Abstract
This review focuses on the most recent advances in the understanding of the electrolyte transport-related mechanisms important for the development of severe inherited renal disorders, autosomal dominant (AD) and recessive (AR) forms of polycystic kidney disease (PKD). We provide here a basic overview of the origins and clinical aspects of ARPKD and ADPKD and discuss the implications of electrolyte transport in cystogenesis. Special attention is devoted to intracellular calcium handling by the cystic cells, with a focus on polycystins and fibrocystin, as well as other calcium level regulators, such as transient receptor potential vanilloid type 4 (TRPV4) channels, ciliary machinery, and purinergic receptor remodeling. Sodium transport is reviewed with a focus on the epithelial sodium channel (ENaC), and the role of chloride-dependent fluid secretion in cystic fluid accumulation is discussed. In addition, we highlight the emerging promising concepts in the field, such as potassium transport, and suggest some new avenues for research related to electrolyte handling.
Keywords: Electrolyte transport, Ion channels, Kidney, Polycystic Kidney Disease, purinergic signaling, Renal Physiology
Introduction
Polycystic kidney disease (PKD) is a group of inherited nephropathies characterized by the formation of fluid-filled sacs along the nephron. There are two main forms of PKD—autosomal dominant (ADPKD) and autosomal recessive (ARPKD). ADPKD is a multifactorial condition characterized by cysts that can originate from any nephron segment; they usually form in the distal nephron and the collecting duct (CD) [1]. ARPKD is typically characterized with dilated renal tubules and is accompanied by hepatic fibrosis [2–4]. ADPKD is most common in adults, while ARPKD is a more rare and severe form of PKD that manifests in early childhood or even perinatally [5,6]. Figure 1 shows the schematic representation of the cystogenesis in ADPKD and ARPKD. Despite the growing body of literature, availability of an FDA-approved medication (tolvaptan, for ADPKD), and relatively successful completed and ongoing clinical trials, many critical questions regarding the pathogenesis and molecular mechanisms of these diseases remain to be answered. Here we summarize most recent data regarding the involvement of various ion channels and transporters in the regulation of ADPKD and ARPKD cystogenesis. For further information on a plethora of other topics important for PKD but not discussed herein, we refer the readers to an excellent collection of review articles in a special issue of Cellular Signalling edited by Dr. Albert Ong and Dr. Vicente Torres [7].
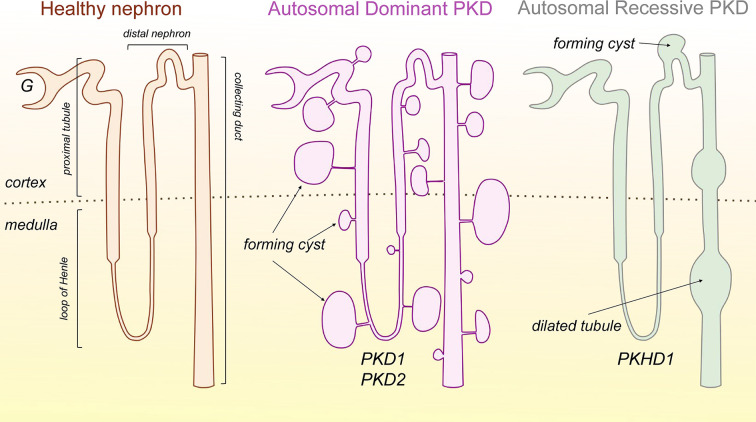
Figure 1. Schematic representation of renal cysts morphology in ADPKD and ARPKD.
A healthy nephron with major segments is shown on the left. In ADPKD (middle image), cysts can form in any nephron segments (including glomeruli); however, they are primarily located in distal regions and the CD. The cysts grow and eventually detach from the rest of the tubule but continue to accumulate fluid. In ARPKD (right image), cysts are formed in the distal nephron, mainly the CDs, and are presented by dilated tubules, which remain connected with the rest of the nephron. Abbreviation: G, glomerulus; CD, collecting duct.
