Abstract
Cancers are highly heterogeneous and typically contain a small subset of drug-resisting cells called tumor initiating cells or cancer stem cells (CSCs). CSCs can self-renew, divide asymmetrically, and often cause tumor invasion and metastasis. Therefore, treatments specifically targeting CSCs are critical to improve patient survival. Recently, we identified a highly specific peptidomimetic (peptoid – PCS2) that selectively binds to the CSC subpopulation of lung cancer over the remaining cancer cells (non-CSCs). Subsequently, we identified plectin as the target of PCS2. Plectin is an intracellular structural protein, which is involved in tumor invasion and metastasis when it appears on cell surface. While PCS2 monomer did not display any anti-cancer activity, we designed a series of homo-dimeric versions of PCS2, and identified PCS2D1.2 optimized homo-dimer that displayed highly specific cytotoxicity towards CSCs over non-CSCs. PCS2D1.2 effectively blocked the in vitro colony formation and cell migration, hallmarks of CSCs. Furthermore, PCS2D1.2 reduced the in vivo tumor formation. In both in vitro and in vivo studies, PCS2D1.2 effectively reduced plectin and/or plectin-rich CSCs, but had no effect on non-CSCs. Therefore, PCS2D1.2 has the potential be developed as a highly CSC specific drug candidate, which can be used in combination with current anti-cancer drugs.
Keywords: Peptidomimetic, Cancer Stem Cells, Lung cancer, Avidity effect, Homo-dimer
Graphical Abstract

1. Introduction:
The highly heterogeneous nature of protein biomarker expression and the extreme complexity of signaling cascades in cancer cells are significant roadblocks for the development of targeted therapies. Within a heterogeneous tumor, this complexity is presented by small sub-population of therapy-resistant cancer cells called tumor initiating cells or cancer stem cells (CSCs) [1-4]. While there are major debates on the origin of such subpopulation of cells, it is clear that these cells can self-renew, differentiate, contribute to cancer metastasis and cancer recurrence following conventional treatments leading to tumor recurrence [4, 5]. Therefore, highly specific drugs that can eliminate the CSC population are needed to effectively treat cancer. For reference, we call these cells CSCs in this study.
Lung cancer is the leading cause for cancer deaths in US [6] and lung CSCs are one of the main drivers in brain and liver metastasis [4, 7, 8]. One of the avenues for CSC specific drug development is to target signaling pathways involved in cell fate, which regulate development and differentiation. These agents have been shown to regulate CSC-related phenotypes [9], including the Wnt, Hedgehog, and Notch pathways. The mechanisms used by these drugs to inhibit these pathways vary considerably. Some, like Vantictumab, Vismodegib or Tarextumab, are inhibitors of the initiating receptor families [10-12]. Others, potential candidates like Demcizumab, target the DLL4 ligand directly [13], or small molecule inhibitors and antagonists to other pathway proteins, like LGK974 [14]. Another approach is to hit the known biomarkers that are found on CSCs, like CD44, ALDH1, or VEGFR, to sensitize tumors to chemotherapy [15, 16]. However, the critical functions of these biomarkers and developmental pathway for normal stem cells pose a risk of significant side effects. Also, the high heterogeneity among those CSC signaling biomarkers once again limit their general use in the clinic [17, 18].
Recently, we applied our unique On-Bead Two-Color (OBTC) combinatorial cell screen [19-22] and unbiasedly identified a peptidomimetic (peptoids: oligo-N-substituted glycines) [23, 24] ligand, PCS2, that specifically targeted to the H358 lung CSC surface, but did not bind to the surface of the non-CSCs from the same H358 cell line [25]. We subsequently identified plectin protein as the PCS2 target [25]. Plectin is a ubiquitous structural protein in cytosol, but is reported to be found on cancer cell surface with functions related to tumor invasion and metastasis [26]. Plectin has a large number of known isoforms with distinct N-terminal domains [27] that have the similar overall size of about 500 kDa and a structure of two globular domains with a large coiled coil of alpha helices (Rod domain) in between [28, 29]. The primary role of plectin is to function as a structural linker between cellular membranes and other cytoskeletal components, including the actin microfilaments, microtubules, and intermediate filaments [30-32]. Through these interactions, plectin plays an important role in cell-cell interactions, cell-extracellular matrix interactions, cell migration, cellular and tissue integrity, and plasticity. Loss of or mutations in plectin are associated with tissue degeneration and a loss of cellular structural stability, and in mice, plectin knockout is lethal in 3 days after birth [33]. Plectin has been associated with cancer progression, mainly via supporting invasion and metastasis [26, 34-38]. While the expected localization of plectin is cytoplasmic, cell surface expression has been identified as a marker of pancreatic ductal adenocarcinoma [26, 34] and oral squamous cell carcinoma [39].
While conventional targeted therapies are directed towards ‘functional proteins’ such as enzymes, hormones, receptors, signaling kinases, or transcription factors that are essential for disease progression, their heterogeneity prevents the common anticancer applications. But the structural proteins that construct the architecture of the cell are highly conserved and targeting those would create commonly applicable cancer drugs. The majority of such cytoskeletal-targeting chemotherapeutic drugs target either actin or microtubules. These drugs function through inhibiting polymerization, stabilizing the filament structure, or depolymerizing the filaments [40, 41]. However, due to the global presence of structural proteins in normal cells, these drugs have significant side effects [42, 43]. Being a structural protein, plectin is unique as a cancer biomarker that moves onto the surface of CSCs, which can be selectively targeted without affecting cytosolic plectin that is expressed in normal cells. So far, exploration of cell surface plectin for cancer therapy is very limited. Cell surface plectin-targeted small molecule reduced tumor invasiveness and growth in colon cancer [44], while few studies used plectin targeted liposomes [45] and nanoparticles [46, 47] to deliver anti-cancer agents.
We have demonstrated recently the correlations of cell surface plectin with CSC functions such as cell survival, colony formation and cell migration in lung cancer [25]. In this report, we have developed an improved homo-dimeric version of PCS2, PCS2D1.2 and show that PCS2D1.2 displays highly specific anti-CSC efficacy in vitro and in vivo, while having no effects on the non-CSCs from the same cell line.
2. Materials and methods:
General:
Rink amide resin, (loading capacity: 0.3-0.6 mmol/g) was purchased from Chem-Impex International, Inc. . All primary amines, all Fmoc-protected amino acids and 2-(1H-Benzotriazole-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate (HBTU), Hydroxybenzotriazole (HOBt), bromoacetic acid, N,N-diisopropylcarbodiimide (DIC), N,N-diisopropylethylamine (DIPEA), piperidine, trifluoroacetic acid (TFA), triisopropylsilane (TIS), α-cyano-4-hydroxycinnamic acid, acetonitrile (ACN), dichloromethane (DCM), N,N-dimethylformamide (DMF), and Trypsin-EDTA solution were obtained from MilliporeSigma . GIBCO enzyme free cell dissociation buffer and Qtracker Cell Labeling Kits were obtained from ThermoFisher Scientific . All chemical reagents and solvents obtained from commercial sources were used without further purification. 5mL disposable reaction columns (Intavis AG) were used as reaction vessels for solid-phase synthesis. Syntheses of peptoids under microwave conditions were performed in a 1000 W microwave oven with 10% power. Analysis of all compounds was carried out on Waters HPLC system, Waters Corporation with Waters 1525 binary HPLC pumps and a 2487 dual λ absorbance detector with C18 reversed phase (RP) HPLC (Kinetex, 5u, 100A, 250 × 4.6 mm) with an automated gradient controller (solvent A: H2O/0.1% TFA; solvent B: acetonitrile/0.1% TFA, gradients, 0-90% B in 30 min, flow rate: 1.0 mL/min). Mass spectra were recorded on an Applied Biosystems Voyager DE Pro mass spectrometer using α-cyano-4-hydroxycinnamic acid as the matrix.
