Abstract
Coronavirus disease-2019 is a serious health threat around the globe. Across the world, approximately 142 million people were infected, and three million deaths happened. The fast propagation is also associated with constant anxiety, mental stress, and discomfort in public and health-care professionals. Lack of approved drugs regimen to combat the pandemic challenge concretely is a challenging project for all who are committed to developing remedial assistance. However, the successful development of three vaccines gives a solid roadmap to combat this disease. In this review, we highlighted the current development and challenges of this pandemic.
Keywords: Corona vaccine, coronavirus, coronavirus disease-2019, epidemiology, pathogenesis, virology
INTRODUCTION
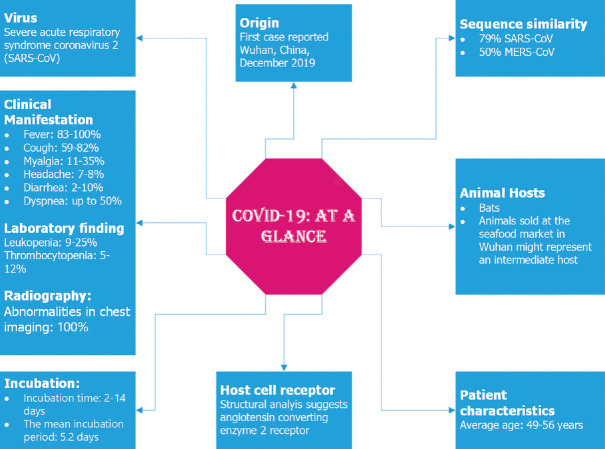
World Health Organization (WHO) confirmed that viral infections are emerging as a serious public health threat. Currently, the virus, coronavirus disease-2019 (COVID-19) has affected so many countries, areas, or territories over 142 million people infected across the world.[1] This disease is caused by a severe acute respiratory syndrome (SARS) that is commonly known as SARS-coronavirus-2 (CoV2).[2] The disease is first started from the city of Wuhan in China in December-2019. Starting from the first identification, it was spread rapidly and a number of cases and death were reported.[3] As of January 30, 2020, the WHO confirmed this outbreak as an international public health emergency.[4] On March 11, 2020, the WHO declared COVID-19 as a pandemic.[5] It has been observed in COVID-19 characteristics that average patients ages are 49–56 years, the mean incubation period is 5.2 days, and the median time of 14 days is noted from the first symptom to death. Male are more than female in hospitalized patients from 54% to 73%. However in the second wave, children are also getting affected by this pandemic. Still, the elders are more susceptible to this disease than the younger. Characteristics of COVID-19 is in Figure 1.[6]
Figure 1.
An outline highlighting its viral characteristic, animal host, incubation time, and clinical manifestation of coronavirus disease-2019 virus
EPIDEMIOLOGY
Pandemic COVID-19 has been spread out globally. As of December 31, the local health authority announced epidemiological alerts and markets were closed all the sudden measures adopted on January 1, 2020. Further, 41 hospitalized patients were identified and confirmed COVID-19 infection on 2nd January.[7] The National Health Commission of China confirmed the death of 17 patients of COVID-19 on January 22, 2020. On January 25, 20202, a total number of 1975 cases and 56 death were confirmed from Mainland China.[8] On January 30, 2020, the cases of this disease increased to 7734. As of February 12, 2020, Taiwan centers for disease control also announced the data comprising the records of 28 countries having 45,167 cases a global update.[9] As of February 23, 2020, it was reported that the number of cases increased 1879 times in comparison to January 10, 2020.[10]
The cases were rapidly increased in aberrant ways throughout the world. At the time of data collection for the manuscript on April 12, 2021, the WHO reported confirmed cases 142,557,268 including 3,037,398 deaths globally. Figures 2 and 3 showed the pattern of the global case of COVID infection and related deaths. The COVID-19 spread to 219 countries, areas, and territories. The WHO also revealed recorded statistics on a continent wise, which comprised as 49,564,187 in Europe, 60,006,538 in America, 8,609,860 in Eastern Mediterranean, 2,258,194 in Western Pacific, 18,562,170 in South East Asia, and 3,236,379 in Africa [Figure 4].[11] However, after the start of the vaccination program in the world, a total of 889,827,023 vaccine doses has also been administered to the world population until April 21, 2021.
Figure 2.