Genetic impetuses for ADPKD and ARPKD: polycystins and fibrocystin
ADPKD
ADPKD most commonly stems from mutations in PKD1 or PKD2, genes which encode for polycystin 1 and polycystin 2 (PC1 and PC2, respectively, also known as transient receptor potential cation channel, subfamily P, members 1 and 2 (TRPP1 and TRPP2)), which are expressed in primary cilia and plasma membrane of renal epithelial cells. Mutations in either of these two genes have been reported in ∼93% of ADPKD patients (PKD1 mutations account for ∼78% and PKD2 mutations account for ∼15% of ADPKD cases) [8]. Interestingly, in ∼7% of ADPKD cases, neither PKD1 nor PKD2 mutations were found [9], which could potentially be explained by mutations in other important genes that have not been identified yet. For instance, recently, ADPKD arising from mutations in the glucosidase II α subunit (GANAB) gene was reported [10–12]. Over 20 years ago, a ‘two-hit’ ADPKD model was proposed to explain why only a small number of the cells in each nephron become cystic. The ‘two-hit’ model implies that the ‘first hit’ is a germline mutation of PKD1 and/or PKD2, while the ‘second hit’ is a somatic mutation in the other allele of the gene in individual epithelial cells [13].
ARPKD
ARPKD results from mutations in PKHD1 (polycystic kidney and hepatic disease 1), which encodes for fibrocystin (FPC, or polyductin). FPC is found in the primary cilia, ciliary basal body, and plasma membrane of renal epithelial cells [14]. In a recent study by Ziegler et al., Pkhd1 silencing was reported to disrupt epithelial morphogenesis (shape and polarization) and cell adhesion in a 3D cell culture model of MDCKII (Madin–Darby Canine Kidney) cells [15]. Pkdh1 knockdown in MDCK line resulted in increased cell invasion through three-dimensional extracellular matrices [14]. These data confirm that loss of FPC contributes to the progression of ARPKD.
Chloride transport in ADPKD
cAMP-centric view of the chloride transport
Current understanding of Cl−-dependent fluid secretion in the accumulation of cyst fluid in ADPKD is based on the classical works performed by Dr. Jared J. Grantham and colleagues in the 1990s. Initially, it was found that in the primary cell culture isolated from cysts of patients with ADPKD, Cl− transport stimulates the secretion of fluid into ADPKD cysts [16]. Subsequently, it was shown that fluid secretion is regulated by 3′,5′-cyclic adenosine monophosphate (cAMP)-dependent Cl− secretion [17]. Grantham et al. established that elevated cAMP constitutes the driving force in the progression of ADPKD cystogenesis [18,19]. Therefore, various regulators of cAMP accumulation, and their effects on fluid secretion, were studied (for example, adenylyl cyclases (ACs), phosphodiesterases (PDEs), hormones, etc) [17,20–23]. The comprehensive overview of the seminal works of Grantham and co-authors is presented in the recent review by Jouret and Devuyst [24].
cAMP is a key player in the process of cystogenesis, which contributes to an increase in the proliferative activity of epithelial cells and fluid secretion [25]. The accumulation of cAMP is regulated by different isoforms of ACs. In ADPKD, increased expression of AC5 and AC6 in the primary cilia was observed to be involved in the regulation of cAMP levels [26,27]. For example, Wang et al. demonstrated that AC5 plays an important role in the up-regulation of cAMP and cyst growth in Pkd2-mutant mice [26], although AC5 knockout did not completely restore cAMP levels to normal in the Pkd2-deletion model. Much attention has been recently devoted to the study of AC6, suggesting that it can be a key mediator of cyst formation [28]. Rees et al. demonstrated that CD-specific deletion of AC6 decreased kidney size and reduced cystogenesis in Pkd1-mutant mice [28]. Other important regulators of the intracellular cAMP level are PDEs: cAMP is hydrolyzed by Ca2+/Calmodulin-dependent PDE1. Inhibition of PDE1 causes an increase in the proliferation of ADPKD cells [29]. In zebrafish, a member of PDE1 subfamily, PDE1A, was demonstrated to be involved in kidney development and cystogenesis, and a functional interaction between PC2 and PDE1A has been suggested [30]. Ye et al. showed that not only PDE1, but also PDE3 can modulate ADPKD development, presumably through the compartmentalization of cAMP pools [31]. Numerous studies have put forward PDE1 as a possible therapeutic target for ADPKD [30,32].
