Abstract
Objective:
It is of interest to investigate the anti-proliferative effect of β-sitosterol (BS) on human hepatocellular carcinoma (HepG2) cell line.
Materials and Methods:
β-sitosterol treatments (0.6 and 1.2 mM/ml) were done in HepG2 and after 24 hr, cell viability was evaluated by MTT assay. Reactive oxygen species (ROS) accumulating potential of BS was assessed by dichloro-dihydro-fluorescein diacetate staining. Morphology related to apoptosis was investigated by acridine orange and ethidium bromide dual staining. Cytochrome c and caspase 3 expressions were evaluated by immunofluorescence and western blot analyses.
Results:
β-sitosterol induced cytotoxicity (p<0.001) and intracellular ROS in HepG2 cells in a dose-dependent manner. BS treatments accumulated induced intracellular ROS accumulation which led to membrane damage and mitochondrial toxicity. At the molecular level, BS treatments induced cytochrome c release from mitochondria and enhanced the protein expressions (p<0.05 vs 0.6 mM/ml and p<0.001 vs 1.2 mM/ml) of both caspase 3 and cleaved caspase 3.
Conclusion:
β-sitosterol induced ROS accumulation which plays a critical role in apoptosis via the intrinsic pathway in HepG2 cells. The present investigation paves the way for further in vivo studies.
Key Words: Liver cancer, β-sitosterol, Reactive oxygen species, Apoptosis, Caspase
Introduction
Natural products such as phytochemical compounds from medicinal plants are one of the best sources of drugs and many chemotherapeutic drugs existing today are plant-derived compounds (Ezhilarasan, 2018 ▶; Rayan et al., 2017 ▶). Phytosterols are structurally similar to cholesterol and are specific phytochemicals found only in plants. However, phytosterols have an extra hydrocarbon chain at the C-24 position which differs from cholesterol (Zaloga, 2015 ▶). β-sitosterol (24a-ethylcholesterol), stigmasterol (D22, 24a-ethylcholesterol), and (campesterol (24a-methylcholesterol) (Bacchetti et al., 2011 ▶) are main plant sterols that have beneficial roles in a variety of chronic diseases including cardio vascular disorders (Jones and AbuMweis, 2009 ▶), diabetes (Misawa et al., 2012 ▶), and cancer (Suttiarporn et al., 2015 ▶). It has been reported that intake of phytosterols-rich diets can reduce the cancer risk by 20% (Ramprasath and Awad, 2015 ▶). Therefore, evaluation of anticancer potential of these phytosterols merits further study. β-sitosterol (BS) is a well-studied plant-derived sterol known for its beneficial effects against liver diseases (Devaraj et al., 2020 ▶). Several experimental studies have shown anticancer potentials of BS (Choi et al., 2003 ▶; Baskar et al., 2012 ▶; Sharmila and Sindhu, 2017 ▶). Particularly in vitro, the anti-proliferative effect of BS has been reported against human colon (HT116) (Choi et al., 2003 ▶), lung (A549) (Rajavel et al., 2018 ▶), gastric adenocarcinoma (Shin et al., 2018 ▶), prostate (PC-3) (Awad et al., 2001 ▶), and breast (MCF-7) cancer cell lines (Awad et al., 2008 ▶). Among phytosterols, BS has been shown to enhance the effectiveness of standard chemotherapeutic agents (Awad et al., 2008 ▶; Cao et al., 2019 ▶).
Hepatocellular carcinoma (HCC) ranks third in causing cancer-related mortality responsible for 600,000 annual deaths globally (Siegel et al., 2016 ▶; Jiang et al., 2017 ▶). Chronic hepatitis B and C virus infections, chronic alcohol consumption, consumption of aflatoxin-contaminated food, certain metabolic liver diseases, and cirrhosis are considered primary risk factors of HCC (Suh et al., 2018). Current cancer treatments include surgical removal and radiotherapy, followed by systemic chemotherapy used for maintenance treatment (Ezhilarasan, 2018 ▶; Gheena and Ezhilarasan, 2019 ▶). The major drawbacks of chemotherapy are recurrence, drug resistance, and severe off-target side effects that limit the use of chemotherapeutic drugs in cancer patients (Solai Prakash and Devaraj E, 2019). Despite significant therapeutic advancements, to date, HCC remains most aggressive cancer type responsible for significant mortality rate worldwide (Hartke et al., 2017 ▶; Shebi et al., 2018 ▶). On the other hand, in a recent systematic meta-analysis, low dietary phytosterol intake has been correlated with high cancer risk (Jiang et al., 2019 ▶). Phytosterols dietary intervention effectively control cancer progression and reduces its risk (Alvarez-Sala et al., 2019 ▶). Therefore, this study investigates the cytotoxic potentials of BS against human HCC (HepG2) cell line.
