Abstract
Herein, chitosan nanoparticles (CS-NPs) were prepared and functionalized chemically with titanium dioxide nanoparticles (TiO2-NPs) to allow on-demand degradation of CS-NPs, using ultraviolet (UV) irradiation as a trigger. This is expected to allow drug release depending on patients' needs or physiological circumstances. Eleven formulations were arranged and their particle size, charge, and polydispersity were determined. The effect of CS-NPs size and the amount of TiO2-NPs, on the system collapse, was studied accordingly. Moreover, the collapse of these systems was examined using a fluorescence microscope after loading CS-NPs with Rhodamine. The formulations showed high monodispersity and had sizes ranged between 170 and 440 nm and charges ranged between +5 and +34 mV. Scanning electron microscope, Fourier-transform infrared spectroscopy, and X-ray diffraction proved the chemical deposition of TiO2-NPs on CS-NPs. The dye test showed that there are two factors that oppose each other and affected the deposition of TiO2-NPs on CS-NPs, the size of CS-NPs, and the amount of TiO2-NPs used. In addition, the dye test showed that the deposition of TiO2-NPs is a saturated process that relies on the amount of TiO2-NPs used initially. Finally, the intensity of Rhodamine released from these systems after illumination with UV light was related to the amount of TiO2-NPs deposited on CS-NPs. In conclusion, functionalization of CS-NPs with TiO2-NPs can be controlled and used to rupture CS-NPs on demand by illumination with UV light.
Keywords: Chitosan nanoparticles, Rhodamine, titanium dioxide nanoparticles, ultraviolet irradiation
INTRODUCTION
Polymeric nanoparticles (PNPs) are widely used in many fields today, including the pharmaceutical industry and drug delivery. PNPs have numerous advantages to be used. First, they use lower drug concentrations, and therefore, less frequent doses are needed. Second, the side effects related to drugs loaded in PNPs are avoided or reduced. Third, they enhance the diffusion through biological membranes and the penetration of drugs into the cells. Fourth, they enhance drug's stability and prolong drugs' activity in vivo and in vitro. Fifth, several methods and polymers are available to prepare biocompatible nanoparticles.[1] Finally, they have a huge potential in the targeted distribution of drugs and biological molecules. This targeting allows the selective transport of any therapeutic agent to its site of action independently on the method of administration.[2]
Recently, numerous polymers were used to formulate nanoparticles. Interestingly, chitosan (CS) is one of the most used polymers to formulate nanoparticles. CS is a natural, linear polysaccharide, biocompatible, biodegradable, nontoxic, and bioadhesive polymer. CS nanoparticles (CS-NPs) have been used as a colloidal drug carrier to target and control the drug delivery to specific sites in the body.[3,4] It is used for gene and vaccine delivery and in cancer therapy.[5] CS-NPs are prepared by different methods, where the method used in the preparation of NPs, parameters, and properties of the starting materials affect the prepared NP physicochemical properties and the drug release profile.[6]
One of the important drawbacks of PNPs, including CS-NPs, is the drug release at a predetermined rate regardless of the patient's needs or the disease physiological circumstances. A system that triggers drug delivery may allow controlling the therapeutic effect depending on time. This on-demand drug release from nanoparticles is very important in chemotherapy, where it maximizes tumor killing and minimizes metastatic spread.[7]
In general, the drug release from PNPs depends on the diffusion through the polymers or on the degradation of the polymeric chain. To allow on-demand drug release from PNPs, these PNPs may be functionalized with materials that respond to specific triggers, such as pH, redox, proteins, temperature, light, or magnetic field. Ultraviolet (UV) light is an important trigger that is used in preparing photo-controlled release systems.[8,9] In such formulations, PNPs are noncovalently or covalently assembled with a material that is photosensitive such as TiO2-NPs. Adding TiO2-NPs to CS-NPs may allow on-demand drug release by photolytic degradation. In previous research, CS/PVA blend was applied as a nanoreactor for Ag and Au nanoparticles and showed promising anticancer applications. Other researchers find that triggering hydrophilic polymers such as CS could control drug release in response to UV irradiation.[10]
TiO2-NPs are inorganic chemicals that are widely used in cosmetics, pollution treatment, food preservation, pharmaceutical, and painting fields. They are also used in biomaterials due to their high stability, antimicrobial, and anticorrosive properties. TiO2-NPs have unique photocatalytic properties that guided many research to be used as disinfectants, biological sensors, antibiotics, and tumor-cell killing agents.[11]
In this study, the chemical functionalization of CS-NPs with TiO2-NPs was studied. CS-NPs were fabricated using TPP as a crosslinker. Subsequently, the nanoparticles were functionalized chemically with TiO2-NPs. The mean particle size (PS), polydispersity index (PDI), charge (ZP), and physical morphology of CS-NPs before and after functionalization were characterized. The deposition of TiO2-NPs on CS-NPs was explored and measured using Fourier-transform infrared spectroscopy (FTIR), X-ray diffraction (XRD), scanning electron microscope (SEM), and dye test. Finally, functionalized CS-NPs were loaded with Rhodamine and the release of Rhodamine after triggering with UV light was studied.