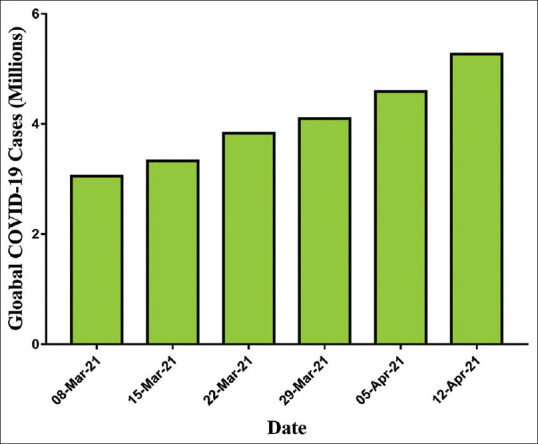
Worldwide accumulative cases of coronavirus disease-2019 pandemic (March–April 2021) as per the WHO report
Figure 3.
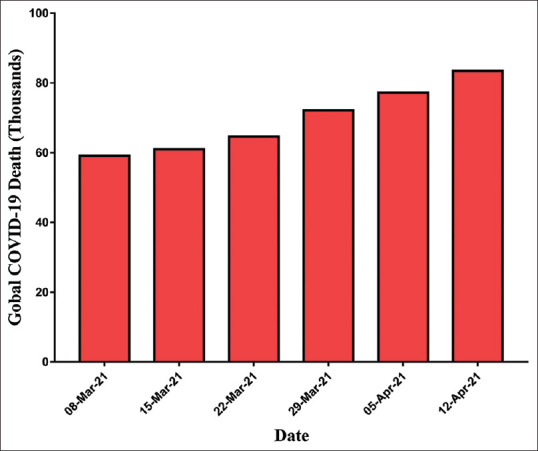
Worldwide accumulative deaths of coronavirus disease-2019 pandemic (March–April 2021) as per World Health Organization report
Figure 4.
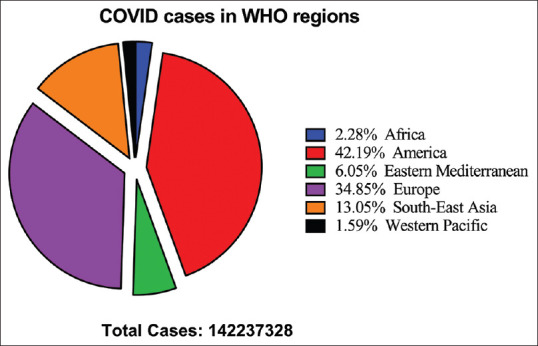
The cases of corona pandemic in World Health Organization region. Data showed that America is most affected by this disease (42.19%) followed by Europe (34.85%), South-East Asia (13.05%), Eastern Mediterranean (6.05%), Africa (2.28%), and Western Pacific (1.59%)
VIROLOGY
Genomic description
COVID-19 virus is a single-stranded RNA virus 30–32 kb genome having a lot of natural roots.[12] The current strain of the virus comprising four subtypes as alpha (α), beta (β), gamma (γ), and delta (δ) categories where α and β were reported to have enough virulence capacities to infect human beings.[13] The structural proteins of the new virus include spike, nucleocapsid, envelope, and membrane, which constitute a complete structure and mechanistic cascades towards binding and proliferation [Figure 5].[14]
Figure 5.
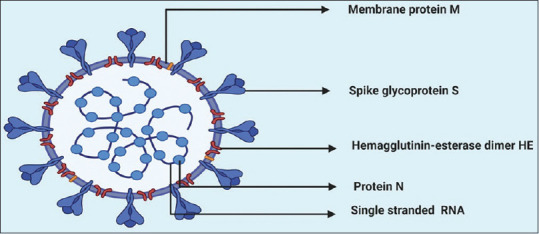
A schematic diagram of coronavirus disease virion showing RNA (single stranded) containing protein N, embedded inside membrane protein (m) with a projection of spike glycoprotein (s) and hemagglutinin-esterase dimer. The figure is made with BioRender (https://biorender.com/)
Physiochemical properties
The appearance of the virus is round in shape, having a diameter of 60–100 nm with deactivating properties either by ultraviolet or heating at 56°C. The virus is believed to be killed after application of the usual disinfectant.[15] Observational studies confirm the stability of the virus on plastic and stainless steel surfaces.[16]
Cellular entry of virus and receptor interaction
Coronavirus and its interaction with the renin–angiotensin–aldosterone system are believed to have a reliable step toward the infection. This interaction between coronavirus and angiotensin-converting enzyme-2 (ACE-2) is also considered a potential stage of infection.[17] The primary role of ACE is converting angiotensin I to produce Ang-(1–9). Moreover, it is also available for binding with the spike protein of CoVs. The binding is due to the presence of N-terminal peptidase domain and C-terminal collectrin domain.[18] COVID-19 is also expressed in other nucleoproteins, polyprotein, and a number of membrane proteins that include RNA polymerase, papain-like protease, and accessory proteins.[19]
CLINICAL MANIFESTATIONS AND DIAGNOSIS
Clinical manifestation
COVID-19 manifestations have been varied from asymptomatic or minor symptoms to severe complaint and finally, they cause death of the patient. Fever, cough, body ache, muscle fatigue myalgia, and dyspnea are the most common symptom while headache, malaise, diarrhea, and rhinitis were reported in this disease.[20]
Diagnosis
Diagnosis of COVID-19 is being carried out through laboratory investigations.[21] The onset of fever, cough, and dyspnea could be primary symptoms for the diagnosis of this disease. The probability of COVID-19 is augmented if patients traveled from the community transmission area or had interacted with a COVID patient as well as the suspected cases in the earlier 14 days. Finally, the suspicious case can be confirmed by performing the various confirmatory tests [Table 1].
