Abstract
We demonstrate a facile approach for the synthesis of gem-disubstituted cyclooctanoids, a motif found in several biologically active compounds. Appropriately substituted 1-alkenyl-5-pentyn-1-ols bearing gem-dialkyl substituents at either the C2, C3, or C4 position serve as useful precursors to a number of cyclooct-4-enone derivatives via a tandem, microwave-assisted oxyanionic 6-exo-dig cyclization/Claisen rearrangement reaction. gem-Dialkyl activation is necessary for these reactions to occur, as unactivated 1-alkenyl-5-pentyn-1-ols fail to undergo 6-exo-dig cyclization under the conditions employed. Further application of the methodology to the corresponding gem-dialkoxy system was also explored to facilitate access to more complex carbocycles.
Graphical Abstract

INTRODUCTION
A variety of biologically active natural and synthetically prepared compounds incorporate 8-membered carbocyclic and heterocyclic rings, underscoring the importance of developing novel strategies for the laboratory synthesis of these systems.1 Indeed, hundreds of structurally diverse cyclooctanoid natural products have been identified and characterized to date from sources such as marine organisms, terrestrial plants, pathogenic fungi, and insects (Figure 1).1 Structurally, cyclooctanoids may be categorized as diterpenoids, sesquiterpenoids, sesterterpenoid systems, dibenzocy-clooctadiene lignans, and polyphenol lignans.1 Despite their prevalence in nature and medicinal potential, the laboratory synthesis of cyclooctanoid systems is challenging due to unfavorable entropic and enthalpic factors associated with traditional annulation strategies inherent in medium-sized ring construction. Currently, the majority of methods available for the synthesis of 8-membered rings involve fragmentation reactions of preinstalled polycyclic structures2,3 and cycloaddition strategies,4 including [4 + 4] and [4 + 2 + 2] cycloadditions.
Figure 1.
Representative examples of cyclooctanoid natural products highlighting the prevalence of gem-dimethyl groups (blue).
We recently reported a novel strategy involving a base-catalyzed, microwave-assisted, tandem 6-exo-dig cyclization/Claisen rearrangement process allowing straightforward access to a variety of 8-membered ring systems from appropriately substituted 1-alkenyl-5-alkyn-1-ols bearing an electron-with-drawing group—preferably a cyano group—at the triple bond terminus (Scheme 1).5 Unlike the analogous 5-exo-dig cyclization/Claisen rearrangement reactions, which occur readily even with unactivated 4-alkyn-1-ol systems to afford a variety of cycloheptenone derivatives,6 simple 5-alkyn-1-ols bearing no activating substituents on the triple bond are resistant to 6-exo-dig cyclization reactions, and we have thus far been unable to utilize them as precursors to cyclooctanoid structures.
Scheme 1. Synthetic Strategies for the Construction of 7- and 8-Membered Ring Systems via Tandem exo-dig Cyclization/Claisen Rearrangement Reactions.
In pursuit of expanding the scope of the 6-exo-dig/Claisen rearrangement strategy to access more diverse cyclooctanoids, we decided to explore the utility of the Thorpe–Ingold effect in promoting the initial anionic cyclization. Such a strategy would obviate the need for an electron-withdrawing group on the triple bond terminus while simultaneously embedding a geminal-dialkyl group in the final 8-membered ring, a moiety present in a number of natural products and bioactive scaffolds (highlighted in Figure 1).1
This strategy relies on the “gem-dialkyl” effect, which was first observed by Thorpe, Ingold, and Beesley more than 100 years ago.7 This effect was initially thought to be based on the idea that replacement of methylene hydrogens with more sterically demanding alkyl groups on an acyclic chain results in an increased bond angle between the gem-dialkyl groups causing compression of the internal bond angle. This, in turn, would allow the reactive termini to become more proximal, thereby increasing the rate of cyclization.
Since Thorpe and Ingold formulated their theory, several other contributing factors have been invoked to explain the theoretical basis for the gem-dialkyl effect. These include the “reactive-rotamer” hypothesis first introduced by Bruce and Pandit8 and further developed by Jung et al.9 In addition, Dolata and co-workers10 proposed the “facilitated transition” hypothesis, which is based on extensive computational experiments, subscribing to the idea that the presence of a gem-dialkyl moiety causes an overall decrease in the activation enthalpy of the cyclization reaction. It is now known that geminal alkyl groups destabilize the extended conformation of open-chain species and populate more folded conformations which favor cyclization.9c This phenomenon, referred to as the Thorpe–Ingold effect, is now well documented and supported by a large number of examples in the chemical literature.11 The practical manifestations of this effect can be quite impressive, often resulting in rate increases by several orders of magnitude.11,12 In addition to accelerating rates of reactions, the gem-dialkyl effect can be advantageous in that it allows the for the formation of products containing quaternary carbon centers, which are common in a large number of natural products (Figure 1).
Since Thorpe and Ingold formulated their theory, several other contributing factors have been invoked to explain the theoretical basis for the gem-dialkyl effect. These include the “reactive-rotamer” hypothesis first introduced by Bruce and Pandit8 and further developed by Jung et al.9 In addition, Dolata and co-workers10 proposed the “facilitated transition” hypothesis, which is based on extensive computational experiments, subscribing to the idea that the presence of a gem-dialkyl moiety causes an overall decrease in the activation enthalpy of the cyclization reaction. The practical manifestations of this effect can be quite impressive, often resulting in rate increases by several orders of magnitude.11,12
RESULTS AND DISCUSSION
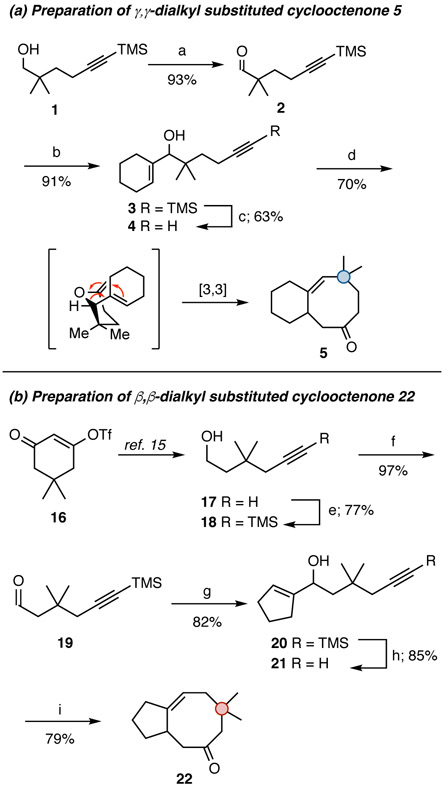
To test the applicability of the Thorpe–Ingold effect for the synthesis of cyclooctenone derivatives via a 6-exo-dig cyclization/Claisen rearrangement sequence, compound 4 was synthesized starting from the known alcohol 1.13 To this end, 1 was oxidized via the Swern protocol14 to afford aldehyde 2 (Scheme 2a).13 Subsequent coupling with cyclohexenyllithium, prepared in situ from the corresponding vinyl iodide via lithium–halogen exchange with t-BuLi, provided the desired alcohol 3. Removal of the TMS group with TBAF gave the desired precursor to cyclization (4). Similar coupling reactions involving aldehyde 2 and several other vinyllithium derivatives (prepared from the corresponding vinyl iodides) were used to access other derivatives of 2,2-dimethyl-4-pentyn-1-ol (6, 8, 10, 12, 14; Table 1). Compounds containing aromatic groups at the triple bond termini were prepared via the Sonogashira reaction (see the Experimental Section for details).15
Scheme 2. Synthesis of Cyclooctenones 5 and 22*.
* Reagents and conditions: (a) (COCl)2, DMSO, then TEA, DCM, −78 °C; (b) cyclohexenyllithium, then NH4Cl, −78 °C, Et2O; (c) TBAF, THF; (d) 10 mol % LHMDS, μwave heating, 210 °C, PhOEt, 1 h; (e) n-BuLi, then TMSCl, then aq HCl, THF, −78 °C; (f) (COCl)2, DMSO, then TEA, DCM, −78 °C;17 (g) cyclopentenyllithium, then NH4Cl, −78 °C, Et2O; (h) TBAF, THF; (i) 10 mol % LHMDS, μwave heating, 210 °C, PhOEt, 1 h.
Table 1.
6-Exo-Dig Cyclization/Claisen Rearrangement of 2,2-, 3,3-, and 4,4-Dimethyl-Substituted 1-Alkenyl-5-hexyn-1-ols

|
Isolated yield of major diastereomer.
Gratifyingly, when exposed to catalytic (10 mol %) LHMDS and microwave irradiation at 210 °C, 4 was converted to the cyclooctenone derivative 5 in 70% isolated yield. Other 2,2-dimethyl-5-hexyn-1-ol derivatives reacted similarly, affording the expected cyclooctenone products (7, 9, 11, 13, 15) in yields ranging from 71 to 87% (Table 1).
In cases where the triple bond is fully substituted (10 and 12), the reaction is highly stereoselective, producing a single detectable diastereomer for both compounds 11 and 13 in high yield. It is also of interest to note that cyclooctanoid 9 is a close analogue of the natural product precapnelladiene (Figure 1). It is envisioned that, starting with an analogue of 8 bearing a methyl group at C5 of the cyclopentenyl ring, this natural product could be prepared in a short number of steps following the key 6-exo cyclization/Claisen rearrangement process.
In order to assess the Thorpe–Ingold effect by alternative positioning of the gem-dimethyl moiety, we also prepared a few 3,3-dimethyl-substituted 1-alkenyl-5-hexyn-1-ol derivatives (21, 23, 25, 27) and one 4,4-dimethyl substituted analogue (29). The requisite acetylenic alcohol starting material 17 for the 3,3-dimethyl-substituted precursors can be prepared in a short sequence from the cyclic vinylogous acyl triflate 16 through a tandem hydride transfer/C–C bond cleaving fragmentation reaction according to the procedure of Dudley and co-workers (Scheme 2b).16 Standard Swern oxidation affords aldehyde 19.17 Coupling of 19 with cyclopentenyllithium, prepared from cyclopentenyl iodide via a low-temperature lithium–halogen exchange reaction with t-BuLi, followed by removal of the TMS group afforded alcohol 21 in 70% yield over two steps (Scheme 2b). Similarly, the analogous 4,4-dimethyl-substituted derivative necessary to access α,α-dialkyl-substituted cyclooctenone 30 was prepared using the same strategy, starting with a vinyl acyl triflate bearing the gem-dimethyl moiety at position C6 of the cyclic triflate.15
When 21 was subjected to our standard cyclization conditions (catalytic base and microwave irradiation) the bicyclic cyclooctenone derivative 22 was produced in 79% yield. Related analogues were synthesized in a similar manner; the results from these experiments are summarized in Table 1. These experiments demonstrate that operation of the Thorpe–Ingold effect is not dependent on specific positioning of the dialkyl moiety along the carbon chain that undergoes the initial 6-exo cyclization.
In addition to taking advantage of the Thorpe–Ingold effect through the use of gem-dialkyl-substituted cyclooctanoid systems, we have also preliminarily explored the use of analogous gem-dimethoxy derivatives. These are particularly attractive synthetically, as this moiety can be unmasked to the corresponding ketone and subsequently functionalized following the 6-exo cyclization/Claisen rearrangement sequence.