Materials and Methods
Chemicals
The cell cultures reagents such as Dulbecco’s minimum essential low glucose medium (DMEM), dimethyl sulfoxide (DMSO), antibiotics (penicillin and streptomycin), fetal bovine serum (FBS), tryspin-EDTA, and 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) were purchased from GIBCO-BRL (Gaithersburg, MD). β-sitosterol was commercially procured from Sigma chemical (Chennai, India).
Cell culture and maintenance
The HepG2 cells were obtained from The NCCS, Pune, India. The DMEM medium containing 10% of FBS, penicillin and streptomycin was used for cell culture. Cells were maintained in a standard culture condition at 37°C with 5% CO2. After reaching enough confluence, 0.25% trypsin-EDTA was added to detach cells and cells were seeded for the treatment. After maintaining couple of passages, cells were used for treatments. The experiments were done once cells reached 70-80% confluence.
MTT assay
Cytotoxicity inducing potential of BS was done by MTT assay (Ponselvi Induja et al., 2018 ▶). After cell counting, 1×104 cell suspension was added to a 96-well plate. After cells adherence for 24 hr, the existing medium was changed to 100 µl of medium with or without BS (0.2, 0.4, 0.6, 0.8 and 1 mM/ml) and left for 24 hr. BS was dissolved in 0.1% DMSO and hence, it was served as internal control. After 24 hr, cells were washed once, 50 μl (0.5 mg/ml in PBS) of MTT was added to wells and cells were incubated at 37°C for 4 hr in CO2 incubator. After incubation, purple formazan product was dissolved by adding DMSO and the intensity of formazan product was measured by spectrophotometer (Biotek, India).
Reactive oxygen species (ROS) level analysis by dichloro-dihydro-fluorescein diacetate (DCFH-DA) staining
BS-induced ROS accumulation in HepG2 cells was observed by a non-fluorescent probe DCFH-DA (Gheena and Ezhilarasan, 2019 ▶). After treatment, cells were detached by trypsin treatment. After counting, 8×106 cells/well was incubated with DCFH-DA (10 µM) at room temperature for 30 min. After incubation, the cells were viewed instantly under an inverted microscope (Nikon Instruments Inc., NY, USA).
Acridine orange/ethidium bromide (AO/EB) staining and fluorescent microscopy
AO/EB staining was performed according to Lakshmi et al. (2017). HepG2 cells were seeded at a concentration of 1×104 in 48-well plates. After treatments with 0.1% DMSO and BS for 24 hr, cells were collected and used for AO/EB staining. Then, 100 μl of AO/EB (1:1) dyes was added to 100 μl of cell suspension, mixed well and investigated instantaneously under a fluorescence microscope (Nikon, Ti series, Japan). Cells from each samples were counted in different fields and the population of apoptotic cells percentage was calculated.
Immunofluorescence analysis of cytochrome c
In a 12-well plate, 5x104 cells were treated with different BS concentrations and incubated for 24 hr. After washing thrice with PBS, and the cells were fixed in 4% formaldehyde exactly for 10-15 min. Cells were rehydrated, blocked with 5% normal goat serum, permeabilized using 0.5% Tween 20 and then incubated with monoclonal cytochrome C primary antibody (ab13575), followed by probing with goat anti-mouse IgG (ab150115) secondary antibody for 2 hr. Then, images were captured using Nikon Eclipse Ti inverted fluorescence microscope (Nikon Instruments Inc., NY, USA).
Western blot analysis of caspase 3
After the treatment, control and BS treated cells were washed with PBS and were collected in radioimmunoprecipitation assay buffer (Sigma Aldrich, USA) and the lysed cells were mixed with sodium dodecyl sulfate (SDS) containing loading buffer (Thermo Scientific, USA). Then, it was incubated at 95oC for 5 min. After protein quantification, 50 µg of each samples was subjected to SDS-polyacrylamide gel electrophoresis (10%) for 90 min. The gel was then transferred onto polyvinylidene difluoride membranes and electro-transferred membrane was blocked with skimmed milk powder for 2 hr at room temperature. The membrane was incubated with anti-cleaved (activated) caspase-3 antibodies (monoclonal, IgG1, 1:100, Cell signaling technology, #9669, USA) overnight at 4oC. After incubation, membranes were incubated with corresponding secondary antibodies conjugated with horseradish peroxidase (1:2000) for 2 hr at room temperature. The membranes were developed by Pierce enhanced chemiluminescence plus western blotting substrate (Thermo Scientific, USA).
