Abstract
Drug control policy can have unintended consequences by pushing existing users to alternative, possibly more dangerous substances. Policies that target only new users may therefore be especially promising. Using commercial insurance claims data, we provide the first evidence on a set of new policies intended to reduce opioid initiation in the form of limits on initial prescription length. We also provide the first evidence on the impact of must-access prescription drug monitoring programs (MA-PDMPs), laws that do not target new users, on initial opioid use. Although initial limit policies reduce the average length of initial prescriptions, they do so primarily by raising the frequency of short prescriptions, resulting in increases in opioids dispensed to new users. In contrast, we find that MA-PDMPs reduce opioids dispensed to new users, even though they do not explicitly set out to do so. Neither policy significantly affects extreme use such as doctor shopping among new patients, because such behavior is very rare.
Keywords: opioids, initial prescription limits, must-access PDMPs, addiction
JEL codes: I18, I12
1. Introduction
Policymakers have a longstanding interest in regulating the use of potentially addictive substances. The use of many drugs is illegal, often through outright prohibition or through age restrictions, as in the case of alcohol and tobacco. Even when legal many substances are heavily taxed, in part to discourage use. Moreover, potentially addictive drugs are often formulated in such a way as to discourage abuse. For example, the long-acting opioid Oxycontin was reformulated in 2010 so that it could not be crushed and snorted to deliver an immediate, potent dose. Although such policies may deter drug and alcohol use, they have potentially harmful unintended consequences. Taxing cigarettes encourages substitution towards higher nicotine-content products (e.g. Evans and Farrelly (1998); Cotti et al. (2016)). When Oxycontin was reformulated, both its use and misuse fell, but many users substituted towards heroin, and opioid-related overdoses skyrocketed (Alpert et al., 2018; Evans et al., 2019). Harmful consequences arise because these policies not only deter new users, but they also target inframarginal users, who end up either bearing higher costs or substituting to more dangerous substances. In this paper, we ask whether substance use policies can deter new users without causing harmful compensatory behavior.
We study the regulation of addictive substances among new users in the context of prescription opioids. Opioid use and misuse are major public health concerns, as reflected in the high and rising number of opioid-related deaths. Opioids were involved in roughly 47,500 deaths in 2017—1.7% of the national total, an increase of 9.6% since 2016 and over 300% since 1999 (Scholl et al., 2019; Ruhm, 2018). Although the U.S. drug overdose death rate had reduced in 2018 (the first reduction in 25 years), data for 2019 show an increase in death rates by 5% over the previous year. Rising mortality, combined with widespread use and misuse of prescription and illegal opioids, has been recognized as a crisis. Policy responses to this crisis have entailed attempts to thwart prescription drug misuse, mitigate harm, and improve access to treatment. Most state policies have been ineffective in reducing opioid prescribing, as Meara et al. (2016) show in their thorough analysis. An exception, however, is must-access prescription drug monitoring programs (MA-PDMPs), which require prescribers to check state databases of controlled substance prescribing before writing an opioid prescription. These laws reduce opioid use and, especially, tail outcomes such as doctor shopping (filling prescriptions from several doctors) and extremely high levels of use, as Buchmueller and Carey (2018) show in an influential paper. However, they may unintentionally increase crime and illicit drug use (Mallatt, 2017; Dave et al., 2018).
In this paper we focus on a set of recently-enacted policies specifically designed to prevent new users from becoming addicted to opioids. These are laws that limit the strength and length of opioid prescriptions for new users with acute, non-cancer pain. Only two states had such laws prior to 2011, but by the end of 2018, 26 states had passed initial prescription limits. The typical law limits an initial prescription for acute pain to seven days supply; prior to such laws, it was possible to write a prescription for 31 days or longer (Davis et al., 2019a). Policymakers had two specific motivations in passing these laws. First, they hoped to limit unintentional addiction among new patients, who diligently take their prescription, not appreciating the risks of developing opioid addiction. For example, former Ohio Governor John Kasich, stated that “by setting aggressive prescription limits, we’re taking big steps to prevent opiate addition from happening in the first place” (Kasich, 2017). Consistent with this concern, observational evidence indicates that the probability of becoming a chronic opioid user rises sharply in the length of the initial prescription (Shah et al., 2017). Second, large initial prescriptions create a stock of drugs available for diversion to non-prescribed users. Not all patients consume the entire prescription and policymakers hoped to reduce diversion possibilities by reducing the number of leftover opiates. Diversion of prescriptions is considered the source of drugs for a large fraction of current misusers (Lipsari and Hughes, 2017), and prior research shows state policies may reduce diversion (Surratt et al., 2014).
We evaluate whether state opioid policy can influence initial prescriptions, including both the hazard and intensity of new use. We estimate difference-in-differences models using health insurance claims data from a national commercial insurer. As our focus is new use, our sample is restricted to cancer-free, initially “opioid-na´’ve” patients, who had not filled an opioid prescription in at least the prior 12 months.
Our focus on new users is novel to the literature and there are at least two reasons to care about this group. First, while harm reduction among all existing users is important, reducing initiation is the most promising avenue to improving opioid use disorders in the longer run. Second, to the extent that opioids are addictive, it may be much harder to discourage existing users than to prevent new use, and efforts to discourage existing users may be more likely to induce substitution towards more dangerous illicit drug use.
Our claims data, which begin in 2007 and run through April of 2018, offer several advantages over previously used data to study opioid use and prescribing. Much of the prior research that has examined the effect of state laws has used state-level aggregate data on dispensed totals derived from DEA records or from Medicaid. It is not possible to study new users with these data, making it difficult to examine laws targeting initial prescriptions. Recent research has also used individual level Medicare claims data. Opioid use is common among Medicare beneficiaries, but those data give an incomplete picture of opioid use in the United States. Moreover, Medicare data are available generally with a 2–3 year lag, which makes it hard to study effects of state initial prescription limit laws.
We begin by describing the characteristics of new opioid use. Every month, about 1 percent of those with no prior opioid use fill an opioid prescription. Our first finding is that initial prescriptions are fairly short, only 6.4 days on average. Even in states without a limit on initial prescription length, 80 percent of initial prescriptions last for 7 days or fewer, and 91 percent are for 14 days or fewer; this is true even in the earliest years of our data.1 As the modal law limits initial prescriptions for acute pain to 7 days or fewer, there is therefore little scope for these laws to bite. Some laws impose tighter limits; however, we find that even in states with 3–5 day limits, initial prescriptions are often for longer than the statutory amount.
Our second finding is that, nonetheless, these laws work to reduce the length of the initial prescription. Initial limit policies reduce the probability of an initial prescription exceeding 7 days by 21 percent, and the laws reduce the average length of the initial prescription by about half a day. However, rather than reduce initial prescription length by reducing the frequency of long prescriptions, initial limit policies increase the frequency of short prescriptions. In particular, we find that the laws induce a 0.12 percentage point increase in the hazard of new prescriptions lasting 1–7 days. The laws also generate a 0.02 percentage point reduction in the hazard of prescriptions lasting longer than 7 days. The net effect, however, is a 8.7 percent increase in the hazard of new prescriptions, as well as an (imprecisely estimated) 9.6 percent increase in the total amount of opioids dispensed to new patients. These results suggest that initial prescription limits are ineffective in reducing opioids dispensed to new users. One possible mechanism for these surprising results is that physicians could interpret the initial prescription limits as signalling the safety or acceptability of short prescriptions.
While limits on initial prescriptions appear ineffective at reducing mean opioid consumption among new users, they could still reduce low-probability events such as dangerous levels of consumption or doctor shopping. We find no evidence to support this view. Limits on initial prescriptions are not associated with reductions in the probability of having high days supply, high daily dose, overlapping claims, or doctor shopping in the initial spell. We caution, however, that such extreme events may take many months or years to develop from the initial prescription, and these laws may be too recently passed to detect effects on these outcomes.
Overall, our evidence suggests that initial prescription limits have not been effective in their goal of reducing opioids dispensed in the initial spell. This finding complements contemporary work by Davis et al. (2019b) and Lowenstein et al. (2019). Davis et al. show that initial prescription limits do not affect overall opioid dispensing, but they do not examine new users, the targeted group. Lowenstein et al. examine outpatient clinics in New Jersey and Pennsylvania and find, like we do, that initial prescription limits reduce the average length of new prescriptions, but they do not consider the extensive margin. Our results, which use national data, show that, rather than reduce the rate of long prescriptions, these laws largely increase the rate of short prescriptions.
Although initial prescription limits appear ineffective at reducing new opioid use, other state prescribing policies that were pursued during these years may be more effective. We therefore also examine the impact of MA-PDMPs on new use. These policies are important to study because they were widely implemented in recent years, reduce dangerous use among existing users, but may induce substitution towards illegal substances. Reducing use among new users would be an important benefit. We find that MA-PDMPs indeed deter new use, decreasing the hazard of initial prescriptions by about 4.7 percent. However, like initial limit policies, they have no impact on markers of dangerous use among new enrollees in the short run. These finding are novel to the literature, which has not considered the impact of MA-PDMPS on new use.2,3 Overall, our findings suggest that initial prescription limits may not be particularly effective for reducing new use, but that MA-PDMPs may be even more influential in affecting opioid prescribing than previously thought, as they reduce both new as well as extreme use.
2. Background
2.1. Opioid prescribing in the United States
The focus of our study is opioid analgesics, which are prescription painkillers. Prior to the 1990s, opioids were prescribed relatively rarely, because physicians were concerned about addiction, and downplayed pain management. Prescribing increased in the 1990s, when the medical community began to define pain as the “fifth vital sign” and increasingly acknowledged the historic under-treatment of pain (Merboth and Barnason, 2000; Mularski et al., 2006; Tompkins et al., 2017). Simultaneously, Oxycontin—a high dose, extended release opioid—was launched for use in the U.S. in 1996 and was subsequently heavily marketed as having a lower potential for abuse than existing (short-acting) opioids (Van Zee, 2009).
These two changes in the culture of pain management were followed by a rapid increase in both opioid prescriptions and opioid-related mortality in the United States. In the recent past, the United States has prescribed an exceptional amount of opioids. For example, from 2007 to 2009 Americans consumed about 15 doses of opioids per person per year, twice as many opioids per capita as did the second ranking nation, Canada, and 7.5 times as many as the average European country (International Narcotics Control Board, 2010). Opioid prescribing in the United States has declined about 10 percent from its 2010 peak, but remains extremely high relative to other countries (Centers for Disease Control and Prevention, 2018).
This high rate of opioid prescribing has alarmed policy makers and public health officials. It raises the possibility that individuals will develop dependence disorder, and that a large volume of opioids may be diverted, i.e. consumed by those without a valid prescription; indeed, 10.7 million people reported misusing prescription pain killers in 2013–2014 (Lipsari and Hughes, 2017). The rise in opioid use is particularly alarming as it has been accompanied by a dramatic rise in opioid related mortality. Deaths from natural and semi-synthetic opioids (including prescription opioids) roughly quadrupled between 2000 and 2016, and deaths from heroin and synthetic opioids (such as fentanyl, which is dramatically stronger than heroin) have grown even more rapidly (Hedegaard et al., 2017; Ruhm, 2018). The increase in opioid-related mortality has been so large that it has led to an increase in overall mortality rates for some cohorts of non-Hispanic white Americans (Case and Deaton, 2015; Kochanek et al., 2016).
High opioid use could increase opioid-related mortality through several channels. First, prescription opioids are potentially addictive, meaning that patients develop tolerance, requiring ever stronger and closer to fatal doses to manage their pain. (White and Irvine, 1999). Second, even at low levels, opioids can be dangerous when taken in combination with benzodiazepines (e.g. Xanax), a commonly prescribed class of sedatives (White and Irvine, 1999; Jones et al., 2012). Benzodiazepines were involved in 3 percent of opioid-analgesic-related overdoses in 2011 (Chen et al., 2014). Third, for some, prescription opioids act as a gateway to stronger and more dangerous drugs such as heroin and fentanyl, which carry a higher risk of overdose, as has been observed with Oxycontin’s reformulation (Alpert et al., 2018; Evans et al., 2019).
2.2. Policy responses
There have been several dimensions of U.S. policy responses to limit opioid prescribing and use. Our focus is on limits on initial prescriptions for acute pain and on MA-PDMPs, two sets of recently enacted laws that we describe in more detail below. Other policy responses include features designed to either reduce access or reduce harm associated with use. Examples of such policies include abuse-deterrent reformulation, and rescheduling of low-strength opioids to make them harder to obtain and refill. There are also a host of state-specific policies that in earlier times, Meara et al. (2016) find to be largely ineffective in reducing opioid prescribing. In addition to state-specific policies, the CDC issued prescribing guidelines in July 2016, urging prescribers to avoid writing long and strong opioid prescriptions (Dowell et al., 2016). Some insurers have also responded by limiting opioid access, for example with prior authorization requirements (Dillender, 2018), as have some individual health care systems.4
Initial prescription limits
In the last few years, many states have enacted laws that limit initial prescriptions for acute pain among new opioid users. Davis et al. (2019a) describe these laws in more detail and report on the dates of implementation. Panel A of Figure 1 reports which states have such limits in effect in each year, and Appendix Table A.1 provides further details. As of 2006, only Missouri had such a law (which was passed decades ago) and from 2007 to 2013 only three additional states enacted initial limits. In 2016 four more states passed initial limits, followed by 17 in 2017, and one in 2018. By the end of 2018, 26 states had some form of limits on initial opioid prescriptions for acute pain.5
Figure 1:
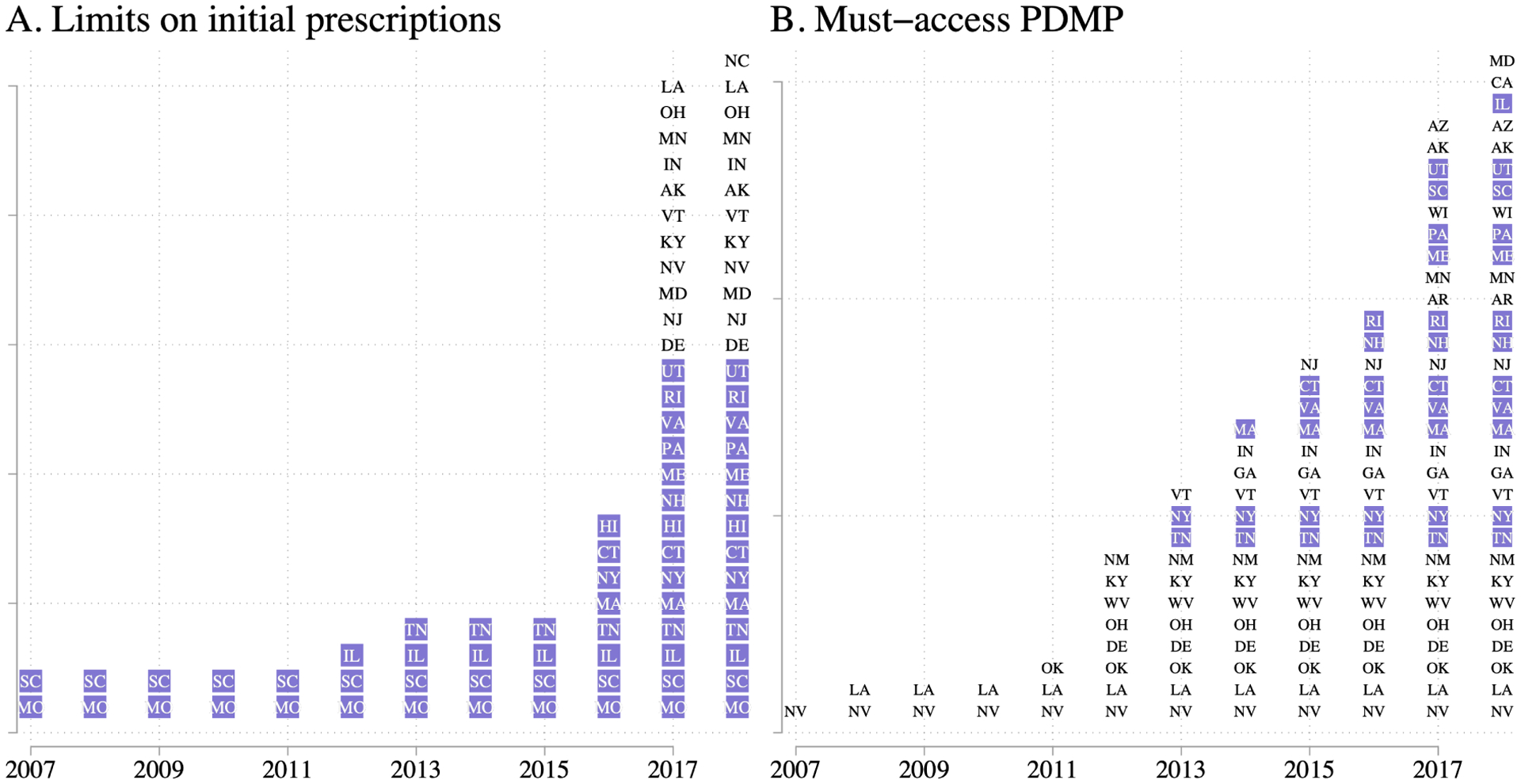
Initial prescription limits and MA-PDMPs in effect, by year
Notes: For each year of our data, we report in Panel A the set of states with limits on the initial prescription in effect, and in Panel B the set of states with MA-PDMPs in effect. Source: Davis et al. (2019a) for panel A; pdaps.org and http://www.namsdl.org/prescription-monitoring-programs.cfm for panel B. The states with the purple background are those that passed their initial prescription limits on or before March 2017. We consider these states to be early adopters. We choose this designation such that our main analysis will have at least one year of data following the implementation of the law.