Table 1.
Diagnostic test for severe acute respiratory syndrome coronavirus 2 (severe acute respiratory syndrome-coronavirus-2)
| Test name | Mechanisms/Procedure | Interpretation |
|---|---|---|
| RT-PCR | The RT PCR is used to detect COVID-19 by collecting nasopharyngeal swab specimen | Positive result of RT-PCR confirms case |
| CT-scan | CT of the chest is recommended in case of the severe pulmonary disease for detection of viral pneumonia infection | CT-scan is being used as a confirmatory test if RT-PCR results could be doubtful |
| Immunoassay | ELISA procedure is being used for the detection of antibodies generated by the immune system of the host | This test will be recommended for the patients who have a history of infection, but RT-PCR is negative |
| Look for other causes | Quick flu investigations and the respiratory viral panel is being used to check the other causes of the symptoms | Persons having physical contact or suspects traveled or residing to a location of COVID-19 transmission within or prior 14 days |
RT: Reverse transcription, PCR: Polymerase chain reaction, CT: Computerized tomography, COVID-19: Coronavirus disease-2019
PATHOGENESIS
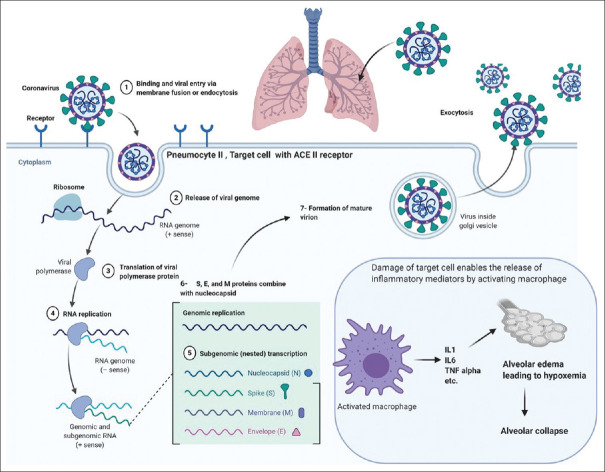
The adherence of COVID-19 virus to ACE-2 receptors in type II pneumocytes forms a complex which stimulates inflammation in the lower respiratory tract.[23] This complex is progressed by type 2 transmembrane enzyme protease (TMPRSS2) in a proteolytic manner leading to the disruption and cleaved out of ACE-2, finally, to activate the s-spike protein.[24] The virus genome is uncoated, transcribed, and translated.[25] Studies suggest that the binding patterns and the mode to trigger up the inflammatory cascade are almost common as in the case of earlier strain (SARS-CoV and novel SARS-CoV-2).[26] This membrane inoculation causes diseased cell outcomes and termination of the cilium normalcy at particular sites.[27] Later on, some specific inflammatory mediators were released. These mediators further stimulate macrophages to release the multiple cytokines interleukin (IL) 1, IL6, and tumor necrosis factor-α. These cytokines get transferred into the bloodstream and cause excessive capillary permeability through the dilatation of smooth muscles [Figure 6]. This vasodilation and increased permeability allow leaking out the plasma and other fluids in interstitial spaces of alveoli leading to alveolar edema hence alveolar collapse and hypoxemia. Due to the release of inflammatory mediators, the multiple organs get influenced to show the abnormalities in the prodromal phase as the major clinical symptoms of high fever, dry cough, high blood pressure, fatigue, myalgia, diarrhea, dyspnea, lymphopenia, RNAaemia, respiratory distress syndrome, secondary superinfections, and acute cardiac injuries.[7]
Figure 6.