Statistical analysis
Values are presented as mean±S.E.M. (n=3) and were subjected to one-way ANOVA and Dunnett’s multiple comparison test was done to determine the statistical differences among groups. A p<0.05 was considered significant (Graph Pad prism 7.0. CA, USA).
Results
Cytotoxic potential of β-sitosterol in HepG2 cells
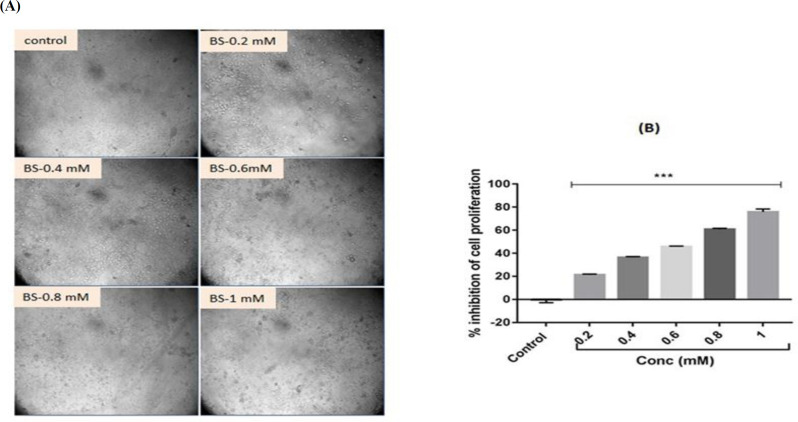
To investigate the cytotoxic potential of BS in HepG2 cells, we performed MTT assay. Firstly, HepG2 cells were exposed to various concentrations of BS (0.2, 0.4, 0.8 and 1 mM/ml) for 24 hr and their cytotoxicity was assessed using MTT assay. The control and BS-treated cells morphology is depicted in Figure 1A. BS treatments significantly (p<0.001) induced dose-dependent cytotoxicity in HepG2 cells. The maximum cytotoxicity was evidenced at 1 mM/ml (Figure 1B). The IC50 value of BS for in HepG2 cells was 0.6 mM/ml at 24 hr. Therefore, further studies were carried out at concentrations of 0.6 and 1.2 mM/ml.
Figure 1.
β-sitosterol (BS)-induced changes in the proliferation of HepG2 cells. A. Morphology of BS-treated HepG2 cells. B. Cytotoxicity analysis by MTT assay. n=3. ***p<0.001 vs control
β-sitosterol treatments induced intracellular ROS accumulation in HepG2 cells
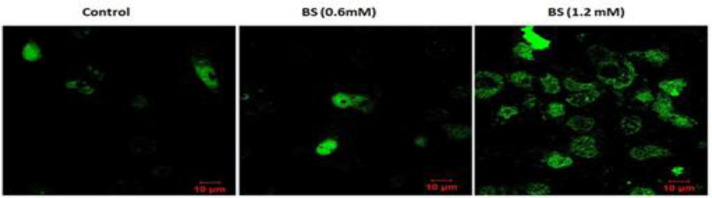
Further, to find out whether BS-induced cytotoxicity was due to the accumulation intracellular ROS, we analyzed ROS by DCFH-DA assay. β-sitosterol at two different concentrations (0.6 and 1.2 mM/ml) for 24 hr, induced ROS expression. The ROS expression was not prominent at low concentration of BS treatment (i.e. 0.6 mM/ml) however, at high concentration, BS treatment prominently increased ROS expression suggesting that a high concentration of BS is required to induce ROS in HepG2 cells (Figure 2).
Figure 2.
Reactive oxygen species inducing potentials of β-sitosterol (BS) in HepG2 cells as assessed by 2', 7'-dichlorodihydrofluorescein diacetate staining
β-sitosterol treatments induced apoptosis-related morphological changes
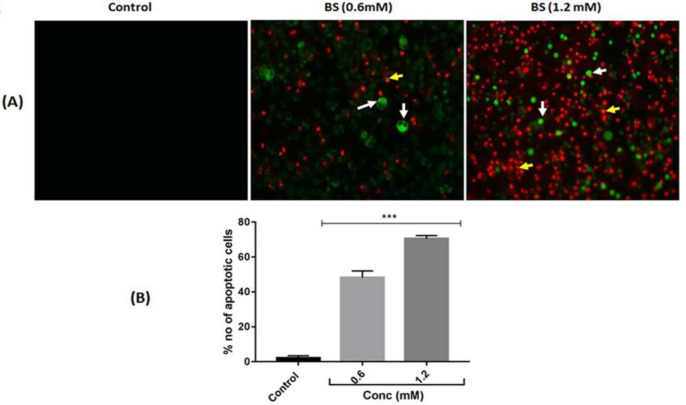
β-sitosterol treated HepG2 cells were exposed to AO/EB dual staining. The dye AO enters the nucleus and stains live cells as intense green color. While EB penetrates the nuclei of dead or late apoptotic cells due to membrane damage and stains as red color. In this study, early apoptotic cell appeared as greenish yellow colored nuclei with condensed or fragmented chromatin and late apoptotic cells nuclei appeared as red color with highly condensed or fragmented chromatin (Figure 3A). BS
Figure 3.
