developed in this study is rapid, specific, and reliable for identification of the 23S rRNA genes of the four Erysipelothrix species and that it can be used for the rapid detection of Erysipelothrix in tissues from diseased swine obtained in slaughterhouses.
REFERENCES
- 1.Isegawa Y, Sheng J, Sokawa Y, Yamanishi K, Nakagomi O, Ueda S. Selective amplification of cDNA sequence from total RNA by cassette-ligation mediated polymerase chain reaction (PCR): application to sequencing 6.5 kb genomic segment of hantavirus strain B-1. Mol Cell Probes. 1992;6:467–475. doi: 10.1016/0890-8508(92)90043-w. [DOI] [PubMed] [Google Scholar]
- 2.Iwahana H, Tsujisawa T, Katashima R, Yoshimoto K, Itakura M. PCR with end trimming and cassette ligation: a rapid method to clone exon-intron boundaries and a 5′-upstream sequence of genomic DNA based on a cDNA sequence. PCR Methods Appl. 1994;4:19–25. doi: 10.1101/gr.4.1.19. [DOI] [PubMed] [Google Scholar]
- 3.Jones D. Bergey's manual of systematic bacteriology. Vol. 2. Baltimore, Md: The Williams & Wilkins Co.; 1986. Genus Erysipelothrix Rosenbach 1909, 367AL; pp. 1245–1249. [Google Scholar]
- 4.Kalf G, White T G. The antigenic components of Erysipelothrix rhusiopathiae. II. Purification and chemical characterization of a type-specific antigen. Arch Biochem Biophys. 1963;102:39–47. doi: 10.1016/0003-9861(63)90317-5. [DOI] [PubMed] [Google Scholar]
- 5.Kucsera G. Serological typing of E. rhusiopathiae strains and the epidemiological significance of the typing. Acta Acad Sci Hung. 1979;27:19–28. [PubMed] [Google Scholar]
- 6.Makino S, Okada Y, Maruyama T. A new method for direct detection of Listeria monocytogenes from foods by PCR. Appl Environ Microbiol. 1995;61:3745–3747. doi: 10.1128/aem.61.10.3745-3747.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Makino S, Okada Y, Ishikawa T, Takahashi K, Nakamura T, Ezaki M, Morita T. Direct and rapid detection of Erysipelothrix rhusiopathiae DNA in animals by PCR. J Clin Microbiol. 1994;32:1526–1531. doi: 10.1128/jcm.32.6.1526-1531.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saiki R K, Scharf S, Faloona F, Mullis K B, Horn G T, Erlich H A, Arnheim N. Enzymatic amplification of γ-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985;230:1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- 9.Schraumen E, Devriese L A, Hoorens J, Takahashi T. Erysipelothrix tonsillarum endocarditis in a dog. A case report. Vlaams Diergeneeskd Tijdschr. 1993;62:160–161. [Google Scholar]
- 10.Takahashi T, Sawada T, Takagi M, Seto K, Kanzaki M, Maruyama T. Serotypes of Erysipelothrix rhusiopathiae strains isolated from slaughter pigs affected with chronic erysipelas. Jpn J Vet Sci. 1984;46:149–153. doi: 10.1292/jvms1939.46.149. [DOI] [PubMed] [Google Scholar]
- 11.Takahashi T, Fujisawa T, Benno Y, Tamura Y, Sawada T, Suzuki S, Muramatsu M, Mitsuoka T. Erysipelothrix tonsillarum sp. nov. isolated from tonsils of apparently healthy pigs. Int J Syst Bacteriol. 1987;37:166–168. [Google Scholar]
- 12.Takahashi T, Fujisawa T, Tamura Y, Suzuki S, Muramatsu M, Sawada T, Benno Y, Mitsuoka T. DNA relatedness among Erysipelothrix rhusiopathiae strains representing all twenty-three serovars and Erysipelothrix tonsillarum. Int J Syst Bacteriol. 1992;42:469–473. doi: 10.1099/00207713-42-3-469. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi T. Swine erysipelas and Erysipelothrix. Jpn J Bacteriol. 1996;51:707–715. doi: 10.3412/jsb.51.707. [DOI] [PubMed] [Google Scholar]
- 14.Wood R L, Shuman R D. Swine erysipelas. In: Scholl E, Leman A D, editors. Diseases of swine. 4th ed. Ames: Iowa State University Press; 1975. pp. 565–620. [Google Scholar]