Synthesis of monomer PCS2:
PCS2 was synthesized on Rink amide resin. 100 mg of resin was taken in 5 mL reaction column, the resin was swelled in DMF for 1.0 h prior to use, and Fmoc group was de-protected by treating the resin with 2.0 mL of 20% piperidine solution in DMF twice for 10 minutes each. The resin was first coupled to Fmoc-Met-OH using 5.0 equivalent (equiv) HBTU and 5.0 equiv HOBt as coupling reagents in the presence of 10.0 equiv of DIPEA in DMF (2 mL solution) for overnight. Fmoc was removed using the method described above. Subsequent amino acid Fmoc-D-Lys(Boc)-OH and Fomc-Lys(Boc)-OH was introduced using the same peptide-coupling protocol (HBTU/HOBt/DIPEA) with a reaction time of 2 hours, washing 10 times with DMF and removing the Fmoc group at each step. After removing the Fmoc group as described above, five peptoid residues were then coupled using a two-step peptoid coupling procedure (acylation and amination) under a microwave assisted synthesis protocol. For the acylation step, beads were treated with 1.0 M bromoacetic acid (1.0 mL) and 1.5 M DIC (1.0 mL), and microwaved at 10% power (2 X 15 seconds) with gentle shaking in between for 30 seconds. After washing with DMF, beads were treated with 1.0 mL of Boc-1,4-diaminobutane (2.0 M), and coupling was performed by shaking at 25 ° C for 2 hours. The procedure was repeated again to attach the remaining four peptoid residues: (R)-(+)-α-Methylbenzylamine, piperonylamine, Boc-1,4-diaminobutane, and 2-methoxyethylamine. In the end, beads were washed with dichloromethane (DCM) (10 X 2 mL) and dried under vacuum before cleavage. Beads were then treated with a cleavage cocktail of TFA/H2O/TIS (95%/2.5%/2.5%) (2 mL) for 2.0 h at 25 ° C. The crude compound was then purified using HPLC and analyzed by MALDI-TOF.
Synthesis of homo-dimer PCS2D1.1:
PCS2D1.1 was synthesized using the protocol similar to PCS2. 100 mg of Rink amide resin was taken in 5 mL reaction column, the resin was swelled in DMF for 1.0 h, and Fmoc group was de-protected using piperidine as described above. The resin was first coupled to Fmoc-DAP(Fmoc)-OH using 5.0 equiv. HBTU and 5.0 equiv. HOBt as coupling reagents in the presence of 10.0 equiv. of DIPEA in DMF (2 mL solution) at 25 °C for overnight. Next, both Fmoc groups were de-protected simultaneously using piperidine (2 X 2mL of 20% piperidine solution in DMF), the Fmoc deprotection of both amine groups produced two NH2 function group to build two copies PCS2 simultaneously to obtain a homo-dimer. The resin was coupled with Fmoc-Met-OH, Fmoc-D-Lys(Boc)-OH and Fmoc-Lys(Boc)-OH using the peptide-coupling protocol (HBTU/HOBt/DIPEA). After removing the Fmoc group, five peptoid residues were then coupled using a two-step peptoid coupling procedure (acylation and amination) under a microwave assisted synthesis protocol as described for PCS2 above. The sequence of peptoid residues are: Boc-1,4-diaminobutane, (R)-(+)-α-Methylbenzylamine, piperonylamine, Boc-1,4-diaminobutane, and 2-methoxyethylamine.
Synthesis of homo-dimer PCS2D1.2:
PCS2D1.2 was synthesized using the protocol similar to PCS2D1.1. The sequence of amino acid residues for the synthesis of PCS2D1.2 was Fmoc-Lys(Fmoc)-OH, Fmoc-Met-OH, Fmoc-D-Lys(Boc)-OH and Fmoc-Lys(Boc)-OH. After removing the Fmoc, five peptoid residues were then coupled, the sequence of peptoid residues are: Boc-1,4-diaminobutane, (R)-(+)-α-Methylbenzylamine, piperonylamine, Boc-1,4-diaminobutane, and 2-methoxyethylamine [Descriptions for the synthesis of homo-dimers PCS2D1.3-1.7 are listed in the Supplementary Materials].
Synthesis of control homo-dimer PC462D1:
PC462D1 was synthesized using the protocol similar to PCS2D1.2. The sequence of amino acid residues for the synthesis of PC462D1 was Fmoc-Lys(Fmoc)-OH, Fmoc-Met-OH, Fmoc-D-Lys(Boc)-OH and Fmoc-Gly-OH. After removing the Fmoc group, five peptoid residues were then coupled, the sequence of peptoid residues are: allylamine, 2-methoxyethylamine , allylamine, 2-methoxyethylamine, and allylamine.
MTS assay:
H358 lung cancer cells (2,500 cells per well) were plated in 96-well plate and allowed to adhere overnight. On day 2 of the plating of cells, wells were treated with compounds from 1 nM to 100 μM prepared in growth media (10%FBS in RPMI). The treatment was repeated on day 3, day 4 and day 5 from the plating of the cells. On day 6, 20 μL/well of CellTiter 96 Aqueous One Solution MTS reagent (Promega, WI) was added, and the samples were incubated for 2 h. The absorbance was read at 490 nm using a plate reader (Spectramax i3; Molecular Devices). When the assay was performed to study PCS2D1.2 direct cytotoxicity effects on ALDH+ CSCs (Fig. 2C), the read out was done after 24 hrs of the single PCS2D1.2 treatment.
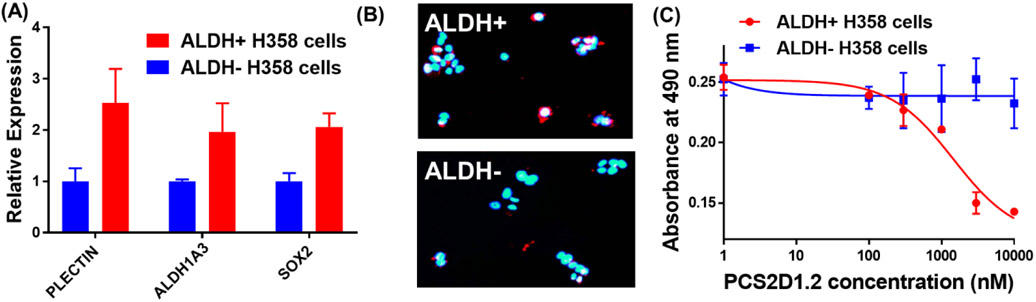
Figure 2:
PCS2D1.2 selectively binds to and inhibits the ALDH+ CSCs in lung cancer. (A) The CSC cells (ALDH+) and non-CSCs (ALDH−) from H358 cells were sorted using ALDH activity based commercial assay kit. qRT-PCR was performed to determine the expression of cancer stem cell markers plectin, ALDH1A3 and SOX2 in ALDH+ vs ALDH− cells. (B) ALDH+ cells and ALDH− cells were incubated with biotinylated-PCS2D1.2 and probed with streptavidin Q-dots 655 and visualized with fluorescent-microscope under long pass DAPI filter. (C) Anti-proliferative effects of PCS2D1.2 on ALDH+ CSCs and ALDH− non-CSCs from H358 cell line were determined using MTS assay.
Time-course MTS assay:
H358 lung cancer cells (2500/well) were plated in triplicate on a 96-well plate and allowed to adhere overnight. For the time-course study, data were recorded for 6 days at 24 hours interval. On day 1, cells were treated with varying concentration of PCS2D1 and with negative control PC462D1. On day 2, 20 μL/well of MTS solution was added to one set of wells and incubated for 2 hours, and absorbance was recorded at 490 nm using a plate reader. On the remaining 4 sets of wells, media were removed and replenished with drug treatment concentrations. Every 24 hours, the process was repeated for the next four days, data were recorded for 5 days for the time course study.
Clonogenicity assay:
[For Day 1 treatment] 300 H358 lung cancer cells were plated in a 24-well plate and allowed to attach for overnight. 24 hours after cells plating, cells were treated with homodimer PCS2D1.2, monomer PCS2 and non-active control PC462D1. On day 13 from cells plating when colonies were visible by naked eyes, wells were washed with PBS and stained with 20% crystal violet solution in methanol (EMD Millipore Sigma) for 10 minutes followed by washing with distilled water three times to remove the excess stain. [For Day 12 treatment] After cells were plated, first those were allowed to form colonies. On day 12 from the plating of cells, wells were treated with PCS2D1.2 for 24 hours, on day 13 wells were washed PBS stained with 20% crystal violet solution in methanol. The number of solid colonies per was counted using ImageJ software.