There are reasons to believe that limiting opioid prescriptions to patients will reduce the likelihood of problematic future opioid use. Using a small-sample survey of non-medical opioid users, Butler et al. (2016) show how an initial medical opioid prescription affects the hazard of future non-medical use; reported median time from initial medical prescription to the first non-medical use was six months for misuse for non-medical reasons, and 18 months for use to avoid withdrawal. Barnett et al. (2017) show that patients quasi-exogenously assigned to emergency department doctors with higher opioid prescribing rates are themselves more likely to get a high-strength opioid, and substantially more likely to engage in long-term opioid use than those assigned to low-prescribing physicians.
These laws are heterogeneous in several dimensions. All but one limits the days supply, with limits ranging from 3 days to 35 days. The most common limit, used in 13 states, is 7 days. In addition to restricting prescription length in days, eight states also limit the strength of the prescription, to a certain morphine-milligram equivalent per day (e.g. 30 or 90). Many of the laws—16—allow for exceptions based on professional judgment or for surgical pain. We show below that the statutory limits set by these laws do not always bind, particularly for states with very short (3–5 day) limits.
Policymakers appear to have two specific goals for these laws, beyond a general desire to reduce opioid addiction, overdose, and mortality. First, they hope to prevent unintentional addictions, which could happen if patients dutifully comply with a long prescription, not realizing the addictive potential of opioids. Axeen et al. (2018) show that the majority of opioid prescriptions originate from office-based visits rather than originating from the ED, underlining how policies geared towards altering primary care or specialist prescriptions may be more effective than setting limits of prescriptions written in ED settings. Second, policymakers hope to reduce the supply of opioids available for diversion. Large initial prescriptions create the potential for diversion because not everyone consumes the entire prescription. Data from the 2015 National Survey of Drug Use and Health show that of those who reported misusing opioids, 60 percent did so without a valid prescription and 40 percent obtained the drugs at no cost from a relative or friend (Han et al., 2017). For both goals, a key question for these policies is whether they actually reduce the total volume of opioids dispensed to new users.
Throughout our main analyses will focus on states that adopted initial prescription limits on or before March 2017. We call these states early adopters. We choose this designation such that our main analyses will have at least one year of data following the implementation of the law (our outcomes data run until April 2018). However, this restriction is not important for our findings as we find similar results regardless of implementation date used for inclusion. We present results including all initial limit restricting states in the Appendix.
MA-PDMPS
PDMPs are state-wide databases recording all prescriptions dispensed (within a certain class, such as schedule II-IV narcotics). PDMPs enable prescribers to better detect drug-seeking behavior (such as a patient with prescriptions from many different providers), as well as potentially dangerous co-prescribing (such as benzodiazepines with opioids). PDMPs are extremely common—every state but Missouri had one by July 2016—but not all states require prescribers to access the database before writing a prescription. Buchmueller and Carey (2018) argue that prescribers rarely access a PDMP unless they are required to do so, and they show that only MA-PDMPs have an effect on opioid use. We therefore focus on must-access provisions of PDMPs.
We code and date MA-PDMPs following the logic of Buchmueller and Carey (2018) as closely as possible; while their data go through mid-2012, we extend the coding of MA-PDMPs through 2018. We begin by using the database provided by pdaps.org, the same source as Buchmueller and Carey (2018). This database records whether each state has a PDMP, the date of PDMP implementation, and the rules around access to PDMP. As the database runs through July 2016, we obtain more recent coding from the National Alliance for Modal State Drug Laws (NAMSDL).6 NAMSDL coding is current for laws passed through January 2018, the last month of initial prescriptions we study. We define a MA-PDMP as a PDMP which requires at least some providers to access it in at least some circumstances, consistent with prior research.7 Panel B of Figure 1 reports which states have MA-PDMPs in effect in each year, and Appendix Table A.1 provides further details. Thirty-two states have enacted MA-PDMP legislation by the end of our sample period; our data thus cover the 10 states enacting MA-PDMPs studied by Buchmueller and Carey (2018), as well as an additional 22 states subsequently enacting MA-PDMPs.
3. Data and empirical strategy
3.1. Data
We use a comprehensive database of commercial insurance claims from a large, national insurer, with both employer-sponsored plans and Affordable Care Act Exchange plans. Our database covers claims from January 2007 through April 2018. Every state is represented in every month. For budgetary reasons we have access to a 20 percent random sample of all enrollees during this period, as well as a 100 percent sample of all patients who ever filled an opioid prescription.8 When studying new fill rates, we use the 20 percent sample; when studying characteristics of opioid use, we use the second sample, to maximize power.
To identify opioid fills, we merged in a database containing the National Drug Code for all prescription opioids (Center for Disease Control and Prevention, 2018). This database also contains a conversion factor to put different opioids on a common scale, morphine-equivalent doses (MED), which converts units of a given opioid into milligrams of morphine. We measure the length of a prescription by its days supply, and the total amount of opioids prescribed as the “morphine-equivalent days” (MED), the product of days supply, morphine equivalent doses, and strength. The modal opioid in the claims data is 5-mg hydrocodone, which has an MME of 1, so a prescription for 7 days supply would have an MED of 35.
Sample construction and initial spells
Our analysis focuses on new users, which we define as enrollees who fill a prescription for an opioid after at least 12 months without any opioid fills, and who were continuously enrolled for at least 12 months at the time of the initial fill. We limit the sample to the continuously enrolled to avoid misclassifying new enrollees as new users. Our sample is also limited to enrollees with both prescription drug and medical coverage. Because we require a look-back period for defining new users, our analysis sample begins in January 2008. Finally, we exclude patient-years that have a cancer diagnoses, because the prescription limits we study do not apply to cancer patients. We are interested in how MA-PDMPs and initial limit policies affect not only the initial prescription but also subsequent use. We therefore consider outcomes during the “initial spell,” which we define as the initial prescription and any prescriptions filled during the subsequent 90 days. We look at the initial spell in addition to the initial prescription because we want to measure whether shorter initial prescriptions are offset by longer subsequent prescriptions. We focus on 90 days because our data run through April 2018, and the last limit on initial prescriptions in our data was passed in December 2017. Thus, 90 days allows us to study all law changes and still have two months of post-period data following each new law. To avoid censored outcomes, we further limit our sample to those who were continuously enrolled for at least three months beyond their initial prescription. We use the same criteria for the construction of both the extensive and intensive margin samples. That is, we only include enrollees without a cancer diagnosis, who are continuously enrolled for one year prior to the policy implementation and for three months following implementation. Appendix Table A.2 shows how the sample size changes as we impose our selection criteria. The final denominator data consists of 6.9 million enrollees, and the final numerator data consists of 17.3 million enrollees with at least one prescription for a controlled substance.9
We aggregate our data to state-month cells, and conduct all analyses at this level, weighting by the appropriate sample size. These cells give the average outcomes among enrollees whose new use begins in the given state-month. For example, the cell California-January-2016 reflects the experiences between January and March of all enrollees in California who initiated use in January 2016, denominated by the number of opioid naive enrollees in California as of January 2016.
Outcomes
We construct several outcomes related to the initial prescription and the initial spell. We start with measures of average use: days supply and days MED in the initial prescription and the initial spell, as well as number of fills in the initial spell. We obtain the spell-level outcomes by aggregating over all prescriptions filled within three months of the initial prescription. For example, if a patient’s initial prescription was for seven days and she obtained an additional seven days supply prescription after eight days, we would say her initial spell had 14 days supply. If each prescription was for 10 mg hydrophone (with an MED of 1), we would say she had 70 days MED for her initial prescription and a daily MED of 1.56 for her initial spell.10
We are interested not only in standard prescription outcomes but also in extreme events which may be indicative of dangerous use or abuse. To examine extreme outcomes, we consider five definitions of potentially problematic opioid use during two different initial spell lengths (90 and 270 days).11. The first is an indicator for greater than 90 (or 270) days supply, meaning that on average over the initial spell, the patient had more than one days supply per day. Next, we define an indicator for greater than 120 MME per day, another measure of potentially dangerous use. Third, we look at doctor shopping, defined as having prescriptions from three or more prescribers, a measure of drug-seeking behavior. Finally, we look at two measures of overlapping claims: overlapping opioid prescriptions (defined as one opioid prescription beginning at least 7 days before the prior one ends), and concurrent benzodiazepine use (defined as at least one day in which a patient has both benzodiazepine and opioid days supply). We include this last outcome because benzodiazepines and opioids are particularly dangerous when taken in combination (e.g. Dasgupta et al. (2016)). These measures largely parallel those in Buchmueller and Carey (2018), but they are less extreme, as appropriate for our focus on new users. For example, Buchmueller and Carey (2018) define doctor shopping as having prescriptions from five or more prescribers. We focus on less extreme outcomes because we find very few signs of potentially dangerous opioid use relative to their Medicare sample, and we have more power with the less extreme outcomes.
Summary statistics
We report summary statistics for new spells in Table 1. Our sample consists of about 10.3 million new spells. The hazard of new spell initiation is just over 0.01, meaning roughly one percent of previous non-users initiate use in a given month. The modal prescription is written by a physician. Primary care providers write about 20 percent of all prescriptions, while pain specialist-written prescriptions are rare (at least among new users). Dentists prescribe 14 percent of initial prescriptions, and nurse practitioners and physicians’ assistants collectively prescribe less than 10 percent.
Table 1:
Summary statistics on new spells
| A. Extensive margin | Hazard rate | ||
|---|---|---|---|
| New fill | 0.011 | ||
| B. Initial prescription characteristics | Mean | Median | 90th percentile |
| Days supply | 6.38 | 4 | 12 |
| Days MED | 49.83 | 25 | 90 |
| Pills per days supply | 6.52 | 6 | 10 |
| Long acting | 0.01 | ||
| Prescriber type | |||
| Physician | 0.57 | ||
| Primary care provider | 0.20 | ||
| Surgeon | 0.13 | ||
| Pain specialist | 0.002 | ||
| Dentist | 0.14 | ||
| Nurse practitioner | 0.03 | ||
| Physicians’ assistant | 0.05 | ||
| Unknown | 0.18 | ||
| C. Spell characteristics | Mean | Median | 90th percentile |
| Days supply | 10.90 | 5 | 26 |
| Daily MED | 114 | 30 | 160 |
| Number of fills | 1.47 | 1 | 3 |
| Greater than 1 fill | 0.27 | ||
| Number of prescribers | 1.18 | 1 | 2 |
| D. Dangerous use indicators (within 90 days of Rx) | Mean | ||
| ≥ 90 days supplied | 0.014 | ||
| ≥ 120 daily MED | 0.001 | ||
| ≥ 3 prescribers | 0.027 | ||
| Overlapping opioid claims | 0.033 | ||
| Concurrent benzodiazepine | 0.071 | ||
| E. Dangerous use indicators (within 270 days of Rx) | Mean | ||
| ≥ 270 days supplied | 0.006 | ||
| ≥ 120 daily MED | 0.001 | ||
| ≥ 3 prescribers | 0.071 | ||
| Overlapping opioid claims | 0.044 | ||
| Concurrent benzodiazepine | 0.080 | ||
| Count of new spells | 10,292,576 |
Table reports summary statistics for new spells. Sample is limited to spells following a period of 12 or more months of non-use, among cancer-free people continuously enrolled for the 12 months prior to the spell starting, and for the three months after the spell starts. We report dangerous use indicators conditional on opioid use. Data in panel E, indicators of dangerous use within 270 days of a prescription, are from those patients that are continuously enrolled for at least ten months following their initial prescription.
The average initial prescription is for slightly less than one week, which foreshadows the limited scope for these laws to affect the modal existing prescription. About a quarter of initial spells involve more than one fill. Looking across all fills in the initial spell, total days supply averages 10.9. This demonstrates the importance of considering a period beyond the initial prescription; as looking just at the initial prescription misses 40 percent of use in the first three months. The average days MED is about 114, roughly equivalent to taking one 5-mg hydrocodone pill every four days over the initial spell. There are few signs of dangerous use among new users, in either the first 90 or 270 days of opioid use and relative to the Medicare opioid users studied by Buchmueller and Carey (2018). For example, about 0.1 percent of new users have a very high daily MED, and about 3 percent have an overlapping claim. The corresponding numbers among all Medicare opioid users are 1.7 and 9 percent (Buchmueller and Carey, 2018).