Binding and replication of coronavirus disease-2019 leading to release and alveolar collapse. The figure is made with BioRender (https://biorender.com/)
TREATMENT AND MANAGEMENT
At the time of preparing this manuscript, there is still no specific treatment available for COVID-19. Despite the facts of minimal recoveries and having no choice, the Food and Drug Administration approved chloroquine and hydroxychloroquine (an antimalarial drug) to be effective somehow as the remedial approach.[28] The treatment is symptomatic; major treatment interventions are mechanical ventilation, hemodynamic support, and oxygen therapy for patients with severe infection. One of the antiviral (lopinavir 400 mg or ritonavir 100 mg BD) treatment approaches has been recommended and aerosol formulation of alpha-interferon twice daily proposed.[29] Other drugs that are widely used around the world to control the complications are fingolimod, methylprednisolone, chloroquine phosphate, hydroxychloroquine sulfate, bevacizumab, leronlimab, ivermectin, and sarilumab. Corona-infected patients can be managed with rehydration therapy, respiratory inhalation therapy, and providing aid to the affected vital organs.[30]
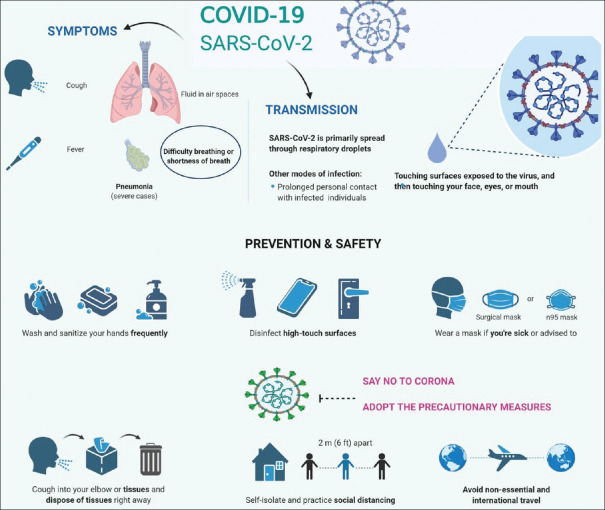
Awareness and dedication can only minimize the spread rate of COVID-19 by strengthening the trust within the communities without having any fear of failure.[31] Strategic recommendations also include the isolation protocols along with the proper use of N95 or FFP3 masks, eye-protective glass, apron, and gloves to prevent the pathogens move [Figure 7].[32]
Figure 7.
An illustration showing the mode of transmission, clinical symptoms and preventive and safety measures for coronavirus disease-2019. The figure is made with BioRender (https://biorender.com/)
SOCIAL DISTANCING IMPACT
Due to the corona pandemic, several countries have declared a state emergency, including developed countries, even having the best infrastructure of the health-care system, which has raised concerns about lasting impacts on civil liberties. Worldwide with social distancing, the most affected system is educational institutes that leads to almost closure of universities, schools, and colleges, which negatively impact learning outcomes. The impact was more distressing for underprivileged children and their families, causing intersperse learning, inadequate nutrition, infant care problems, and subsequent economic expenditure to family members who could not work. This pandemic also affected the financial markets.
PSYCHOLOGICAL IMPACT
This pandemic has a severe psychological impact including a significant degree of mental stress, fear, anxiety, and worry in most of the public, health caregivers, as well as in a specific group of comorbid diseased populations. Stress during this pandemic can comprises concern and fear about health and health condition of relatives, changing and diet practices, trouble in sleeping or concentration, deterioration of chronic health issues, and deteriorating psychological health situations, increase consumption of alcohol, tobacco, or other drugs.[33]
VACCINE DEVELOPMENT
Research scientists throughout the world have been struggling to develop powerful vaccines against COVID-19. Inactivated or weakened virus vaccine, protein-based vaccines, RNA and DNA vaccines, and viral vector vaccines are the types of potential vaccines that are in development. Several vaccines are currently available to overcome this pandemic which include Pfizer-BioNTech, Sinopharm (China), Johnson and Johnson, Novavax (UK), Astrazeneca, Sinovac (China), CanSinoBio, and Gamaleya Research Institute (Russia) [Table 2]. The vaccine for the COVID-19 was first started in December 2020 and until February 15, 2021, 175.3 million vaccine doses have been given. The emergency use licenses were also issued for some vaccines such as Pfizer, AstraZeneca/Oxford, and vaccine developed by Johnson and Johnson. The side effects of the vaccines have also been reported. Thirty-three suspected adverse drug reactions have been found in Norway including some fatal responses after the use of BioNTech and Pfizer vaccines as per the Norwegian Medicines Agency.[34]
Table 2.