(A) Morphological analysis of apoptosis by acridine orange/ethidium bromide dual staining. (B). Quantification of early and late apoptotic cells. n=3. ***p<0.001 vs control
treatments dose-dependently (p<0.001) increased the number of apoptotic cells as compared to control (Figure 3B).
β-sitosterol-induced cytochrome c dislocation
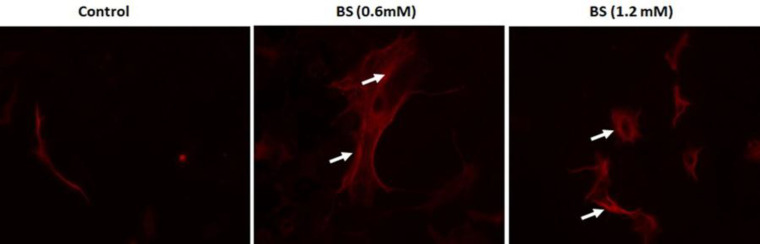
β-sitosterol-treated HepG2 cells were analyzed for cytochrome c expression as its
cytosolic expression plays a significant role in the intrinsic mitochondrial pathway. Cytochrome c was prominently expressed in BS-treated cells as compared to control cells. The maximum expression was noticed for 1.2 mM/ml of BS (Figure 4).
Figure 4.
Immunofluorescence analysis of cytochrome c dislocation in control and β-sitosterol (BS)-treated HepG2 cells
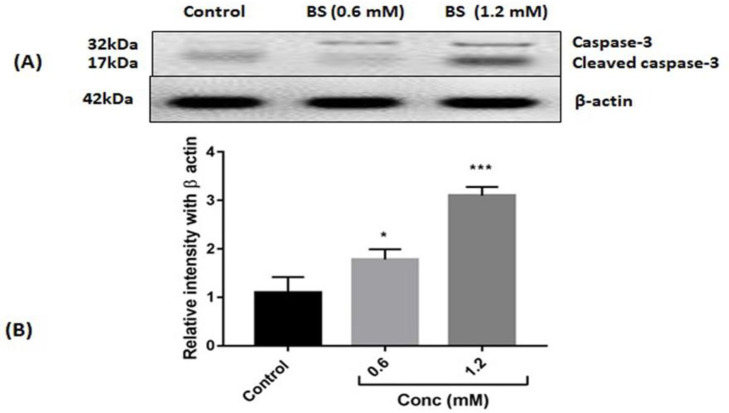
β-sitosterol-induced caspase 3 and cleaved caspase expression
Further to confirm the downstream target of cytochrome c, we analyzed caspase 3 expressions in BS-treated HepG2 cells. BS treated cells were significantly expressed cleaved caspase-3 and caspase-3. There was a prominent expression of both caspase 3 and cleaved caspase 3 in HepG2 cells treated with the high concentration of BS as compared to control (Figure 5A). Quantitative analysis by densitometry confirmed a significant increase of caspase expression p<0.05 vs 0.6 mM/ml and p<0.001 vs 1.2 mM/ml upon BS treatment in HepG2 cells (Figure 5B).
Figure 5.
(A) Western blot expression of caspase 3 and its cleaved fraction. (B). Quantification of caspase protein expression by densitometry analysis. n=3. ***p<0.001 vs control
Discussion
β-sitosterol and its oxy-derivatives have been reported to offer protection against cancer via induction of apoptosis, cytotoxicity, and cell cycle arrest and inhibition of adhesion metastasis, angiogenesis, and cell invasion (Ramprasath and Awad, 2015 ▶; Baskar et al., 2012 ▶; Li et al., 2016 ▶; Raj et al., 2020). In adjuvant therapy, the efficacy of gemcitabine, a standard chemotherapeutic agent was evidently increased when it was combined with BS (Cao et al., 2019 ▶). In previous studies, BS treatments inhibited the proliferation of lung, prostate, breast, gastric and colon cancer cell lines (Awad et al., 2001 ▶; Choi et al., 2003 ▶; Awad et al., 2008 ▶; Shin et al., 2018 ▶). Consistent with above studies, in this study, BS treatments dose-dependently caused cytotoxicity in HepG2 cells and these results suggest that regardless of cancer cell line, BS induces cytotoxicity.