The summary statistics suggest that initial prescriptions are fairly short, meaning limits on initial prescriptions may not bind often. We show this more clearly in Figure 2, which plots the distribution of days supply in states and months that do not have initial prescription limits (in unshaded bars) and in early-adopting state and months with limits of 7 days or fewer (in shaded bars). The figure shows that even in states and months without any limits, most initial prescriptions are short. Indeed, 80 percent are for 7 days or fewer, 90 percent for 14 days or fewer, and 95 percent for 20 days or fewer.12 Thus on their face it is unlikely initial prescription limits will have an effect for most new users, at least unless the limits are fairly tight. In Figure 3, we show further that initial prescriptions do not always conform to the statutory limits on days supply. The figure plots, for each early-adopting state with an initial prescription limit, the share of initial prescriptions exceeding the limit (in months when the limits are in effect, and separately in months when the limits are not in effect). Prescriptions are more likely to be conforming when limits are in effect. However, for states with low limits, as many as half of initial prescriptions exceed the limits. These exceptions may well be legitimate (as the laws often allow for exceptions for those with non-acute pain such as cancer), but they provide further evidence that the laws often have limited bite.
Figure 2:
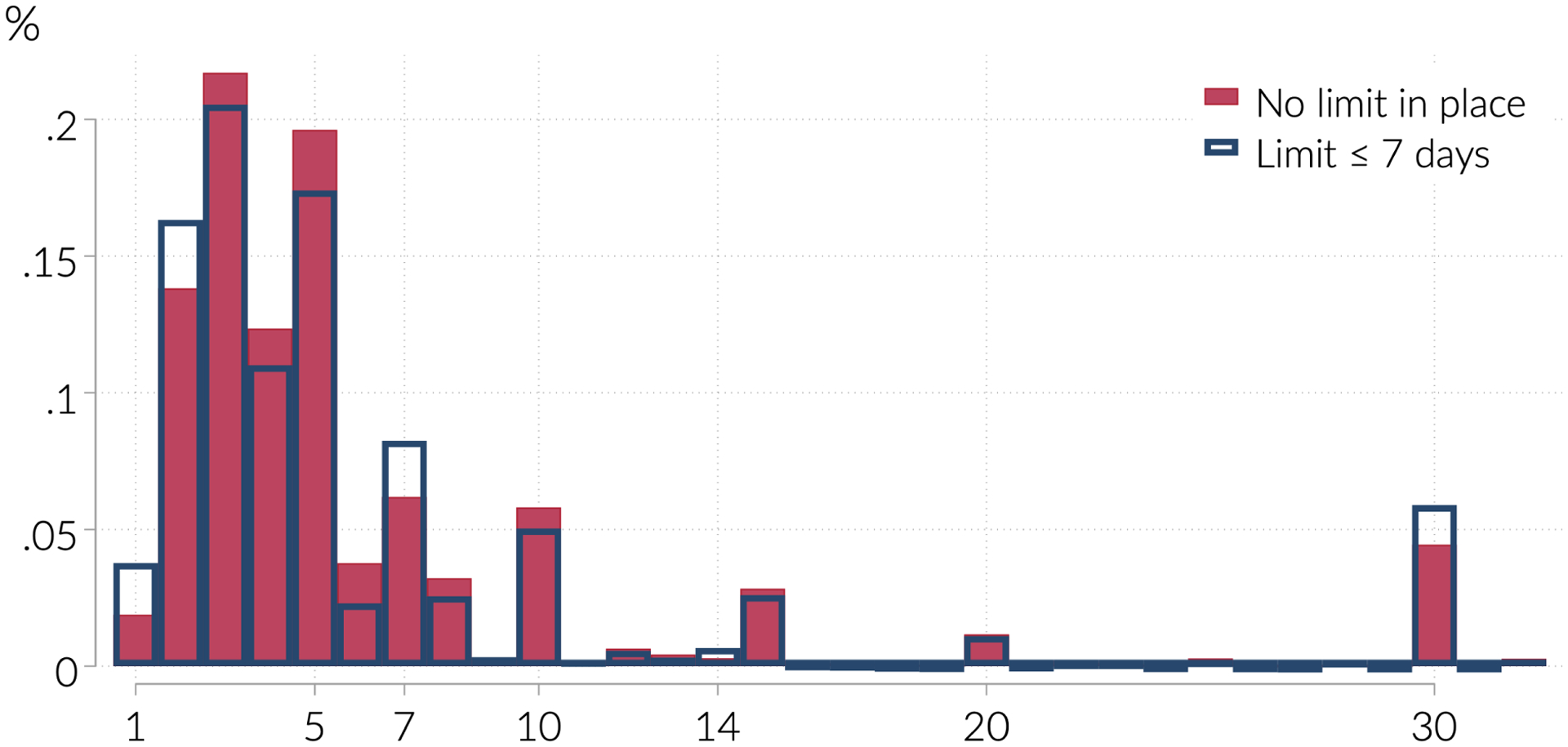
Distribution of days supply in initial prescription
Notes: Figure shows the proportion of initial prescriptions with the indicated number of days supply, for states which never or have not yet implemented limits on days supply (hollow bars) and for states which have implemented limits of 7 days supply or fewer (solid bars) by March of 2017. Including all initial prescription states does not meaningfully alter the distribution.
Figure 3:
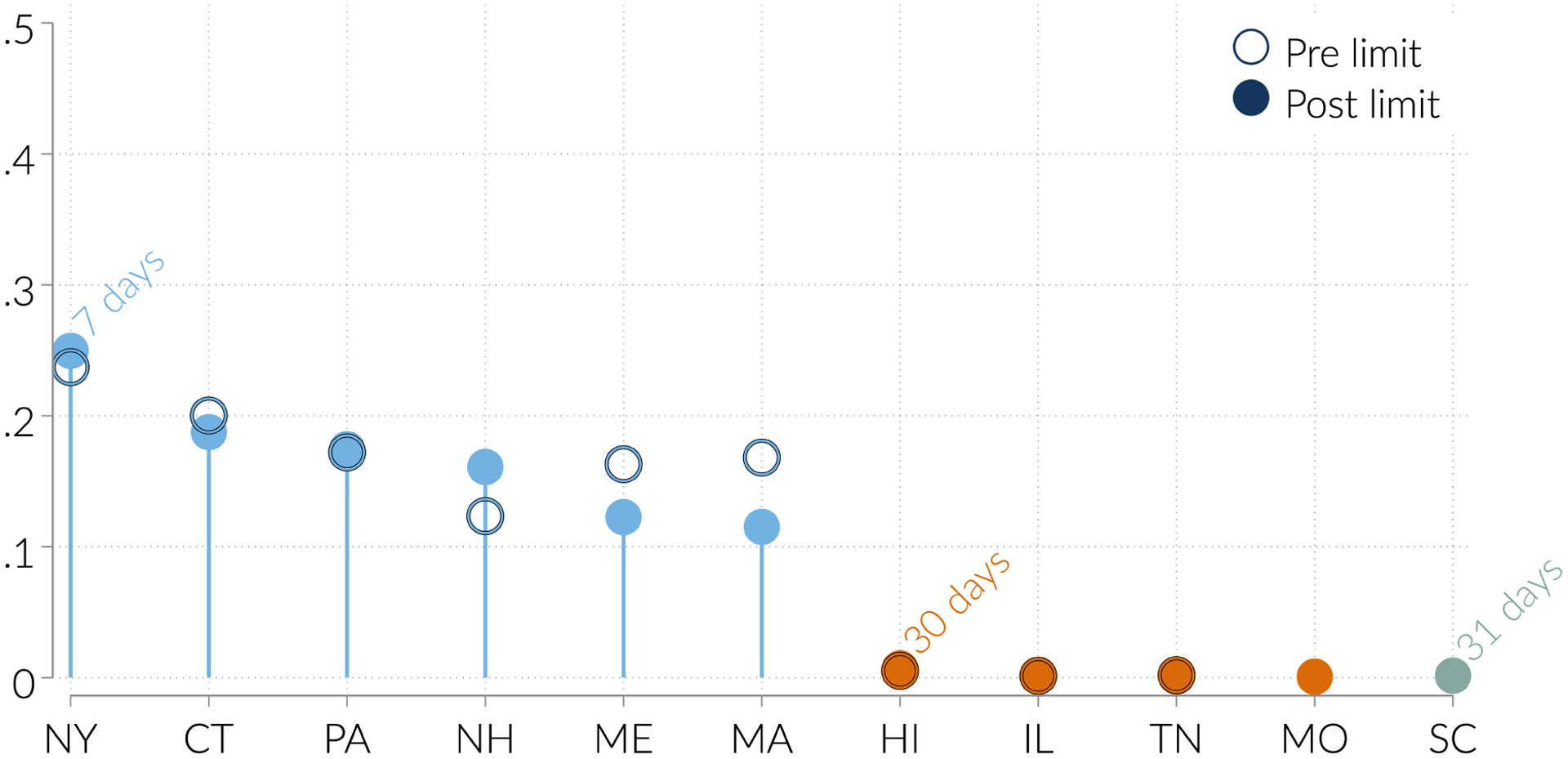
Share of initial prescriptions above days supply limit, by state
Notes: For each state with a limit on days supply in effect by March 2017, we calculate the fraction of initial prescriptions above the statutory limit, using the sample described in the data section.
3.2. Empirical strategy
Estimating equation
Our goal is to estimate the effect of limits on initial prescriptions and MA-PDMPs on opioid utilization among new users. We therefore estimate regressions of the following form:
| (1) |
The specification controls for state and time (month-by-year) fixed effects. Our interest is in α and β, which give the differential change in yst among states implementing limits on initial prescriptions or MA-PDMPs, relative to the change for control states.
Limitst indicates if a state limits prescriptions for new opioid users to 14 days supply or fewer. This definition excludes 7 states with a limit on the initial prescription longer than 14 days.13 We chose this cutoff because the longer limits appear to rarely bind. (See Figure 2.) In principle we could analyze each limit separately, but power concerns motivated us to pool all limits 14 days and shorter. In our main analysis, we focus on states that adopted initial prescription limits by March of 2017. We consider robustness to narrower and wider definitions of Limit, including removing the early-adopter restriction in results in the appendix.
MAPDMPst is an indicator for states requiring at least some providers to check a PDMP in at least some circumstances, consistent with Buchmueller and Carey (2018). However some states require checking only in limited (such as only pain clinics) or discretionary (such as with a suspicion of abuse) circumstances. In robustness checks, we omit states with limited or discretionary access requirements.
The key coefficients, α and β, have a causal interpretation if changes in limits on initial prescriptions and MA-PDMPs are uncorrelated with other state-level changes in opioid use. Our time and state fixed effects control for many potential confounds. The time fixed effects, for example, control for national policies and guidelines like Oxycontin’s reformulation and the CDC’s prescribing guidelines. The state fixed effects control for permanent differences across states in propensity to prescribe opioids.
One concern is that initial prescription limits and MA-PDMPs are passed in conjunction with other opioid-related regulations. Our main specification does not include additional state-policy controls because prior research has found that other opioid-related policies have no effect on opioid prescribing (Meara et al., 2016). In robustness tests, however, we include as additional controls a set of indicators for non-opioid policies that might affect opioid prescribing: indicators for state Medicaid expansions, and for legal prescribing of medical or recreational marijuana.14
Threats to identification
We remain particularly concerned with two broad threats to our identifying assumptions. First, some states implemented packages of reforms, so there many other law changes at the same time that PDMPs become must-access or limits on initial prescriptions are introduced. Second, states hit hardest by the opioid epidemic may have been most likely to implement these reforms. While our state fixed effects control for time-invariant cross-state heterogeneity, we remain concerned about differential trends. Specifically, we are worried that high-use states have experienced particularly large declines in opioid use since the middle of our sample period. This is because policy makers and physicians have become aware of the dangers of opioid prescribing, prescribing has fallen, and it has had the most room to fall in high use states.
We address these concerns in turn. To address the first concern, we control for MA-PDMPs; other policies are not robustly associated with declines in opioid use (Meara et al., 2016; Buchmueller and Carey, 2018). We also note that we end up finding positive coefficients on the initial prescription limits. The concern that limits are implemented along with other legal changes would imply a downward bias in our estimate, and so, if anything, would suggest that we have underestimated the degree to which initial prescription limits increase opioid prescribing.
We address the second concern by estimating event study models which allow us to explore if there are differential trends in the pre-period. We do not find evidence for such pre-trends. This is reassuring because it suggests that slow-moving trends in opioid use or prescribing do not drive our estimates. Nonetheless it is possible that these tests are underpowered to detect fast-moving trend shifts that could drive both opioid prescribing and state lawmaking.
4. Results
We begin by showing that initial prescription limits increase use on the “extensive margin,” raising the probability of receiving an initial opioid prescription among the opioid naive and the total amount dispensed to opioid-naive enrollees. MA-PDMPs reduce use on this margin. We turn to the intensive margin to show that neither policy affects the amount received conditional on receiving an initial prescription. Next, we show that neither policy has an effect on extreme or dangerous use in the short run among new users. Finally, we present a variety of evidence establishing the validity and robustness of our our findings.
4.1. Extensive margin
Table 2 reports our extensive margin estimates. The first column shows the effect on the hazard of new use. Whereas we might have expected that initial prescription limits simply reduce the length of written prescriptions, with no extensive margin effect, we find that these limits are associated with an 8.7 percent increase in the hazard of new fills. MA-PDMPs are associated with a decrease in this outcome that is slightly smaller in magnitude, about 4.7 percent. Both estimates are statistically significantly different from zero and from each other.
Table 2:
Extensive margin effects
| Any initial prescription (×100) (1) |
Days MED in initial prescription (2) |
|
|---|---|---|
| Limits | 0.100 (0.022) |
0.055 (0.035) |
| PDMP | −0.054 (0.015) |
−0.035 (0.053) |
| α = β | 0.000 | 0.303 |
| Mean DV | 1.155 | 0.593 |
Notes: Table reports estimates of the coefficients on indicators for “initial prescription limits” and “must access PDMPs”, obtained from estimating OLS regressions of the indicated variables against policy indicators, state fixed effects, and month-year fixed effects. MED is morphine equivalent days. The row labelled α = β reports p-values of the hypothesis that the coefficients on MA–PDMP and Limit are equal. Robust standard errors, clustered on state, in parentheses.
Although initial prescription limits therefore increase the number of new prescriptions, they could reduce the total amount of opioids dispensed to new patients if they result in substantially shorter prescriptions. In Column (2) we show that that morphine-equivalent days dispensed to new patients does not decrease following the enactment of initial prescription limits. The coefficient, which is not statistically significant, implies a 9.3 percent increase in the quantity of opioids dispensed. Note that this specification is unconditional in that it includes patients who do not receive prescriptions. Intensive margin results are presented in Table 4.