MATERIALS AND METHODS
Materials
Sodium tripolyphosphate (TPP) was purchased from AZchm (China). CS (50–190kDa, 75%DDA), titanium (IV) oxide (TiO2-NPs) (99.5%,<100 nm), and Rhodamine B (95%) were purchased from Sigma-Aldrich (St. Louis, MO). All chemicals used were of analytical grade.
Preparation and functionalization of chitosan nanoparticles
CS-NPs were prepared according to the ionic gelation method, and the experimental conditions were chosen according to our previous findings to control the sizes of the NPs.[6] Two solutions were prepared: CS (0.5 mg\mL) in 1% acetic acid solution and TPP solution in water. TPP was added to the CS solution. Part of these NPs was purified by placing the dispersion into a dialysis bag with a cutoff of 12–14 KD. Consequently, CS-NPs were freeze-dried and stored in a tightly closed container in the fridge.
The other portion was functionalized by adding TiO2-NPs. TiO2-NPs were dispersed in 1% acetic acid solution and added to the CS-NPs dispersion at 25s by a syringe pump. The flow rate was 2.5 ml/min and the speed of stirring of 700 rpm. The final mixture was purified and dried. To study the effect of the size of the NPs on the functionalization, CS-NPs with sizes around 200, 250, and 400 nm were prepared. The different parameters applied are described in Table 1.
Table 1.
Weight ratios and parameters used in preparing chitosan nanoparticles
| CS: TPP | CS: TiO2-NPs | Flow rate (ml/min) | |
|---|---|---|---|
| F0 | 1:2 | NA | 0.25 |
| F1 | 1:2 | 3:1 | 0.25 |
| F2 | 1:2 | 1:1 | 0.25 |
| F3 | 1:2 | 1:3 | 0.25 |
| F4 | 2:1 | NA | 2.5 |
| F5 | 2:1 | 3:1 | 2.5 |
| F6 | 2:1 | 1:1 | 2.5 |
| F7 | 2:1 | 1:3 | 2.5 |
| F8 | 1:7.5 | NA | 2.5 |
| F9 | 1:7.5 | 3:1 | 2.5 |
| F10 | 1:7.5 | 1:1 | 2.5 |
| F11 | 1:7.5 | 1:3 | 2.5 |
CS: Chitosan, TPP: Tripolyphosphate, TiO2: Titanium dioxide, NPs: Nanoparticles, NA: Not applicable
Characterization of chitosan nanoparticles before and after functionalization
The PS, PDI, and ZP of CS-NPs before and after functionalization with TiO2-NPs were determined using a Zetasizer Nano ZS90 (Malvern, UK). SEM (Thermo scientific, Germany) was used to study the morphologies of all formulations after being coated with carbon film.
Shimadzu IR Spectrophotometer (Shimadzu, Kyoto, Japan) and Ultima IV X-Ray Diffractometer (Rigaku, Japan) were used to study the formulations.
Dye test
Functionalized CS-NPs were mixed with 50 mg/L Direct Blue 78 (DB78) (50 mg/L) at pH~2 under magnetic stirring at 200rpm for 60 min. The changes in the absorbance at λmax~600nm of solution samples were determined at predetermined intervals.[12] The percentage absorption reduction was calculated as follow:
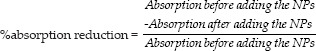
Rhodamine thin film fluorescence
The formulations were loaded with Rhodamine to study the rupture of the systems upon exposure to UV light. Rhodamine was dissolved in the CS solution in the preparation step and the particles were characterized. A drop of a solution of ethanol/water (50:50) was added to each formulation on a slide and covered with a coverslip and analyzed by fluorescence microscope (Motic AE31E, USA). The formulations were illuminated by UV light at λ~370 nm. The fluorescence of Rhodamine was detected after 30 min of illumination at λ~520 nm. Fluorescent intensities of each sample were normalized and evaluated using ImageJ software.