As shown in Scheme 3, commercially available 4-pentynoic acid can be converted to the corresponding acyl chloride and then subjected to modified Wissner conditions,18b affording hydroxyl ketone 32 in nearly quantitative yield. Subsequent benzoyl protection and treatment with trimethyl orthoformate in methanol gave acetal 34 in 88% yield. Protection of the terminal triple bond and removal of the benzoyl group afforded 35, which was then oxidized, and the resulting aldehyde was coupled with cyclopentenyllithium according to our standard protocol. Removal of the TMS protecting group provided acetal 37.
Scheme 3. Preparation of Cyclooctenone 38.A.
AReagents and conditions: (a) (COCl)2, DMF, DCM, rt; (b) tris(trimethylsilyloxy)ethylene, TEA, THF, 0 °C, then μwave heating, 100 °C, 10 min; (c) BzCl, TEA, cat. DMAP, DCM, 0 °C; (d)trimethyl orthoformate, cat. TsOH, MeOH, rt; (e) LHMDS, then TMSCl, THF, −78 C; (f) MeLi, Et2O, −78 C; (g) (COCl)2, DMSO, then TEA, DCM, −78 °C; (h) cyclopentenyllithium, then H2O, −78 °C, Et2O; (i) TBAF, THF; (j) 20 mol % LHMDS, 10 mol % TEA, μwave heating, 210 °C, PhOEt, 2.5 h.
Unlike the gem-dimethyl-substituted 1-alkenyl-5-hexyn-1-ols investigated, the 2,2-dimethoxy derivative 37 was found to be somewhat unstable. Our initial attempts at cyclization of this acetal on a 40 mg scale at 210 °C for 1 h showed mostly decomposition of the starting material. However, when the reaction was reattempted at a slightly lower temperature (180–190 °C) with 10 mol % triethylamine as an additive, 19% conversion to the desired product (38) was observed (Scheme 3). Despite the low yield obtained thus far with 37, this proof-of-concept result is encouraging. Accordingly, our laboratory is currently working to optimize this result and extend our methodology to other geminal diheteroatom-containing moieties.
In summary, we have demonstrated that 1-alkenyl-5-alkyn-1-ols bearing gem-dimethyl or -dimethoxy moieties undergo anionic 6-exo-dig cyclization/Claisen rearrangement reactions to provide various cyclooctenone derivatives generally in up to 87% yield. These processes benefit from operation of the Thorpe–Ingold effect, which accelerates the initial cycloisomerization reaction. This 6-exo cyclization affords the requisite tetrahydropyranyl intermediate, poised for the subsequent in situ [3,3]-sigmatropic rearrangement to afford a range of cyclooctanoid systems. Our new strategy, employing the Thorpe–Ingold effect, enables the synthesis of more biologically relevant and functional group rich 8-membered rings. Application of this methodology for the synthesis of cyclooctanoid natural products is currently underway in our laboratory and results from these studies will be reported in due course.
EXPERIMENTAL SECTION
General Experimental Methods.
All commercially available reagents used for experiments were obtained from Sigma–Aldrich, Acros, or Strem and used without further purification unless otherwise noted. Bulk solvents were obtained from Fisher or VWR. Anhydrous diethyl ether (Et2O), tetrahydrofuran (THF), dichloromethane (DCM), and N,N-dimethylformamide (DMF) were obtained using an Innovative Technology Pure Solv solvent purification system. Phenetole (PhOEt), chlorotrimethylsilane (TMSCl), and triethylamine (Et3N) were freshly distilled prior to use from calcium hydride under N2 atmosphere. All glassware used for nonaqueous reactions was flame-dried prior to reactions under N2 atmosphere. All reactions were carried out under an inert N2 atmosphere unless otherwise indicated. Reaction products were purified by column chromatography on silica gel using a glass column or by flash chromatography using a Biotage Isolera One system or Isco CombiFlash SG100C LC system. A Biotage Initiator Classic microwave synthesizer was used for the cyclization/Claisen rearrangement sequence. NMR spectral analyses were obtained from an Agilent DD2-500 NMR spectrometer and recorded in units of parts per million (ppm) relative to tetramethylsilane at δ = 0.00 ppm. NMR spectral data are reported with the following conventions: chemical shift (multiplicity [singlet (s), broad singlet (br s), doublet (d), triplet (t), quartet (q), heptet (hept), multiplet (m), doublet of doublets (dd), doublet of doublet of doublets (ddd), doublet of doublet of doublet of doublets (dddd), doublet of triplets (dt), and doublet of triplet of triplets (dtt)], coupling constants (J, Hz), integration). High-resolution mass spectrometry (HRMS) for previously unreported compounds was performed at the University of Illinois Urbana–Champaign Mass Spectrometry Lab.
Synthesis of 5-Hexyn-1-ols.
1-(Cyclohex-1-enyl)-2,2-dimethyl-6-(trimethylsilyl)hex-5-yn-1-ol (3). 1-Iodocyclohexene19 (0.53 g, 2.5 mmol) was dissolved in diethyl ether (30 mL) and cooled to −78 °C. A solution of t-BuLi (2.7 mL, 1.9 M in pentane, 5.13 mmol) was then added dropwise and the resulting mixture allowed to stir for 15 min.20 The reaction mixture was warmed to 0 °C while stirring for an additional 1 h to destroy any excess t-BuLi. The solution was then cooled back to −78 °C, and a solution of aldehyde 2 (0.21 g, 1.05 mmol) in 10 mL of diethyl ether was added dropwise. The reaction mixture was allowed to stir for 30 min and then quenched with 1 mL of water. Saturated aq NH4Cl (10 mL) was added, the layers were separated, and the aqueous layer was extracted with ether (3 × 50 mL). The combined organic layers were washed with water and brine and then dried over magnesium sulfate. The organic solution was filtered and the solvent removed under reduced pressure. The residue was purified by column chromatography (3% EtOAc in hexanes) to afford 0.32 g (91%) of alcohol 3 as a yellow oil. 1H NMR (500 MHz, CDCl3): δ 5.63, (bs, 1H), 3.71 (d, J = 3.4 Hz, 1H), 2.26–2.22 (m, 2H), 2.07–2.04 (m, 3H), 1.69–1.63 (m, 4H), 1.55–1.49 (m, 4H), 1.44 (d, J = 3.9 Hz, 1H), 0.89 (s, 3H), 0.85 (s, 3H), 0.14 (s, 9H) ppm. 13C{1H} NMR (126 MHz, CDCl3): δ 138.9, 125.5, 108.5, 83.9, 82.6, 38.6, 38.0, 31.6, 27.2, 25.1, 23.1, 22.9, 22.6, 15.1, 0.14 ppm. HRMS (ESI-TOF): m/z [M + Na]+ calcd for C17H30OSiNa 301.1964, found 301.1958.
1-(6,6-Dimethylcyclohex-1-enyl)-2,2-dimethylhex-5-yn-1-ol (4).
Compound 3 (0.32 g, 1.1 mmol) and TBAF (0.01 mL, 1 M solution in THF, 0.01 mmol) were dissolved in THF (5 mL) and stirred for 30 min. The reaction was terminated with the addition of 1 mL of water. The organic solution was rinsed with water and brine, dried over magnesium sulfate, and filtered. The solvent was removed under reduced pressure. The residue was further purified by column chromatography eluting with 6% ethyl acetate in hexanes to afford 0.15 g (63%) of 4 as a pale-yellow oil. 1H NMR (500 MHz, CDCl3): δ 5.64 (bs, 1H), 3.70 (s, 1H), 2.22–2.19 (m, 2H), 2.16–2.04 (m, 3H), 1.94 (t, J = 2.5 Hz, 1H), 1.71–1.61 (m, 3H), 1.56–1.50 (m, 3H), 1.43 (bs, 1H) 0.90 (s, 3H), 0.87 (s, 3H) ppm. 13C{1H} NMR (126 MHz, CDCl3): δ 138.9, 125.6, 85.6, 82.7, 67.8, 38.5, 38.0, 27.2, 23.3, 23.3, 22.9, 22.6, 13.6 ppm. HRMS (ESI-TOF): m/z [M + H]+ calcd for C14H23O 207.1749, found 207.1749.
1-(6,6-Dimethylcyclohex-1-enyl)-2,2-dimethyl-6-(trimethylsilyl)-hex-5-yn-1-ol (S1).
Compound S1 was prepared from 1-iodo-6,6-dimethylcyclohex-1-ene19 (0.740 g, 0.313 mmol) and aldehyde 213 (0.309 g, 0.157 mmol) according to the procedure described for the preparation of 3 (Scheme 2a). The crude product was purified by column chromatography eluting with 3% ethyl acetate in hexanes to afford 0.430 g (89%) of S1. 1H NMR (500 MHz, CDCl3): δ 5.94 (t, J = 4.4 Hz, 1H), 3.87 (d, J = 4.9 Hz, 1H), 2.26 (ddd J = 8.8, 7.1, 1.2 Hz, 2H), 2.06–1.99 (m, 2H), 1.74 (ddd, J = 13.6, 8.9, 7.3 Hz, 1H), 1.60–1.41 (m, 6H), 1.11 (s, 3H), 0.98 (d, J = 4.4, Hz, 6H), 0.87 (s, 3H), 0.13 (s, 9H) ppm. 13C{1H} NMR (126 MHz, CDCl3): δ 148.4, 125.5, 108.6, 84.0, 74.0, 39.8, 39.5, 38.7, 34.5, 28.9, 26.0, 23.7, 23.3, 18.9, 15.2, 0.1 ppm. HRMS (ESI-TOF): m/z [M + Na]+ calcd for C19H34OSiNa 329.2277, found 329.2271.
1-(6,6-Dimethylcyclohex-1-en-1-yl)-2,2-dimethylhex-5-yn-1-ol (6).
Compound S1 (0.43 g, 1.4 mmol) and TBAF (0.015 mL, 1 M in THF, 0.015 mmol) were stirred in THF (5 mL) in a scintillation vial for 30 min. The reaction was terminated by the addition of 1 mL of water. The organic solution was rinsed with water and brine, dried over magnesium sulfate, and filtered. The solvent was removed under reduced pressure. The crude product was purified by column chromatography over silica gel, eluting with 5% ethyl acetate in hexanes to afford 0.15 g (47%) of 6 as a white semisolid. 1H NMR (500 MHz, CDCl3): δ 5.94 (t, J = 4.2 Hz, 1H), 3.85 (d, J = 4.9 Hz, 1H), 2.23 (td, J = 8.2, 2.7 Hz, 2H), 2.10–2.01 (m, 2H), 1.94 (t, J = 2.5 Hz, 1H), 1.90–1.72 (m, 1H), 1.60–1.47 (m, 6H), 1.23 (d, J = 4.9 Hz, 1H), 1.11 (s, 3H), 0.99 (d, J = 45.9 Hz, 6H), 0.90 (s, 3H) ppm. 13C{1H} NMR (126 MHz, CDCl3) : δ 148.7, 125.5, 109.7, 85.6, 74.7, 67.8, 39.8, 39.2, 34.5, 29.0, 26.0, 23.5, 18.9, 13.8 ppm. HRMS (ESI-TOF): m/z [M + H]+ calcd for C16H27O 235.2062, found 235.2069.
1-(1-Cyclopentenyl)-2,2-dimethyl-4-hexyn-1-ol (S2).