Table 4:
Intensive margin effects
| Initial Prescription | Initial Spell | |||||
|---|---|---|---|---|---|---|
| > 7 Days supply (1) |
Days supply (2) |
Days MED (3) |
# Fills (4) |
Days supply (5) |
Days MED (6) |
|
| −0.044 (0.011) |
−0.477 (0.220) |
2.247 (3.001) |
0.034 (0.019) |
−0.458 (0.481) |
5.789 (9.461) |
|
| PDMP | −0.006 (0.009) |
−0.091 (0.159) |
−0.091 (3.841) |
0.023 (0.009) |
0.046 (0.348) |
−1.866 (13.124) |
| α = β | 0.007 | 0.106 | 0.708 | 0.493 | 0.340 | 0.728 |
| Mean DV | 0.206 | 6.531 | 51.311 | 1.475 | 11.164 | 118.354 |
Notes: “DS” stands for days supply, and “MED” is morphine equivalent doses. Table reports estimates of the coefficients on indicators for “initial prescription limits” and “must access PDMPs”, obtained from estimating OLS regressions of the indicated variables against policy indicators, state fixed effects, and month-year fixed effects. The sample is limited to initial prescriptions, the unit of observation is a state-month, and all estimates are weighted by the number of initial prescriptions. The row labelled α = β reports p-values of the hypothesis that the coefficients on MustAccessPDMP and Limits are equal. Robust standard errors, clustered on state, in parentheses.
We further investigate the surprising increase in prescribing in Figure 4. To construct the figure, we estimate Equation 1 with “filled an initial prescription for d days supply” as the dependent variable, and plot the estimated coefficients on Limits and MustAccessPDMP. The figure shows large clear increases in the hazard of having a short initial prescription induced by initial limit policies, with increases at each days supply up to seven. Aggregating over all the shorter responses, we find that the initial prescription limits increase the hazard of initial prescriptions for 1–7 days supply by 0.12 percentage points (s.e = 0.02). There are small decreases in the hazard of having a long prescription which collectively amount to a reduction in the hazard of longer initial prescriptions of 0.02 percentage points (s.e. = 0.01). The decreases in longer prescriptions are small enough that they do not nearly offset the increase in short prescriptions. Initial prescription limits therefore do not decrease opioids dispensed to new patients, because they increase short prescriptions, without sufficiently reducing long prescriptions. MA-PDMPs, by contrast, reduce initial prescriptions written throughout the days supply distribution.
Figure 4:
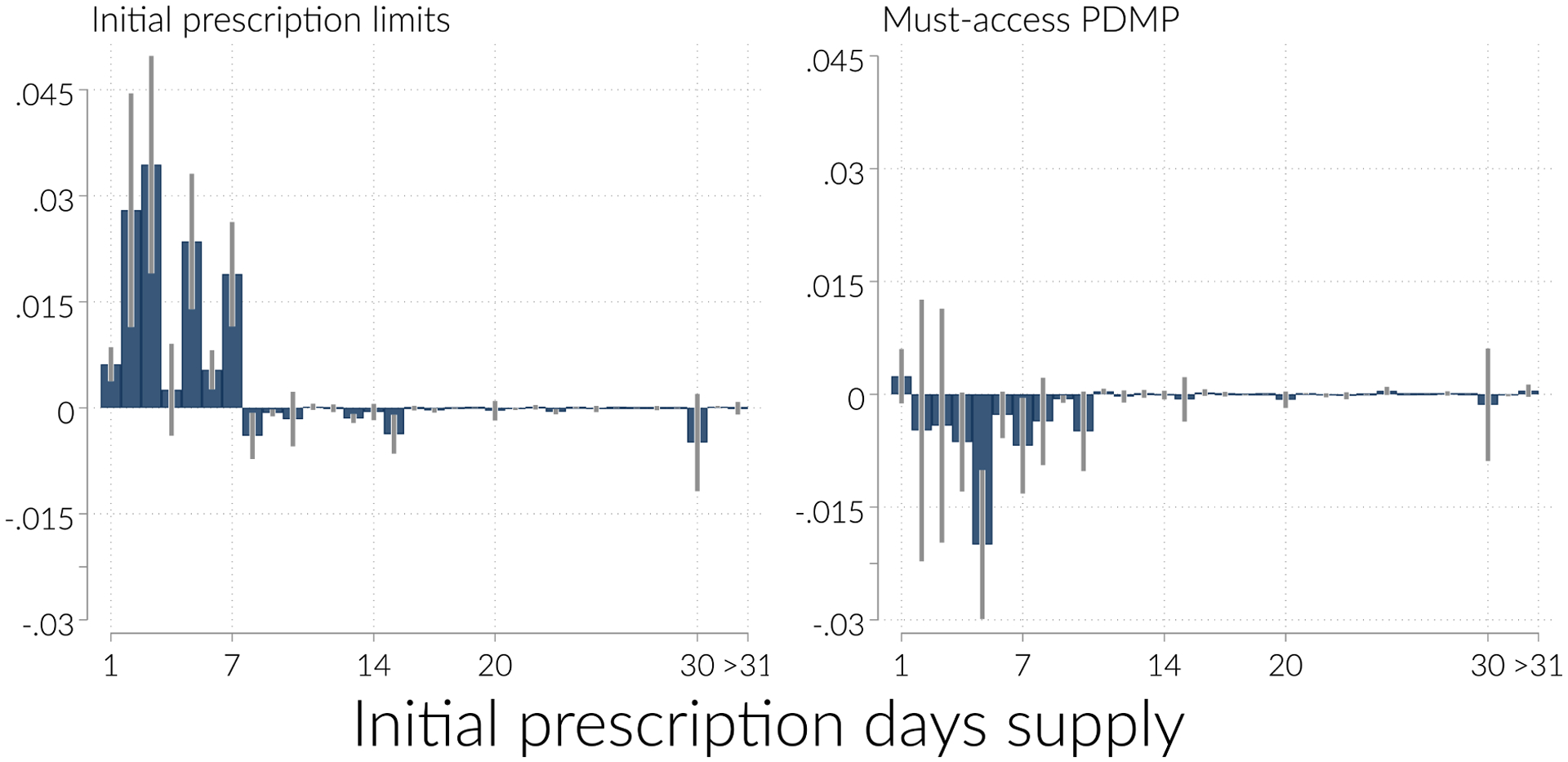
Effect distribution of days supply
Notes: Figure shows the estimated coefficient on initial prescription limits (left panel) and MA-PDMP (right panel), obtained from Equation 1 with indicators for “initial prescription is for d days supply” as the dependent variable. Each point in each panel is from a separate regression.
Who drives this extra prescribing? In Table 3, we look at the hazard of filling a new opioid prescription from specific types of providers. We find that physicians explain most of the response, as initial prescription limits induce a 0.088 increase in the hazard of new physician prescribing. Among physicians, primary care providers are especially responsive, as they account for 67 percent of the overall response (0.059/0.088) but 20 percent of opioid prescribing. Other provider types are all relatively unresponsive to the initial prescription limits. Although other factors such as physician education may influence physician response to these policies (e.g. Schnell and Currie (2018)), we lack data on physician characteristics to explore this question further.15
Table 3:
Extensive margin effects by provider type
| Filled a prescription written by a: (×100) | ||||||
|---|---|---|---|---|---|---|
| Physician (1) |
PCP (2) |
Surgeon (3) |
Pain MD (4) |
Dentist (5) |
NP or PA (6) |
|
| Limits | 0.088 (0.014) |
0.059 (0.010) |
−0.001 (0.003) |
−0.000 (0.000) |
−0.008 (0.006) |
−0.001 (0.012) |
| PDMP | −0.031 (0.020) |
−0.017 (0.014) |
0.001 (0.003) |
−0.000 (0.000) |
−0.010 (0.010) |
−0.002 (0.007) |
| α = β | 0.000 | 0.000 | 0.560 | 0.191 | 0.873 | 0.941 |
| Mean DV | 0.747 | 0.261 | 0.168 | 0.002 | 0.172 | 0.082 |
Notes: PCP is primary care provider, NP nurse practitioner, PA physician’s assistant. Table reports estimates of the coefficients on indicators for “initial prescription limits” and “must access PDMPs”, obtained from estimating OLS regressions of the indicated variables against policy indicators, state fixed effects, and month-year fixed effects. MED is morphine equivalent days. The row labelled α = β reports p-values of the hypothesis that the coefficients on MustAccessPDMP and Limits are equal. Robust standard errors, clustered on state, in parentheses.
4.2. Intensive margin
We now turn to examine outcomes for patients who receive an initial prescription for an opioid. In Table 4, we present estimates of our main equation, looking at characteristics of the initial prescription (in Columns 1–3) and the initial spell (in Columns 4–6), conditional on filling an initial opioid prescription.
The results in Columns (1) and (2) show that both initial prescription limits and MA-PDMPs reduce the length of the initial prescription, conditional on getting an initial prescription. Initial prescription limits reduce the length of the initial prescription received by about 7 percent, and reduce the probability that the initial prescription exceeds 7 days supply by 21 percent. On their own, these estimates might suggest that initial prescription limits succeed in their goal of shortening initial prescriptions. Combined with the extensive margin results, however, they indicate only that the limits increased the number of short prescriptions.
We might have feared that prescribers or patients responded to initial prescription limits with stronger or more frequent prescriptions. We do not see much evidence of such offsetting behavior. Column (3) shows that conditional on getting an initial prescription, limits do not significantly affect the days MED (a measure of opioid quantity). Columns (4) shows that patients fill 2% more prescriptions in response to the law. Columns (5) and (6) show that patients also do not obtain substantially greater overall days supply, or greater days MED over the course of their initial spell. Thus conditional on getting an initial prescription, initial prescription limits appear to have minimal effects for the average patient—their main effect is simply increasing the frequency of short prescriptions.
Of course, the small average effects reported in Table 4 may mask meaningful responses in the tails of the opioid use distribution. We investigate tail behavior in Table 5. We consider five measures of extreme use (and all measures condition on receiving an initial prescription). We examine each outcome across two different lengths of time following an initial prescription, 90 (Panel A) and 270 days (Panel B). In Panel A, Columns (1) and (2), we look at high levels of use: filling prescriptions for more than 90 days supply total, or averaging more than 120 daily morphine-equivalent doses. In Columns (3) and (4), we look at two measures of drug-seeking behavior: obtaining opioids from at least 3 prescribers, or filling overlapping opioid prescriptions. In Column (5) we look at an alternative measure of dangerous behavior: concurrent days supply of opioids and benzodiazepines. Neither policy affects the rate of high days supply, daily MED, or doctor shopping. Initial prescription limits reduce the rate of opioid overlap. However, given that we do not find a reduction in the rate of high days supply, this is likely a mechanical consequence of the fact that initial prescription limits induce shorter prescriptions, rather than a reduction in dangerous or drug seeking behavior. Across all our outcomes in both panels, the standard errors are large enough (and base rates low enough) that we cannot detect positive or negative effects of MA PDMPs, we do find that MA PDMPs have a marginally significant effect on the rate of concurrent benzodiazepine prescribing.
Table 5:
Effects on indicators of dangerous use during the initial spell
| A. 90 days after initial prescription | |||||
|---|---|---|---|---|---|
| Outcome ×100 | ≥90 Days supply (1) |
≥ 120 Daily MED (2) |
At least 3 Prescribers (3) |
Opioid Overlap (4) |
Concurrent Benzodiazepine (5) |
| Limits | 0.017 (0.190) |
0.003 (0.017) |
0.102 (0.138) |
−0.262 (0.169) |
0.411 (0.337) |
| PDMP | 0.058 (0.144) |
−0.012 (0.024) |
0.027 (0.065) |
0.039 (0.176) |
−0.463 (0.326) |
| α = β | 0.875 | 0.703 | 0.617 | 0.183 | 0.088 |
| Mean DV | 1.434 | 0.106 | 2.746 | 3.331 | 7.183 |
| B. 270 days after initial prescription | |||||
| Outcome ×100 | ≥270 Days supply (1) |
≥ 120 Daily MED (2) |
At least 3 Prescribers (3) |
Opioid Overlap (4) |
Concurrent Benzodiazepine (5) |
| Limits | 0.131 (0.102) |
0.003 (0.018) |
0.326 (0.264) |
−0.213 (0.149) |
0.496 (0.452) |
| PDMP | −0.075 (0.124) |
−0.023 (0.023) |
−0.188 (0.225) |
−0.145 (0.261) |
−0.763 (0.403) |
| α = β | 0.344 | 0.531 | 0.075 | 0.814 | 0.075 |
| Mean DV | 0.622 | 0.069 | 7.365 | 4.584 | 8.131 |
Notes: “MED” is morphine equivalent doses. All outcomes are multiplied by 100. Table reports estimates of the coefficients on indicators for “initial prescription limits” and “must access PDMPs”, obtained from estimating OLS regressions of the indicated variables against policy indicators, state fixed effects, and month-year fixed effects. The sample is limited to people with an initial prescription and all estimates are at the state-month level, weighted by the number of initial prescriptions. The row labelled α = β reports p-values of the hypothesis that the coefficients on MA–PDMP and Limits are equal. Robust standard errors, clustered on state, in parentheses.
Results are similar when looking at the longer period of 270 days following the initial prescription.16 For the longer follow-up period, there is some evidence of increased doctor shopping. However we caution against interpreting this as clear evidence of dangerous behavior as this may be mechanical. That is, the likelihood of seeing more than three prescribers certainly increases as larger time periods are considered, so it is not obvious that this larger coefficient reflects more problematic use.
4.3. Validity tests
We investigate the validity of our estimates in four sets of exercises. First, we show that trends appear roughly parallel in the pre-period. Second, we conduct an analysis where we leave out each early-adopting state to ensure that no single state is driving our results. Third, we show that our results are robust to alternative ways of coding the key policies. Finally, we show that our results are robust to including all initial prescription states, not just early adopters.
Stacked difference-in-differences
We test for parallel trends—our key identifying assumption—by estimating stacked difference-in-differences models. These stacked models resemble those in Deshpande and Li (Forthcoming). To estimate these models, we divide the states data into “timing groups,” defined by the quarter in which they passed initial prescription limits. States which passed their limits in the same quarter are in the same timing group. For each timing group, we create a control group consisting of all the states which never adopted an initial prescription limit. We examine the trend in event time rather than calendar time. In each of these data sets we can then define event time τ as quarters since the initial prescription limit went into effect. Importantly, event time is defined for both the treatment and control states. We then stack these data sets together into one long data set. Each “treated” states appears once but each “control” state appears once for each timing group. Looking in event time is useful both for assessing parallel trends and for estimating heterogeneous treatment effects that vary by time since the law went into effect (Goodman-Bacon, 2019).17
One difficulty in implementing this approach is that some states have very short policy post-periods, making the composition of the treatment group change as event time grows. We therefore only present event-time periods that have a stable set of states across event-time. A second difficulty is that some states implement MA-PDMPs at roughly the same time as initial prescription limits, confounding an event study. We therefore also exclude nine states who implement an MA-PDMP in the window from 12 months before implementing initial prescription limits to 6 months after. These restrictions allow us to examine trends around prescription limit implementation without the confounding effect of MA-PDMP implementation, but they result in a smaller sample size.