Indeed, phytocompounds are capable of inducing ROS in cancer cells as compared to normal cells. Mitochondria act as both source and target for intracellular ROS. ROS can induce dissipation of mitochondrial membrane potential and cytochrome c release and it is one of the early events in apoptosis (Rajavel et al., 2018 ▶; Ezhilarasan et al., 2019 ▶; Gheena and Ezhilarasan, 2019 ▶; Rohit and Ezhilarasan, 2019 ▶). Therefore, we analyzed ROS expression to investigate whether oxidative stress was responsible for cytotoxicity induced by BS. The present study showed that BS could induce intracellular ROS level in HepG2 cells. In previous studies, BS treatments were shown to effectively induce ROS-mediated cytotoxicity in various cancer cell lines (Baskar et al., 2010 ▶; Kazłowska et al., 2013 ▶; Yang et al., 2013 ▶; Rajavel et al., 2018 ▶) and therefore, in light of these studies, the ROS inducing potential of BS could be hold accountable for its cytotoxic potential in HepG2 cells.
Phytocompounds selectively target cancer cells as they have a different stress response compared to normal cells. Phytocompounds have selective pro-oxidant effects on cancer cells (Chirumbolo et al., 2018 ▶). For instance, BS from Grewia tiliaefolia induced cytotoxicity only in human lung cancer cell lines (A549, MCF-7, PC3 and L-132) but not in normal human lung (L132) and peripheral blood mononuclear cells (Rajavel et al., 2017 ▶). Phytoderived cytotoxic drugs like paclitaxel, vincristine, vinblastine, and podophyllotoxin analogues are currently in clinical use against a variety of cancers (Hosseini and Ghorbani, 2015 ▶). The intracellular ROS accumulation is one of the direct causes of apoptosis induction and morphological damage induced by plant-derived chemotherapeutic drugs (Ezhilarasan et al., 2019 ▶; Rohit and Ezhilarasan, 2019 ▶) and therefore, we studied the BS-induced morphological changes related to apoptosis by AO/EB staining. In this study, BS-induced apoptotic cell death in HepG2 cells. AO/EB staining is a gold standard technique to detect cells undergoing apoptosis (Kasibhatla et al., 2006 ▶; Liu et al., 2015 ▶; Shebi et al., 2019 ▶). AO stain only enters live cells as they have intact cell membrane and emit intense green fluorescence in nuclei. EB enters cells which lost cytoplasmic membrane and intercalate with DNA thus, nuclei appear red. Early apoptotic cells with fragmented chromatin will have bright yellowish-green nuclei (Kasibhatla et al., 2006 ▶). Nuclear fragmented and chromatin condensed nuclei appear as bright green patches and late apoptotic cells show orange to red nuclei (Byczkowska et al., 2013 ▶). Consistent with previous studies, in this study, BS-treated cells showed red-colored nuclei which indicate cells with DNA damage which are in the late apoptotic phase. The appearance of intense red-colored nuclei after treatment with BS 1.2 mM/ml attributed to plasma membrane blebbing, and chromatin and nuclear condensation. These changes strongly indicated that BS-treated cells were undergoing apoptosis.
Apoptosis plays a crucial role in elimination of unwanted cells and maintaining homeostasis (Pfeffer and Singh, 2018 ▶; Li et al., 2019 ▶). Previous studies showed ROS-mediated apoptosis-inducing potential of phytochemicals in cancer cells (Ezhilarasan, 2018 ▶; Hong et al., 2019 ▶). Particularly, mitochondria are one of the main target of intracellular ROS and are also responsible for the generation of ROS intracellularly (Yang et al., 2016 ▶). The cytochrome c release from mitochondria is a key and early intracellular event during the mitochondrial apoptotic pathway (Jan and Chaudhry, 2019 ▶). In the cytosol, cytochrome c combines with Apaf–1 and procaspase-9 and form apoptosome. Then apoptosome subsequently activates caspase 9 and -3 signaling cascade to trigger apoptosis (Jan and Chaudhry, 2019 ▶). In the present investigation, BS caused an enhanced cytosolic expression of cytochrome c and caspase 3 and this could contribute to apoptosis due to the mitochondrial dysfunction and increased mitochondrial membrane permeability.
In summary, our current results suggest that ROS production by BS plays a crucial role in apoptosis induction via intrinsic pathway in HepG2 cells. Therefore, these findings suggest that BS may be useful as an adjuvant drug in cancer chemotherapy for liver cancer patients. However, future in vivo studies and clinical trials are warranted to explore the detailed molecular mechanisms responsible for ROS generation and growth inhibitory effects of BS in liver cancer.