Coronavirus disease-2019 candidate vaccines in clinical evaluation
| COVID-19 Vaccine developer/manufacturer | Vaccine platform | Type of candidate vaccine | Number of doses | Timing of doses | Route of Administration |
|---|---|---|---|---|---|
| Sinovac | Inactivated | Inactivated | 2 | 0, 14 days | IM |
| Wuhan Institute of Biological Products/Sinopharm | Inactivated | Inactivated | 2 | 0,21 days | IM |
| Beijing Institute of Biological Products/Sinopharm | Inactivated | Inactivated | 2 | 0,21 days | IM |
| University of Oxford/AstraZeneca | Non- Replicating Viral Vector | ChAdOx1-S | 1 | IM | |
| CanSino Biological Inc./Beijing Institute of Biotechnology | Non- Replicating Viral Vector | Adenovirus Type 5 Vector | 1 | IM | |
| Gamaleya Research Institute | Non- Replicating Viral Vector | Adeno-based (rAd26-S + rAd5-S) | 2 | 0,21 days | IM |
| Janssen Pharmaceutical Companies | Non- Replicating Viral Vector | Ad26COVS1 | 2 | 0, 56 days | IM |
| Novavax | Protein Subunit | Full length recombinant SARS CoV-2 glycoprotein nanoparticle vaccine adjuvanted with Matrix M | 2 | 0, 21 days | IM |
| Moderna/NIAID | RNA | LNP-encapsulated mRNA | 2 | 0, 28 days | IM |
| BioNTech/Fosun Pharma/Pfizer | RNA | 3 LNP-mRNAs | 2 | 0, 28 days | IM |
| Anhui Zhifei Longcom Biopharmaceutical/Institute of Microbiology, Chinese Academy of Sciences | Protein Subunit | Adjuvanted recombinant protein (RBD-Dimer) | 2 or 3 | 0,28 or 0,28, 56 days | IM |
| Curevac | RNA | mRNA | 2 | 0, 28 days | IM |
| Institute of Medical Biology, Chinese Academy of Medical Sciences | Inactivated | Inactivated | 2 | 0, 28 days | IM |
| Research Institute for Biological Safety Problems, Rep of Kazakhstan | Inactivated | Inactivated | 2 | 0, 21 days | IM |
| Inovio Pharmaceuticals/International Vaccine Institute | DNA | DNA plasmid vaccine with electroporation | 2 | 0, 28 days | ID |
| Osaka University/AnGes/Takara Bio | DNA | DNA plasmid vaccine + Adjuvant | 2 | 0, 14 days | IM |
| Cadila Healthcare Limited | DNA | DNA plasmid vaccine | 3 | 0, 28, 56 days | ID |
| Genexine Consortium | DNA | DNA Vaccine (GX-19) | 2 | 0, 28 days | IM |
| Bharat Biotech | Inactivated | Whole-Virion Inactivated | 2 | 0, 14 days | IM |
| Kentucky Bioprocessing, Inc | Protein Subunit | RBD-based | 2 | 0, 21 days | IM |
| Sanofi Pasteur/GSK | Protein Subunit | S protein (baculovirus production) | 2 | 0, 21 days | IM |
| Arcturus/Duke-NUS | RNA | mRNA | IM | ||
| SpyBiotech/Serum Institute of India | VLP | RBD-HBsAg VLPs | 2 | 0, 28 days | IM |
| ReiThera/LEUKOCARE/Univercells | Non- Replicating Viral Vector | Replication defective Simian Adenovirus (GRAd) encoding S | 1 | IM | |
| Institute of Biotechnology, Academy of Military Medical Sciences, PLA of China | Non- Replicating Viral Vector | Ad5-nCoV | 2 | 0, 28 days | IM/mucosal |
| Vaxart | Non- Replicating Viral Vector | Ad5 adjuvanted Oral Vaccine platform | 2 | 0, 28 days | Oral |
| Ludwig-Maximilians - University of Munich | Non- Replicating Viral Vector | MVA-SARS-2-S | 2 | 0, 28 days | IM |
| Clover Biopharmaceuticals Inc./GSK/Dynavax | Protein Subunit | Native like Trimeric subunit Spike Protein vaccine | 2 | 0, 21 days | IM |
| Vaxine Pty Ltd/Medytox | Protein Subunit | Recombinant spike protein with Advax™ adjuvant | 1 | IM | |
| University of Queensland/CSL/Seqirus | Protein Subunit | Molecular clamp stabilized Spike protein with MF59 adjuvant | 2 | 0, 28 days | IM |
| Medigen Vaccine Biologics Corporation/NIAID/Dynavax | Protein Subunit | S-2P protein + CpG 1018 | 2 | 0, 28 days | IM |