We plot the average outcome in treatment and control states, in both calendar (left-panel) and event time (right-panel), in Figure 5, for three outcomes: the hazard of new fills, new morphine-equivalent days, and days supply in the first fill of a new prescription. Quarter 0 is the quarter of implementation. Because most of the law changes happen relatively late in our sample. The figures all show roughly parallel trends until the quarter before implementation. We take this to be reassuring evidence for our parallel trends assumption.
Figure 5:
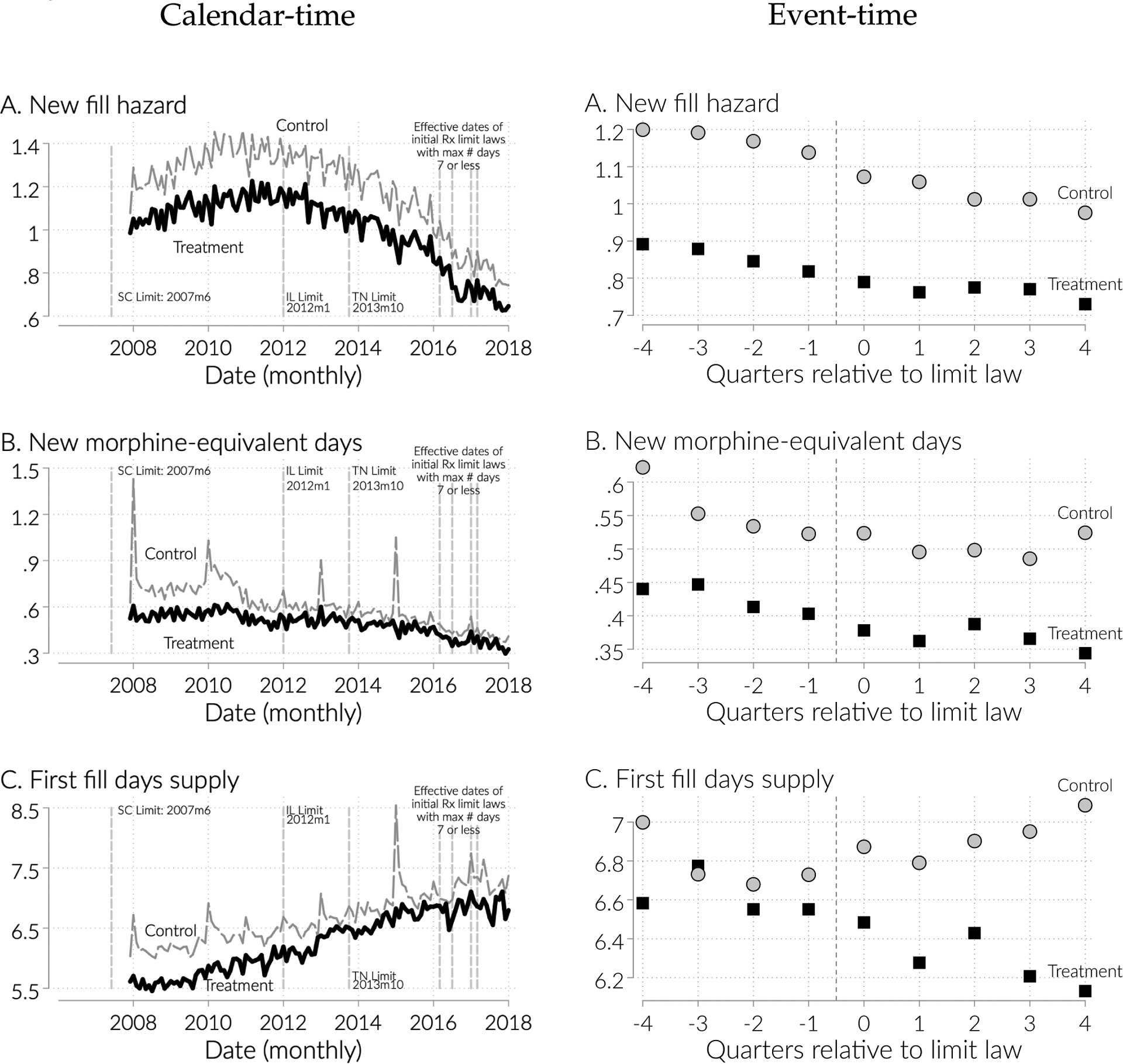
Trends in calendar (left) event-time around initial limit implementation (right) for key outcomes for control and treated states.
Note: Left panel shows the mean of the indicated outcomes in calendar time (months) for treated states (early-adopters of limits) and control states (never adopters). Right panel shows the mean of indicated outcome by treatment status, but is reported in quarters relative to initial prescription limits enactment (controls are matched in event time as described in the text).
To plot the difference in outcomes, rather than the levels, we estimate event study models using the stacked data, with specifications of the form:
| (2) |
and
| (3) |
In these models we control for fixed effects for state s, calendar time t, and event time τ (measured in quarters), as well as post-MA PDMP (or intitial limit) implementation dummy variable MAPDMP (Limit). The objects of interest are the ωτ terms, the coefficients on interactions between event time dummies and treatment dummies. These coefficients trace out the differential trend in the outcome around the time of initial limit enactment, relative to non-enacting states, and adjusting for the general trend and for MA-PDMPs. We cluster standard errors at the state level; this accounts for the fact that the same control state shows up multiple times in the stacked data set as well as for the usual serial correlation and heteroskedasticity concerns.
We plot the estimated γs and their 95 percent confidence intervals in Figure 6. The figure reveals several important patterns. First, we γ−2 coefficient is always statistically insignificant, as are all but a few of the pre-period coefficient. Second, the pre-trends are relatively flat and centered around zero. Third, the quarter specific estimates are noisy; this motivates our focus on the pooled estimates from the traditional DID specification. Finally, the effect of initial prescription limits on both new fill hazard and days supply (conditional on having an initial prescription) shows up almost immediately, whereas the effects on new morphine equivalent days takes longer to materialize if it does at all.
Figure 6:
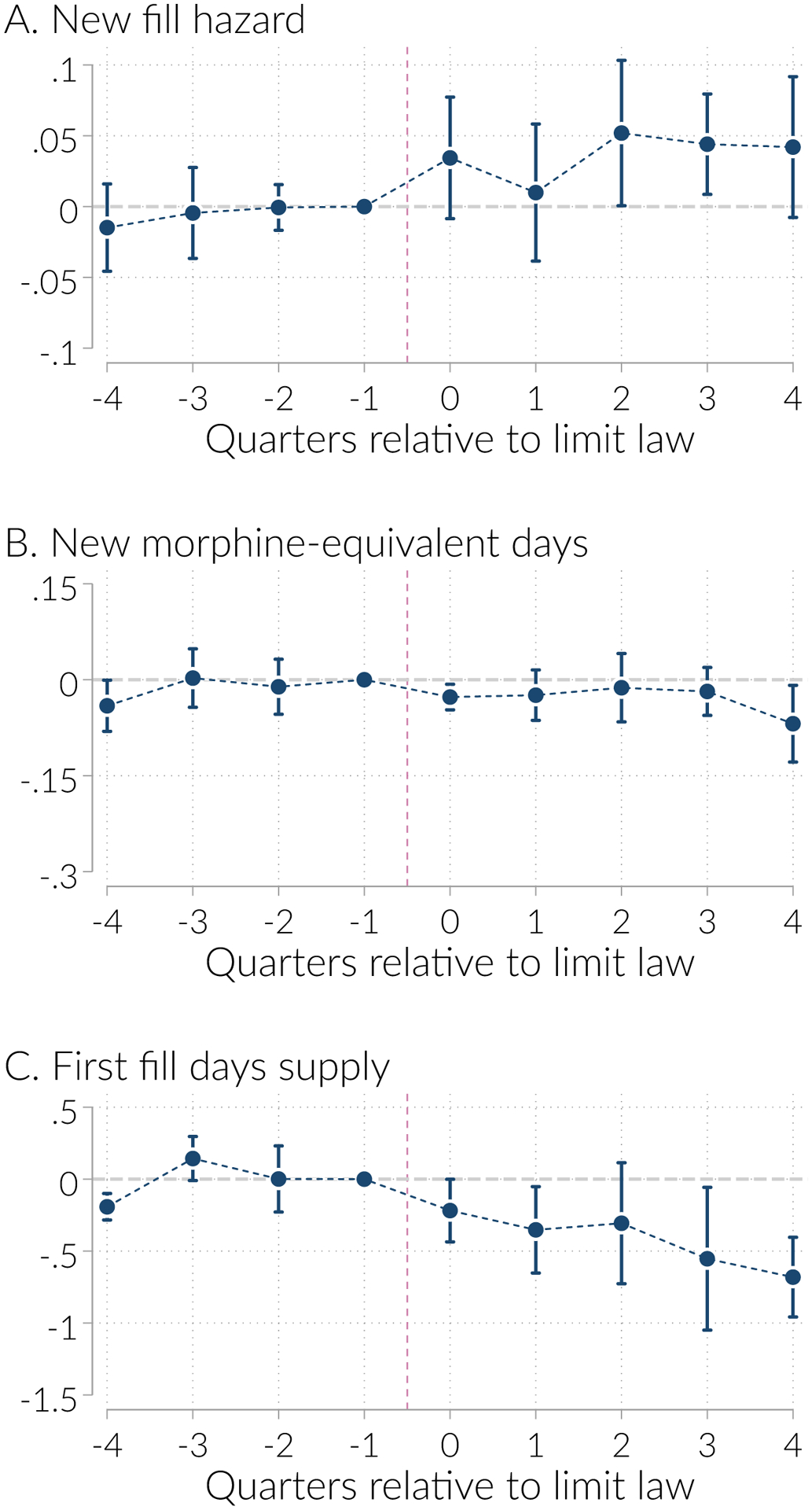
Differential trends in event time around initial prescription limits enactment
Notes: Figure plots the γs from Equation 2. These represent the estimated difference in the indicated outcome, in event time, net of state and time fixed effects and adjusting for MAPDMP enactment. The vertical lines are the 95% confidence intervals, estimated from standard errors clustered on state.
Overall these results show that opioid-related outcomes in states adopting and not adopting initial prescription limits moved roughly in parallel prior to implementation of initial prescription limits and diverged thereafter. However, noisiness in the quarterly estimates precludes us from making strong conclusions. Thus we take this as modestly reassuring evidence in support of our parallel trends assumption.
Robustness
We demonstrate several robustness checks. First, we demonstrate that our results are not sensitive to the inclusion of any single state in Figure A.4. Here we leave out each treated state with a binding policy and re-estimate our analysis without that state. The extensive margin effects do not change in any meaningful way as we omit each state. The removal of New York has the biggest effect on our estimates. However, with the exception of the intensive margin # fills, this has a minor effect in every case.
Second, we demonstrate that our results are not sensitive to the use of early-adopters as the treated unit. Table A.4 re-presents the main results using early adopters in panel A; presents the same analysis, but including any initial limit adopting state as treated in panel B; and displays results from an analysis where early adopters are allowed to have a differential effect of the policy than late adopters in Panel C. Throughout the control group remains never adopting states. In all we find similar effects between the analysis in our original manuscript and the new analysis that focuses only on early adopters. Importantly the treatment effect estimates are larger in magnitude and more precisely estimated for early adopters. For the most part late adopters have consistent signs and magnitudes as early adopters, although these effects are more imprecisely estimated.
Finally, we examined the robustness of our findings to several alternative specification choices. We present the results of robustness tests in Appendix Table A.3. Panel A re-presents our main estimates. In Panel B we adopt a stricter coding of initial prescription limits, only counting limits of 7 days or fewer. The resulting estimates for initial prescription limits are generally somewhat larger in magnitude, although never substantially so. In Panel C, we report the results of using a looser coding, treating all laws reported in Davis et al. (2019a) as limits on initial prescriptions. This adds another 5 states to those with limits: all which limit days supplied to between 20 and 31, and one which does not limit days supply but does limit the initial prescription to the weakest effective dose. Consistent with our expectation that looser limits are less likely to bind, we find that under this alternative coding, limits on initial prescriptions have an even smaller impact on days supply, and we continue to find slight offsetting effects later in the initial spell, resulting in higher daily MED.
We also considered an alternative coding of MA-PDMPs. Our baseline defines MA-PDMPs as those which require at least some providers to access the PDMP in at least some circumstance. As Buchmueller and Carey (2018) note, the weakest such PDMPs require providers to access only in limited (e.g., for Methadone prescriptions) or discretionary (e.g. only when the provider suspects abuse) circumstances. We therefore re-estimated our models assuming a stricter definition of must-access which treats such limited or discretionary requirements as non-must-access. The alternative coding and dating of MA-PDMPs may be seen in Appendix Table A.1.
The new difference-in-difference estimates are in Panel D of Appendix Table A.3. This alternative coding of MA-PDMPs produces very similar estimates, in part because many of the states with limited or discretionary access requirements subsequently passed stricter must-access requirements, making the laws highly correlated.
As a final robustness check, in Panel E we include controls for a set of state level policy variables: a dummy variable for Medicaid expansion, and dummy variables categorizing state marijuana regulation. Specifically, we include dummy variables indicating legalization of medical marijuana and, separately, recreational marijuana legalization. Because research has indicated that the presence of dispensaries is particularly important (over and above the legalization effect (Chan et al., 2019)), we also include dummy variables indicating that at least one medical dispensary is open and, separately, at least one recreational dispensary.
Including these state-level policy variables does not substantially change our estimates. The extensive margin response becomes slightly larger, and the initial prescription effects become, in some cases, less precise and statistically insignificant. Thus our finding that MA-PDMPs but not initial prescription limits reduce opioids dispensed to new users is robust to these state-level policy variables.
5. Mechanisms
In this section, we discuss the possible mechanisms that could generate the finding that initial prescription limits increase the hazard of initial opioid use. We caution that this discussion is speculative as we are ex post rationalizing a surprising result.
5.1. Explaining the initial prescription limits results
We consider three possible mechanisms for the finding that initial prescription limits increase the hazard of initial prescriptions. Our leading explanation is that the limit acts as a reference point for prescribers, signaling a safe or acceptable level, and therefore increasing prescribing below that level. We rule out two alternative explanations: that compensatory behavior such as the laws leading to more refills by patients or providers ends up undermining initial prescription limits, and that lower out-of-pocket prices for shorter prescriptions explains the higher rate of observed fills in the claims data.
The most likely mechanism that we believe explains these results is that initial prescription limits act as reference points. The hypothesis here is that physicians, who have recently been exposed to many warnings urging caution in opioid prescribing, see a law with a limit of 7 days supply and come to think that 7 days is relatively safe or socially acceptable. Thus they end up writing a 7 day supply for a patient on the margin of receiving a prescription, who would otherwise receive no opioid. This reference point hypothesis does not necessarily imply that some physicians increased their prescribing immediately when states passed these laws. Rather, these laws were passed in a context of falling opioid prescribing and rising skepticism about opioids. This hypothesis implies only that initial prescription limits could reduce the decline in prescribing.