RESULTS AND DISCUSSION
Characterization of chitosan-TiO2 nanoparticles
The conditions applied in this work to prepare CS-NPs were chosen depending on our previous work to get three different sizes of CS-NPs. The targeted sizes were around 200, 250, and 400 nm. The ratio of CS to TPP and the rate of adding TPP to CS were controlled to realize the sizes. The PS of all formulations is summarized in Table 2.
Table 2.
Particle size (nm), polydispersity index, and charge (mV) for the formulations prepared (mean±standard deviation, n=3)
| PS | PDI | ZP | |
|---|---|---|---|
| F0 | 170.67±1.35 | 0.451±0.021 | 19.54±0.56 |
| F1 | 220.25±3.41 | 0.513±0.085 | 9.62±0.44 |
| F2 | 395.23±4.00 | 0.373±0.111 | 5.97±0.86 |
| F3 | 376.43±8.41 | 0.411±0.033 | 6.61±1.01 |
| F4 | 234.37±2.13 | 0.521±0.005 | 26.33±1.04 |
| F5 | 290.37±3.00 | 0.379±0.028 | 15.50±1.21 |
| F6 | 439.33±5.39 | 0.408±0.060 | 18.33±0.52 |
| F7 | 442.80±5.99 | 0.714±0.303 | 16.72±0.33 |
| F8 | 391.17±2.47 | 0.618±0.0615 | 34.04±0.88 |
| F9 | 344.93±3.25 | 0.547±0.004 | 8.71±0.34 |
| F10 | 408.43±10.21 | 0.392±0.010 | 11.63±0.11 |
| F11 | 420.56±3.70 | 0.481±0.002 | 7.46±0.18 |
PS: Particle size, PDI: Polydispersity index
F0, F4, and F8 were functionalized using different amounts of TiO2-NPs. The effect of the amount of TiO2-NPs used on the NP properties is shown in Table 2. Unfunctionalized CS-NPs have smaller sizes in comparison to functionalized ones, which may prove the accumulation of TiO2-NPs on the surface of CS-NPs. Further, when the amount of TiO2-NPs used in the coating increased from 3:1 to 1:1, a huge increase in the sizes can be noticed, while increasing the amount of TiO2-NPs further from 1:1 to 1:3 has a smaller effect on the particles' size. This behavior is observed in the three groups of sizes prepared. This may indicate saturation of the sites of interaction between CS-NPs and TiO2-NPs at certain concentrations. The PS increased after loading CS-NPs with Rhodamine in F0, F4, and F8 and the EE was around 35%, as shown in Table 3. All systems prepared carried positive charges, but it is obvious that functionalization decreases the charges.
Table 3.
Particle size (nm), polydispersity index, charge (mV), and EE % of the chitosan nanoparticles loaded with Rhodamine before functionalization (mean±standard deviation, n=3)
| PS | PDI | ZP | EE (%) | |
|---|---|---|---|---|
| F0 | 175.12±2.50 | 0.321±0.101 | 18.70±0.55 | 37.81 |
| F4 | 241.41±1.98 | 0.494±0.113 | 24.47±0.75 | 35.29 |
| F8 | 412.05±4.16 | 0.700±0.0835 | 31.07±1.07 | 33.76 |
PS: Particle size, PDI: Polydispersity index, EE: Entrapment Efficiency
SEM confirmed that CS-NPs prepared in this study are spherical. The coated CS-NPs showed small particles on their surfaces, which may be related to the precipitated TiO2-NPs. Figure 1 shows a representative SEM image of CS-NPs before and after functionalization (F0 and F1).
Figure 1.

Scanning electron microscope image of chitosan nanoparticles (F1) (a) before functionalization (F0) (b) after functionalization
FTIR of CS, TiO2-NPs, CS-NPs, and functionalized CS-NPs is demonstrated in Figure 2. The spectrum of CS was compared to that of CS-NPs to confirm the cross-linking in CS-NPs. In CS spectrum, a characteristic band related to NH2 and OH groups stretching was observed at 3447 cm−1. This band gets shallower in CS-NPs. Further, a new double peak at 2360 cm−1 related to–NH2 in CS-NPs spectrum appeared.[13]
Figure 2.