| Instituto Finlay de Vacunas, Cuba | Protein Subunit | RBD + Adjuvant | 2 | 0, 28 days | IM |
| FBRI SRC VB VECTOR, Rospotrebnadzor, Koltsovo | Protein Subunit | Peptide | 2 | 0, 21 days | IM |
| West China Hospital, Sichuan University | Protein Subunit | RBD (baculovirus production expressed in Sf9 cells) | 2 | 0, 28 days | IM |
| University Hospital Tuebingen | Protein Subunit | SARS-CoV-2 HLA-DR peptides | 1 | SC | |
| COVAXX | Protein Subunit | S1-RBD-protein | 2 | 0, 28 days | IM |
| Institute Pasteur/Themis/Univ. of Pittsburg CVR/Merck Sharp & Dohme | Replicating Viral Vector | Measles-vector based | 1 or 2 | 0, 28 days | IM |
| Beijing Wantai Biological Pharmacy/Xiamen University | Replicating Viral Vector | Intranasal flu-based-RBD | 1 | IM | |
| IMperial College London | RNA | LNP-nCoVsaRNA | 2 | IM | |
| People’s Liberation Army (PLA) Academy of Military Sciences/Walvax Biotech. | RNA | mRNA | 2 | 0, 14 or 0, 28 days | IM |
| Medicago Inc. | VLP | Plant-derived VLP adjuvanted with GSK or Dynavax adjs. | 2 | 0, 21 days | IM |
|
| |||||
| COVID-19 Vaccine developer/manufacturer | Clinical Stage | ||||
|
| |||||
| Phase 1 | Phase 1/2 | Phase 2 | Phase 3 | ||
|
| |||||
| Sinovac |
NCT04383574 NCT04352608 NCT04551547 |
NCT04456595 669/UN6.KEP/EC/2020 | |||
| Wuhan Institute of Biological Products/Sinopharm | ChiCTR2000031809 Interim Report |
ChiCTR2000034780 | |||
| Beijing Institute of Biological Products/Sinopharm | ChiCTR2000032459 | ChiCTR2000034780 NCT04560881 |
|||
| University of Oxford/AstraZeneca | PACTR202006922165132 2020-001072-15 NCT04568031 Interim Report |
2020-001228-32 | ISRCTN89951424 NCT04516746 NCT04540393 CTRI/2020/08/027170 | ||
| CanSino Biological Inc./Beijing Institute of Biotechnology | ChiCTR2000030906 Study Report | ChiCTR2000031781 Study Report |
NCT04526990 NCT04540419 |
||
| Gamaleya Research Institute | NCT04436471 NCT04437875 Study Report | NCT04530396 NCT04564716 | |||
| Janssen Pharmaceutical Companies | NCT04436276 | NCT04505722 | |||
| Novavax | NCT04368988 Study Report | NCT04533399 (phase 2b) | 2020-004123-16 | ||
| Moderna/NIAID |
NCT04283461 Interim Report Final Report |
NCT04405076 | NCT04470427 | ||
| BioNTech/Fosun Pharma/Pfizer | 2020-001038-36 ChiCTR2000034825 NCT04537949 Study Report | NCT04368728 | |||
| Anhui Zhifei Longcom Biopharmaceutical/Institute of Microbiology, Chinese Academy of Sciences | NCT04445194 | NCT04550351 | NCT04466085 | ||
| Curevac | NCT04449276 | NCT04515147 | |||
| Institute of Medical Biology, Chinese Academy of Medical Sciences | NCT04412538 | NCT04470609 | |||
| Research Institute for Biological Safety Problems, Rep of Kazakhstan | NCT04530357 | ||||
| Inovio Pharmaceuticals/International Vaccine Institute | NCT04447781 NCT04336410 | ||||
| Osaka University/AnGes/Takara Bio | NCT04463472 NCT04527081 | ||||
| Cadila Healthcare Limited | CTRI/2020/07/026352 | ||||
| Genexine Consortium | NCT04445389 | ||||
| Bharat Biotech | NCT04471519 CTRI/2020/09/027674 | ||||
| Kentucky Bioprocessing, Inc | NCT04473690 | ||||
| Sanofi Pasteur/GSK | NCT04537208 | ||||
| Arcturus/Duke-NUS | NCT04480957 | ||||
| SpyBiotech/Serum Institute of India | ACTRN12620000817943 | ||||
| ReiThera/LEUKOCARE/Univercells | NCT04528641 | ||||
| Institute of Biotechnology, Academy of Military Medical Sciences, PLA of China | NCT04552366 | ||||
| Vaxart | NCT04563702 | ||||
| Ludwig-Maximilians - University of Munich | NCT04569383 | ||||
| Clover Biopharmaceuticals Inc./GSK/Dynavax | NCT04405908 | ||||