This mechanism is also consistent with other evidence that physicians can react sharply to guidelines and diagnoses. For example, Almond et al. (2010) show that newborns with birthweight just below 1500 grams—putting them below a commonly used threshold for very low birthweight—have higher survival rates than newborns with birthweight just above 1500 grams. Relatedly, Alalouf et al. (2019) find that people with blood sugar level just above the diagnostic threshold for diabetes receive discontinuously more medical care than people with blood sugar just below the threshold. These examples show that sharp numeric guidelines can generate strong responses among physicians, resulting in extra health care utilization.
The reference point hypothesis is consistent with several features of the data. First, it implies that initial prescription limits increase only prescriptions perceived as safe, i.e. short prescriptions, which is what we see in the data. It is also arguably consistent with the effect being concentrated among primary care providers, who may need to keep track of many different prescribing guidelines, and may therefore especially rely on heuristics. Third, it is consistent with the non-immediate extensive margin response to the limits evident in Figure 6, the event study figure, as a reference point would likely take time to develop.
Other plausible mechanisms do not fit the data as well. One possible way for initial prescription limits to raise opioid dispensed to new users and in general is through compensatory behavior. For example, given a short initial prescription, patients might seek or physicians might write stronger prescriptions or more subsequent prescriptions. We find no evidence for that view, as the strength of prescribing does not increase and neither does the number of prescriptions in the initial spell.18
Still another possibility is that initial prescription limits increase initial use by changing the number of filled prescriptions rather than the number of written prescriptions. To understand this hypothesis, note that we only observed filled prescriptions in our claims data, not unfilled prescriptions written by physicians. If initial prescription limits induce physicians to write shorter prescriptions that are cheaper for patients, then perhaps we observe more fills because there are fewer unfilled, longer duration and expensive prescriptions.
Although at first this seems a potential explanation, we find this mechanism implausible because there are not substantial cost differences between short and long prescriptions. Appendix Figure A.3 shows that the copay per days supply of a given drug actually declines in prescription length, so longer prescriptions are cheaper on a per day basis.19 The total copay (not per days supply) increases with days supply, from about $6 for a 7 days supply to about $10 for a 20 days supply. Extremely tight liquidity constraints might suffice to prevent some patients from filling a 20 days supply but not a 7 day supply. We think it unlikely that this extra $4 would be decisive, however, because we are studying commercially insured patients who likely have some liquidity, and because we expect that patients seeking an opioid for acute pain are unlikely to be deterred by a few dollars.
5.2. Explaining the MA-PDMP results
We find that MA-PDMPs reduce prescribing to new patients. Although the event study estimates are not as clear as those for initial prescription limits, we hypothesize two possible mechanisms for this result. First, MA-PDMPs create several fixed costs of prescribing opioids even to new patients, because they require prescribers to register with the system and check it upon writing each prescription. The laws may also generate a sense that the prescribers’ actions are being scrutinized. Second, MA-PDMPs inform physicians that opioids are more dangerous or more frequently abused than they had believed, by showing them the frequency with which patients engage in doctor shopping.
Although we cannot fully separate these mechanisms, we find the fixed cost of prescribing mechanism more plausible. This hypothesis is consistent with the evidence in Buchmueller et al. (2018), who find that when Kentucky introduced its MA-PDMP, opioid prescribing fell, and many low-volume prescribers stopped prescribing entirely, relative to a control group of Indiana prescribers. Buchmueller et al. indicate that a likely reason for this extensive margin response is that Kentucky’s must-access provisions required prescribers to register with the database, creating a fixed cost of prescribing any opioid. On the other hand, we think it is unlikely that MA-PDMPs informed prescribers about the general dangers of opioid prescribing to first-time users. Among new patients, our evidence suggests that opioid rarely show patterns of misuse, at least in the short period we follow in this analysis. Prescribers would have had to have very low priors on the probability of misuse for this information to push them to prescribe less.
6. Discussion and conclusions
In response to the opioid epidemic, many states have now passed laws limiting the length of initial opioid prescriptions written for patients with acute pain. Policy makers hope that these laws reduce the quantity of opioids dispensed to new users, to prevent unintentional dependence and to cut down the stock of opioids available for diversion. We investigate the effects of these limits using difference-in-difference models and a sample of new opioid users derived from claims data of a large commercial health insurer. Our findings suggest that these laws have not achieved their goal. Although they are effective in the narrow sense that they reduce the length of the initial prescription, they are less effective in reducing overall opioid dispensing to new users. We find that initial limit policies induce a greater number of initial prescriptions, resulting in more opioids dispensed to new users. By contrast, MA-PDMPs are effective in reducing opioids dispensed to new users in terms of days supply (as well as reducing the extreme outcomes they were primarily intended to target): they reduce the hazard of initial prescription as well as the length of the initial prescription, as expected, and we do not observe an increase in the subsequent number of prescriptions.
Our results have implications for policy makers hoping to slow the rate of new opioid use. First, initial prescription laws may be too narrowly targeted, or allow too many exceptions to be effective. On most measures these laws do not appear to reduce use, and so it is unlikely that they prevent development of addiction disorders or dependence. Second, MA-PDMPs may be a more effective tool even at reducing initial prescriptions. Thus it may be worthwhile for future research to examine implications of further modifications within MA-PDMPs, such as integration with electronic health records.
However, our results are subject to at least two limitations. First, our sample consists of commercially insured patients, thus we are not able to claim representativeness of results for the population as a whole. However, we believe our focus on a commercially insured population is a valuable complement to the existing literature, especially because it encompasses more age groups affected by high opioid overdose mortality, relative to the more-often-studied Medicare population. Second, because we study laws which were enacted only recently, we are unable to examine outcomes beyond about three months after the initial prescription. It may be that initial limit policies reduce adverse outcomes at a greater time horizon; the laws likely aim to reduce misuse at much greater time horizons.
Our results may have broader implications for substance use policy. We found that a policy that explicitly targeted new use was not effective and had negative unintended consequences, but a policy that raised the cost of prescribing has desirable effects beyond the intended ones. We speculate that, in general, limits on new use or overall use are unlikely to be an effective tool. Such limits are only appealing for substances whose harms and benefits are ambiguous. But it is in exactly that situation when a limit may signal a safe or acceptable level, generating unintentionally higher usage. Other policies such as explicit requirements for step therapy may be more effective, as may be policies that raise the cost of prescribing.
Acknowledgments
We are grateful to Dhaval Dave, Catherine Maclean, Justine Mallatt, and audiences at University of Georgia, University of Illinois, Indiana University and the Midwest Health Economics Conference for helpful comments. This research was supported in part by National Institute on Drug Abuse, R01 DA039928 (PI Brea Perry), “Doctor shopping for controlled substances: Insights from two-mode social network analysis,” and the Indiana University Addictions Grand Challenge Initiative. We acknowledge the Indiana University Pervasive Technology Institute for providing HPC resources that have contributed to the research results reported in this paper (https://pti.iu.edu).
Table A.1:
Policy implementation dates and notes, by state
| Initial prescription limits | Must access PDMP | ||||
|---|---|---|---|---|---|
| State | Effective date | Max days | Other limits | Effective date | Alternative date |
| AK | 2017m7 | 7 | None | 2017m7 | |
| AL | |||||
| AR | 2017m1 | ||||
| AZ | 2017m10 | ||||
| CA | 2018m4 | ||||
| CO | |||||
| CT | 2016m7 | 7 | None | 2015m10 | |
| DC | |||||
| DE | 2017m4 | 7 | None | 2012m3 | No must access PDMPa |
| FL | |||||
| GA | 2014m7 | ||||
| HI | 2016m7 | 30 | None | ||
| IA | |||||
| ID | |||||
| IL | 2012m1 | 30 | None | 2018m1 | |
| IN | 2017m7 | 7 | None | 2014m7 | |
| KS | |||||
| KY | 2017m6 | 3 | None | 2012m7 | |
| LA | 2017m8 | 7 | None | 2008m1 | No must access PDMPb |
| MA | 2016m3 | 7 | None | 2014m7 | |
| MD | 2017m5 | Lowest effective dose | 2018m7 | ||
| MI | |||||
| MN | 2017m7 | 4 | None | 2017m1 | |
| MO | 1988m12 | 30 | None | ||
| MS | |||||
| MT | |||||
| NC | 2018m1 | 5 | None | ||
| ND | |||||
| NE | |||||
| NH | 2017m1 | 7 | Lowest effective dose | 2016m1 | |
| NJ | 2017m5 | 5 | Lowest effective dose | 2015m11 | |
| NM | 2012m9 | ||||
| NV | 2017m6 | 14 | 90 MME per day | 2007m10 | 2015m10c |
| NY | 2016m7 | 7 | None | 2013m8 | |
| OH | 2017m8 | 7 | 30 MME per day | 2012m3 | 2015m12c |
| OK | 2011m3 | 2015m11d | |||
| OR | |||||
| PA | 2017m1 | 7 | None | 2017m1 | |
| RI | 2017m3 | 20 | 30 MME per day | 2016m6 | |
| SC | 2007m6 | 31 | None | 2017m5 | |
| SD | |||||
| TN | 2013m10 | 30 | None | 2013m7 | |
| TX | 2019m9 | ||||
| UT | 2017m3 | 7 | None | 2017m5 | |
| VA | 2017m3 | 7 | None | 2015m7 | |
| VT | 2017m7 | 7 | Varies by Pain Level | 2015m5 | 2013m11e |
| WA | |||||
| WI | 2017m4 | ||||
| WV | 2012m6 | ||||
| WY | |||||
Notes: Table reports the effective date of states’ limits on initial prescriptions and of MA-PDMP laws. For states with limits, we report the maximum allowed days supply (if limited) and any other limits on the prescription (if any). We note ambiguities in the dating of MA-PDMPs and provide alternative dates where such ambiguity exists.
PDMP requires access only if providers have a reasonable suspicion of abuse.
Only pain clinics must access.
Initial PDMP required access only with a reasonable suspicion of abuse; access laws later strengthened.
Only methadone clinics must access (until 12/2015).
Until 5/2015, access required only for patients reporting their prescription was lost or destroyed.
Table A.2:
Sample construction and sample size
| Enrollees | Enrollee-Months | |
|---|---|---|
| A. 20 percent sample of all enrollees | ||
| All | 11,916,182 | 371,597,845 |
| Enrolled t − 11 to t | 8,269,520 | 245,493,810 |
| Enrolled t − 11 to t + 3 | 7,144,063 | 228,452,677 |
| In 50 states + DC | 7,050,728 | 225,762,240 |
| No cancer | 6,949,041 | 211,041,311 |
| B. 100 percent sample of all controlled substance recipients | ||
| All | 22,264,546 | 1,029,010,777 |
| Enrolled t − 11 to t | 19,259,589 | 753,973,937 |
| Enrolled t − 11 to t + 3 | 17,695,352 | 712,189,870 |
| In 50 states + DC | 17,636,425 | 707,568,491 |
| No cancer | 17,343,695 | 653,119,778 |
Notes: Table shows how our sample size changes as we impose our sample restrictions. The 20 percent sample is a random sample of all enrollees, and the 100 percent sample consists of all enrollees who filled a prescription for at least one controlled substance.
Table A.3:
Robustness of main difference-in-differences estimates to multiple specifications
| Extensive margin | Initial prescription | Initial spell | Extreme outcomes | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard (1) |
New Days MED (2) |
>7 DS (3) |
Days Supply (4) |
Days MED (5) |
# Fills (6) | Days supply (7) |
Days MED (8) |
> 90 DS (9) |
> 120 Daily MED (10) |
≥ 3 prescribers (11) |
Opioid overlap (12) |
Concurrent benzo. (13) |
|
| A. Main Estimates | |||||||||||||
| Limits | 0.100 (0.022) |
0.055 (0.035) |
−0.044 (0.011) |
− 0.477 (0.220) |
2.247 (3.001) |
0.034 (0.019) |
−0.458 (0.481) |
5.789 (9.461) |
0.017 (0.190) |
0.003 (0.017) |
0.102 (0.138) |
−0.262 (0.169) |
0.411 (0.337) |
| PDMP | −0.054 (0.015) |
−0.035 (0.053) |
−0.006 (0.009) |
−0.091 (0.159) |
−0.091 (3.841) |
0.023 (0.009) |
0.046 (0.348) |
−1.866 (13.124) |
0.058 (0.144) |
−0.012 (0.024) |
0.027 (0.065) |
0.039 (0.176) |
−0.463 (0.326) |
| B. Tight initial prescription limits only | |||||||||||||
| Limits | 0.107 (0.021) |
0.066 (0.039) |
−0.044 (0.010) |
−0.459 (0.210) |
2.679 (3.382) |
0.032 (0.019) |
−0.411 (0.465) |
7.559 (10.984) |
0.045 (0.191) |
0.006 (0.020) |
0.092 (0.138) |
−0.254 (0.172) |
0.485 (0.341) |
| PDMP | −0.057 (0.016) |
−0.039 (0.061) |
−0.002 (0.009) |
−0.058 (0.171) |
−0.184 (4.585) |
0.025 (0.010) |
0.081 (0.392) |
−3.041 (15.551) |
0.038 (0.167) |
−0.013 (0.028) |
0.032 (0.073) |
0.086 (0.199) |
−0.596 (0.352) |
| C. Loose initial prescription limits | |||||||||||||
| Limits | 0.069 (0.018) |
0.068 (0.035) |
−0.011 (0.017) |
−0.153 (0.203) |
2.801 (2.502) |
0.007 (0.009) |
−0.122 (0.329) |
7.576 (8.627) |
−0.042 (0.104) |
0.014 (0.016) |
0.113 (0.055) |
0.043 (0.180) |
0.183 (0.222) |
| PDMP | −0.052 (0.015) |
−0.041 (0.054) |
−0.009 (0.010) |
−0.116 (0.166) |
−0.402 (3.994) |
0.025 (0.010) |
0.017 (0.368) |
−2.741 (13.587) |
0.068 (0.156) |
−0.014 (0.024) |
0.016 (0.070) |
−0.001 (0.181) |
−0.452 (0.327) |
| D. Alternative coding of must–access PDMPs | |||||||||||||
| Limits | 0.100 (0.022) |
0.055 (0.035) |
−0.044 (0.011) |
− 0.477 (0.220) |
2.247 (3.001) |
0.034 (0.019) |
−0.458 (0.481) |
5.789 (9.461) |
0.017 (0.190) |
0.003 (0.017) |
0.102 (0.138) |
−0.262 (0.169) |
0.411 (0.337) |
| PDMP | −0.054 (0.015) |
−0.035 (0.053) |
−0.006 (0.009) |
−0.091 (0.159) |
−0.091 (3.841) |
0.023 (0.009) |
0.046 (0.348) |
−1.866 (13.124) |
0.058 (0.144) |
−0.012 (0.024) |
0.027 (0.065) |
0.039 (0.176) |
−0.463 (0.326) |
| E. Control for Medicaid expansion and Marijuana Access | |||||||||||||
| Limits | 0.063 (0.024) |
−0.014 (0.017) |
−0.050 (0.014) |
−0.604 (0.221) |
−3.252 (1.566) |
0.022 (0.020) |
−0.812 (0.450) |
−8.666 (5.842) |
−0.159 (0.157) |
−0.014 (0.012) |
0.051 (0.161) |
−0.462 (0.222) |
0.208 (0.316) |
| PDMP | −0.053 (0.013) |
−0.035 (0.041) |
−0.009 (0.009) |
−0.151 (0.145) |
0.194 (2.885) |
0.022 (0.006) |
−0.045 (0.303) |
−1.355 (11.095) |
0.043 (0.126) |
−0.011 (0.022) |
0.040 (0.067) |
0.007 (0.161) |
−0.534 (0.337) |
Notes: “DS” stands for days supply, and “MED” is morphine equivalent doses. Hazard rate and extreme outcomes are multiplied by 100. Table reports estimates of the coefficients on indicators for “initial prescription limits” and “must access PDMPs”, obtained from estimating OLS regressions of the indicated variables against policy indicators, state fixed effects, and month-year fixed effects. In Panel A we use our baseline coding of the policies, treating limits for 14 days or fewer as effective. In Panel B we code initial prescriptions only if they are for 7 days or fewer. In Panel C, we use a loose coding of initial prescription limits that treats all limits as effective. In Panel D we use an alternative coding of must-access PDMPs. In Panel E we include indicators for post Medicaid expansion, legalization of medical marijuana, legalization of recreational marijuana, at least one medical marijuana dispensaries open, and at least one recreational marijuana dispensary open. Robust standard errors, clustered on state, in parentheses
Figure A.1:
Trends in event time around MA-PDMP implementation, key outcomes
Notes: Figure shows the mean of the indicated outcomes in quarters relative to MA-PDMP enactment (in the right panels), for treated states (enacting MA-PDMP) and control states (not enacting them but matched in event time as described in the text).