Compound S2 was prepared from 1-iodocyclopentane19 (8.30 g, 42.8 mmol) and 2,2-dimethylhex-4-ynal21 (2.66 g, 21.4 mmol) according to the procedure described for the preparation of 3. The crude product was purified by column chromatography eluting with 2.6% ethyl acetate and 15% dichloromethane in hexanes to afford 3.19 g (77%) of the desired product S2 as a clear oil. 1H NMR (500 MHz, CDCl3): δ 5.63–5.64 (m, 1H), 4.18 (d, J = 4.4 Hz, 1H), 2.38–2.46 (m, 1H), 2.29–2.38 (m, 3H), 2.20 (dq, J = 16.4, 2.8 Hz, 1H), 2.04 (dq, J = 16.4, 2.7 Hz, 1H), 1.78–1.90 (m, 3H), 1.79 (t, J = 2.7 Hz, 3H), 0.96 (s, 3H), 0.94 (s, 3H) ppm. 13C{1H} NMR (126 MHz, CDCl3): δ 128.3, 77.7, 77.5, 76.7, 38.3, 33.9, 32.0, 29.8, 24.0, 22.9, 3.5 ppm. HRMS (ESI-TOF): m/z [M]+ calcd for C13H20O 192.1514, found 192.1522.
1-(1-Cyclopentenyl)-2,2-dimethyl-5-hexyn-1-ol (8).
A slurry of 30% potassium hydride in mineral oil (7.18 g, 53.68 mmol) was added to a preweighed 50 mL Erlenmeyer flask equipped with a septum and a stir bar. The slurry was then washed sequentially with anhydrous diethyl ether (5 mL × 4), each time removing the resulting clear solution with a syringe and a Teflon cannula inserted through the septum. The flask was then flushed with nitrogen gas to remove any remaining solvent, leaving a fluffy gray powder. Freshly distilled 1,3-diaminopropane (50 mL) was then added to the flask at a quick dropwise pace while stirring. After 1.5 h of stirring, the solution finished bubbling and appeared very dark. Alcohol S2 (3.04 g, 15.8 mmol), which had been dried over molecular sieves prior to use, was added in one aliquot, and the resulting solution was allowed to stir for 2 h. The reaction mixture was then quenched by a slow addition of crushed ice and water (50 mL). Ether (50 mL) was added, the layers were separated, and the aqueous layer was extracted with ether (30 mL × 12). The combined organic layers were then washed sequentially with distilled water, satd aq NaHCO3, and brine, and then dried over magnesium sulfate. The solution was filtered, and the solvent was removed under reduced pressure. The crude product was purified by column chromatography eluting with 5% ethyl acetate in hexanes to afford 2.58 g (85%) of alcohol 8 as a pale-yellow oil. 1H NMR (500 MHz, CDCl3): δ 5.62–5.63 (m, 1H), 4.02 (d, J = 3.9 Hz, 1H), 2.36–2.44 (m, 1H), 2.28–2.35 (m, 3H), 2.17–2.22 (m, 2H), 1.93 (t, J = 2.45 Hz, 1H), 1.78–1.91 (m, 2H), 1.64–1.71 (m, 1H), 1.48–1.55 (m, 1H), 1.48 (d, J = 3.9 Hz, 1H), 0.90 (s, 3H), 0.87 (s, 3H) ppm. 13C{1H} NMR (126 MHz, CDCl3): δ 145.6, 128.5, 85.5, 78.0, 67.8, 38.1, 37.8, 34.0, 32.0, 23.9, 23.0, 13.6 ppm. HRMS (ESI-TOF) m/z [M + H]+ calcd for C13H21 193.1592, found 193.1583.
1-(1-Cyclopentenyl)-2,2-dimethyl-6-phenyl-5-hexyn-1-ol (10).
A solution of alcohol 8 (0.12 g, 0.61 mmol), iodobenzene (0.17 g, 0.82 mmol), triphenylphosphine (0.0020 g, 0.008 mmol), copper iodide (0.0023 g, 0.01 mmol), and triethyl amine (8 mL) in dimethylformamide was briefly sparged with nitrogen at which point PdCl2(PPh3)2 (0.0054 g, 0.0080 mmol) was added. After 3 h, the reaction was judged complete by TLC. Most of the DMF solvent was removed under reduced pressure, and the residue was taken up in diethyl ether. Water was added (15 mL), the layers were separated, and the aqueous layer was extracted with diethyl ether (3 × 10 mL). The combined organic layers were washed brine, dried over magnesium sulfate, filtered, and concentrated under reduced pressure to give a brown residue that was purified by column chromatography eluting with 5% ethyl acetate in hexanes to afford 0.14 g (87%) of the desired product as a pale-yellow oil. 1H NMR (500 MHz, CDCl3): δ 7.37–7.40 (m, 2H), 7.25–7.28 (m, 3H), 5.65–5.66 (m, 1H), 4.08 (s, 1H), 2.41–2.45 (m, 3H), 2.30–2.34 (m, 3H), 1.83–1.92 (m, 2H), 1.73–1.77 (m, 1H), 1.57–2.64 (m, 1H), 1.52 (br s, 1H), 0.95 (s, 3H), 0.93 (s, 3H) ppm. 13C{1H} NMR (126 MHz, CDCl3) δ 145.6, 131.5, 128.5, 128.2, 127.5, 123.4, 91.0, 80.3, 78.1, 38.1, 37.9, 34.1, 32.0, 23.9, 23.1, 14.6 14.6 ppm. HRMS (ESI-TOF): m/z [M − H]+ calcd for C19H25O 267.1749, found 267.1746.
1-(1-Cyclopentenyl)-6-(2,5-dimethoxyphenyl)-2,2-dimethyl-5-hexyn-1-ol (12).
A solution of alcohol 8 (0.12 g, 0.60 mmol), 2-bromo-1,4-dimethoxybenzene (0.17 g, 0.8 mmol), triphenylphosphine (0.0020 g, 0.008 mmol), copper iodide (0.0023 g, 0.01 mmol), and Et3N (8 mL) in DMF was briefly sparged with nitrogen at which point PdCl2(PPh3)2 (0.002 g, 0.003 mmol) was added. The reaction mixture was then heated to 80 °C and stirred for 5 h. Most of the DMF solvent was then removed under reduced pressure, and the residue was taken up in diethyl ether. Water was added (15 mL), the layers were separated and the aqueous layer was extracted with diethyl ether (3 × 10 mL). The combined organic layers were washed brine, dried over magnesium sulfate, filtered and concentrated under reduced pressure to give a brown residue that was purified by column chromatography eluting with 5% ethyl acetate in hexanes to afford 0.17 g (84%) of the desired product as a viscous pale-yellow oil. 1H NMR (500 MHz, CDCl3): δ 6.92 (d, J = 2.4 Hz, 1H), 6.80–6.75 (m, 2H), 5.64–5.62 (m, 1H), 4.13 (s, 1H), 3.82 (s, 3H), 3.75 (s, 3H), 2.48 (t, J = 7.80 Hz, 2 H), 2.38–2.45 (m, 1H), 2.31 (t, J = 7.80 Hz, 2H), 2.30–2.36 (m, 1H), 1.76–1.91 (m, 4H), 1.55–1.62 (m, 1H), 0.95 (s, 3H), 0.92 (s, 3H) ppm. 13C{1H} NMR (126 MHz, CDCl3): δ 153.2, 145.7, 128.3, 118.3, 114.7, 113.5, 111.7, 95.4, 77.6, 76.2, 56.3, 55.7, 38.2, 38.0, 34.1, 32.0, 23.9, 23.2, 23.1, 14.9 ppm. HRMS (ESI-TOF): m/z [M + H]+ calcd for C21H29O3 329.2117, found 329.2122.
2,4,4-Trimethyl-8-(trimethylsilyl)oct-1-en-7-yn-3-ol (S3).
Compound S3 was prepared from commercially available 2-bromopropene (0.098 g, 0.812 mmol) and aldehyde 213 (0.399 g, 0.203 mmol) according to the procedure described for the preparation of 3. The crude product was purified by column chromatography eluting with 5% ethyl acetate in hexanes to afford 0.470 g (97%) of S2 as a pale-yellow oil. 1H NMR (500 MHz, CDCl3): δ 4.97 (br s, 1H), 4.91 (br s, 1H), 3.85 (s, 1H), 2.25 (t, J = 7.6 Hz, 2H), 1.79 (s, 3H), 1.73–1.65 (m, 1H), 1.57 (br s, 1H), 1.55–1.48 (m, 1H), 0.90 (s, 3H), 0.87 (s, 3H), 0.13 (s, 9H) ppm. 13C{1H} NMR (126 MHz, CDCl3): δ 146.2, 114.1, 108.4, 84.1, 81.6, 38.4, 37.8, 23.4, 22.9, 20.9, 15.0, 0.12 ppm. HRMS (ESI-TOF): m/z [M + H]+ calcd for C14H27OSi 239.1831, found 239.1827.
2,4,4-Trimethyloct-1-en-7-yn-3-ol (14).
Compound S3 (0.45 g, 1.9 mmol) and TBAF (0.015 mL, 1 M in THF, 0.015 mmol) were dissolved in THF (6 mL) in a scintillation vial for 40 min. The reaction was terminated by the addition of 1 mL of water. The organic solution was rinsed with water and brine, dried over magnesium sulfate, and filtered. The solvent was removed under reduced pressure. The crude product was purified by column chromatography eluting with 5% ethyl acetate in hexanes to afford 0.20 g (64%) of the desired product as a pale-yellow liquid. 1H NMR (500 MHz, CDCl3): δ 4.98 (app. quin, J = 1.6 Hz, 1H), 4.94–4.92 (m, 1H), 3.85 (s, 1H), 2.24–2.19 (m, 2H), 1.94 (t, J = 2.7 Hz, 1H), 1.8 (s, 3H), 1.74–1.68 (m, 1H), 1.57–1.51 (m, 2H), 0.92 (s, 3H), 0.90 (s, 3H) ppm. 13C{1H} NMR (126 MHz, CDCl3) : δ 146.2, 114.2, 85.4, 81.7, 67.9, 38.3, 37.7, 23.3, 23.0, 20.9, 13.6 ppm. HRMS (ESI-TOF): m/z [M]+ calcd for C11H18O 166.1358, found 166.1361.
1-(1-Cyclopent-1-en-1-yl)-3,3-dimethyl-6-(trimethylsilyl)hex-5-yn-1-ol (20).
This route is shown in Scheme 2b. To a −78 °C solution of oxalyl chloride (0.463 g, 3.65 mmol) in DCM (15 mL) was added DMSO (0.570 mL, 7.29 mmol) in DCM (4 mL) dropwise, and the resulting mixture was stirred at this temperature for 30 min. A solution of 1823 (0.295 g, 1.460 mmol) in 10 mL of DCM was then added dropwise via syringe. After 30 min, freshly distilled trimethylamine (2.76 mL, 19.8 mmol) was added, and the resulting solution was allowed to warm to room temperature. The reaction mixture was then sequentially washed with 10% HCl (10 mL), H2O, satd aq NaHCO3, and brine before the organic layer was separated. The organic layer was separated, dried over MgSO4, filtered, and concentrated under reduced pressure to yield aldehyde 196 as a yellow oil, which was used without further purification for the next step.