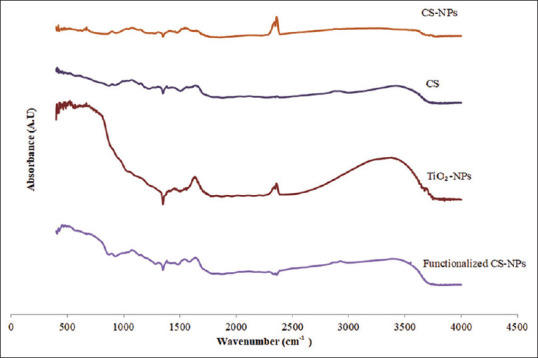
Fourier-transform infrared spectroscopy of chitosan, chitosan nanoparticles, titanium dioxide nanoparticles, and functionalized chitosan nanoparticles
The spectrums of TiO2-NPs, CS-NPs, and functionalized CS-NPs showed the following differences: for TiO2-NPs, the characteristic band at 3500 cm−1 that corresponds to OH stretching gets shallower in the functionalized CS-NPs. The second peak in TiO2-NPs around 1630cm−1 that corresponds to bending modes of water Ti-OH, disappeared in the functionalized NPs. In addition, the peak at 2368 cm−1 in CS-NPs disappeared in the spectrum of functionalized NPs. These differences in the spectrums of TiO2-NPs, CS-NPs, and the functionalized NPs give a clear proof of the interaction between TiO2-NPs and CS-NPs.[14]
XRD was carried out for CS, CS-NPs, TiO2-NPs, and functionalized CS-NPs to explore any interactions. Figure 3 shows that CS exhibited an amorphous structure with one large broad peak. CS-NPs showed a spectrum without any peaks that is somehow different from the spectrum of CS, which reveals the formation of CS-NPs. The spectrum of TiO2-NPs showed a crystalline behavior as indicated by the sharp peaks. When the spectrums of TiO2-NPs, CS-NPs, and the functionalized CS-NPs are compared, it can be noticed that the functionalized CS-NPs spectrum is similar to TiO2-NPs. This however indicates that TiO2-NPs precipitated on the surface of CS-NPs.[15]
Figure 3.
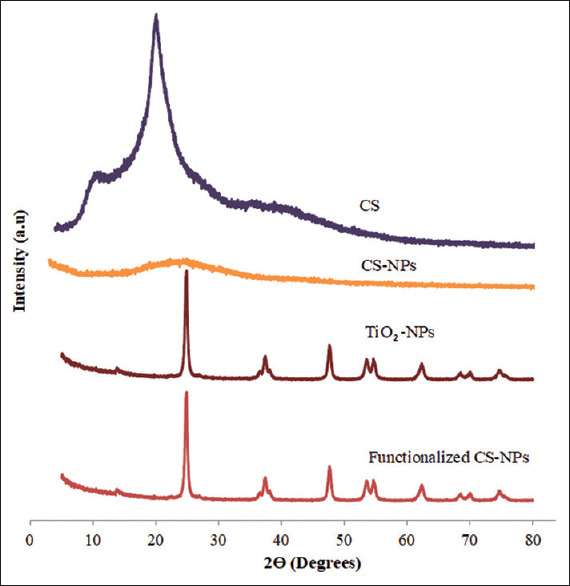
X-ray diffraction of chitosan, chitosan nanoparticles, titanium dioxide-nanoparticles, and functionalized chitosan nanoparticles
Dye test
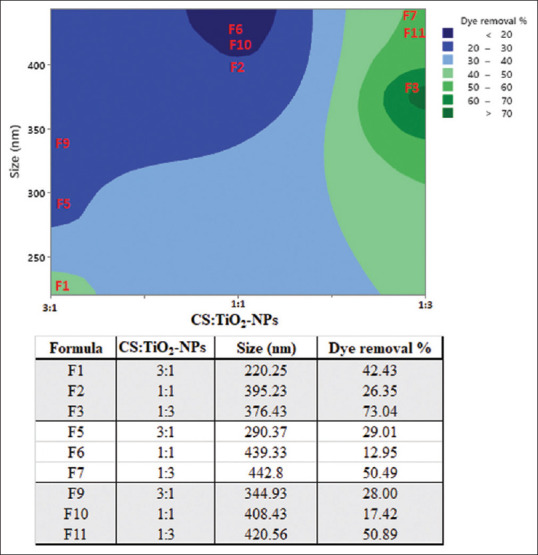
Since the dye adsorption is a mass transfer process, then the higher the reduction of the absorption indicates the higher amount of TiO2-NPs available to interact with the dye. This indirectly indicates the higher deposition of TiO2-NPs on CS-NPs. The percentage reduction in the dye absorbance was measured and referred to as dye removal percentage and the results are shown in Figure 4.[12]
Figure 4.