Cystic fibrosis transmembrane conductance regulator
Overall, Cl− transport is clearly involved in both ADPKD and ARPKD progression, as Cl− can move via paracellular and transcellular pathways in the CDs. Cl− is driven from the cell across the apical membrane due to its electrochemical gradient via a Cl− channel—namely, the cystic fibrosis transmembrane conductance regulator, or CFTR (see Table 1). CFTR, a member of ATP-binding cassette family of transporters, is a cAMP-dependent Cl− channel expressed at the apical surface of polarized epithelia including kidney, lung, intestine, pancreas, and other organs [33]. Dysfunction of CFTR channel affects anion secretion that eventually alters water homeostasis and plays a critical role in the pathogenesis of cystic fibrosis, despite the absence of significant changes in kidney function in patients with cystic fibrosis [34].
Table 1. The expression and function of ion channels in various ARPKD and ADPKD models vs healthy renal epithelia.
| ADPKD or ARPKD | Model/species | Effector/force | Action/effect | Changes in expression level of mRNA/protein for the channel/transporter of interest | Reference |
|---|---|---|---|---|---|
| ENaC | |||||
| ADPKD | Pkd1-knockout mice/mouse | VX-809, a corrector of CFTR | VX-809 ↓cyst growth | ↓ αENaC protein reduced in Pkd1-null mice; VX-809 restored αENaC apical membrane localization | [39] |
| M1 renal monolayers/mouse | P2X7 (αβmeATP) | P2X7 stimulation ↓ ENaC activity | N/A | [122] | |
| ARPKD | Immortalized cells of ARPKD renal cysts/human | ↑ amiloride-sensitive Na+ absorption compared with control cells | ↑ αENaC both mRNA and protein vs control cells | [133] | |
| PCK rat kidney; primary cultured PCK CD cells/rat | Mislocalization of E3 ubiquitin-protein ligase Nedd 4-2 | ↑ Amiloride-sensitive Na+ reabsorption vs control rats | ↑ α, β, γ ENaC protein vs control rats | [133] | |
| Cilium-deficient orpk CCD principal cell monolayers/mouse | Tg737orpk mutation | ↑ Amiloride and benzamil-sensitive ENaC-mediated Na+ absorption vs control mouse | N/A | [134] | |
| CD principal cells from Bpk ARPKD mice/mouse | N/A | ↓ Amiloride-sensitive ENaC-mediated Na+ absorption vs normal mice | αENaC mRNA detected, changes vs normal mice not tested | [135] | |
| Primary monolayer cultures of cystic CD principal cells/mouse | Mislocalization of apical EGF receptors; EGF | ↓ Na+ transport | ↓ αENaC mRNA vs non-cystic cells | [136] | |
| PCK rats/rat | Salt-deficient diet (SD) | ↑ cyst growth, ↑ miR-9a-5p in SD diet-fed group vs normal and high salt-fed groups | ↑ α, β, γ ENaC mRNA, ↓ αENaC protein in SD diet fed group vs normal and high salt-fed groups | [130] | |
| ARPKD cysts from PCK rats/rat | Benzamil | ↓ ENaC activity; ↑ cyst growth in 4- or 12-week benzamil-treated PCK rats | ↓ β-ENaC protein | [129] | |
| PCK rats/rat | P2x7 receptor knockout | ↓ ENaC activity; P2rx7 knockout ↑ENaC activity, ↓cyst growth vs littermate PCK rats | N/A | [123] | |
| Pannexin-1 | |||||
| ADPKD | Pkd1 knockout mice (iKsp-Pkd1−/−)/mouse | Fluid shear stress (FSS) | ↑FSS-sensitive ATP release | ↑mRNA expression | [124] |
| Pkd1−/− distal convoluted tubule 15 (mDCT15) cells/mouse | FSS; Pannexin-1 inhibition by BB-FCF or knockout | ↑FSS-sensitive ATP release; Pannexin-1 inhibition (by BB-FCF) or knockout ↓FSS sensitive ATP release | ↑mRNA expression | [124] | |