A solution of 1-iodocyclopentene (0.587 g, 3.03 mmol) in Et2O (18 mL) was dried over activated molecular sieves and then was cooled to −78 °C followed by dropwise addition of t-BuLi (1.9M, 3.18 mL, 6.05 mmol), and the resulting solution was stirred for 15 min. The reaction mixture was then warmed to 0 °C and stirred at this temperature for an additional 1 h to destroy any excess t-BuLi. The solution was then cooled to −78 °C, and a solution of crude aldehyde 19 (0.297g, 1.51 mmol) in Et2O (10 mL) was added dropwise. After 30 min, no more aldehyde starting material remained as judged by TLC, and the reaction was quenched by the addition of 5 mL of H2O. A solution of satd aq NH4Cl (15 mL) was then added, and the layers were separated. The aqueous layer was extracted with Et2O (3 × 10 mL), and the combined ethereal layers were dried over MgSO4/K2CO3. The solution was filtered and concentrated under reduced pressure to obtain a crude clear and colorless oil, which was further purified by column chromatography (7% ethyl acetate in hexanes) to afford 0.325 g (82% over two steps) of 20 as a clear, colorless oil. 1H NMR (500 MHz, CDCl3): δ 5.59–5.58 (m, 1H), 4.42–4.40 (m, 1H), 2.35–2.30 (m, 4H), 2.22 (s, 2H), 1.87 (app. quin, J = 6.85 Hz,2H), 1.62 (dd, J = 14.7, 3.4 Hz, 1H), 1.65–1.53 (m, 2H), 1.05 (s, 3H), 1.03 (s, 3H), 0.14 (s, 9H) ppm. 13C{1H} NMR (126 MHz, CDCl3): δ 148.5, 124.5, 105.9, 86.7, 68.8, 46.4, 33.3, 33.2, 32.1, 31.2, 27.8, 27.5, 23.4, 0.1 ppm. HRMS (ESI-TOF): m/z [M]+ calcd for C16H28OSi 264.1910, found 264.1901.
1-(Cyclopent-1-en-1-yl)-3,3-dimethylhex-5-yn-1-ol (21).
Following the general procedure described for the preparation of 4, 20 (0.275 g, 1.04 mmol) was treated with TBAF (0.01 mL, 1.0 M solution in THF, 0.01 mmol) and then purified via column chromatography (6.5% ethyl acetate in hexanes) to afford 21 (0.170 g, 85%) as a clear oil. 1H NMR (500 MHz, CDCl3): δ 5.59–5.58 (m, 1H), 4.42–4.40 (t, J = 5.9 Hz,1H), 2.27–2.36 (m, 4H), 2.22 (dd, J = 16.6, 2.4 Hz, 1H),2.17 (dd, J = 16.6, 2.9 Hz, 1H), 2.00 (t, J = 2.4 Hz, 1H), 1.88 (app quin, J = 6.85 Hz, 2H), 1.57–1.65 (m, 3H), 1.06 (s, 3H), 1.05 (s, 3H) ppm. 13C{1H} NMR (126 MHz, CDCl3) : δ 148.5, 124.6, 82.8, 70.1, 68.8, 46.2, 33.1, 32.1, 32.0, 31.2, 27.7, 27.2, 23.4 ppm. HRMS (ESI-TOf): m/z [M + H]+ calcd for C13H21O 193.1592, found 193.1600.
2,5,5-Trimethyl-8-(trimethylsilyl)oct-1-en-7-yn-3-ol (S4).
Following the procedure described for the synthesis of 20, alcohol S4 was prepared in two steps from 19 (0.400 g, 2.02 mmol), 2-bromopropene (0.740 mL, 1.01 g, 8.34 mmol), and t-BuLi (1.9M, 8.50 mL, 16.1 mmol) to give S4 (0.198 g, 41% over two steps) as a pale-yellow oil following purification by column chromatography (7% ethyl acetate in hexanes). 1H NMR (500 MHz, CDCl3): δ 4.96 (m, 1H), 4.79 (m, 1H), 4.19–4.21 (m, 1H), 2.22 (s, 2H), 1.75 (s, 3H), 1.50–1.75 (m, 3H), 1.05 (s, 3H), 1.04 (s, 3H), 0.15 (s, 9H) ppm. 13C{1H} NMR (126 MHz, CDCl3): δ 149.2, 109.9, 105.8, 86.8, 73.2, 46.2, 33.3, 33.1, 27.9, 27.5, 17.9, 0.1 ppm. HRMS (ESI-TOF): m/z [M + H]+ calcd for C14H27OSi 239.1831, found 239.1831.
2,5,5-Trimethyloct-1-en-7-yn-3-ol (23).
Following the general procedure described for the preparation of 4, S4 (0.158 g, 0.663 mmol) was treated with TBAF (0.01 mL, 1.0 M solution in THF, 0.01 mmol) to afford 23 (0.101 g, 92%) as a clear oil following column chromatography (11% ethyl acetate in hexanes). 1H NMR (500 MHz, CDCl3): δ 4.96 (m, 1H), 4.79 (m, 1H), 4.20 (t, J = 5.87 Hz, 1H), 2.17–2.26 (m, 2H), 2.01 (t, J = 2.93 Hz, 1H), 1.74 (s, 3H), 1.55–1.57 (m, 2H), 1.06 (s, 3H), 1.48 (br s, 1H), 1.07 (s, 3H) ppm. 13C{1H} NMR (126 MHz, CDCl3): δ 149.2, 110.1, 82.7, 73.3, 70.2, 46.1, 33.1, 31.9, 27.7, 27.2, 17.9 ppm. HRMS (ESI-TOF): m/z [M − OH]+ calcd for C16H27Si 247.1882, found 247.1889.
3,3-Dimethyl-6-phenyl-5-hexyn-1-ol (S5).
A solution of alcohol 17 (0.34 g, 2.7 mmol), iodobenzene (0.77 g, 3.8 mmol), triphenylphosphine (0.0020 g, 0.008 mmol), copper iodide (0.025 g, 0.13 mmol), and triethylamine (10 mL) was briefly sparged with nitrogen at which point Pd(PPh3)4 (0.062 g, 0.054 mmol) was added. After 4 h of stirring at room temperature, the reaction was judged complete by TLC. Most of the Et3N solvent was removed under reduced pressure, and the residue was taken up in diethyl ether. A 10% HCl solution (15 mL) of was added, the layers were separated, and the aqueous layer was extracted with diethyl ether (3 × 10 mL). The combined organic layers were washed brine, dried over magnesium sulfate, filtered, and concentrated under reduced pressure to give a brown residue that was purified by column chromatography eluting with 5% to 40% ethyl acetate in hexanes to afford 0.435 g (80%) of S5 as a pale-yellow oil. 1H NMR (500 MHz, CDCl3): δ 7.41–7.37 (m, 2H), 7.30–7.25 (m, 3H), 3.76 (t, J = 7.30 Hz, 2H), 2.33 (s, 2H), 1.69 (t, J = 7.30 Hz, 2H), 1.30 (br s, 1H), 1.06 (s, 6H) ppm. 13C{1H} NMR (126 MHz, CDCl3): δ 131.5, 128.2, 127.6, 123.9, 88.0, 82.6, 59.8, 43.9, 33.2, 33.1, 27.3 ppm. HRMS (ESI-TOF): m/z [M + H]+ calcd for C14H19O 203.1436, found 203.1432.
3,3-Dimethyl-8-phenyl-1-octen-7-yn-3-ol (25).
To a −78 °C solution of oxalyl chloride (0.640 g, 5.08 mmol) in DCM (10 mL) was added DMSO (0.797 g, 10.2 mmol) in DCM (5 mL) dropwise, and the resulting mixture was stirred at this temperature for 30 min. A solution of S5 (0.414 g, 2.05 mmol) in 10 mL of DCM was then added dropwise via syringe. After 30 min, freshly distilled Et3N (3.90 mL, 28.0 mmol) was added, and the resulting solution was allowed to warm to room temperature. The reaction mixture was then sequentially washed with 10% HCl (10 mL), H2O, satd aq NaHCO3, and brine before the organic layer was separated. The organic layer was separated and dried over MgSO4, filtered, and concentrated under reduced pressure to yield 3,3-dimethyl-6-phenyl-5-hexynal (S6) as a yellow oil, which was used without further purification for the next step.
A solution of crude S6 in diethyl ether (4 mL) was briefly dried over molecular sieves and then transferred to a 100 mL round-bottom flask, rinsing with more diethyl ether (30 mL total), and cooled to −78 °C. Once at this temperature, a solution of vinylmagnesium bromide (1.0 M in THF, 8.0 mL, 8.0 mmol) was added dropwise. After 20 min, the reaction was judged to be complete by TLC. The cooling bath was then removed and the reaction was quenched by the addition of 5 mL of 10% aq K2CO3 solution. The organic layer was decanted off, and the remaining white slurry was washed several times with diethyl ether (4 × 20 mL). The combined organic layers were washed with brine and dried over MgSO4. The solution was filtered and concentrated under reduced pressure to obtain a pale-yellow oil, which was further purified by column chromatography (Biotage Isolera, 2% to 20% ethyl acetate/hexanes) to afford 25 as a clear oil (0.339 g, 72% over two steps). 1H NMR (500 MHz, CDCl3): δ 7.41–7.37 (m, 2H), 7.29–7.25 (m, 3H), 5.92 (ddd, J = 17.1, 10.3, 6.4 Hz, 1H), 5.23 (app. dt, J = 17.1, 1.5 Hz, 1H), 5.07 (app. dt, J = 10.3, 1.5 Hz, 1H), 4.35–4.29 (m, 1H), 2.42 (d, J = 16.7 Hz, 1H), 2.39 (d, J = 16.7 Hz, 1H), 1.64 (d, J = 16.7 Hz, 1H), 1.61 (d, J = 16.7 H, 1H), 1.45 (app. d, J = 4.0 Hz, 1H), 1.21 (s, 3H), 1.11 (s, 3H) ppm. 13C{1H} NMR (126 MHz, CDCl3): δ 142.6, 131.5, 128.2, 127.6, 123.9, 113.8, 88.4, 82.7, 70.8, 47.9, 33.7, 33.0, 28.0, 27.6 ppm. HRMS (ESI-TOF): m/z [M-H]+ calcd for C16H20O 227.1436, found 227.1439.
2,5,5-Trimethyl-8-phenyloct-1-en-7-yn-3-ol (27).