The %dye removal by functionalized chitosan nanoparticles versus time measured at 600 nm (mean ± % relative standard deviation, n = 3)
Adsorption is affected by many factors including temperature, pH, size, surface area, and the amounts of the adsorbent and adsorbate. Herein, the effect of the particles size and the amount of TiO2-NPs used was studied.
First, it is obvious that as the size of the NPs increases the dye removal decrease (adsorption decrease). This behavior can be observed when F3, F7, and F11 are compared, F2, F6, and F10 are compared, and when F1, F5, and F9 are compared.
Formulations prepared using the highest amount of TiO2-NPs showed the highest dye removal (1:3). For the ratios 1:1 and 3:1, the dye removal from the ratio 3:1 was higher than that of 1:1. For example, F1 showed a high %dye removal in comparison to F2. Furthermore, F5 showed a high %dye removal in comparison to F6. In addition, F9 showed a high %dye removal in comparison to F10. This may be due to the lower sizes recorded for F1, F5, and F9 that were prepared using lower amounts of TiO2-NPs. These results indicate that the effect of the two factors, the size of CS-NPs and the amount of TiO2-NPs, are controverting each other. Increasing the particles sizes decreases the surface area, which is expected to decrease the adsorption, while increasing the amount of TiO2-NPs used is expected to increase the sites available for dye adsorption.[16]
Rhodamine thin film fluorescence
Figure 5a shows the Rhodamine release from F5 after illustration with UV light for 30 min. Figure 5b shows the intensity of Rhodamine released from the 11 formulations prepared. From these optical micrographs, it is clear that the lower intensities of Rhodamine were recorded for the unfunctionalized CS-NPs. This indicates that TiO2 NPs are a crucial part of the system to start the on-demand NP degradation. For the functionalized CS-NPs, the intensities were related somehow to the dye removal pattern described previously in the dye removal section. As we mentioned previously, the higher the dye removal, the higher the TiO2-NPs are attached to CS-NPs. Therefore, it is expected that the amount of Rhodamine released will be higher as the amount of TiO2-NPs that is attached to CS-NPs increases.
Figure 5.
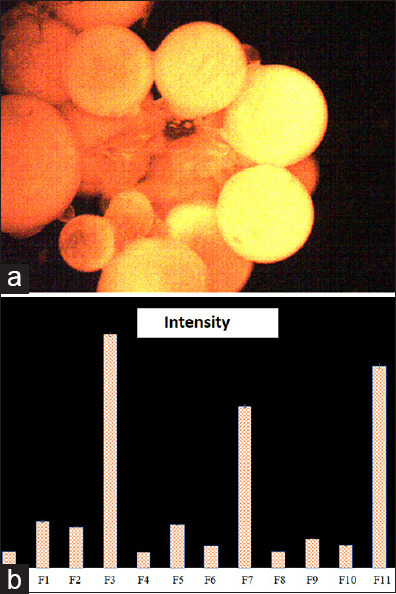
(a) Rhodamine released from F5 after illustration with ultraviolet light for 30 min (b) The intensity of Rhodamine released from the formulations
CONCLUSION
In this study, CS-NPs were prepared and functionalized with TiO2-NPs to allow on-demand degradation of CS-NPs using UV irradiation as a trigger. Monodispersed nanoparticulate systems were prepared successfully with sizes and charges related to the size of CS-NPs and the amount of TiO2-NPs used in the preparation. Further, the sizes of the NPs and the amount of TiO2-NPs used have a great effect on NPs collapse upon irradiated with UV light. Moreover, the deposition of TiO2-NPs increases as the size of CS-NPs decreases and as the amount of TiO2-NPs used in the formulation increases. Finally, the fluorescence intensity test showed a significant difference in the release of Rhodamine as the functionalized and unfunctionalized CS-NPs were irradiated with UV light. Therefore, we can conclude that the chemical deposition of TiO2-NPs on PNPs could allow on-demand remotely controlled drug release that depends on therapy and patient circumstances.
Financial support and sponsorship
The deanship of research at Jordan University of Science and Technology: proposal number 20200495.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors are thankful to the deanship of research at Jordan University of Science and Technology for the generous fund in the proposal number of 20200495.