| Vaxine Pty Ltd/Medytox | NCT04453852 | ||||
| University of Queensland/CSL/Seqirus | ACTRN12620000674932p ISRCTN51232965 | ||||
| Medigen Vaccine Biologics Corporation/NIAID/Dynavax | NCT04487210 | ||||
| Instituto Finlay de Vacunas, Cuba | IFV/COR/04 | ||||
| FBRI SRC VB VECTOR, Rospotrebnadzor, Koltsovo | NCT04527575 | ||||
| West China Hospital, Sichuan University | ChiCTR2000037518 | ||||
| University Hospital Tuebingen | NCT04546841 | ||||
| COVAXX | NCT04545749 | ||||
| Institute Pasteur/Themis/Univ. of Pittsburg CVR/Merck Sharp & Dohme | NCT04497298 | ||||
| Beijing Wantai Biological Pharmacy/Xiamen University | ChiCTR2000037782 | ||||
| IMperial College London | ISRCTN17072692 | ||||
| People’s Liberation Army (PLA) Academy of Military Sciences/Walvax Biotech. | ChiCTR2000034112 | ||||
| Medicago Inc. | NCT04450004 | ||||
COVID-19: Coronavirus disease-2019, PLA: People’s liberation army, SARS: Severe acute respiratory syndrome, CoV-2: Coronavirus-2, HLA: Human leukocyte antigen, DR: D related, RBD: Receptor-binding domain
CONCLUSION
More than 15 months passed of this pandemic, some nonpharmacological approaches have been adopted to combat the symptoms of the disease. As a result of this, only social distancing, quarantine, and isolation methods are advised to keep away from the infections. It also leads to a negative impact on the psychological behavior of human beings.
The scientific community has led to the development more than 40 vaccines that are undergoing the clinical trials, including more than 10 in phase III trials and three of them is ended with the positive results. The world is expecting that this vaccination program will be a significant measure to eradicate this pandemic. Other challenges that still need to be addressed are the multiple variants of this virus that is emerging day by day.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Cascella M, Rajnik M, Cuomo A, Dulebohn SC, Di Napoli R. Features, Evaluation and Treatment Coronavirus (COVID-19) In: StatPearls. FL, USA: StatPearls Publishing; 2020. [PubMed] [Google Scholar]
- 2.Chan JF, Kok KH, Zhu Z, Chu H, To KK, Yuan S, et al. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microbes Infect. 2020;9:221–36. doi: 10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hui DS, Azhar EI, Madani TA, Ntoumi F, Kock R, Dar O, et al. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health–The latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis. 2020;91:264. doi: 10.1016/j.ijid.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mahtani S, Berger M, O'Grady S, Iati M. Hundreds of Evacuees to be Held on Bases in California; Hong Kong and Taiwan Restrict Travel from Mainland China. The Washington Post. 2020. [[Last accessed on 2020 Feb 11]]. Available from: https://www.washingtonpost.com/world/asia_pacific/coronavirus-china-live-updates/2020/02/05/114ced8a-479c-11eabc78-8a18f7afcee7_story.html .
- 5.WHO. WHO Director-General's Opening Remarks at the Media Briefing on COVID-19”. World Health Organization (WHO) (Press Release) [[Last accessed on 2020 Mar 11]]. https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefingoncovid-19-11-march-2020.
- 6.Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu H. Drug treatment options for the 2019-new coronavirus (2019-nCoV) Biosci Trends. 2020;14:69–71. doi: 10.5582/bst.2020.01020. [DOI] [PubMed] [Google Scholar]
- 9.Anonymous. [[Last accessed on 2020 Mar 05]]. Available from: https://www.cdc.gov.tw/En .