Iodobenzene (0.390 mL, 0.711 g, 0.348 mmol) was dissolved in Et3N (13 mL). In a separate flask, 23 (0.460 g, 2.77 mmol) was dissolved in Et3N (4 mL), and both solutions were sparged with nitrogen for 10 min. Copper(I) iodide (0.0302 g, 0.159 mmol) and Pd(PPh3)4 (0.0733 g, 0.0634 mmol) were added to the iodobenzene solution and then combined with the solution of 23 and Et3N. The resulting mixture was stirred at room temperature overnight at which point the reaction was deemed complete by TLC. The reaction mixture was then treated with satd aq NH4Cl (1 mL) and washed sequentially with H2O and brine. The aqueous layer was extracted with ether (3 × 10 mL) and the combined organic layers were washed again with brine and then dried over MgSO4. The organic layer was filtered and concentrated under reduced pressure to afford the crude product as a pale-yellow oil, which was purified by Isco CombiFlash SG100C LC System (Interchim puriFlash 4 g column, 0–20% ethyl acetate in hexanes) to afford 27 (0.564 g, 84%) as a viscous, pale-yellow oil. 1H NMR (500 MHz, CDCl3): δ 7.40–7.26 (m, 5H), 4.98 (br s, 1H), 4.81–4.97 (m, 1H), 4.27–4.24 (m, 1H), 2.42 (s, 2H), 1.76 (s, 3H), 1.65 (dd, J = 14.67, 2.93 Hz, 1H), 1.59 (dd, J = 14.67, 8.34 Hz, 1H), 1.56 (s, 1H), 1.13 (s, 3H), 1.12 (s, 3H) ppm. 13C{1H} NMR (126 MHz, CDCl3): δ 131.5, 128.2, 127.5, 110.1, 88.4, 82.7, 73.4, 46.4, 33.7, 32.8, 29.4, 27.9, 27.5, 17.9 ppm. HRMS (ESI-TOf): m/z [M + Na]+ calcd for C17H22ONa 265.1568, found 265.1574.
4,4-Dimethyl-6-(trimethylsilyl)hex-5-yn-1-ol (S7).
To a −78 °C solution of 4,4-dimethylhex-5-yn-1-ol16 (1.11 g, 8.77 mmol) in THF (20 mL) was added n-BuLi (1.6 M in hexanes, 11.4 mL, 18.3 mmol) dropwise. The resulting solution was stirred at this temperature for 30 min and TMSCl (2.03 g, 18.6 mmol) was added dropwise. The solution was then warmed to room temperature, and 10 mL of H2O was added slowly. The solvents were then evaporated, and the crude product was redissolved in diethyl ether (20 mL), followed by the addition of 10% aq HCl (10 mL). The biphasic solution was stirred vigorously for 1 h. The organic layer was separated and washed successively with satd aq NaHCO3 and brine and dried over MgSO4. The solution was then filtered and the solvent evaporated under reduced pressure to give a clear oil that was subjected to purification by Biotage Isolera Prime (5 to 30%, ethyl acetate in hexanes) to afford 1.27 g (73%) of alcohol S7 as a clear oil. 1H NMR (500 MHz, CDCl3): δ 3.66 (t, J = 6.6 Hz, 2H), 1.67–1.74 (m, 2H), 1.41–1.45 (m, 3H), 1.18 (s, 6H), 0.12 (s, 9H) ppm. 13C{1H} NMR (126 MHz, CDCl3): δ 114.4, 83.6, 63.3, 39.3, 31.5, 29.2, 28.7, 0.3 ppm. HRMS (ESI-TOF): m/z [M + H]+ calcd for C11H23OSi 199.1518, found 199.1519.
2,6,6-Trimethyl-8-(trimethylsilyl)oct-1-en-7-yn-3-ol (S9).
To a solution of oxalyl chloride (1.27 g, 10.1 mmol) in DCM (20 mL) at −78 °C was added DMSO (1.57 g, 20.2 mmol) in DCM (10 mL) dropwise, and the resulting mixture was stirred at this temperature for 30 min. A solution of S7 (0.800 g, 4.03 mmol) in DCM (10 mL) was then added dropwise via syringe. After 30 min, freshly distilled Et3N (2.81 mL, 20.2 mmol) was added, and the resulting solution was allowed to warm to room temperature. The reaction mixture was then sequentially washed with 10% HCl (10 mL), H2O, satd aq NaHCO3, and brine before the organic layer was separated. The combined organics were dried over MgSO4, filtered, and concentrated under reduced pressure to yield aldehyde S8 as a yellow oil, which was used without further purification for the next step.
A solution of 2-bromopropene (1.46 g, 12.09 mmol), which had been dried previously over activated molecular sieves, in Et2O (30 mL) was cooled to −78 °C followed by dropwise addition of t-BuLi (1.9M, 12.7 mL, 24.20 mmol), and the resulting solution was stirred for 15 min. The reaction mixture was then warmed to 0 °C and stirred at this temperature for an additional 1 h to destroy any excess t-BuLi. The solution was then cooled to −78 °C, and a solution of crude aldehyde S8 in Et2O (16 mL) was added dropwise. After 30 min, no more aldehyde starting material remained as judged by TLC, and the reaction was quenched by the addition of 5 mL of H2O. A solution of satd aq NH4Cl (15 mL) was then added, and the layers were separated. The aqueous layer was extracted with Et2O (10 mL × 3), and the combined ethereal layers were dried over MgSO4. The solution was filtered and concentrated under reduced pressure to obtain a crude oil, which was further purified by column chromatography (12% ethyl acetate in hexanes) to afford alcohol S9 (0.614 g, 64% over two steps) as a clear, colorless oil. 1H NMR (500 MHz, CDCl3): δ 4.93–4.94 (m, 1H), 4.84–4.85 (m, 1H), 4.07 (t, J = 6.35 Hz, 1H), 1.72–1.73 (m, 3H), 1.64–1.79 (overlapping patterns, 2H), 1.55 (br s, 1H), 1.40–1.46 (m, 1H), 1.27–1.33 (m, 1H), 1.17 (s, 3H), 1.16 (m, 3H), 0.11 (s, 9H) ppm. 13C{1H} NMR (126 MHz, CDCl3): δ 147.3, 114.4, 111.3, 83.6, 76.1, 38.8, 31.6, 30.5, 29.4, 28.9, 17.5, 0.3 ppm. HRMS (ESI-TOF): m/z [M + H]+ calcd for C14H27OSi 239.1831, found 239.1832.
2,6,6-Trimethyloct-1-en-7-yn-3-ol (29).
Following the general procedure described for the preparation of 4, S9 (0.427 g, 1.79 mmol) was treated with TBAF (0.05 mL, 1.0 M solution in THF, 0.05 mmol) to afford 29 (0.258 g, 80%) as a clear, colorless oil following column chromatography (12% ethyl acetate in hexanes). 1H NMR (500 MHz, CDCl3): δ 4.93–4.94 (m, 1H), 4.83–4.84 (m, 1H), 4.06 (t, J = 6.36 Hz, 1H), 2.07 (s, 1H), 1.72–1.73 (m, 3H), 1.64–1.79 (overlapping patterns, 2H), 1.57 (br s, 1H), 1.45–2.52 (m, 1H), 1.30–1.36 (m, 1H), 1.21 (s, 3H), 1.20 (s, 3H) ppm. 13C{1H} NMR (126 MHz, CDCl3): δ 147.3, 111.3, 91.5, 76.1, 68.0, 38.7, 30.7, 30.5, 29.2, 29.0, 17.5 ppm. HRMS (ESI-TOF): m/z [M + H]+ calcd for C11H19O 167.1436, found 167.1437.
Pent-4-ynoyl Chloride (31).
To a 100 mL round-bottom flask containing DCM was added 4-pentynoic acid (5.0 g, 51.0 mmol), and then oxalyl chloride (6.0 mL, 70.0 mmol) was added in one aliquot. The reaction mixture was cooled to 0 °C, and then DMF (0.2 mL, 2.6 mmol), which had been previously dried under molecular sieves, was added dropwise slowly, resulting in gas evolution. The reaction progress was monitored via NMR for 4 h, upon which it was deemed complete. The solvent was removed under reduced pressure, keeping the crude product under a nitrogen atmosphere. The crude oil was distilled using a Kugelrohr apparatus at 30 mmHg and ~100 °C, yielding 3.64 g of 31 as a colorless oil (61% yield). This compound has been previously reported by Crossey et al.22
1 Hydroxyhex-5-yn-2-one (32).
To a flame-dried 20 mL microwave vial under a nitrogen atmosphere was added dry THF (1 M solution), 31 (0.67 g, 5.7 mmol), Wissner reagent18b (2.1 mL, 6.3 mmol), and freshly distilled Et3N (0.80 mL, 5.7 mmol) in succession at 0 °C. Formation of a white solid was observed, and the reaction was stirred vigorously at this temperature for 5 min. The reaction vessel was then heated in a microwave oven (100 °C) for 10 min. The contents of the microwave vial were then transferred to a round-bottom flask, and 8 mL of 2 M HCl was added. Decarboxylation progress was monitored by TLC for 30 min, upon which the solvent was removed under reduced pressure. The crude oil was redissolved in ethyl acetate and combined with brine. The organics were extracted with ethyl acetate (×3), combined, and washed with satd aq NaHCO3. The organics were washed a final time with brine and dried over magnesium sulfate. The solution was filtered, and the solvent was removed in vacuo to give 0.638 g (99% yield) of 31 as a clear oil. 1H NMR (500 MHz, CDCl3): δ 4.28 (s, 1H), 3.05 (s, 2H), 2.64 (t, J = 7.1 Hz, 2H), 2.52 (td, J = 2.6 Hz, 6.9 Hz, 2H), 1.97 (t, J = 2.7 Hz, 1H) ppm. 13C{1H} NMR (126 MHz, CDCl3) δ 207.6, 82.1, 69.4, 68.4, 37.1, 12.9 ppm. HRMS (ESI-TOF): m/z [M + Na]+ calcd for C6H8O2Na 135.0422, found 135.0422.
2-Oxo-5-hexyn-1-yl Benzoate (33).
Compound 32 (0.604 g, 5.39 mmol) was dissolved in 50 mL of anhydrous DCM, followed by the addition of NEt3 (1.50 mL, 10.8 mmol, 2.0 equiv) along with a catalytic amount of DMAP, and the resulting solution was cooled to 0 °C. Benzoyl chloride (0.750 mL, 6.46 mmol) was then added dropwise, resulting in darkening of the solution. The reaction was allowed to proceed for 1 h, upon which it was quenched by the slow addition of 15 mL of distilled water. The layers were separated, and the aqueous layer was extracted with DCM (15 mL × 3). The combined organics were washed with 15 mL portions of saturated sodium carbonate solution, followed by brine, and dried over magnesium sulfate. Filtration and removal of solvent under reduced pressure gave a beige solid, which was purified by Biotage Isolera One chromatography system (3–24% ethyl acetate in hexanes) to afford 0.882 g (76%) of 33 as a white fluffy solid. mp = 75–78 °C. 1H NMR (500 MHz, CDCl3): δ 8.09 (d, J = 8.59 Hz, 2H), 7.59 (t, J = 7.77 Hz, 1H), 7.46 (t, J = 7.77 Hz, 2H), 4.90 (s, 2H), 2.77 (t, J = 7.75 Hz, 2H), 2.52 (td, J = 7.75 Hz, 2.80 Hz, 2H), 1.97 (t, J = 2.91 Hz, 1H) ppm. 13C{1H} NMR (126 MHz, CDCl3): δ 202.1, 165.8, 133.5, 129.9, 129.0, 128.5, 82.4, 69.1, 68.4, 37.8, 12.5 ppm. HRMS (ESI-TOF): m/z [M + Na]+ calcd for C13H12O3Na 239.0684, found 239.0696.
2,2-Dimethoxy-5-hexyn-1-yl Benzoate (34).