Scratch/Wound healing assay:
150,000 H358 lung cancer cells were plated in a 24-well plate and allowed to incubate for 48 hours to form a monolayer. A cross-section scratch was made in each well, and after washing with PBS, wells were treated with increasing concentrations of PCS2D1.2, PCS2 and control PC462D1 prepared in growth media. 48 hours of the treatment cells were stained with 20% crystal violet solution in methanol, and photographs were taken at 10X microscope. Area of the scratch was calculated using ImageJ software.
Cell surface plectin detection using Q-dots:
H358 cells were sorted in ALDH+ and ALDH− cells (see Supplementary Materials Fig. S10). To these cells, 500 nM of biotinylated-PCS2D1.2 was incubated for 1 hour. Cells were washed three times and incubated with Qdot 655 Streptavidin Conjugate as per manufacturer instructions (Thermofischer Scientific, https://assets.thermofisher.com/TFS-Assets/LSG/manuals/mp19000.pdf ) for 30 mins. Cells were washed three times and visualized under a fluorescent microscope (Olympus).
Gene expression analysis:
Relative gene expression was performed through RT-qPCR. Cells were lysed in 100 μL iScript RT-qPCR Sample Prep Reagent (Bio-Rad), vortexed for 30 seconds and then centrifuged. 12 μL of the lysate was added to iScript cDNA synthesis kit (Bio-Rad) to a total volume of 20 μL and run for 1 hour at 42°C. 2 μL of each cDNA was added to a mixture containing: 17.5 μL Fast Start Essential DNA Probes Master, 13.75 μL water, 1.75 μL TaqMan GAPDH (VIC) probe (Thermo Fisher), and 1.75 μL of either Hs00167476_m1 TaqMan ALDH1A3 (FAM), Hs01053049_s1 TaqMan SOX2 (FAM), or Hs00356986_g1 TaqMan PLEC (FAM) probe (Thermo Fisher). Each sample was then mixed, pipetted in triplicate at a volume of 10 μL per well, and run on a Lightcycler 96 (Roche) under the program: 95°C for 10 minutes and 40 cycles of 95°C for 10 sec and 60°C for 30 seconds. Relative expression was calculated as an average of the triplicate using the ddCT method, with GAPDH expression as the normalization control, and standard deviation calculated and noted by error bars.
Tumor xenograft study:
Six to eight weeks old male nu/nu nude mice were purchased from Charles River (Wilmington, MA). The mice were maintained in pathogen-free conditions and used following the protocol approved by the Institutional Animal Care and Use Committee (IACUC) of University of Houston. H358 cells (1 x 106) were subcutaneously injected into the right flanks of the mice to establish tumor xenografts. PCS2D1.2 (40mg/Kg) and saline (as control) were administered by i.p. injection every other day for 13 days. Day 15 was recorded as the drug withdrawal point. Weights were record every other day for 15 days. Two axes of the tumor (L, longest axis; W, shortest axis) were measured using a caliper. Tumor volume was calculated as: V = L × W2/2.
Immunofluorescence study:
Slides were fixed in cold acetone (−20 °C) for 10 minutes, followed by washing with PBST (0.05% tween-20 in PBS) three times. The tissues were blocked with 2% BSA (in PBS) for 1 hour at room temperature (RT). After removing the BSA, Plectin (Biotin conjugate) antibody (1/100 dilution) (Bioss antibodies) was added and incubated for 2 hours at RT. Slides were washed five times with PBST for 5 mins each and incubated with Streptavidin Q-dots 655 (1/200 dilution) for 1 hour. Slides were washed five times with PBST for 5 min and visualized under a fluorescent microscope using Long-pass filter.
3. Results:
3.1. Identification of tumor suppressing PCS2 dimer with optimized linker length
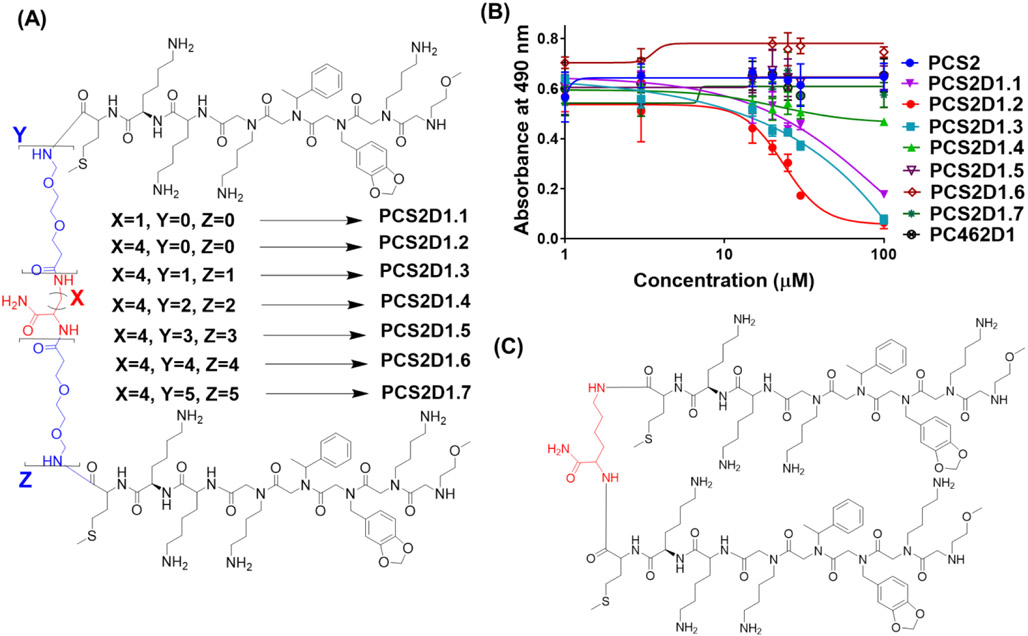
While our previous study found that PCS2 has highly specific affinity towards plectin, standard MTS cell cytotoxicity assay indicated that PCS2 has no cytotoxic effect (Fig. 1B). Since cell surface biomarkers are displayed with large number of copies, simple multimerization strategy has been successfully applied for ligand optimizations to extract the ‘avidity effect’ leading to improved affinity and activity [48-50]. We have previously utilized multimerization strategies to significantly improve our lead compounds, developing much improved homo [20, 21, 51-53] and hetero-dimeric [54, 55] peptoids with significantly higher potency than their monomeric units. While the length, geometry (i.e. rigidity/flexibility) and physiochemical properties of the linker connecting two monomeric components play crucial roles [48, 49, 56], we first connected two PCS2 monomeric units at the C-terminus with varying linker lengths to develop series of dimers. The 2,3-Diaminopropionic acid (DAP), Lysine (Lys) and Polyethylene glycol (PEG) moieties were opted to design linkers. These homo-dimeric compounds were synthesized using the fully on bead synthesis protocol that we developed [57] by applying the Fmoc solid-phase peptide synthesis (Fmoc-SPPS) approach. Briefly, a central residue that has two amine functionalities (Fig 1A – residue in red, e.g. DAP or lysine) was first loaded onto the resin, the linker region on both arms was extended (i. e. on amines) by adding varying number of linker moieties (Fig 1A – moieties depicted in blue, e.g. PEG), and finally two monomeric PCS2 units were grown simultaneously on those two arms. On each step, both Fmoc protection groups were removed to get a pair of NH2 groups opened to continue adding next two moieties. First, we synthesized the shortest linker containing compound PCS2D1.1 having DAP as the central residue (Figure 1A, red residue, X=1 atoms), and complete C-terminal linker. Next, we used Lysine as the central residue (Figure 1A, red residue, X=4 atoms), which has total of 3 carbons more than DAP as a longer linker to synthesize PSC2D1.2. From this point onwards, we used Lysine as the central linker and the linker length was further extended using increasing number of PEG moieties (Figure 1A, blue moieties), synthesizing total of five homo-dimers. Those homo-dimers had 2-10 PEG moieties as extended linker, to obtain PCS2D1.3, PCS2D1.4, PCS2D1.5, PCS2D1.6, and PCS2D1.7 compounds, respectively (Fig. 1A). These seven homo-dimers have a linker distance ranging between 05-110 Å.
Figure 1:
Chemical structure of PCS2 homo-dimers linked via a central linker to their C-terminal and their anti-proliferation activities. (A) PCS2D1.1 having DAP as its central linker, PCS2D1.2 having lysine as its central linker, and then PCS2D1.3, PCS2D1.4, PCS2D1.5, PCS2D1.6 and PCS2D1.7 having two, four, six, eight and ten PEG linkers respectively added to the central lysine. (B) Anti-proliferative effect was measured using MTS assay for homodimers, monomeric PCS2 and control PC462D1 on H358 lung cancer cells. (C) Chemical structure of the PCS2D1.2.