- 10.Wang W, Tang J, Wei F. Updated understanding of the outbreak of 2019 novel coronavirus (2019-nCoV) in Wuhan, China. J Med Virol. 2020;92:441–7. doi: 10.1002/jmv.25689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anonymous. [[Last accessed on 2020 Mar 10]]. https://www.who.int/docs/default-source/coronaviruse/situationreports.
- 12.Weiss SR, Leibowitz JL. Coronavirus pathogenesis. Adv Virus Res. 2011;81:85–164. doi: 10.1016/B978-0-12-385885-6.00009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu F, Zhao S, Yu B, Chen YM, Wang W, Hu Y, et al. Complete genome characterisation of a novel coronavirus associated with severe human respiratory disease in Wuhan, China. BioRxiv. 2020 https://doi.org/10.1101/2020.01.24.919183. [Google Scholar]
- 14.Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet. 2020;395:565–74. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin Y, Yang H, Ji W, Wu W, Chen S, Zhang W, et al. Virology, epidemiology, pathogenesis, and control of COVID-19. Viruses. 2020;12:1–17. doi: 10.3390/v12040372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN, et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N Engl J Med. 2020;382:1564–7. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vaduganathan M, Vardeny O, Michel T, McMurray JJ, Pfeffer MA, Solomon SD. Renin-angiotensin-aldosterone system inhibitors in patients with COVID-19. N Engl J Med. 2020;382:1653–9. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, et al. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ Res. 2000;87:E1–9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 19.Xu X, Chen P, Wang J, Feng J, Zhou H, Li X, et al. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci China Life Sci. 2020;63:457–60. doi: 10.1007/s11427-020-1637-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin X, Lian JS, Hu JH, Gao J, Zheng L, Zhang YM, et al. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. 2020;69:1002–9. doi: 10.1136/gutjnl-2020-320926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anonymous. 2020. [[Last accessed on 2020 Mar 20]]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/laboratory-guidance .
- 22.Ai T, Yang Z, Hou H, Zhan C, Chen C, Lv W, et al. Correlation of chest CT and RT-PCR testing for coronavirus disease 2019 (COVID-19) in China: A report of 1014 cases. Radiology. 2020;296:E32–40. doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11:875–9. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glowacka I, Bertram S, Müller MA, Allen P, Soilleux E, Pfefferle S, et al. Evidence that TMPRSS2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response. J Virol. 2011;85:4122–34. doi: 10.1128/JVI.02232-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mousavizadeh L, Ghasemi S. Genotype and phenotype of COVID-19: Their roles in pathogenesis. J Microbiol Immunol Infect. 2021;54:159–63. doi: 10.1016/j.jmii.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rabi FA, Al Zoubi MS, Kasasbeh GA, Salameh DM, Al-Nasser AD. SARS-CoV-2 and Coronavirus Disease 2019: What We Know So Far. Pathogens. 2020;9:1–14. doi: 10.3390/pathogens9030231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–33. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–71. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ratia K, Pegan S, Takayama J, Sleeman K, Coughlin M, Baliji S, et al. A noncovalent class of papain-like protease/deubiquitinase inhibitors blocks SARS virus replication. Proc Natl Acad Sci U S A. 2008;105:16119–24. doi: 10.1073/pnas.0805240105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fisher D, Heymann D. Q and A: The novel coronavirus outbreak causing COVID-19. BMC Med. 2020;18:57. doi: 10.1186/s12916-020-01533-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alexander N, Gibbons K, Marshall S, Rodriguez M, Sweitzer J, Varma K. Implementing Principles of Reimagine Minnesota in a Period of Remote Teaching and Learning: Education Equity in the Age of COVID-19; Minneapolis Foundation, MN, US. 2020 [Google Scholar]
- 32.Ahmad MD, Wahab S, Ali Ahmad F, Intakhab Alam M, Ather H, Siddiqua A, et al. A novel perspective approach to explore pros and cons of face mask in prevention the spread of SARS-CoV-2 and other pathogens. Saudi Pharm J. 2021;29:121–33. doi: 10.1016/j.jsps.2020.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang C, Pan R, Wan X, Tan Y, Xu L, Ho CS, et al. Immediate psychological responses and associated factors during the initial stage of the 2019 coronavirus disease (COVID-19) epidemic among the general population in China. Int J Environ Res. 2020;17:1729. doi: 10.3390/ijerph17051729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Torjesen I. Covid-19: Norway investigates 23 deaths in frail elderly patients after vaccination. BMJ. 2021;372:n149. doi: 10.1136/bmj.n149. [DOI] [PubMed] [Google Scholar]