Compound 33 (0.938 g, 4.34 mmol) was dissolved in 20 mL of anhydrous methanol, followed by the addition of trimethyl orthoformate (1.2 mL, 10.9 mmol) along with a catalytic amount of p-toluenesulfonic acid monohydrate (0.083 g, 0.43 mmol). After 24 h, an additional 0.5 equiv (0.25 mL, 2.0 mmol) of trimethyl orthoformate was added, and the reaction was allowed to proceed for an additional 24 h. The reaction was terminated by the slow addition of 3 mL of satd aq NaHCO3. The solvent was then removed under reduced pressure, and the residue was redissolved in ethyl acetate (50 mL). The layers were separated, the aqueous layer was extracted with ethyl acetate (10 mL × 3), and the combined organics were washed with brine (10 mL). The organic solution was dried over magnesium sulfate anhydrous and filtered, and the solvent was removed under reduced pressure. Purification was performed using silica column chromatography eluting with 12% ethyl acetate in hexanes, affording 1.00 g (88%) of 34 as a yellow oil. 1H NMR (500 MHz, CDCl3): δ 8.04 (dt, J = 7.58, 1.31 Hz, 2H), 7.56 (tt, J = 7.58, 1.62 Hz, 1H), 7.44 (t, J = 7.82 Hz, 2H), 4.31 (s, 2H), 3.25 (s, 6H), 2.28 (td, J = 7.89, 2.91 Hz, 2H), 2.05 (t, J = 7.89 Hz, 2H), 1.92 (t, J = 2.50 Hz, 1H) ppm. 13C{1H} NMR (126 MHz, CDCl3) δ 165.8, 133.2, 129.7, 129.7, 128.4, 100.3, 83.6, 68.4, 61.4, 48.3, 31.4, 13.1 ppm. HRMS (ESI-TOF): m/z [M + Na]+ calcd for C15H18O4Na 285.1103, found 285.1096.
2,2-Dimethoxy-6-trimethylsilyl-5-hexyn-1-yl Benzoate (S10).
Compound 34 (0.836 g, 3.19 mmol) was dissolved in 40 mL of anhydrous THF and cooled to −78 °C. LHMDS (3.8 mL, 1 M) was added to the yellow solution dropwise, and the resulting orange solution was stirred for 10 min. Freshly distilled trimethylsilyl chloride (0.6 mL, 4.78 mmol) was then added dropwise, and the reaction mixture was allowed to warm to room temperature. The reaction was terminated by the addition of 5 mL of distilled water, and the solvent was removed under reduced pressure. The residue was redissolved in diethyl ether (40 mL), and the aqueous layer was extracted with diethyl ether (10 mL × 3). The combined organic solution was washed with brine (15 mL), dried over magnesium sulfate, and filtered, and the solvent was removed under reduced pressure. Purification was performed using column chromatography (10% ethyl acetate in hexanes with 1% Et3N to minimize decomposition), affording 0.970 g (91%) of S10 as a yellow oil. 1H NMR (500 MHz, CDCl3): δ 8.05 (app. dt, J = 7.63, 1.53 Hz, 2H), 7.57 (tt, J = 7.25, 1.14 Hz, 1H), 7.45 (t, J = 7.82 Hz, 2H), 4.32 (s, 2H), 3.24 (s, 6H), 2.32 (t, J = 8.23 Hz, 2H), 2.04 (t, J = 7.72 Hz, 2H), 0.00 (s, 9H) ppm. 13C{1H} NMR (126 MHz, CDCl3): δ 165.8, 133.2, 129.9, 128.5, 106.3, 100.4, 84.7, 61.6, 48.3, 31.3, 14.50, 0.0 ppm. HRMS (ESI-TOF): m/z [M + Na]+ calcd for C18H26O4SiNa 357.1498, found 357.1508.
2,2-Dimethoxy-6-trimethylsilyl-5-hexyn-1-ol (35).
Compound S10 (1.07 g, 3.18 mmol, 1.0 equiv) was dissolved in 50 mL of anhydrous diethyl ether and cooled to −78 °C. A solution of methyllithium/lithium iodide (6.50 mL, 1.0 M in Et2O) was then added dropwise, and the resulting cloudy solution was allowed to stir at this temperature for 10 min. The reaction was terminated by the slow addition of 5 mL of distilled water, and the solution was allowed to warm to room temperature. Additional distilled water (5 mL) was added until two layers were clearly visible. The aqueous layer was extracted three times with 10 mL portions of diethyl ether, and the combined organics were washed with 10 mL of brine. The organic solution was dried over magnesium sulfate and filtered, and the solvent was removed in vacuo. The crude product was further purified by Biotage Isolera One chromatography system (7–60% ethyl acetate in hexanes) affording 0.594 g (81%) of the desired product as a yellow oil. 1H NMR (500 MHz, CDCl3): δ 3.59 (br s, 2H), 3.21 (s, 6H), 2.25 (t, J = 7.56 Hz, 2H), 1.93 (t, J = 7.56 Hz, 2H), 1.59 (br s, 1H), 0.13 (s, 9H) ppm. 13C{1H} NMR (126 MHz, CDCl3): δ 107.03, 101.4, 84.7, 61.0, 48.3, 30.7, 14.4, 0.0 ppm. HRMS (ESI-TOF): m/z [M + Na]+ calcd for C11H22O3NaSi 253.1236, found 253.1243.
1-(1-Cyclopentenyl)-2,2-dimethoxy-6-trimethylsilyl-5-hexyn-1-ol (36).
Oxalyl chloride (1.85 g, 14.7 mmol) was dissolved in 70 mL of anhydrous dichloromethane at −78 °C followed by the dropwise addition of DMSO (2.27 g, 29.0 mmol). The colorless solution was then allowed to stir at this temperature for 30 min. Compound 35 (1.12 g, 4.86 mmol), previously dried over molecular sieves and dissolved in 15 mL of DCM, was added dropwise to the solution. The reaction was terminated after 75 min by the addition of Et3N (8.19 mL, 58.0 mmol), and the solution was allowed to warm to room temperature. Diethyl ether (60 mL) was then added, and the solvents were removed in vacuo until a white slurry remained. This process was repeated two more times. The slurry was then filtered through a pad of Celite, rinsing with 100 mL of 20% diethyl ether in hexanes. The remaining solvent was removed in vacuo, and the yellow remaining oil (0.830 g) was used directly for the subsequent vinyllithium coupling reaction. In a separate flask, cyclopentenyl iodide (1.63 g, 8.40 mmol), previously dried over molecular sieves, was dissolved in 40 mL of anhydrous diethyl ether at −78 °C. tert-Butyllithium (1.9 M in pentane, 8.95 mL, 17.0 mmol) was then added dropwise, and the resulting solution was warmed to room temperature to destroy any excess t-BuLi and recooled to −78 °C. Compound 35a (0.830 g, 3.63 mmol), previously dried over molecular sieves in 10 mL of diethyl ether, was then added dropwise. The reaction was allowed to proceed for 30 min, upon which it was terminated by the dropwise addition of 5 mL distilled water. The solution was warmed to room temperature, and the aqueous layer was extracted three times with 10 mL portions of diethyl ether. The combined organics were washed with 10 mL of brine, and the organic solution was dried over magnesium sulfate anhydrous. The solution was filtered, and the solvent was removed in vacuo. The crude product was purified by column chromatography over silica gel, eluting with 15% ethyl acetate with 1% Et3N in hexanes, affording 0.657 g (46% over two steps) of the desired product as a yellow oil. 1H NMR (500 MHz, CDCl3): δ 5.82 (m, 1H), 4.34 (br s, 1H), 3.29 (s, 3H), 3.25 (s, 3H), 2.49–2.16 (m, 6H), 1.92–1.76 (m, 4H), 0.12 (s, 9H) ppm. 13C{1H} NMR (126 MHz, CDCl3: δ 142.1, 127.7, 107.4, 84.0, 72.5, 60.4, 39.0, 33.3, 32.5, 32.2, 23.6, 14.8, 0.1 ppm. HRMS (ESI-TOF): m/z [M + Na]+ calcd for C16H28O3NaSi 319.1705, found 319.1720.
1-(Cyclopent-1-en-1-yl)-2,2-dimethoxyhex-5-yn-1-ol (37).
Following the general procedure described for the preparation of 4, compound 37 was prepared by treating 36 (0.657 g, 2.22 mmol) with TBAF (0.22 mL, 1.0 M solution in THF, 0.22 mmol) to afford 0.395 g (79%) of the desired desilylated product as a clear oil following column chromatography using the Biotage Isolera One chromatography system (6 to 50% ethyl acetate in hexanes containing 1% Et3N). 1H NMR (500 MHz, CDCl3): δ 5.83–5.81 (m, 1H), 4.35 (br s, 1H), 3.30 (s, 3H), 3.25 (s, 3H), 2.48–2.17 (m, 6H), 1.93–1.80 (m, 4H), 1.57 (br s, 1H) ppm. 13C{1H} NMR (126 MHz, CDCl3): δ 142.0, 127.7, 101.5, 84.6, 72.5, 67.9, 48.9, 39.1, 33.3, 32.2, 31.6, 23.5, 13.3 ppm. HRMS (ESI-TOF): m/z [M + Na]+ calcd for C13H20O3Na 247.1310, found 247.1305.
General Procedure for the Cyclization/Claisen Rearrangement.
The desired 5-hexyn-1ol was dissolved in anhydrous phenetole in a 5 mL microwave vial followed by addition of a catalytic amount of LHMDS (0.02 mL, 1 M in THF, 0.02 mmol). The vial was sealed, and the reaction was then allowed to proceed for 1 h at 210 °C in the microwave oven. Once complete, the phenetole solvent was removed under reduced pressure using a Kugelrohr apparatus to afford the crude product.
9,9-Dimethyl-1,3,4,4a,5,7,8,9-octahydrobenzo[8]annulen-6(2H)-one (5).
The general procedure was followed using alcohol 4 (0.049 g, 0.24 mmol). The crude material was purified by column chromatography eluting with 4% ethyl acetate in hexanes to afford 0.0345 g (70%) of 5 as a yellow oil. 1H NMR (500 MHz, CDCl3): δ 5.00 (d, J = 2.4 Hz, 1H), 3.71–3.67 (m, 1H), 2.61 (d, J = 9.3 Hz, 1H), 2.27–2.21 (m, 1H), 2.14–2.01 (m, 3H), 1.94–1.91 (m, 1H), 1.70–1.52 (m, 7H), 1.46–1.40 (m, 1H), 1.24 (s, 3H), 1.10 (s, 3H) ppm. 13C{1H} NMR (126 MHz, CDCl3): δ 212.4, 140.0, 132.7, 48.7, 39.8, 39.7, 35.8, 35.0, 33.7, 32.4, 31.3, 29.8, 26.2, 20.7 ppm. HRMS (ESI-TOF): m/z [M + Na]+ calcd for C14H22ONa 229.1568, found 229.1575.
1,1,9,9-Tetramethyl-1,3,4,4a,5,7,8,9-octahydrobenzo[8]annulen-6(2H)-one (7).
The general procedure was followed using alcohol 6 (0.051 g, 0.22 mmol). The crude product obtained was purified by column chromatography eluting with 5% ethyl acetate in hexanes to afford 0.034 g (72%) of 7 as a white semisolid. 1H NMR (500 MHz, CDCl3): δ 5.15 (s, 1H), 3.76–3.72 (m, 1H), 2.73–2.64 (m, 2H), 2.52–2.47 (m, 1H), 2.39–2.29 (m, 2H), 1.69–1.65 (m, 1H), 1.57–1.53 (m, 3H), 1.43–1.40 (m, 2H), 1.29–1.26 (m, 1H), 1.15 (s, 3H), 1.02 (s, 3H), 1.00 (s, 6H) ppm. 13C{1H} NMR (126 MHz, CDCl3): δ 212.6, 146.1, 131.1, 50.8, 50.6, 40.7, 40.1, 37.9, 35.5, 35.4, 33.4, 31.6, 30.7, 29.9, 29.1, 17.2 ppm. HRMS (ESI-TOF): m/z [M + Na]+ calcd for C16H26ONa 257.1881, found 257.1884.