We determined the effects of the peptoid dimers on H358 Lung cancer cell proliferation using MTS assay, with monomeric unit PCS2 and non-active compound PC462D1 as controls. As shown in Figure 1B, the homodimer compounds inhibited cell proliferation in a dose-dependent manner, while the monomeric PCS2 and control PC462D1 had no effects. The PCS2D1.2 homo-dimer with lysine as the central linker (Fig. 1C) displayed the highest anti-proliferation activity with IC50 of ~ 18 μM. Homo-dimers having shorter linker or longer linkers than the lysine showed an incremental loss in the inhibition activity, and the longest linker containing homo-dimers were completely inactive.
3.2. PCS2D1.2 specifically binds to and inhibits the proliferation of Aldehyde Dehydrogenase (ALDH) positive CSCs.
ALDH activity has been used as a detection and isolation method for CSCs in lung tumors and cell line experiments [18]. To validate PCS2D1.2’s specificity towards CSCs, we sorted the H358 cells using ALDH assay kit (STEMCELL Technologies, Cambridge, MA). We quantified stem cell specific markers ALDH1A3 and SOX2, along with plectin and found that ALDH+ cells have significantly higher expression levels of these CSCs markers in comparison to ALDH− cells (Fig. 2A). Next, we incubated ALDH+ CSCs and ALDH− cells with biotinylated-PCS2D1.2 and probed with streptavidin red Q-dots 655 under fluorescent microscope. The data in Figure 2B shows that PCS2D1.2 specifically binds with ALDH+ CSCs and not to ALDH− cells. Further, we performed MTS assay to study the effects of PCS2D1.2 on sorted ALDH+ CSCs group alone (it is important to note that Fig. 1B MTS study is on unsorted H358 cell population, which has only about 17% of ALDH+ CSCs [58]. PCS2D1.2 was added to sorted ALDH+ CSCs and ALDH− cell groups separately (seeded 2,500 cells per well, plated to a 96 well plate) with varying concentration. MTS reagent was added to the wells after 24 hours, and absorbance data was recorded at 490 nm. As shown in Figure 2C, PCS2D1.2 exhibited a significant cytotoxicity on ALDH+ CSCs (IC50 ~0.85 μM) (24 hour treatment MTS), whereas PCS2D1.2 has no effects on ALDH− cells. More strikingly, this is a 20-fold better anti-cancer activity when PCS2D1.2 treatment on sorted ALDH+ CSCs in comparison to its anti-proliferative effects on the total cell population (Comparing Figures 1B and 2C).
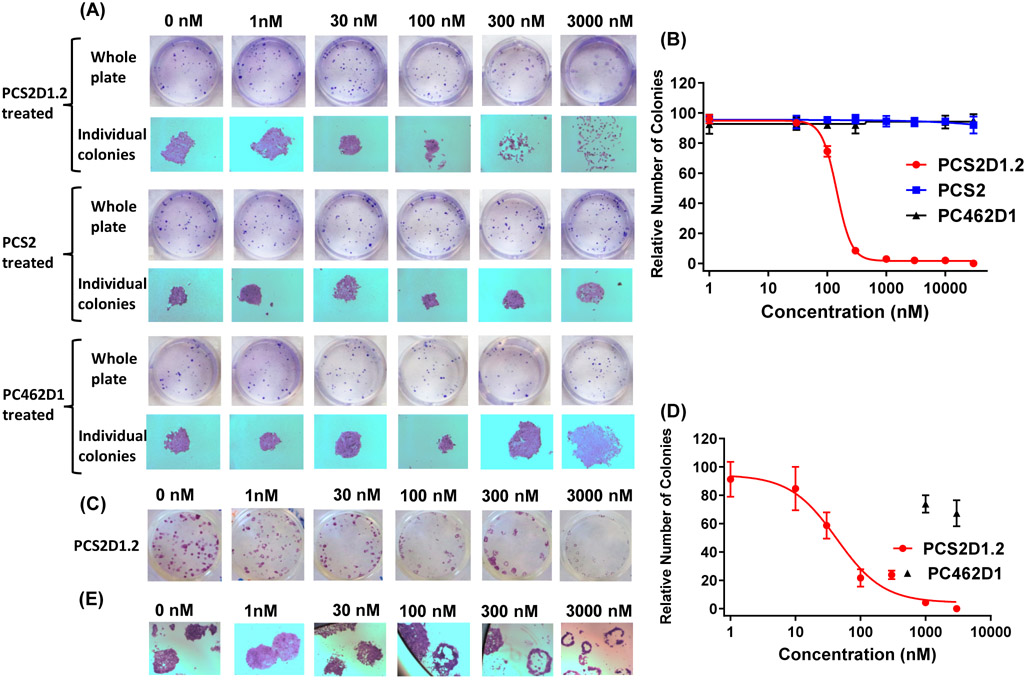
3.3. PCS2D1.2 disrupts in vitro colony formation of CSCs.
Clonogenicity assay is one of the standard assays to evaluate CSC activity. When cells are grown in low numbers and cell-cell communications are lost, CSCs survive and form colonies while non-CSCs will die. We performed the colony formation assay in two ways. In the first approach, we seeded H358 cells and treated with PCS2D1.2 from the day 1, with PCS2 and PC462D1 as controls. The properly formed colonies were counted and plotted. As shown in Figure 3A and Figure 3B, PCS2D1.2 disrupted the colony formation with an IC50 value of 144 nM. PCS2 and PC462D1 did not affect colony formation. In the second approach, we left the colonies to establish for 12 days and treated with PCS2D1.2, PCS2, or PC462D1, where we could assess the effects of PCS2D1.2 on CSCs within each colony. A careful examination of the individual colonies indicates that PCS2D1.2 effectively eliminated cells in the center of the colonies, which are mostly considered CSCs (Fig. 3C and 3E). The IC50 was found to be 60 nM (Fig. 3D). Both these approaches indicate that PCS2D1.2 specifically targets the CSCs over non-CSCs.
Figure 3:
PCS2D1.2 disrupts CSC lead colony formation. (A) Sparsely seeded H358 cells were treated with PCS2D1.2, PCS2 and PC462D1 on day 1. Colony formation assay was performed as described in Methods. (B) Graph-plot of number of properly formed colonies with concentrations of PCS2D1.2, PCS2 and PC462D1. The dispersed colonies were not counted. (C) Sparsely seeded H358 cells were first allowed to form colonies and treated with PCS2D1.2 on day 12. (D) Graph-plot of the number of solid colonies with concentrations of PCS2D1.2 and PC462D1. The colonies with no cells in the center area were not counted. (E) PCS2D1.2 depletes cells from the center of the individual colonies.
3.4. PCS2D1.2 decreases the expression of plectin and CSC markers, and disrupts cell proliferation over the time.
We studied how PCS2D1.2 affect H358 cell proliferation over a 6-day time course. As shown in Figure 4A, PCS2D1.2 significantly inhibited cell proliferation at 30 μM and 100 μM. We harvested the cells on day 6 and quantified known CSC markers ALDH1A3 and SOX2 along with plectin using qRT-PCR (Fig. 4B). PCS2D1.2 inhibited the expression of all 3 genes dose dependently. Interestingly, the effect on plectin started as low as 1 μM and the decrease of ALDH1A3 and SOX2 began at 10 μM, despite the overall anti-proliferative effect starting at 30 μM, indicating PCS2D1.2 specifically affects CSCs and has no effect on non-CSCs. In order to investigate the broader applicability of plectin targeting in lung cancer, we performed the same MTS studies in multiple other cell models as well. We picked diverse set of cell lines that include, H1693 (adenocarcinoma, NSCLC, stage 3B, female origin), H1299 (NSCLC carcinoma, male origin), H441 (papillary adenocarcinoma, male origin) and HCC4017 (large cell lung cancer, female). The data indicates a similar anti-prliferative effects as PCS2D1.2 did on H358 cells on all tested cancer cell types and is listed in supplementary Figure S11. Importantly, PCS2D1.2 had no effect on normal bronchial epithelial HBEC3KT cells (Supplementary Figure S11).