Conflicts of interest
The authors have declared that there is no conflict of interest.
References
- Alvarez-Sala A, Attanzio A, Tesoriere L, Garcia-Llatas G, Barberá R, Cilla A. Apoptotic effect of a phytosterol-ingredient and its main phytosterol (β-sitosterol) in human cancer cell lines. Int J Food Sci Nutr. 2019;70:323–334. doi: 10.1080/09637486.2018.1511689. [DOI] [PubMed] [Google Scholar]
- Solai Prakash AK, Devaraj E. Cytotoxic potentials of S cumini methanolic seed kernel extract in human hepatoma HepG2 cells. Environ Toxicol. 2019;34:1313–1319. doi: 10.1002/tox.22832. [DOI] [PubMed] [Google Scholar]
- Awad AB, Barta SL, Fink CS, Bradford PG. Beta-sitosterol enhances tamoxifen effectiveness on breast cancer cells by affecting ceramide metabolism. Mol Nutr Food Res. 2008;52:419–426. doi: 10.1002/mnfr.200700222. [DOI] [PubMed] [Google Scholar]
- Awad AB, Fink CS, Williams H, Kim U. In vitro and in vivo (SCID mice) effects of phytosterols on the growth and dissemination of human prostate cancer PC-3 cells. Eur J Cancer Prev. 2001;10:507–513. doi: 10.1097/00008469-200112000-00005. [DOI] [PubMed] [Google Scholar]
- Bacchetti T, Masciangelo S, Bicchiega V, Bertoli E, Ferretti G. Phytosterols, phytostanols and their esters: from natural to functional foods. Med J Nutrition Metab. 2011;4:165–172. [Google Scholar]
- Baskar AA, Al Numair KS, Gabriel Paulraj M, Alsaif MA, Muamar MA, Ignacimuthu S. β-sitosterol prevents lipid peroxidation and improves antioxidant status and histoarchitecture in rats with 1,2-dimethylhydrazine-induced colon cancer. J Med Food. 2012;15:335–343. doi: 10.1089/jmf.2011.1780. [DOI] [PubMed] [Google Scholar]
- Baskar AA, Ignacimuthu S, Paulraj GM, Al Numair KS. Chemopreventive potential of beta-Sitosterol in experimental colon cancer model-an in vitro and In vivo study. BMC Complement Altern Med. 2010;10:24. doi: 10.1186/1472-6882-10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byczkowska A, Kunikowska A, Kaźmierczak A. Determination of ACC-induced cell-programmed death in roots of Vicia faba ssp minor seedlings by acridine orange and ethidium bromide staining. Protoplasma. 2013;250:121–128. doi: 10.1007/s00709-012-0383-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao ZQ, Wang XX, Lu L, Xu JW, Li XB, Zhang GR, Ma ZJ, Shi AC, Wang Y, Song YJ. β-sitosterol and gemcitabine exhibit synergistic anti-pancreatic cancer activity by modulating apoptosis and inhibiting epithelial-mesenchymal transition by deactivating Akt/GSK-3β signaling. Front Pharmacol. 2019;9 doi: 10.3389/fphar.2018.01525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirumbolo S, Bjørklund G, Lysiuk R, Vella A, Lenchyk L, Upyr T. Targeting cancer with phytochemicals via their fine tuning of the cell survival signaling pathways. Int J Mol Sci. 2018;19:3568. doi: 10.3390/ijms19113568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YH, Kong KR, Kim YA, Jung KO, Kil JH, Rhee SH, Park KY. Induction of Bax and activation of caspases during beta-sitosterol-mediated apoptosis in human colon cancer cells. Int J Oncol. 2003;23:1657–1662. [PubMed] [Google Scholar]
- Devaraj E, Roy A, Royapuram Veeraragavan G, Magesh A, Varikalam Sleeba A, Arivarasu L, Marimuthu Parasuraman B. β-Sitosterol attenuates carbon tetrachloride-induced oxidative stress and chronic liver injury in rats. Naunyn Schmiedebergs Arch Pharmacol. 2020;393:1067–1075. doi: 10.1007/s00210-020-01810-8. [DOI] [PubMed] [Google Scholar]
- Ezhilarasan D, Apoorva VS, Ashok Vardhan N. Syzygium cumini extract induced reactive oxygen species-mediated apoptosis in human oral squamous carcinoma cells. J Oral Pathol Med. 2019;48:115–121. doi: 10.1111/jop.12806. [DOI] [PubMed] [Google Scholar]
- Ezhilarasan D. Prakash Srinivasan, Timiri Shanmugam. Understanding Cancer Therapies: CRC Press; 2018. Herbal therapy for cancer; pp. 129–166. [Google Scholar]