Figure A.2:
Event study coefficients around MA-PDMP implementation for key outcomes
Note: Figure plots the γs from Equation 3. These represent the estimated difference in the indicated outcome, in event time, net of state and time fixed effects and adjusting for initial limit enactment. The vertical lines are the 95% confidence intervals, estimated from standard errors clustered on state.
Figure A.3:
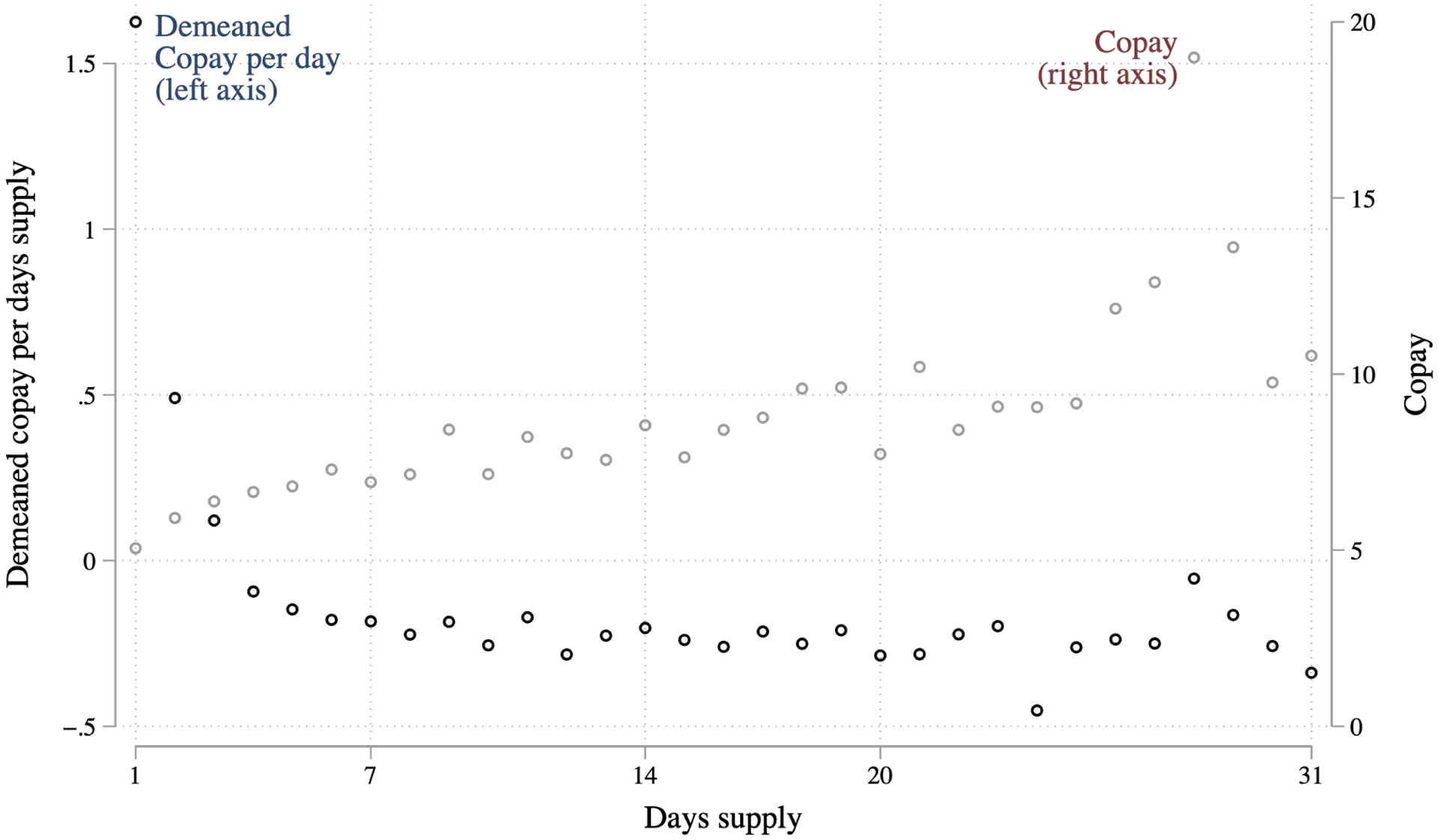
Copay per days supply falls with days supply
Notes: Figure shows the average copay per days supply (demeaned relative to the NDC-year average) as a function of days supply (left axis), and the average copay of prescribed drugs (right axis), among new opioid fills.
Table A.4:
Robustness of main difference-in-differences estimates to different treatment definition
| Extensive margin | Initial prescription | Initial spell | Extreme outcomes | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard (1) |
New Days MED (2) |
>7 DS (3) |
Days Supply (4) |
Days MED (5) |
# Fills (6) | Days supply (7) |
Days MED (8) |
> 90 DS (9) |
> 120 Daily MED (10) |
≥ 3 prescribers (11) |
Opioid overlap (12) |
Concurrent benzo. (13) |
|
| A. Effects with only early adopters | |||||||||||||
| Limits | 0.100 (0.022) |
0.055 (0.035) |
−0.044 (0.011) |
−0.477 (0.220) |
2.247 (3.001) |
0.034 (0.019) |
−0.458 (0.481) |
5.789 (9.461) |
0.017 (0.190) |
0.003 (0.017) |
0.102 (0.138) |
−0.262 (0.169) |
0.411 (0.337) |
| PDMP | −0.054 (0.015) |
−0.035 (0.053) |
−0.006 (0.009) |
−0.091 (0.159) |
−0.091 (3.841) |
0.023 (0.009) |
0.046 (0.348) |
−1.866 (13.124) |
0.058 (0.144) |
−0.012 (0.024) |
0.027 (0.065) |
0.039 (0.176) |
−0.463 (0.326) |
| B. Effects with all adopters | |||||||||||||
| Limits | 0.051 (0.021) |
0.034 (0.023) |
−0.041 (0.007) |
−0.469 (0.126) |
0.381 (1.867) |
0.008 (0.014) |
−0.683 (0.298) |
0.597 (5.872) |
−0.095 (0.117) |
0.002 (0.011) |
0.166 (0.174) |
−0.322 (0.124) |
0.065 (0.235) |
| PDMP | −0.035 (0.015) |
−0.022 (0.035) |
−0.010 (0.008) |
−0.162 (0.138) |
−0.413 (2.484) |
0.012 (0.009) |
−0.098 (0.283) |
0.462 (8.935) |
0.053 (0.103) |
−0.005 (0.016) |
0.081 (0.101) |
−0.016 (0.139) |
− 0.471 (0.250) |
| C. Effects with an interaction for early adopters | |||||||||||||
| Limits | 0.031 (0.018) |
0.021 (0.021) |
−0.042 (0.009) |
−0.490 (0.129) |
−0.385 (1.193) |
0.004 (0.012) |
−0.759 (0.259) |
−0.931 (4.012) |
−0.090 (0.103) |
−0.000 (0.009) |
0.150 (0.211) |
−0.398 (0.123) |
−0.010 (0.210) |
| Limits × early adopter | 0.050 (0.019) |
0.052 (0.025) |
0.008 (0.013) |
0.126 (0.151) |
3.321 (1.345) |
0.013 (0.010) |
0.365 (0.252) |
7.660 (4.937) |
0.049 (0.086) |
0.012 (0.012) |
0.025 (0.159) |
0.252 (0.137) |
0.285 (0.210) |
Notes: “DS” stands for days supply, and “MED” is morphine equivalent doses. Hazard rate and extreme outcomes are multiplied by 100. Table reports estimates of the coefficients on indicators for “initial prescription limits” and “must access PDMPs”, obtained from estimating OLS regressions of the indicated variables against policy indicators, state fixed effects, and month-year fixed effects. In Panel A we use our baseline coding of the policies, treating limits for 14 days or fewer as effective and focusing only on those states who adopted initial prescription limits before March 2017. In Panel B we consider all initial prescription limit states regardless of implementation date. In Panel C, we allow early adopting states to have a different effect than late adopting states. Robust standard errors, clustered on state, in parentheses
Figure A.4:
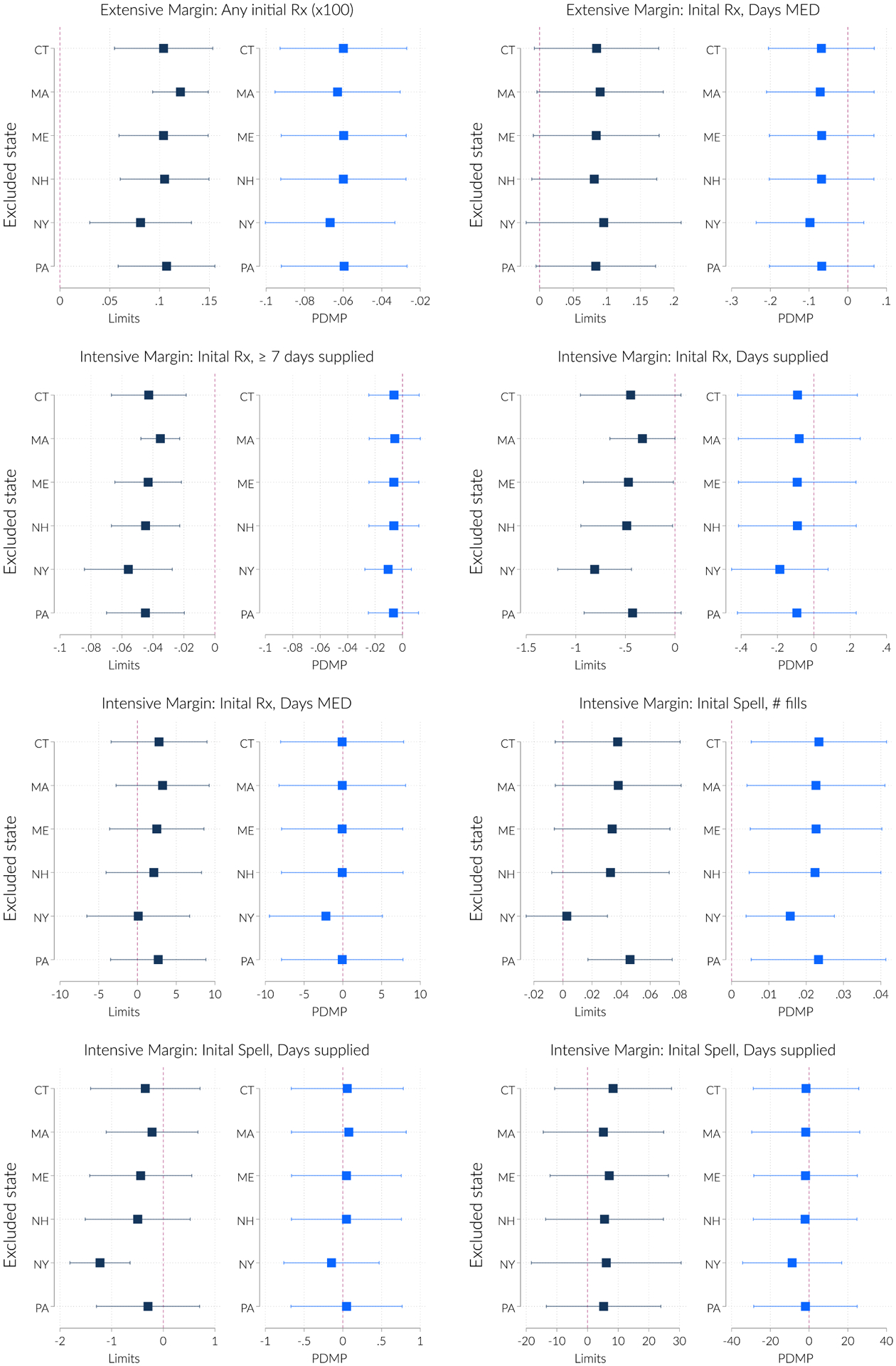
Results are largely robust to excluding each treated state.
Footnotes
Shah et al. (2017) similarly find that 70 percent of initial prescriptions are for 7 days or fewer.
Kilby (2015) estimates the impact of PDMPs on new opioid use, but does not consider MA-PDMPs in particular. She finds quantitatively small effects, about 40 percent of our estimate, which is consistent with the evidence in Buchmueller and Carey (2018) that non-must-access PDMPs have limited impacts on opioid use.
It should be noted that event studies do not show as clear an effect for MA-PDMPs as we find for initial prescription limits. It is entirely possible that it may still may be too soon to know the full extent of the initial fill policies.
See, for example, https://www.wfyi.org/news/articles/how-one-hospital-drastically-cut-opioid-prescriptions.
These initial prescription limits are not among the many opioid-control laws studied by Meara et al. (2016). They study different prescription restrictions as well as tamper resistant prescription forms, ID requirements, doctor shopping, physician exams, pharmacist verification, PDMPs, and pain clinics. Their prescription restrictions do not specifically apply to new prescriptions. As they note, only Iowa and Vermont imposed quantitative prescription limits during their analysis periods, which runs through 2013, whereas most of the laws we studied were passed in 2016 and beyond.