Figure 4:
PCS2D1.2 decreases plectin expression and cell proliferation. (A) H358 cells were treated with various concentrations of PCS2D1.2 and cell proliferation was assessed at an interval of every 24 hours for six days using MTS assay. (B) qRT-PCR analysis of CSC markers ALDH1A3, SOX2 and plectin from the harvested cells at the day 6.
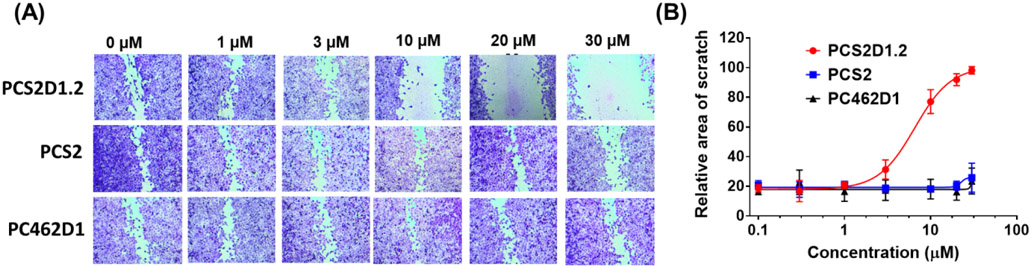
3.5. PCS2D1.2 disrupts the in vitro cell migration.
The wound healing assay indicates the mobility/motility of cells, which is another hallmark of CSCs. We studied the effects of PCS2D1.2 on cell migration using the wound healing assay. We found that PCS2D1.2 inhibited H358 cell migrations starting from 3 μM and significantly inhibited the process at 10 μM, 20 μM and 30 μM concentrations (Figure 5). In contrast, PCS2 and PC462D1 had no effect on cell migration.
Figure 5:
PCS2D1.2 inhibited cell migration. (A) H358 cells were cultured to form a monolayer in 24 well plate. A scratch was made and wells were treated with PCS2D1.2, PCS2 and control PC462D1 in concentrations between 1 – 30 μM. (B) Quantification of the distance of the scratch area with the treatment concentrations.
3.6. PCS2D1.2 decreases plectin expression and inhibited tumor xenograft growth in nude mice.
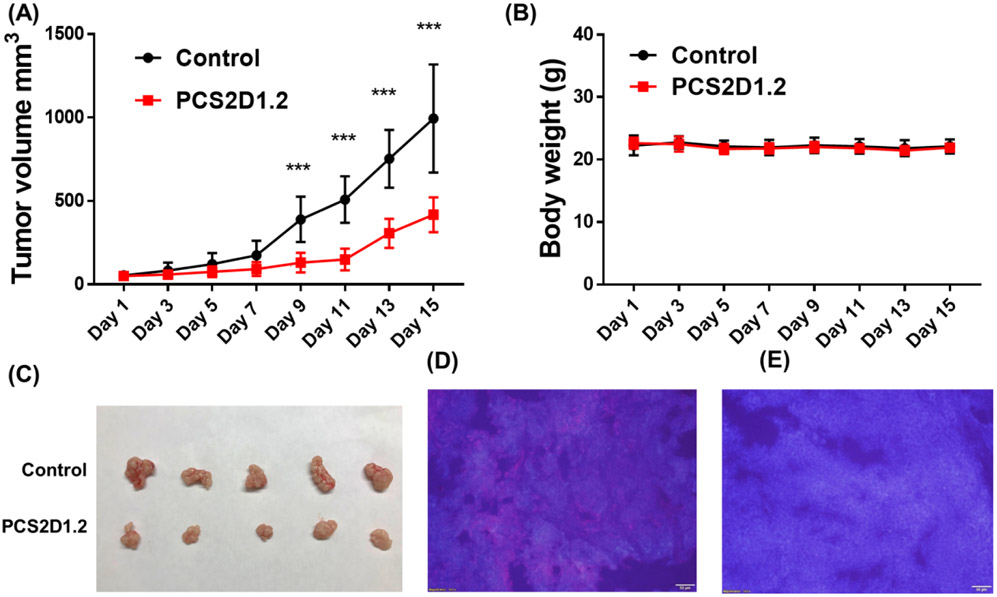
We determined the in vivo therapeutic efficacy of PCS2D1.2 in H358 xenografts in nude mice. After establishing subcutaneous tumor xenografts in nude mice, PCS2D1.2 and control-saline were administered to the mice through intraperitoneal injection every other day and the tumor volumes were measured using a caliper. At the dose of 40mg/kg of body weight, PCS2D1.2 significantly suppressed tumor growth in mice in comparison to the control compound (Fig. 6A). There was no effect on body weight of the mice during the treatment, indicating no toxicities to the host (Fig. 6B). The tumor tissues harvested from the mice showed a significant reduction in size with PCS2D1.2 treatment in comparison to the control (Fig. 6C). The tumor sections were subjected to immunohistochemical (IHC) studies using commercially available biotinylated plectin antibody and probed with streptavidin coated quantum dot (SA-Qdot 655). The red stain was only found in the untreated tumors (Fig. 6D) indicating the depletion of cell surface plectin by PCS2D1.2 in vivo (Fig. 6E).
Figure 6:
PCS2D1.2 inhibited tumor growth in vivo. (A) Nude mice carrying H358 xenografts were treated with PCS2D1.2 (red line) or control-saline (black line). Tumor volume measurements from day 1 −15 were done with a caliper. (B) The bodyweight measurement of mice during the treatment period. (C) The size difference of tumors treated with PCS2D1.2 and control-saline harvested after day 15. (D) and (E) IHC studies of control and PCS2D1.2 treated tumor sections, respectively. The plectin antibody bound streptavidin-red-Qdot 655 signal was probed using long pass DAPI filter (cells are indicated in blue color). Plectin was found only on control-saline treated tumors (D), and disappeared in PCS2D1.2 treated tumors (E).
4. Discussion:
In this study, we evaluated the therapeutic potential of our plectin-targeted CSC-specific peptoid PCS2 and its homo-dimeric derivatives. Although the monomeric PCS2 has no anti-cancer activity, we found significant anti-proliferative activity upon homo-dimerization of PCS2. The idea of testing seven dimers with a linker distance ranging between ~5 −110 Å was to allow monomers to bind intra- or inter-hots spots of plectin found on the cell surface. The optimum linker size of PCS2D1.2 is found to be around 9 Å, and this may indicate “intra-plectin binding”. Here, each monomer may bind to a binding site that is common for two of the repeat domains within the plectin C-terminal domain. The reduction of the activity upon shortening or increasing the linker (Fig. 1A-B) supports this hypothesis, as if PCS2D1.2 binds to such two adjscent repeat domains, a precise linker length is needed. If this hypothesis is correct, there are 6 of those similar repeat domains available within the 500 kDa plectin for future multimerizations, probably allowing to develop multimers up to hexamers.
We previously reported that plectin is found on the surface of CSCs from H358 cells and PCS2 specifically binds to the cell surface plectin [25]. Therefore, in this study, we first showed that sorted ALDH+ CSCs from H358 cells indeed express plectin (and other CSC markers) (Fig. 2A) and PCS2D1.2 has specific binding to ALDH+ CSCs and not to the remaining ALDH− H358 cancer cells (Fig. 2B). Our single treatment MTS studies indicated a strong cytotoxic effect of PCS2D1.2 on ALDH+ CSCs, but not on remaining ALDH− H358 cancer cells (Fig. 2C). More strikingly, we observed a 20-fold improved activity on CSCs in comparison to the treatment on overall H358 cell population (Fig. 1B). These data suggest that PCS2D1.2 explicitly targets plectin positive CSCs and has no effect on the non-CSC cancer cells. This was further and directly confirmed by the colony formation (Fig. 3) and wound-healing assay (Fig. 5) results. It is striking that PCS2D1.2 either destroyed or affected the integrity of the surviving colonies when treated from the beginning (Day 1), with an IC50 of about 144 nM. Those survived colonies become highly dispersed collection of cells in morphology (Fig. 3A – second row, last 2 panels). This indicates that most CSCs were not present upon PCS2D1.2 treatment to build a well-defined colony and those remaining dispersed cells may be differentiated cells. Given the high tumor heterogeneity, small number of CSCs that may not express cell surface plectin would have survived and initiated those dispersed colonies. The morphology of delayed (12 days) PCS2D1.2 treated colonies display even more striking feature, with those losing all cells in the center of the colony upon PCS2D1.2 treatment with an IC50 of about 60 nM (Fig. 3E). Typically, when the colony is built from a single CSC, the majority of center cells are still CSCs and outer cells become more differentiated. Our data suggests that PCS2D1.2 affect CSCs in the center area and have no effect on differentiated cancer cells (non-CSCs) on the periphery of the colony.