8,8-Dimethyl-1,2,3,3a,4,6,7,8-octahydro-5H-cyclopenta[8]-annulen-5-one (9).
The general procedure was followed using alcohol 8 (0.18 g, 0.93 mmol). The crude product obtained was purified by column chromatography eluting with 5% ethyl acetate in hexanes to afford 0.15 g (87%) of 9 as a clear oil. 1H NMR (500 MHz, CDCl3): δ 5.15 (s, 1H), 3.54–3.52 (m, 1H), 2.85 (dd, J = 13.2, 7.8 Hz, 1H), 2.55 (dd, J = 10.3, 6.8 Hz, 1H), 2.40 (q, J = 17.1, 10.3 Hz, 2H), 2.20–2.09 (m, 2H), 1.83–1.77 (m, 2H), 1.66–1.50 (m, 4H), 1.06 (s, 3H), 1.00 (s, 3H) ppm. 13C{1H} NMR (125 MHz, CDCl3): δ 212.4, 142.5, 129.8, 50.3, 40.6, 36.6, 36.6, 36.5, 34.8, 34.2, 30.6, 30.4, 22.3 ppm. HRMS (ESI-TOF): m/z [M + H]+ calcd for C13H21O 193.1592, found 193.1591.
8,8-Dimethyl-4-phenyl-1,2,3,3a,4,6,7,8-octahydro-5H-cyclopenta-[8]annulen-5-one (11).
The general procedure was followed using alcohol 10 (0.044 g, 0.16 mmol). The crude product obtained was purified by column chromatography eluting with 5% ethyl acetate in hexanes to afford 0.037 g (85%) of 11 as a pale-yellow oil. 1H NMR (500 MHz, CDCl3): δ 7.40–7.35 (m, 4H), 7.29–7.27 (m, 1H), 5.26 (s, 1H), 3.97–3.93 (ddd, J = 11.7, 7.3, 1.5 Hz, 1H), 3.54–3.47 (m, 1H), 2.76–2.71 (m, 1H), 2.49–2.29 (m, 2H), 2.27–2.24 (m, 1H), 2.16–2.11 (m, 1H), 1.86–1.78 (m, 1H), 1.67–1.54 (m, 4H), 1.13 (s, 3H), 1.05 (s, 3H) ppm. 13C{1H} NMR (126 MHz, CDCl3): δ 211.8, 141.6, 137.2, 130.7, 128.9, 128.1, 127.3, 66.7, 39.4, 37.2, 36.8, 36.6, 34.5, 32.3, 30.5, 30.3, 22.3 ppm. HRMS (ESI-TOF): m/z [M + H]+ calcd for C19H25O 269.1905, found 269.1895.
4-(2,5-Dimethoxyphenyl)-8,8-dimethyl-1,2,3,3a,4,6,7,8-octahydro-5H-cyclopenta[8]annulen-5-one (13).
The general procedure was followed using alcohol 12 (0.031 g, 0.094 mmol). The crude product obtained was purified by column chromatography eluting with 5% ethyl acetate in hexanes to afford 0.027 g (87%) of 13 as a pale-yellow oil. 1H NMR (500 MHz, CDCl3): δ 7.11 (d, J = 2.9 Hz, 1H), 6.81–6.76 (m, 2H), 5.23 (s, 2H), 3.81 (s, 3H), 3.74 (s, 3H), 2.77–2.72 (m, 1H), 2.49–2.40 (m, 1H), 2.36–2.21 (m, 1H), 2.14 (td, J = 12.7, 4.4 Hz, 1H), 1.84–1.54 (m, 7H), 1.10 (s, 3H), 1.04 (s, 3H) ppm. 13C{1H} NMR (126 MHz, CDCl3): δ 210.6, 153.8, 152.8, 141.3, 130.6, 127.6, 115.2, 111.9, 111.3, 59.5, 56.2, 55.7, 39.7, 37.2, 37.0, 36.6, 34.4, 32.6, 30.5, 30.5, 22.4 ppm. HRMS (ESI-TOF): m/z [M + Na]+ calcd for C21H28O3Na 351.1936, found 351.1943.
4,6,6-Trimethylcyclooct-4-en-1-one (15).
The general procedure was followed using alcohol 14 (0.036 g, 0.22 mmol). The crude product obtained was purified by column chromatography eluting with 5% ethyl acetate in hexanes to afford 0.031 g (86%) of 15 as a pale-yellow oil. This compound has been reported previously by Gadwood et al.23
(Z)-7,7-dimethyl-2,3,3a,4,7,8-hexahydro-1H-cyclopenta[8]-annulen-5(6H)-one (22).
The general procedure was followed using alcohol 21 (0.0425 g, 0.2210 mmol). After 1 h of microwave irradiation, TLC indicated the reaction was not complete. Another aliquot of LHMDS was added, and the reaction was irradiated at 220 °C for another 1 h. The solvent was then removed under reduced pressure and the resulting crude product was purification by column chromatography (6.5% ethyl acetate in hexanes) to afford 0.034 g (79%) of 22 as a clear, yellow oil. 1H NMR (500 MHz, CDCl3): δ 0.93 (s, 2H), 1.08 (s, 2H), 1.39–1.43 (m, 2H), 1.55–1.79 (m, 4H), 1.87–2.00 (m, 3H), 2.26–2.35 (m, 2H), 2.39–2.50 (m, 2H), 2.53–2.55 (m, 2H), 2.82–2.83 (m, 1H), 5.55–5.58 (m, 1H) ppm. 13C{1H} NMR (126 MHz, CDCl3): δ 212.6, 148.2, 118.6, 54.2, 52.0, 40.9, 36.8, 34.2, 33.8, 33.3, 29.1, 28.5, 24.3 ppm. HRMS (ESI-TOF): m/z [M + H]+ calcd for C13H21O 193.1592, found 193.1595.
4,7,7-Trimethylcyclooct-4-en-1-one (24).
The general procedure was followed using alcohol 23 (0.0455 g, 0.273 mmol). The crude product obtained was purified by column chromatography eluting with 11% ethyl acetate in hexanes to afford 0.0378 g (83%) of 24 as a pale-yellow oil. 1H NMR (500 MHz, CDCl3): δ 5.40–5.35 (m, 1H), 2.48 (app t, J = 6.36 Hz, 2H), 2.44–2.34 (m, 2H), 2.27 (s, 2H), 1.96 (app. d, J = 7.34 Hz, 2H), 1.75 (s, 3H), 0.98 (s, 6H) ppm. 13C{1H} NMR (126 MHz, CDCl3): δ 212.3, 138.4, 123.3, 52.8, 46.1, 40.5, 35.9, 29.7, 26.8, 23.5 ppm. HRMS (ESI-TOF): m/z [M]+ calcd for C11H18O 166.1358, found 166.1355.
7,7-Dimethyl-2-phenylcyclooct-4-en-1-one (26).
The general procedure was followed using alcohol 25 (0.0447 g, 0.196 mmol). The crude product obtained was purified by purification by Biotage Isolera One chromatography system (0 to 6% ethyl acetate in hexanes) to afford 0.0361 g (81%) of 26 as a clear yellow oil. 1H NMR (500 MHz, CDCl3): δ 7.35–7.26 (m, 5H), 5.86–5.82 (m, 1H), 5.78–5.76 (m, 1H), 3.78 (dd, J = 12.2, 3.9 Hz, 1H), 3.13 (app. q, J = 12.7 Hz, 1H), 2.56 (d, J = 12.3 Hz, 1H), 2.51–2.34 (overlapping patterns, 3H), 1.92–1.83 (m, 2H), 1.02 (s, 3H), 1.01 (s, 3H) ppm. 13C{1H} NMR (126 MHz, CDCl3): δ 210.8, 137.7, 130.2, 129.9, 128.6, 127.4, 127.2, 61.9, 50.4, 39.9, 36.3, 32.5, 26.7, 25.6 ppm. HRMS (ESI-TOF): m/z [M + H]+ calcd for C16H21O 229.1592, found 229.1593.
4,7,7-Trimethyl-2-phenylcyclooct-4-en-1-one (28).
The general procedure was followed using alcohol 27 (0.0585 g, 0.241 mmol). The crude product obtained was purified by purification by Biotage Isolera One chromatography system (5–25% ethyl acetate in hexanes) to afford 0.0485 g (83%) of 28 as a clear, yellow oil. 1H NMR (500 MHz, CDCl3): δ 7.36–7.26 (m, 5H), 5.45 (t, J = 8.31 Hz, 1H), 3.87 (dd, J = 3.92, 13.2 Hz, 1H), 3.36 (t, J = 12.7 Hz, 1H), 2.58 (d, J = 11.3 Hz, 1H), 2.33 (dd, J = 8.3, 13.2 Hz, 1H), 2.19 (dd, J = 3.91, 12.7 Hz, 1H), 1.81 (s, 3H), 1.82–1.75 (m, 1H), 0.99 (s, 6H) ppm. 13C{1H} NMR (126 MHz, CDCl3): δ 210.3, 137.3, 128.7, 127.3, 124.4, 60.5, 50.3, 41.0, 37.9, 33.2, 31.1, 27.4, 25.0, 23.4 ppm. HRMS (ESI-TOF): m/z [M + H]+ calcd for C17H23O 243.1749, found 243.1752.
4,8,8-Trimethylcyclooct-4-en-1-one (30).
The general procedure was followed using alcohol 29 (0.072 g, 0.433 mmol), with the exception that anhydrous dimethoxyethane (DME) was used as the solvent instead of phenetole. The crude product was purified by column chromatography eluting with 5% ethyl acetate in hexanes to afford 0.061 g (84%) of 30 as a clear, pale yellow oil. 1H NMR (500 MHz, CDCl3): δ 5.31 (tq, J = 7.83 Hz, 1.50 Hz, 1H), 2.67 (t, J = 7.10 Hz, 2H), 2.42 (t, J = 7.10 Hz, 2H), 2.08–2.12 (m, 2H), 1.68 (t, J = 1.50 Hz, 3H), 1.48–1.50 (m, 2H), 1.18 (s, 6H) ppm. 13C{1H} NMR (126 MHz, CDCl3): δ 216.7, 137.3, 124.7, 46.6, 41.8, 40.5, 27.2, 26.0, 23.8, 23.5 ppm. HRMS (ESI-TOF): m/z [M + H]+ calcd for C11H19O 167.1436, found 167.1438.
8,8-Dimethoxy-1,2,3,3a,4,6,7,8-octahydro-5H-cyclopenta[8]-annulen-5-one (38).