- Gheena S, Ezhilarasan D. Syringic acid triggers reactive oxygen species-mediated cytotoxicity in HepG2 cells. Hum Exp Toxicol. 2019;38:694–702. doi: 10.1177/0960327119839173. [DOI] [PubMed] [Google Scholar]
- Hartke J, Johnson M, Ghabril M. The diagnosis and treatment of hepatocellular carcinoma. Semin Diagn Pathol. 2017;34:153–159. doi: 10.1053/j.semdp.2016.12.011. [DOI] [PubMed] [Google Scholar]
- Hong SH, Cha HJ, Hwang-Bo H, Kim MY, Kim SY, Ji SY, Cheong J, Park C, Lee H, Kim GY, Moon SK, Yun SJ, Chang YC, Kim WJ, Choi YH. Anti-proliferative and pro-apoptotic effects of licochalcone A through ROS-mediated cell cycle arrest and apoptosis in human bladder cancer cells. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20153820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini A, Ghorbani A. Cancer therapy with phytochemicals: evidence from clinical studies. Avicenna J Phytomed. 2015;5:84–97. [PMC free article] [PubMed] [Google Scholar]
- Jan R, Chaudhry GE. Understanding apoptosis and apoptotic pathways targeted cancer therapeutics. Adv Pharm Bull. 2019;9:205–218. doi: 10.15171/apb.2019.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang JF, Lao YC, Yuan BH, Yin J, Liu X, Chen L, Zhong JH. Treatment of hepatocellular carcinoma with portal vein tumor thrombus: advances and challenges. Oncotarget. 2017;8:33911–33921. doi: 10.18632/oncotarget.15411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Zhao X, Xu J, Li C, Yu Y, Wang W, Zhu L. The protective effect of dietary Phytosterols on cancer risk: A systematic meta-analysis. J Oncol. 2019;2019:7479518. doi: 10.1155/2019/7479518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PJ, AbuMweis SS. Phytosterols as functional food ingredients: linkages to cardiovascular disease and cancer. Curr Opin Clin Nutr Metab Care. 2009;12:147–151. doi: 10.1097/mco.0b013e328326770f. [DOI] [PubMed] [Google Scholar]
- Kasibhatla S, Amarante-Mendes GP, Finucane D, Brunner T, Bossy-Wetzel E, Green DR. Acridine orange/ethidium bromide (AO/EB) staining to detect apoptosis. CSH Protoc. 2006;2006:pdb. doi: 10.1101/pdb.prot4493. [DOI] [PubMed] [Google Scholar]
- Kazłowska K, Lin HT, Chang SH, Tsai GJ. In vitro and in vivo anticancer effects of sterol fraction from red algae porphyra dentata. Evid Based Complement Alternat Med. 2013;2013:493869. doi: 10.1155/2013/493869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshmi T, Ezhilarasan D, Vijayaragavan R, Bhullar SK, Rajendran R. Acacia catechu ethanolic bark extract induces apoptosis in human oral squamous carcinoma cells. J Adv Pharm Technol Res. 2017;8:143–149. doi: 10.4103/japtr.JAPTR_73_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Jiang Z, Chai W, Xu Y, Wang Y. Autophagy activation alleviates nonylphenol-induced apoptosis in cultured cortical neurons. Neurochem Int. 2019;122:73–84. doi: 10.1016/j.neuint.2018.11.009. [DOI] [PubMed] [Google Scholar]
- Li X, Wu Q, Bu M, Hu L, Du WW, Jiao C, Pan H, Sdiri M, Wu N, Xie Y, Yang BB. Ergosterol peroxide activates Foxo3-mediated cell death signaling by inhibiting AKT and c-Myc in human hepatocellular carcinoma cells. Oncotarget. 2016;7:33948–33959. doi: 10.18632/oncotarget.8608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K, Liu PC, Liu R, Wu X. Dual AO/EB staining to detect apoptosis in osteosarcoma cells compared with flow cytometry. Med Sci Monit Basic Res. 2015;21:15–20. doi: 10.12659/MSMBR.893327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misawa E, Tanaka M, Nomaguchi K, Nabeshima K, Yamada M, Toida T, Iwatsuki K. Oral ingestion of aloe vera phytosterols alters hepatic gene expression profiles and ameliorates obesity-associated metabolic disorders in zucker diabetic fatty rats. J Agric Food Chem. 2012;60:2799–2806. doi: 10.1021/jf204465j. [DOI] [PubMed] [Google Scholar]