While PCS2D1.2 has strong cytotoxic effect on its direct target - CSCs with IC50’s of 60-144 nM, it needed higher doses to exhibit overall anti-cancer effects such as cell migration (IC50 ~ 6.5 μM - Fig. 5) and anti-proliferation (IC50 ~ 18 μM Fig. 1). This is understandable as such anti-cancer effects are secondary effects of rescinding CSCs and those assays measure the ‘overall’ effect on a population that has majority of non-CSCs (where our compound has no effect). In particular, H358 has only 17% of CSCs (ALDH+)[58] and PCS2D1.2 only affect that population, leaving large majority of non-CSC (ALDH−) cancer cells unaffected. However, the qRT-PCR studies indicates that expression of plectin and two CSC markers start to decrease at 1 μM and 10 μM of PCS2D1.2 treatments, respectively (Fig. 4B). This means the majority of CSCs are affected at these concentrations of PCS2D1.2 and again confirms the very high specificity of our PCS2D1.2 for plectin positive CSCs over non-CSCs.
Although the overall micromolar in vitro activity typically discourages moving into in vivo testing, we are encouraged to observe a significant in vivo anti-tumor activity of PCS2D1.2 in nude mice (Fig. 6). Importantly, there was no toxicity observed in the mice. In addition, IHC studies indicated that plectin was significantly reduced on the cancer cells and this suggests that the antitumor effect was due to PCS2D1.2 targeting plectin containing cells. Although an in vitro micromolar level small molecule usually does not display significant in vivo activity, the previous studies of our peptoid compounds uniquely display a pattern of showing strong in vivo antitumor effects despite in vitro IC50’s at micromolar levels [19-21, 51]. We believe that the unique features of peptoids such as high serum stability [24, 59], tissue and cell permeability [60, 61] and moderate clearance [62, 63] altogether provide better pharmacokinetic and pharmacodynamic effects to ultimately achieve stronger in vivo antitumor activities.
It has been shown that plectin plays an important role in the apical extrusion of KRAS V12 transformed cells from normal epithelial cells as part of a plectin-EPLIN (epithelial protein lost in neoplasm)-microtubule complex (in KRASV12 transformed cells), and the activation of the plectin complex may increase removal of newly emerging transformed cells from the epithelium [64]. This indicates that plectin could be a target for both early cancer prevention as well as elimination of CSCs in established cancers. There are multiple reports indicating that plectin is overexpressed in highly metastatic cells correlating with cancer progression and metastasis [26, 37, 38], which are generally considered as CSCs. More importantly, we recently showed the direct relationship of plectin expression in CSCs [25]. Therefore, targeting plectin for therapeutic intervention may be a promising anticancer strategy, as evidenced by Plecstatin-1, a metal-based plectin specific compound displaying significant inhibition of tumor invasion and in vivo antitumor activity against invasive B16 melanoma after oral administration [44]. However, the presence of plectin in cytosol of every cell calls for question whether plectin is a suitable therapeutic target. To this end, our recent study [25] and some other studies [26, 34, 39, 45] found that plectin indeed moves onto the cell surface in cancer cells, making a clear distinction from the cytosolic plectin that is universally present in normal cells. Two previous therapeutic studies also used plectin-directed nanoparticles that specifically targeted cell surface plectin to deliver gemcitabine in pancreatic cancer [46] and quinazolinediones to pancreatic ductal adenocarcinoma (PDAC) [47]. A peptide-conjugated liposomes formulation was also used to deliver chemotherapeutic drugs specifically targeting cell surface plectin to increase PARP inhibition in OVCAR8 tumors [45].
Our MTS studies in multiple other diverse set of lung cancer cell models such as H1693 (adenocarcinoma, NSCLC, stage 3B, female origin), H1299 (NSCLC carcinoma, male origin), H441 (papillary adenocarcinoma, male origin) and HCC4017 (large cell lung cancer, female) indicates the broader applicability of PCS2D1.2 in targeting plectin. In particular, H1693 and H1299 cells that are highly rich in CSCs[58, 65] displayed higher anti-prilferative effects. Therefore, we believe that PCS2D1.2 can be developed into a better and safer drug-lead that can specifically target plectin on the cell surface of CSCs. PCS2D1.2 could be developed into a drug that can be very useful in combination with chemotherapy, where CSCs often remain unaffected. While PCS2D1.2 selectively inhibits the CSCs, combining with traditional chemotherapy (such as carboplatin and/or paclitaxel, which kills the non-CSC cells) may prove to be an effective strategy to eliminate all cancer cells. Such combination therapy will be invaluable for overall patient survival at the clinic.
Supplementary Material
Highlights:
Optimization of plectin targeted, cancer stem cell (CSC) specific peptoid PCS2D1.2.
PCS2D1.2 display highly specific cytotoxicity towards CSCs over non-CSCs.
PCS2D1.2 effectively blocked the in vitro colony formation and cell migration.
PCS2D1.2 reduced the in vivo tumor formation.
In both in vitro and in vivo studies, PCS2D1.2 reduced plectin-rich CSCs.
Acknowledgments:
This research was funded by National Institute of Health grants NIH SPORE 5P50 CA70907 and P20CA221731 and University of Houston GEAR grants (Udugamasooriya) and National Institute of Health grant number CA186100 (Bin Guo). We thank Dr. John Minna at UT-Southwestern Medical Center for providing cell lines and important guidance on experiments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supporting Information Available: This material is available free of charge via the Internet.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References:
- [1].Aubry M, de Tayrac M, Etcheverry A, Clavreul A, Saikali S, Menei P, Mosser J, From the core to beyond the margin: a genomic picture of glioblastoma intratumor heterogeneity, Oncotarget 6(14) (2015) 12094–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Burrell RA, McGranahan N, Bartek J, Swanton C, The causes and consequences of genetic heterogeneity in cancer evolution, Nature 501(7467) (2013) 338–45. [DOI] [PubMed] [Google Scholar]
- [3].Grove O, Berglund AE, Schabath MB, Aerts HJ, Dekker A, Wang H, Velazquez ER, Lambin P, Gu Y, Balagurunathan Y, Eikman E, Gatenby RA, Eschrich S, Gillies RJ, Quantitative computed tomographic descriptors associate tumor shape complexity and intratumor heterogeneity with prognosis in lung adenocarcinoma, PloS one 10(3) (2015) e0118261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Liu H, Lathia JD, Editors, Cancer Stem Cells: Targeting The Roots of Cancer, Seeds Of Metastasis, And Sources Of Therapy Resistance, Elsevier Ltd.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Fulawka L, Donizy P, Halon A, Cancer stem cells--the current status of an old concept: literature review and clinical approaches, Biol Res. 47 (2014) 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Siegel RL, Miller KD, Jemal A, Cancer statistics, 2016, CA Cancer J Clin. 66(1) (2016) 7–30. [DOI] [PubMed] [Google Scholar]
- [7].Koren A, Motaln H, Cufer T, Lung cancer stem cells: a biological and clinical perspective, Cell Oncol. 36(4) (2013) 265–75. [DOI] [PubMed] [Google Scholar]