Compound 37 (0.084 g, 0.36 mmol) was dried over molecular sieves and dissolved in 2.0 mL of anhydrous phenetole in a 5 mL microwave vial. Triethylamine (6.0 μL, 0.04 mmol) was then added, followed by catalytic LHMDS (0.036 mL, 1 M in THF). The reaction was allowed to proceed in the microwave for 1 h at 180 °C and checked for completion by TLC, which revealed the presence of some starting material. Another 0.1 equiv of LHMDS (0.036 mL, 1 M in THF) was added, and the reaction was allowed to proceed in the microwave oven for an additional 90 min. The reaction mixture was then directly transferred to a SNAP Ultra 10 g column and purified by the Biotage Isolera One chromatography system (5 to 85% ethyl acetate in hexanes), affording 0.016 g of 38 as a pale-yellow oil (19% yield). 1H NMR (500 MHz, CDCl3): δ 5.26 (d, J = 2.07 Hz, 1H) 3.48–3.56 (m, 1H) 3.19 (s, 3H) 3.09 (s, 3H) 2.68 (ddd, J = 11.62, 9.42, 2.45 Hz, 1H) 2.59 (dd, J = 12.47, 6.11 Hz, 1H) 2.44–2.52 (m, 1H) 2.18–2.36 (m, 4H) 1.83–1.93 (m, 1H) 1.62–1.73 (m, 3H) 1.53 (m, 1H) ppm. 13C{1H} NMR (126 MHz, CDCl3) δ 212.3, 150.5, 122.2, 102.3, 52.6, 48.3, 48.1, 36.1, 35.9, 33.2, 32.9, 31.6, 22.4 ppm. HRMS (ESI-TOF): m/z [M + Na]+ calcd for C13H20O3Na 247.1310, found 247.1311.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by a grant from the National Institutes of Health NIGMS. T.V.O. also gratefully acknowledges support from the Hans and Ella McCollum-Vahlteich ’21 endowment and W.C.D. gratefully acknowledges support from Organic Syntheses, Inc. in the form of a Summer Undergraduate Fellowship.
Footnotes
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.joc.0c01132.
1H and 13C NMR spectra for all new compounds (PDF)
The authors declare no competing financial interest.
REFERENCES
- (1) (a).Mehta G; Singh V Progress in the Construction of Cyclooctanoid Systems: New Approaches and Applications to Natural Product Syntheses. Chem. Rev 1999, 99, 881–930. [DOI] [PubMed] [Google Scholar]; (b) Hu Y-J; Li L-X; Han J-C; Min L; Li C-C Recent Advances in the Total Synthesis of Natural Products Containing Eight-Membered Carbocycles (2009–2019). Chem. Rev 2020, 120, 5910. [DOI] [PubMed] [Google Scholar]
- (2).Petasis NA; Patane MA The synthesis of carbocyclic eight-membered rings. Tetrahedron 1992, 48, 5757–5821. [Google Scholar]
- (3) (a).Paquette LA; Ham WH Total synthesis of the marine sesquiterpenes dactylol and africanol. De novo construction of a cyclooctanoid natural product from cycloheptane precursors. J. Am. Chem. Soc 1987, 109, 3025–3036. [Google Scholar]; (b) Birch AM; Pattenden G Total synthesis of epi-precapnelladiene. J. Chem. Soc., Chem. Commun 1980, 1195–1197. [Google Scholar]; (c) Mehta G; Murthy AN A General Stereocontrolled Approach to the 5–8 Fused Ring System. Applications to the Total Synthesis of Marine Natural Product (±)-Precapnelladiene. J. Org. Chem 1987, 52, 2875–2881. [Google Scholar]; (d) Feldman KS; Wu MJ; Rotella DP Total synthesis of (±)-dactylol and related studies. J. Am. Chem. Soc 1990, 112, 8490–8496. [Google Scholar]
- (4).For a review, see Ojima I; Tzamarioudaki M; Li Z; Donovan RJ Transition Metal-Catalyzed Carbocyclizations in Organic Synthesis. Chem. Rev 1996, 96, 635–662. [DOI] [PubMed] [Google Scholar]
- (5).Feldman AW; Ovaska SI; Ovaska TV Facile access to cyclooctanoid ring systems via microwave-assisted tandem 6-exo dig cyclization–rearrangement sequence. Tetrahedron 2014, 70, 4147–4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6) (a).Li X; Kyne RE; Ovaska TV Synthesis of Seven-Membered Carbocyclic Rings via a Microwave-Assisted Tandem Oxyanionic 5-exo dig Cyclization–Claisen Rearrangement Process. J. Org. Chem 2007, 72, 6624–6627. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Li X; Keon AE; Sullivan JA; Ovaska TV Studies toward Frondosin A and Its Analogues. Formal Total Synthesis of (±)-Frondosin A. Org. Lett 2008, 10, 3287–3290. [DOI] [PubMed] [Google Scholar]; (c) Ovaska TV; Sullivan JA; Ovaska SI; Winegrad JB; Fair JD Asymmetric Synthesis of Seven-Membered Carbocyclic Rings via a Sequential Oxyanionic 5-Exo-Dig Cyclization/Claisen Rearrangement Process. Total Synthesis of (−)-Frondosin B. Org. Lett 2009, 11, 2715–2718. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Li X; Ovaska TV Total Synthesis of (±)-Frondosin B. Org. Lett 2007, 9, 3837–3840. [DOI] [PubMed] [Google Scholar]; For additional recent examples of the synthesis of seven-membered rings, see: (e) Pflästerer D; Rettenmeier E; Schneider S; de las Heras Ruiz E; Rudolph M; Hashmi ASK Highly Efficient Gold Catalyzed Synthesis of Dibenzocycloheptatrienes. Chem. - Eur. J 2014, 20, 6752–6755. [DOI] [PubMed] [Google Scholar]; (f) Pflästerer D; Schumacher S; Rudolph M; Hashmi ASK Mechanistic Insights into the Post-Cyclization Isomerization in Gold-Catalyzed 7-exo-dig-Hydroarylations. Chem. - Eur. J 2015, 21, 11585–11589. [DOI] [PubMed] [Google Scholar]; (g) Pflästerer D; Rudolph M; Yates BF; Ariafard A; Hashmi ASK Total Synthesis of (±)-Dihydroisosubamol. Adv. Synth. Catal 2017, 359, 866–874. [Google Scholar]; For recent syntheses of eight-membered rings, see: (h) Cervantes-Reyes A; Rominger F; Rudolph M; Hashmi ASK Gold(I) Complexes with Eight-Membered NHC Ligands: Synthesis, Structures and Catalytic Activity. Adv. Synth. Catal 2020, 362, 2523–2533. [Google Scholar]
- (7).Beesley RM; Ingold CK; Thorpe JF CXIX.—The formation and stability of spiro-compounds. Part I. spiro-Compounds from cyclohexane. J. Chem. Soc., Trans 1915, 107, 1080–1106. [Google Scholar]
- (8).Bruice TC; Pandit UK The Effect of Geminal Substitution Ring Size and Rotamer Distribution on the Intramolecular Nucleophilic Catalysis of the Hydrolysis of Monophenyl Esters of Dibasic Acids and the Solvolysis of the Intermediate Anhydrides. J. Am. Chem. Soc 1960, 82, 5858–5865. [Google Scholar]
- (9) (a).Jung ME; Gervay J gem-Dialkyl effect in the intramolecular Diels-Alder reaction of 2-furfuryl methyl fumarates: the reactive rotamer effect, the enthalpic basis for acceleration, and evidence for a polar transition state. J. Am. Chem. Soc 1991, 113, 224–232. [Google Scholar]; (b) Jung ME; Kiankarimi M Substituent Effects in the Intramolecular Diels–Alder Reaction of 6-Furylhexenoates. J. Org. Chem 1998, 63, 2968–2974. [Google Scholar]; (c) Jung ME Substituent and Solvent Effects in Intramolecular Diels-Alder Reactions. Synlett 1990, 1990, 186–190. [Google Scholar]
- (10).The “Facilitated Transition” hypothesis as an explanation for the gem-dialkyl effect: Parrill AL; Dolata DP J. Mol. Struct.: THEOCHEM 1996, 370, 187–202. [Google Scholar]
- (11).For a review, see: Jung ME; Piizzi G gem-Disubstituted Effect: Theoretical Basis and Synthetic Applications. Chem. Rev 2005, 105, 1735–1766. [DOI] [PubMed] [Google Scholar]
- (12).Kaneti J; Kirby AJ; Koedjikov AH; Pojarlieff IG Thorpe–Ingold effects in cyclizations to five-membered and six-membered rings containing planar segments. The rearrangement of N(1)-alkyl-substituted dihydroorotic acids to hydantoinacetic acids in base. Org. Biomol. Chem 2004, 2, 1098–1103. [DOI] [PubMed] [Google Scholar]
- (13).Trost BM; Surivet J-P; Toste DF Ruthenium-Catalyzed Enyne Cycloisomerizations. Effect of Allylic Silyl Ether on Regioselectivity. J. Am. Chem. Soc 2004, 126, 15592–155602. [DOI] [PubMed] [Google Scholar]
- (14).Omura K; Swern D Oxidation of alcohols by “activated” dimethyl sulfoxide. a perspective, steric and mechanistic study. Tetrahedron 1978, 34, 1651–1660. [Google Scholar]
- (15).Sonogashira K Development of Pd–Cu catalyzed cross-coupling of terminal acetylenes with sp2-carbon halides. J. Organomet. Chem 2002, 653, 46–49. [Google Scholar]
- (16).Kamijo S; Dudley GB Tandem Nucleophilic Addition/Fragmentation Reactions and Synthetic Versatility of Vinylogous Acyl Triflates. J. Am. Chem. Soc 2006, 128, 6499–6507. [DOI] [PubMed] [Google Scholar]
- (17).Bogen S; Fensterbank L; Malacria M Study of a Radical Cyclizations Cascade Leading to Bicyclo[3.1.1]heptanes. J. Org. Chem 1999, 64, 819–825. [DOI] [PubMed] [Google Scholar]
- (18) (a).Wissner A 2-Hetero Substituted Silylated Ketene Acetals: Reagents for the Preparation of α-Functionalized Methyl Ketones from Carboxylic Acid Chlorides. J. Org. Chem 1979, 44, 4617–4622. [Google Scholar]; (b) Vaismaa MJP; Leskinen MV; Lajunen MK Microwave-Assisted One-Carbon Chain Extension in the Preparation of Terminal α-Hydroxy Ketones. Synth. Commun 2009, 39, 2042–2052. [Google Scholar]
- (19).Barton DH; Bashiardes G; Fourrey J-L An improved preparation of vinyl iodides. Tetrahedron Lett. 1983, 24, 1605–1608. [Google Scholar]
- (20).Lithium–halogen exchange reactions were conducted using 2.05 equiv of t-BuLi for every 1.0 equiv of a vinyl halide.
- (21).Helmboldt H; Hiersemann M Synthetic Studies toward Jatrophane Diterpenes from Euphorbia characias. Enantioselective Synthesis of (−)-15-O-Acetyl-3-O-propionyl-17-norcharaciol. J. Org. Chem 2009, 74, 1698–1708. [DOI] [PubMed] [Google Scholar]
- (22).Crossey K; Migaud ME Solventless synthesis of acyl phosphonamidates, precursors to masked bisphosphates. Chem. Commun 2015, 51, 11088–11091. [DOI] [PubMed] [Google Scholar]
- (23).Gadwood RC; Lett RM Preparation and rearrangement of 1,2-dialkenylcyclobutanols. A useful method for synthesis of substituted cyclooctenones. J. Org. Chem 1982, 47, 2268–2275. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.