REFERENCES
- 1.Kumari A, Yadav SK, Yadav SC. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf B Biointerfaces. 2010;75:1–18. doi: 10.1016/j.colsurfb.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 2.Palanikumar L, Al-Hosani S, Kalmouni M, Nguyen VP, Ali L, Pasricha R, et al. pH-responsive high stability polymeric nanoparticles for targeted delivery of anticancer therapeutics. Commun Biol. 2020;3:95. doi: 10.1038/s42003-020-0817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Nemrawi NK, Alshraiedeh NH, Zayed AL, Altaani BM. Low molecular weight Chitosan-Coated PLGA nanoparticles for pulmonary delivery of tobramycin for cystic fibrosis. Pharmaceuticals (Basel) 2018;11:E28. doi: 10.3390/ph11010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Nemrawi NK, Alsharif SS, Alzoubi KH, Alkhatib RQ. Preparation and characterization of insulin chitosan-nanoparticles loaded in buccal films. Pharm Dev Technol. 2019;24:967–74. doi: 10.1080/10837450.2019.1619183. [DOI] [PubMed] [Google Scholar]
- 5.Tran P, Lee SE, Kim DH, Pyo YC, Park JS. Recent advances of nanotechnology for the delivery of anticancer drugs for breast cancer treatment. J Pharm Investig. 2020;50:261–70. [Google Scholar]
- 6.Al-Nemrawi NK, Alsharif SS, Dave RH. Preparation of chitosan-TPP nanoparticles: The influence of chitosan polymeric properties and formulation variables. Int J Appl Pharm. 2018;10:60–5. [Google Scholar]
- 7.Moses MA, Brem H, Langer R. Advancing the field of drug delivery: Taking aim at cancer. Cancer Cell. 2003;4:337–41. doi: 10.1016/s1535-6108(03)00276-9. [DOI] [PubMed] [Google Scholar]
- 8.Alvarez-Lorenzo C, Bromberg L, Concheiro A. Light-sensitive intelligent drug delivery systems. Photochem Photobiol. 2009;85:848–60. doi: 10.1111/j.1751-1097.2008.00530.x. [DOI] [PubMed] [Google Scholar]
- 9.Al-Nemrawi NK, Marques J, Tavares CJ, Oweis RJ, Al-Fandi MG. Synthesis and characterization of photocatalytic polyurethane and poly(methyl methacrylate) microcapsules for the controlled release of methotrexate. Drug Dev Ind Pharm. 2018;44:2083–8. doi: 10.1080/03639045.2018.1513023. [DOI] [PubMed] [Google Scholar]
- 10.Çeşmeli S, Avci CB. Application of titanium dioxide (TiO2) nanoparticles in cancer therapies. J Drug Target. 2019;27:762–6. doi: 10.1080/1061186X.2018.1527338. [DOI] [PubMed] [Google Scholar]
- 11.Ziental D, Czarczynska-Goslinska B, Mlynarczyk DT, Glowacka-Sobotta A, Stanisz B, Goslinski T, et al. Titanium dioxide nanoparticles: Prospects and applications in medicine. Nanomaterials (Basel) 2020;10:E387. doi: 10.3390/nano10020387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salehi R, Arami M, Mahmoodi NM, Bahrami H, Khorramfar S. Novel biocompatible composite (chitosan-zinc oxide nanoparticle): Preparation, characterization and dye adsorption properties. Colloids Surf B Biointerfaces. 2010;80:86–93. doi: 10.1016/j.colsurfb.2010.05.039. [DOI] [PubMed] [Google Scholar]
- 13.Lawrie G, Keen I, Drew B, Chandler-Temple A, Rintoul L, Fredericks P, et al. Interactions between alginate and chitosan biopolymers characterized using FTIR and XPS. Biomacromolecules. 2007;8:2533–41. doi: 10.1021/bm070014y. [DOI] [PubMed] [Google Scholar]
- 14.Nandiyanto AB, Oktiani R, Ragadhita R. How to read and interpret FTIR spectroscope of organic material. Indones J Sci Technol. 2019;4:97–118. [Google Scholar]
- 15.Arsalani N, Nezhad-Mokhtari P, Jabbari E. Microwave-assisted and one-step synthesis of PEG passivated fluorescent carbon dots from gelatin as an efficient nanocarrier for methotrexate delivery. Artif Cells Nanomed Biotechnol. 2019;47:540–7. doi: 10.1080/21691401.2018.1562460. [DOI] [PubMed] [Google Scholar]
- 16.Balarak D, Khatibi AD, Chandrika K. Antibiotics removal from aqueous solution and pharmaceutical wastewater by adsorption process: A review. Int J Pharm Investig. 2020;10:106–11. [Google Scholar]