- [8].Leon G, MacDonagh L, Finn SP, Cuffe S, Barr MP, Cancer stem cells in drug resistant lung cancer: Targeting cell surface markers and signaling pathways, Pharmacol Ther. 158 (2016) 71–90. [DOI] [PubMed] [Google Scholar]
- [9].Reya T, Clevers H, Wnt signalling in stem cells and cancer, Nature 434(7035) (2005) 843–50. [DOI] [PubMed] [Google Scholar]
- [10].Beachy PA, Karhadkar SS, Berman DM, Tissue repair and stem cell renewal in carcinogenesis, Nature 432(7015) (2004) 324–31. [DOI] [PubMed] [Google Scholar]
- [11].Gurney A, Axelrod F, Bond CJ, Cain J, Chartier C, Donigan L, Fischer M, Chaudhari A, Ji M, Kapoun AM, Lam A, Lazetic S, Ma S, Mitra S, Park IK, Pickell K, Sato A, Satyal S, Stroud M, Tran H, Yen WC, Lewicki J, Hoey T, Wnt pathway inhibition via the targeting of Frizzled receptors results in decreased growth and tumorigenicity of human tumors, Proc Natl Acad Sci U S A. 109(29) (2012) 11717–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yen WC, Fischer MM, Axelrod F, Bond C, Cain J, Cancilla B, Henner WR, Meisner R, Sato A, Shah J, Tang T, Wallace B, Wang M, Zhang C, Kapoun AM, Lewicki J, Gurney A, Hoey T, Targeting Notch signaling with a Notch2/Notch3 antagonist (tarextumab) inhibits tumor growth and decreases tumor-initiating cell frequency, Clin Cancer Res. 21(9) (2015) 2084–95. [DOI] [PubMed] [Google Scholar]
- [13].Smith DC, Eisenberg PD, Manikhas G, Chugh R, Gubens MA, Stagg RJ, Kapoun AM, Xu L, Dupont J, Sikic B, A phase I dose escalation and expansion study of the anticancer stem cell agent demcizumab (anti-DLL4) in patients with previously treated solid tumors, Clin Cancer Res. 20(24) (2014) 6295–303. [DOI] [PubMed] [Google Scholar]
- [14].Liu J, Pan S, Hsieh MH, Ng N, Sun F, Wang T, Kasibhatla S, Schuller AG, Li AG, Cheng D, Li J, Tompkins C, Pferdekamper A, Steffy A, Cheng J, Kowal C, Phung V, Guo G, Wang Y, Graham MP, Flynn S, Brenner JC, Li C, Villarroel MC, Schultz PG, Wu X, McNamara P, Sellers WR, Petruzzelli L, Boral AL, Seidel HM, McLaughlin ME, Che J, Carey TE, Vanasse G, Harris JL, Targeting Wnt-driven cancer through the inhibition of Porcupine by LGK974, Proc Natl Acad Sci U S A. 110(50) (2013) 20224–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Deonarain MP, Kousparou CA, Epenetos AA, Antibodies targeting cancer stem cells: a new paradigm in immunotherapy?, mAbs 1(1) (2009) 12–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Okamoto OK, Perez JF, Targeting cancer stem cells with monoclonal antibodies: a new perspective in cancer therapy and diagnosis, Expert Rev Mol Diagn. 8(4) (2008) 387–93. [DOI] [PubMed] [Google Scholar]
- [17].Elkin SR, Bendris N, Reis CR, Zhou Y, Xie Y, Huffman KE, Minna JD, Schmid SL, A Systematic Analysis Reveals Heterogeneous Changes in the Endocytic Activities of Cancer Cells, Cancer Res. 75(21) (2015) 4640–4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sullivan JP, Minna JD, Tumor oncogenotypes and lung cancer stem cell identity, Cell Stem Cell. 7(1) (2010) 2–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gocke AR, Udugamasooriya DG, Archer CT, Lee J, Kodadek T, Isolation of antagonists of antigen-specific autoimmune T cell proliferation, Chem Biol. 16(11) (2009) 1133–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Matharage JM, Minna JD, Brekken RA, Udugamasooriya DG, Unbiased Selection of Peptide-Peptoid Hybrids Specific for Lung Cancer Compared to Normal Lung Epithelial Cells, ACS Chem Biol. 10(12) (2015) 2891–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Udugamasooriya DG, Dineen SP, Brekken RA, Kodadek T, A peptoid "antibody surrogate" that antagonizes VEGF receptor 2 activity, J Am Chem Soc. 130(17) (2008) 5744–52. [DOI] [PubMed] [Google Scholar]
- [22].Udugamasooriya DG, Kodadek T, On-Bead Two-Color (OBTC) Cell Screen for Direct Identification of Highly Selective Cell Surface Receptor Ligands, Curr Protoc Chem Biol. 4 (2012) 35–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Simon RJ, Kania RS, Zuckermann RN, Huebner VD, Jewell DA, Banville S, Ng S, Wang L, Rosenberg S, Marlowe CK, et al. , Peptoids: a modular approach to drug discovery, Proc Natl Acad Sci U S A. 89(20) (1992) 9367–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zuckermann RN, Kodadek T, Peptoids as potential therapeutics, Curr Opin Mol Ther. 11(3) (2009) 299–307. [PubMed] [Google Scholar]
- [25].Raymond AC, Gao B, Girard L, Minna JD, Gomika Udugamasooriya D, Unbiased peptoid combinatorial cell screen identifies plectin protein as a potential biomarker for lung cancer stem cells, Sci Rep. 9(1) (2019) 14954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Shin SJ, Smith JA, Rezniczek GA, Pan S, Chen R, Brentnall TA, Wiche G, Kelly KA, Unexpected gain of function for the scaffolding protein plectin due to mislocalization in pancreatic cancer, Proc Natl Acad Sci U S A. 110(48) (2013) 19414–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Fuchs P, Zorer M, Rezniczek GA, Spazierer D, Oehler S, Castanon MJ, Hauptmann R, Wiche G, Unusual 5' transcript complexity of plectin isoforms: novel tissue-specific exons modulate actin binding activity, Hum Mol Genet. 8(13) (1999) 2461–72. [DOI] [PubMed] [Google Scholar]
- [28].Ortega E, Manso JA, Buey RM, Carballido AM, Carabias A, Sonnenberg A, de Pereda JM, The Structure of the Plakin Domain of Plectin Reveals an Extended Rod-like Shape, J Biol Chem. 291(36) (2016) 18643–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Winter L, Wiche G, The many faces of plectin and plectinopathies: pathology and mechanisms, Acta Neuropathol. 125(1) (2013) 77–93. [DOI] [PubMed] [Google Scholar]
- [30].Bouameur JE, Favre B, Fontao L, Lingasamy P, Begre N, Borradori L, Interaction of plectin with keratins 5 and 14: dependence on several plectin domains and keratin quaternary structure, J Invest Dermatol. 134(11) (2014) 2776–2783. [DOI] [PubMed] [Google Scholar]
- [31].Favre B, Schneider Y, Lingasamy P, Bouameur JE, Begre N, Gontier Y, Steiner-Champliaud MF, Frias MA, Borradori L, Fontao L, Plectin interacts with the rod domain of type III intermediate filament proteins desmin and vimentin, Eur J Cell Biol. 90(5) (2011) 390–400. [DOI] [PubMed] [Google Scholar]
- [32].Osmanagic-Myers S, Rus S, Wolfram M, Brunner D, Goldmann WH, Bonakdar N, Fischer I, Reipert S, Zuzuarregui A, Walko G, Wiche G, Plectin reinforces vascular integrity by mediating crosstalk between the vimentin and the actin networks, J Cell Sci. 128(22) (2015) 4138–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Andra K, Lassmann H, Bittner R, Shorny S, Fassler R, Propst F, Wiche G, Targeted inactivation of plectin reveals essential function in maintaining the integrity of skin, muscle, and heart cytoarchitecture, Genes Dev. 11(23) (1997) 3143–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Bausch D, Thomas S, Mino-Kenudson M, Fernandez-del CC, Bauer TW, Williams M, Warshaw AL, Thayer SP, Kelly KA, Plectin-1 as a novel biomarker for pancreatic cancer, Clin Cancer Res. 17(2) (2011) 302–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Cheng CC, Liu YH, Ho CC, Chao WT, Pei RJ, Hsu YH, Yeh KT, Ho LC, Tsai MC, Lai YS, The influence of plectin deficiency on stability of cytokeratin18 in hepatocellular carcinoma, J Mol Histol. 39(2) (2008) 209–16. [DOI] [PubMed] [Google Scholar]
- [36].Hu L, Wu Y, Guan X, Liang Y, Yao X, Tan D, Bai Y, Xiong G, Yang K, Germline copy number loss of UGT2B28 and gain of PLEC contribute to increased human esophageal squamous cell carcinoma risk in Southwest China, Am J Cancer Res. 5(10) (2015) 3056–71. [PMC free article] [PubMed] [Google Scholar]