- Pfeffer CM, Singh ATK. Apoptosis: A target for anticancer therapy. Int J Mol Sci. 2018;19:E448. doi: 10.3390/ijms19020448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PonselviInduja M, Ezhilarasan D, Ashok Vardhan N. Evolvulus alsinoides methanolic extract triggers apoptosis in HepG2 cells. Avicenna J Phytomed. 2018;8:504–512. [PMC free article] [PubMed] [Google Scholar]
- Kathiswar R, Ezhilarasan D, Rajeshkumar S. β-Sitosterol-assisted silver nanoparticles activates Nrf2 and triggers mitochondrial apoptosis via oxidative stress in human hepatocellular cancer cell line. J Biomed Mater Res A. 2020;108:1899–1908. doi: 10.1002/jbm.a.36953. [DOI] [PubMed] [Google Scholar]
- Rajavel T, Packiyaraj P, Suryanarayanan V, Singh SK, Ruckmani K, Pandima Devi K. β-sitosterol targets Trx/Trx1 reductase to induce apoptosis in A549 cells via ROS mediated mitochondrial dysregulation and p53 activation. Sci Rep. 2018;8:2071. doi: 10.1038/s41598-018-20311-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajavel T, Mohankumar R, Archunan G, Ruckmani K, Devi KP. Beta sitosterol and Daucosterol (phytosterols identified in Grewia tiliaefolia) perturbs cell cycle and induces apoptotic cell death in A549 cells. Sci Rep. 2017;7:3418. doi: 10.1038/s41598-017-03511-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramprasath VR, Awad AB. Role of phytosterols in cancer prevention and treatment. J AOAC Int. 2015;98:735–738. doi: 10.5740/jaoacint.SGERamprasath. [DOI] [PubMed] [Google Scholar]
- Rayan A, Raiyn J, Falah M. Nature is the best source of anticancer drugs: Indexing natural products for their anticancer bioactivity. PLoS One. 2017;12:e0187925. doi: 10.1371/journal.pone.0187925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohit Singh T, Ezhilarasan D. Ethanolic extract of Lagerstroemia Speciosa (L ) Pers induces apoptosis and cell cycle arrest in HepG2 cells. Nutr Cancer. 2019;72:146–156. doi: 10.1080/01635581.2019.1616780. [DOI] [PubMed] [Google Scholar]
- Sharmila R, Sindhu G. Modulation of angiogenesis, proliferative response and apoptosis by BS in rat model of renal carcinogenesis. Indian J Clin Biochem. 2017;32:142–152. doi: 10.1007/s12291-016-0583-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shebi S, Ezhilarasan D, Thomas J, Chandrasekaran N, Mukherjee A. Gracilaria foliifera (Forssk ) Børgesen ethanolic extract triggers apoptosis via activation of p53 expression in HepG2 cells. Phcog Mag. 2019;15:259–263. [Google Scholar]
- Shin EJ, Choi HK, Sung MJ, Park JH, Chung MY, Chung S, Hwang JT. Anti-tumour effects of beta-sitosterol are mediated by AMPK/PTEN/HSP90 axis in AGS human gastric adenocarcinoma cells and xenograft mouse models. Biochem Pharmacol. 2018;152:60–70. doi: 10.1016/j.bcp.2018.03.010. [DOI] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- Suh JK, Lee J, Lee JH, Shin S, Tchoe HJ, Kwon JW. Risk factors for developing liver cancer in people with and without liver disease. PLoS One. 2018;13:e0206374. doi: 10.1371/journal.pone.0206374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suttiarporn P, Chumpolsri W, Mahatheeranont S, Luangkamin S, Teepsawang S, Leardkamolkarn V. Structures of phytosterols and triterpenoids with potential anti-cancer activity in bran of black non-glutinous rice. Nutrients. 2015;7:1672–1687. doi: 10.3390/nu7031672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Chen ZY, Wong SL, Liu J, Liang YT, Lau CW, Lee HK, Huang Y, Tsang SY. β-Sitosterol oxidation products attenuate vasorelaxation by increasing reactive oxygen species and cyclooxygenase-2. Cardiovasc Res. 2013;97:520–532. doi: 10.1093/cvr/cvs370. [DOI] [PubMed] [Google Scholar]
- Yang Y, Karakhanova S, Hartwig W, D'Haese JG, Philippov PP, Werner J, Bazhin AV. Mitochondria and mitochondrial ROS in cancer: Novel targets for anticancer therapy. J Cell Physiol. 2016;231:2570–2581. doi: 10.1002/jcp.25349. [DOI] [PubMed] [Google Scholar]
- Zaloga GP. Phytosterols, lipid administration, and liver disease during parenteral nutrition. JPEN J Parenter Enteral Nutr. 2015;39:39S–60S. doi: 10.1177/0148607115595978. [DOI] [PubMed] [Google Scholar]