| zebrafish pkd2 morphants (pkd2-MO) | BB-FCF (Pannexin-1 inhibitor) | Pannexin-1 inhibition attenuated cyst growth | N/A | [124] | |
| Pkd1RC/RC mouse kidneys/mouse; M1 renal monolayers/mouse | 50 μM probenecid (PANX1 blocker) | ↑ cyst growth; probenecid treatment of M-1 cells ↓shear-stress-stimulated ATP release | ↑protein | [122] | |
| CFTR | |||||
| ADPKD | ADPKD kidneys and primary cultured ADPKD cells/human | N/A | N/A | mRNA; protein, apical localization detected in cystic cells | [38] |
| CD-derived mCCD-N21 cells/mouse | Forskolin, CPT-cAMP, a membrane-permeable cAMP analog (cAMP-elevating agents); NPPB, 50–100 μM or CFTRinh172, 10–20 μM (CFTR Inhibitors) | ↑tubule enlargement and cyst formation; NPPB ↓ tubule enlargement and cyst formation, in part inhibited aldosterone- and/or vasopressin-induced Isc | mRNA detected | [51] | |
| Primary cultures of ADPKD cells/human | Forskolin; CPT-cAMP, a membrane-permeable cAMP analog; glibenclamide, 200 μM; DPC, 500 μM (CFTR channel inhibitors) | ↑ activity of CFTR channel; glibenclamide, DPC inhibit Cl− currents | protein, apical membrane localization detected | [35] | |
| MDCK cell model/dog embryonic kidney cyst model (Pkd1 knockout)/mouse | Thiazolidinone tetrazolo-CFTRinh172, glycine hydrazide Ph-GlyH-101 (CFTR inhibitors), IC50 > 3 μM | ↓ 8-Br-cAMP-induced cyst number and growth | N/A | [44] | |
| Embryonic kidney organ culture model of PKD/mouse | Pyrimido-pyrrolo-quinoxalinedione PPQ-102, IC50 ∼ 90 nM | ↓ 8-Br-cAMP-induced cyst number and growth | N/A | [43] | |
| PKD1−/− metanephric kidneys; embryonic kidneys with 8-Br-cAMP-induced cysts/mouse | Thiazolidinone CFTRinh172, 100 μM | ↓ cyst formation, Cftr−/− genotype completely blocked cyst formation in Pkd−/− kidneys | N/A | [52] | |
| PKD1-knockout mice/mouse | VX-809, a corrector of CFTR | VX-809 increases CFTR at the apical membrane, but most strongly at the basolateral membrane | Protein, apical membrane localization detected | [39] | |
| Principal-like (pl)MDCK cyst model/canine | Pkd1-knockout cells; CFTRinh172, 10 μM (CFTR inhibitor) | ↑Cl− secretion, cyst size; ↓ cyst growth in the presence of forskolin by inhibition of CFTR | ↑Protein | [40] | |
| MDCK cyst model/canine | Knockdown of CFTR, CFTRinh172, 10 μM | ↓ cyst growth | N/A | [50] | |
| ARPKD | BPK ARPKD mice/mouse | CFTR knockout | The loss of CFTR does not alter the course of ARPKD cystic disease | N/A | [44] |
| Pkhd1del4/del4 cholangiocytes/mouse | VX-809 (CFTR corrector), heat shock proteins: ↑ HSP27 or ↓HSP70 or HSP90 | ↑ forskolin-induced cyst growth, altered colocalization of CFTR with both apical and basolateral membranes; VX-809 and HSPs ↓cyst growth and restored CFTR localization toward normal values | ↓ protein | [43] | |
| TMEM16A (anoctamin 1) | |||||
| ADPKD | Principal-like MDCK cyst model/dog; ADPKD tissue/human; Metanephric kidney cyst model/mouse | Forskolin (adenylyl cyclase agonist), UTP, ATP; tannic acid AO1 (selective inhibitor of anoctamin 1) Knockdown of anoctamin 1 | UTP ↑ Cl− secretion; AO1 or knockdown of anoctamin 1 ↓ Ca2+-dependent Cl− secretion; in metanephric kidney: knockdown and inhibitors ↓ forkolin-induced cyst growth | Forskolin ↑protein in the apical membrane, mRNA detected | [47] |