- [37].Katada K, Tomonaga T, Satoh M, Matsushita K, Tonoike Y, Kodera Y, Hanazawa T, Nomura F, Okamoto Y, Plectin promotes migration and invasion of cancer cells and is a novel prognostic marker for head and neck squamous cell carcinoma, J Proteomics. 75(6) (2012) 1803–15. [DOI] [PubMed] [Google Scholar]
- [38].McInroy L, Maatta A, Plectin regulates invasiveness of SW480 colon carcinoma cells and is targeted to podosome-like adhesions in an isoform-specific manner, Exp Cell Res. 317(17) (2011) 2468–78. [DOI] [PubMed] [Google Scholar]
- [39].Chaudhari PR, Charles SE, D'Souza ZC, Vaidya MM, Hemidesmosomal linker proteins regulate cell motility, invasion and tumorigenicity in oral squamous cell carcinoma derived cells, Exp. Cell Res 360(2) (2017) 125–137. [DOI] [PubMed] [Google Scholar]
- [40].Jordan MA, Wilson L, Microtubules as a target for anticancer drugs, Nat Rev Cancer. 4(4) (2004) 253–65. [DOI] [PubMed] [Google Scholar]
- [41].Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT, Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia, J Am Chem Soc. 93(9) (1971) 2325–7. [DOI] [PubMed] [Google Scholar]
- [42].Hayot C, Debeir O, Van Ham P, Van Damme M, Kiss R, Decaestecker C, Characterization of the activities of actin-affecting drugs on tumor cell migration, Toxicol Appl Pharmacol. 211(1) (2006) 30–40. [DOI] [PubMed] [Google Scholar]
- [43].Senderowicz AM, Kaur G, Sainz E, Laing C, Inman WD, Rodriguez J, Crews P, Malspeis L, Grever MR, Sausville EA, et al. , Jasplakinolide's inhibition of the growth of prostate carcinoma cells in vitro with disruption of the actin cytoskeleton, J Natl Cancer Inst. 87(1) (1995) 46–51. [DOI] [PubMed] [Google Scholar]
- [44].Meier SM, Kreutz D, Winter L, Klose MHM, Cseh K, Weiss T, Bileck A, Alte B, Mader JC, Jana S, Chatterjee A, Bhattacharyya A, Hejl M, Jakupec MA, Heffeter P, Berger W, Hartinger CG, Keppler BK, Wiche G, Gerner C, An Organoruthenium Anticancer Agent Shows Unexpected Target Selectivity For Plectin, Angew Chem Int Ed. 56(28) (2017) 8267–8271. [DOI] [PubMed] [Google Scholar]
- [45].Dasa SSK, Diakova G, Suzuki R, Mills AM, Gutknecht MF, Klibanov AL, Slack-Davis JK, Kelly KA, Plectin-targeted liposomes enhance the therapeutic efficacy of a PARP inhibitor in the treatment of ovarian cancer, Theranostics 8(10) (2018) 2782–2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Pal K, Al-Suraih F, Gonzalez-Rodriguez R, Dutta SK, Wang E, Kwak HS, Caulfield TR, Coffer JL, Bhattacharya S, Multifaceted peptide assisted one-pot synthesis of gold nanoparticles for plectin-1 targeted gemcitabine delivery in pancreatic cancer, Nanoscale 9(40) (2017) 15622–15634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Sanna V, Nurra S, Pala N, Marceddu S, Pathania D, Neamati N, Sechi M, Targeted Nanoparticles for the Delivery of Novel Bioactive Molecules to Pancreatic Cancer Cells, J Med Chem. 59(11) (2016) 5209–20. [DOI] [PubMed] [Google Scholar]
- [48].Kiessling LL, Gestwicki JE, Strong LE, Synthetic multivalent ligands in the exploration of cell-surface interactions, Curr. Opin. Chem. Biol 4(6) (2000) 696–703. [DOI] [PubMed] [Google Scholar]
- [49].Kiessling LL, Gestwicki JE, Strong LE, Synthetic multivalent ligands as probes of signal transduction, Angew. Chem., Int. Ed 45(15) (2006) 2348–2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Mourez M, Kane RS, Mogridge J, Metallo S, Deschatelets P, Sellman BR, Whitesides GM, Collier RJ, Designing a polyvalent inhibitor of anthrax toxin, Nat. Biotechnol 19(10) (2001) 958–961. [DOI] [PubMed] [Google Scholar]
- [51].Desai TJ, Toombs JE, Minna JD, Brekken RA, Udugamasooriya DG, Identification of lipid-phosphatidylserine (PS) as the target of unbiasedly selected cancer specific peptide-peptoid hybrid PPS1, Oncotarget 7(21) (2016) 30678–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Rustagi V, Udugamasooriya DG, Identification of side arm-modified DOTA scaffolds as multi-site binding ligands for cancer cells over normal cells, Bioorg Med Chem Lett. 29(19) (2019) 126619. [DOI] [PubMed] [Google Scholar]
- [53].Shukla SP, Manarang JC, Udugamasooriya DG, A unique mid-sequence linker used to multimerize the lipid-phosphatidylserine (PS) binding peptide-peptoid hybrid PPS1, Eur J Med Chem. 137 (2017) 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Kedika SR, Shukla SP, Udugamasooriya DG, Design of a dual ERK5 kinase activation and autophosphorylation inhibitor to block cancer stem cell activity, Bioorg Med Chem Lett. 30(23) (2020) 127552. [DOI] [PubMed] [Google Scholar]
- [55].Kedika SR, Udugamasooriya DG, Converting a weaker ATP-binding site inhibitor into a potent hetero-bivalent ligand by tethering to a unique peptide sequence derived from the same kinase, Org Biomol Chem. 16(35) (2018) 6443–6449. [DOI] [PubMed] [Google Scholar]
- [56].Vagner J, Xu L, Handl HL, Josan JS, Morse DL, Mash EA, Gillies RJ, Hruby VJ, Heterobivalent ligands crosslink multiple cell-surface receptors: the human melanocortin-4 and delta-opioid receptors, Angew Chem Int Ed. 47(9) (2008) 1685–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Hooks JC, Matharage JP, Udugamasooriya DG, Development of homomultimers and heteromultimers of lung cancer-specific peptoids, Biopolymers 96(5) (2011) 567–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Sullivan JP, Spinola M, Dodge M, Raso MG, Behrens C, Gao B, Schuster K, Shao C, Larsen JE, Sullivan LA, Honorio S, Xie Y, Scaglioni PP, DiMaio JM, Gazdar AF, Shay JW, Wistuba II, Minna JD, Aldehyde Dehydrogenase Activity Selects for Lung Adenocarcinoma Stem Cells Dependent on Notch Signaling, Cancer Res. 70(23) (2010) 9937–9948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Fowler SA, Blackwell HE, Structure-function relationships in peptoids: recent advances toward deciphering the structural requirements for biological function, Org Biomol Chem. 7(8) (2009) 1508–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Kwon YU, Kodadek T, Quantitative evaluation of the relative cell permeability of peptoids and peptides, J Am Chem Soc. 129(6) (2007) 1508–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Tan NC, Yu P, Kwon YU, Kodadek T, High-throughput evaluation of relative cell permeability between peptoids and peptides, Bioorg Med Chem. 16(11) (2008) 5853–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].De Leon-Rodriguez LM, Lubag A, Udugamasooriya DG, Proneth B, Brekken RA, Sun X, Kodadek T, Dean Sherry A, MRI detection of VEGFR2 in vivo using a low molecular weight peptoid-(Gd)8-dendron for targeting, J Am Chem Soc. 132(37) (2010) 12829–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Spicer SK, Subramani A, Aguila AL, Green RM, McClelland EE, Bicker KL, Toward a clinical antifungal peptoid: Investigations into the therapeutic potential of AEC5, Biopolymers 110(6) (2019) e23276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Kadeer A, Maruyama T, Kajita M, Morita T, Sasaki A, Ohoka A, Ishikawa S, Ikegawa M, Shimada T, Fujita Y, Plectin is a novel regulator for apical extrusion of RasV12-transformed cells, Sci Rep. 7 (2017) 44328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Gibbons DL, Lin W, Creighton CJ, Rizvi ZH, Gregory PA, Goodall GJ, Thilaganathan N, Du L, Zhang Y, Pertsemlidis A, Kurie JM, Contextual extracellular cues promote tumor cell EMT and metastasis by regulating miR-200 family expression, Genes & development 23(18) (2009) 2140–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.