We accessed these data in March, 2019, from a now defunct website. The data may be seen on https://web.archive.org/web/20190320022501/http://www.namsdl.org/prescription-monitoring-programs.cfm..
We make one exception, consistent with Buchmueller and Carey (2018). Until 2015, Vermont’s PDMP required access only when a patient requested a replacement prescription for one that had been lost or destroyed. We code this as a non-must-access.
We obtained all records over the time period January 2007- April 2018 for a randomly chosen 20 percent of enrollees who appeared at least once during this time period. Some individuals are present only for a subset of the time period, reflecting the churn in private insurance coverage, but our sample remains representative of privately insured enrollees and has a potentially long panel dimension.
The difference in size is because the numerator comes from a 20% sample and the denominator comes from a 100% sample. When adjusted by a factor of 5, the denominator becomes 34.8 million. This implies that a little less than 50% of enrollees have at least one opioid prescription over our sample period.
Note that we work with MED for the initial prescription but daily MED for the initial spell. The reason for this is that the days of the initial spell is fixed at 90, making it easy to define daily MED. But for the initial prescription, the denominator would be days supply, meaning that a lower daily MED could be due to a strong but short prescription.
We consider a longer spell length in addition to the 90 day spell for two reasons. First the increased length makes it more likely to observe rare events and second because it may take time for dangerous behavior to develop. For the longer 270 day analysis, we ensure that patients are continuously enrolled for the entire period. Thus the sample of patients is slightly smaller for this analysis
This figure pools surgical and non-surgical prescriptions. Even among prescriptions written by surgeons, long prescriptions are rare: 79 percent are for 7 days or fewer, and 95 percent for 14 days or fewer.
The states are Rhode Island (20 days); Hawaii, Illinois, Rhode Island, and Tennessee (all 30 days); South Carolina (31 days); and Maryland (strength is limited to the lowest effective dose, no limit on days supply).
A large literature examines the association between both medical and recreational marijuana legalization and opioid use, finding that marijuana access is associated with reductions in opioid prescriptions (e.g. Wen and Hockenberry (2018); Bradford et al. (2018)) as well as reductions in opioid-related mortality (Bachhuber et al., 2014; Powell et al., 2018; Chan et al., 2019).
Our physician identifiers are encrypted so we cannot merge in external information.
As in the 90 day analysis, we ensure that enrollees are continuously enrolled for the entire period. For the 270 day case, we look at those continuously enrolled for 10 months following the initial prescription.
We present analogous plots for MA-PDMPs in Appendix Figure A.1 and Appendix Figure A.2. These figures show some evidence of anticipatory behavior, with effects showing up as soon as two quarters before MA-PDMP implementation. This likely reflects a response to the passage of laws authorizing MA-PDMP.
We also looked at whether initial prescription limits increased the number of pills per prescription, to facilitate stretching the prescription out. Our (unreported) estimates indicate that they do not.
Every drug claim in our data records a copay, which we interpret as the total out-of-pocket cost to the patient of filling the prescription.
Contributor Information
Daniel W. Sacks, Indiana University, Kelley School of Business
Alex Hollingsworth, Indiana University, O’Neill SPEA.
Thuy Nguyen, University of Michigan, School of Public Health.
Kosali Simon, Indiana University, O’Neill SPEA and NBER.
References
- Alalouf Mattan, Miller Sarah, and Wherry Laura R., “What difference does a diagnosis make? Evidence from Marginal Patients,” October 2019. NBER Working Paper No. 26363.
- Almond Douglas, Doyle Joseph J., Kowalski Amanda E., and Williams Heidi, “Estimating Marginal Return to Medical Care: Evidence from At-Risk Newborns,” The Quarterly Journal of Economics, 2010, 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpert Abby, Powell David, and Pacula Rosalie Liccardo, “Supply-Side Drug Policy in the Presence of Substitutes: Evidence from the Introduction of Abuse-Deterrent Opioids,” American Economic Journal: Economic Policy, 2018, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axeen Sarah, Seabury Seth A, and Menchine Michael, “Emergency Department Contribution to the Prescription Opioid Epidemic,” Annals of Emergency Medicine, June 2018, 71 (6), 659–667.e3. [DOI] [PubMed] [Google Scholar]
- Bachhuber Marcus A., Saloner Brendan, Cunningham Chinazo O., and Barry Colleen L., “Medical Cannabis Laws and Opioid Analgesic Overdose Mortality in the United States, 1999–2010,” JAMA Internal Medicine, 2014, 174, 1668–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett Michael L., Olenski Andrew R., and Jena Anupam B, “Opioid-Prescribing Patterns of Emergency Physicians and Risk of Long-Term Use,” New England Journal of Medicine, February 2017, 376 (7), 663–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford Ashley C., Bradford W. David, Abraham Amanda, and Adams Grace Bagewell, “Association Between US State Medical Cannabis Laws and Opioid Prescribing in the Medicare Part D Population,” JAMA Internal Medicine, 2018, 178, 667–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmueller Thomas C. and Carey Colleen, “The effect of prescription drug monitoring programs on opioid utilization in Medicare,” American Economic Journal: Economic Policy, 2018, 10, 77–112. [Google Scholar]
- —, Carey Colleen M., and Meille Giacomo, “How Well Do Doctors Know Their Patients? Evidence from a Mandatory Access Prescription Drug Monitoring Program,” October 2018. Unpublished working paper. [DOI] [PubMed] [Google Scholar]
- Butler Megan M, Ancona Rachel M, Beauchamp Gillian A, Yamin Cyrus K, Win-stanley Erin L, Hart Kimberly W, Ruffner Andrew H, Ryan Shawn W., Ryan Richard J, Lindsell Christopher J, and Lyons Michael S, “Emergency Department Prescription Opioids as an Initial Exposure Preceding Addiction,” Annals of Emergency Medicine, August 2016, 68 (2), 202–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case Anne and Deaton Angus, “Rising morbidity and mortality in midlife among white non-Hispanic Americans in the 21st century,” Proceedings of the National Academy of Sciences, 2015, 112, 15078–15083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Disease Control and Prevention, “Data Resources - Oral MMEs,” 2018. https://www.cdc.gov/drugoverdose/resources/data.html, Accessed September 13, 2019.
- Centers for Disease Control and Prevention, “2018 Annual Surveillance Report of Drug-Related Risks and Outcomes,” Technical Report August 2018. Available at https://www.cdc.gov/drugoverdose/pdf/pubs/2018-cdc-drug-surveillance-report.pdf. [Google Scholar]
- Chan Nathan W., Burkhardt Jesse, and Flyr Matthew, “The Effects of Recreational Marijuana Legalization and Dispensing on Opioid Mortality,” Economic Inquiry, 2019. doi: 10.1111/ecin.12819. [DOI] [Google Scholar]
- Chen Li Hui, Hedegaard Molly, and Warner Margaraet, “Drug-poisoning deaths involving opioid analgesics: United States, 1999–2011,” Technical Report September 2014. NCHS Data Brief. No. 166. [PubMed] [Google Scholar]
- Cotti Chad, Nesson Erik, and Tefft Nathan, “The Effects of Tobacco Control Policies on Taboacco Products, Tar, and Nicotine Purchases Among Adults: Evidence from Household Panel Data,” American Economic Journal: Economic Policy, 2016, 8 (4), 103–123. [Google Scholar]
- Dasgupta N, Funk MJ, Proescholdbell S, Hirsch A, Ribisl KM, and Marshall S, “Cohort study of the impact of high-dose opioid analgesics on overdose mortality,” Pain Medicine (United States), 2016, 17 (1), 85–98. [DOI] [PubMed] [Google Scholar]
- Dave Dhaval, Deza Monica, and Horn Brady P., “Prescription Drug Monitoring Programs, Opioid Abuse, and Crime,” August 2018. NBER Working Paper No. 24975.
- Davis Corey S., Liberman Amy Judd, Herandez-Delgado Hector, and Suba Carli, “Laws limiting the initial prescribing or dispensing of opioids for acute pain in the United States: A national systematic legal review,” Drug and Alcohol Dependence, 2019, 194, 166–172. [DOI] [PubMed] [Google Scholar]
- —, Piper Brian J., Gertner Alex K., and Rotter Jason S., “Opioid Prescribing Laws Are Not Associated with Short-term Declines in Prescription Opioid Distribution,” Pain Medicine, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande Manasi and Li Yue, “Who is screened out? Application costs and the targeting of disability programs,” American Economic Journal: Economic Policy, Forthcoming. [Google Scholar]
- Dillender Marcus, “What happens when the insurer can say no? Assessing prior authorization as a tool to prevent high-risk prescriptions and to lowerÂăcosts,” Journal of Public Economics, 2018, 165, 170–200. [Google Scholar]
- Dowell Deborah, Haegerich Tamara M., and Chou Roger, “CDC Guideline for Prescribing Opioids for Chronic Pain âĂŤ United States, 2016,” MMWR Recomm Rep, 2016, 65, 1–49. 10.15585/mmwr.rr6501e1External. [DOI] [PubMed] [Google Scholar]
- Evans William N. and Farrelly Matthew C., “The compensating Behavior of Smokers: Taxes, Tar, and Nicotine,” RAND Journal of Economics, 1998, 29 (3), 578–95. [PubMed] [Google Scholar]
- —, Lieber Ethan, and Power Patrick, “How the reformulation of Oxycontin ignited the heroin epidemic,” Review of Economics and Statistics, 2019, 101. [Google Scholar]
- Goodman-Bacon Andrew, “Difference-in-differneces with variation in treatment timing,” July 2019. https://cdn.vanderbilt.edu/vu-my/wp-content/uploads/sites/2318/2019/07/29170757/ddtiming_7_29_2019.pdf.
- Han Beth, Compton Wilson M., Blanco Carlos, Crane Elizabeth, Lee Jinhee, and Jones Christopher M., “Prescription Opioid Use, Misuse, and Use Disorders in U.S. Adults: 2015 National Survey on Drug Use and HealthPrescription Opioid Use, Misuse, and Use Disorders in U.S. Adults,” Annals of Internal Medicine, 09 2017, 167 (5), 293–301. [DOI] [PubMed] [Google Scholar]
- Hedegaard Holly, Warner Matgaret, and Mini Arialdi M. no, “Drug overdose deaths in the United States, 1999–2016,” Technical Report December 2017. NCHS Data Brief. No. 294. [Google Scholar]
- International Narcotics Control Board, “Report of the International Narcotics Control Board on the availability of internaionally controlled drugs: Ensuring adequate access for medical and scientific purposes.,” June 2010. Available at http://www.incb.org/documents/Publications/AnnualReports/AR2010/Supplement-AR10_availability_English.pdf.
- Jones Jermaine D., Mogali Shanthi, and Comer Sandra D., “Polydrug abuse: A review of opioid and benzodiazepine combination use,” Drug and Alcohol Dependence, 2012, 125, 8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasich John., “(@JohnKasich),” Tweet, April 2017. [Google Scholar]
- Kilby Angela E., “Opioids for the masses: Welfare Tradeoffs in the Regulation of Narcotic Pain Medications,” November 2015. Unpublished working paper.
- Kochanek Kenneth D., Arias Elizabeth, and Bastian Brigham, “The effect of changes in selected age-specific causes of death,” Technical Report June 2016. NCHS Data Brief. No. 250. [PubMed] [Google Scholar]
- Lipsari Rachel N. and Hughes Arthur, “How people obtain the prescription pain relievers they misuse,” January 2017. The CBHSQ Report: January 12, 2017. Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration, Rockville, MD. [PubMed] [Google Scholar]
- Lowenstein Margaret, Hossain Erik, Yang Wei, Grande David, Perrone Jeanmarie, Neuman Mark D., Ashburn Michael, and Delgado M. Kit, “Impact of a State Opioid Prescribing Limit and Electronic Medical Record Alert on Opioid Prescriptions: a Difference-in-Differences Analysis,” Journal of General Internal Medicine, 2019. doi: 10.1007/s11606-019-05302-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallatt Justine, “The Effect of Prescription Drug Monitoring Programs on Opioid Prescriptions and Heroin Crime Rates,” October 2017. Unpublished working paper.
- Meara Ellen, Horowitz Jill R., Powell Wilson, McClelland Lynn, Zhou Weiping, O’Malley A. James, and Morden Nancy E., “State legal restrictions and precription opioid use among disabled adults,” New England Journal of Medicine, 2016, 375, 44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merboth MK and Barnason Susan Ann, “Managing Pain: The Fifth Vital Sign,” The Nursing Clinics of North America, 2000, 35, 375–383. [PubMed] [Google Scholar]
- Mularski Richard A., White-Chu Foy, Overbay Devorah, Miller Lois, Asch Steven M., and Ganzini Linda, “Measuing Pain as the 5th Vital Sign Does Not Improve Quality of Pain Management,” Journal of General Internal Medicine, 2006, 21, 607–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell David, Pacula Rosalie Liccardo, and Jacobson Mireille, “Do medical marijuana laws reduce addictions and deaths related to pain killers?,” Journal of Health Economics, March 2018, 58, 29–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhm Christopher J., “Corrected US opioid-involved drug poisoning deaths and mortality rates, 1999–2015,” Addiction, 2018, 113. [DOI] [PubMed] [Google Scholar]
- Schnell Molly and Currie Janet, “Addressing the Opioid Epidemic: Is There a Role for Physician Education?,” American Journal of Health Economics, 2018, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl Laurence, Seth Puja, Kariisa Mbabazi, Wilson Nana, and Baldwin Grant, “Drug and Opioid-Involved Overdose Deaths: United States, 2013–2017,” Morbidity and Mortality Weekly Report, 2019, 67, 1419–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah Anuj, Hayes Corey J., and Martin Braddley C., “Characteristics of initial prescription episodes and likelihood of long-term opioid use - United States, 206–2015,” Morbidity and Mortality Weekly Report, 2017, 10, 265–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surratt Hilary L., O’Grady Catherine, Kurtz Steven P., Stivers Yamilka, Cicer Theodore J., Dart Richard C., and Chen Minxing, “Reductions in prescription opioid diversion following recent legislative interventions in Florida,” Phamacoepidemiology and Drug Safety, 2014, 23, 317–320. [DOI] [PubMed] [Google Scholar]
- Tompkins D Andrew J, Hobelmann Greg, and Compton Peggy, “Providing chronic pain management in the “Fifth Vital Sign” Era: Historical and treatment perspectives on a modern-day medical dilemma,” Drug and Alcohol Dependence, 2017, 173, S11–S21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zee Van, Art, “The promotion and marketing of oxycontin: Commercial triumph, public health tragedy.,” American Journal of Public Health, 2009, 99, 221–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Hefei and Hockenberry Jason M., “Association of Medical and Adult-Use Marijuana Laws With Opioid Prescribing for Medicaid Enrollees,” JAMA Internal Medicine, 2018, 178, 673–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White Jason M. and Irvine Rodney J., “Mechanisms of fatal opioid overdose,” Addiction, July 1999, 94, 961–972. [PubMed] [Google Scholar]