| Renal CD principle cells from dog (MDCK)/canine and M1/mouse | Knockdown of Pkd1 or Pkd2 ATP, UTP | ↑ intracellular Ca2+ ([Ca2+]i) signals, purinergic Ca2+ release from ER, Cl− secretion and cyst cell proliferation | ↑mRNA, ↑protein | [47] | |
| Principal-like MDCK cyst model/dog | Pkd1-knockout cells; niclosamide (TMEM16A inhibitor) | ↑Cl− secretion, [Ca2+]i and cyst cell proliferation; ↓ cyst growth in the presence of niclosamide | ↑protein | [40] | |
| Pkd1 knockout mice/mouse | Niclosamide benzbromarone Ani9 (TMEM16A specific inhibitor) | ↓ cyst growth and cyst cell proliferation | ↑protein | [40] | |
| Pkd1/Tmem16a double knockout mice/mouse | Pkd1/Tmem16a double knockout | ↓ cyst growth and cyst cell proliferation | ↓protein | [40] | |
| Tissue samples from patients with ADPKD/human, embryonic PKD1−/− kidney cultures/mouse, MDCK cyst model/dog | ROS, peroxidation of plasma membrane phospholipids (e.g. tert-butyl hydroperoxide (tBHP)), ATP, UTP; scavengers of ROS glutathione, coenzyme Q10; ferrostatin-1; idebenone, AO1; knockdown of T16A | ↑activity of TMEM16A, cyst growth, Ca2+ signaling, Ca2+-sensitive adenylate cyclase ADCY1; scavengers of ROS delay cyst enlargement, ↓activation of TMEM16A | ↑protein | [50] | |
| Primary renal epithelial cells isolated from Pkd1 knockout mice/mouse | Gender | Basal [Ca2+]i higher in males compared with females, ↑basal and ATP-stimulated Cl− currents, ↑ cell proliferation and cyst development in male kidneys | Protein level not different between male and female | [126] | |
| NKCC1 | |||||
| ADPKD | ADPKD kidneys and primary cultured ADPKD cells/human | N/A | N/A | mRNA detected; protein-basolateral localization (in same cells where CFTR is also expressed) | [38] |
| CD-derived mCCD-N21 cells/mouse | Bumetanide or ethacrynic acid (NKCC1 inhibitors) | ↓ AVP- and aldosterone-stimulated short-circuit current, tubular enlargement and cyst formation | Aldosterone ↑ mRNA expression, forskolin didn't change mRNA level | [51] | |
| PKD1−/− metanephric kidneys, Embryonic kidneys with cAMP-induced cysts/mouse | Bumetanide | ↓ Cyst formation | Protein detected | [52] | |
| Kir 6.2 (KCNJ11) | |||||
| ADPKD | Cells derived from the cysts of patients with ADPKD/human | Glibenclamide, 25–100 μM (inhibitor of ATP-sensitive K+ channels and CFTR) | Glibenclamide modestly decreased forskolin-stimulated current at the apical membrane. Its basolateral application ↓Isc to a greater extent. ↓ cyst growth and proliferation | mRNA detected | [138] |
| Ca2+-dependent KCa3.1 (SK4, KCNN4) | |||||
| ADPKD | MDCK/dog; kidney cells derived from patients with ADPKD/human | Forskolin, DCEBIO (KCa3.1 activator); TRAM-34, overexpression of myotubularin-related protein-6 (specific KCa3.1 blockers) | ↑anion secretion, KCa3.1 channel activity, in vitro cyst growth; ↓anion secretion, in vitro cyst formation and enlargement | mRNA detected | [139] |
| Principal-like MDCK cyst model/canine | Clotrimazole (SK4 inhibitor) | ↓ ATP, UTP-induced Cl− secretion | N/A | [47] | |
| TRPV4 | |||||
| ADPKD | Primary ADPKD cells/human | Flow | ↓TRPV4 activity, basal [Ca2+]i, loss of flow-induced [Ca2+]i signaling compared with normal kidney (NK) cells | Protein expression did not change vs NK cells; TRPV4 glycosylation was reduced in ADPKD cells | [62] |
| ARPKD | CD cysts derived from PCK rats/rat | Flow; GSK1016790A (selective TRPV4 activator) | ↓TRPV4 activity, basal [Ca2+]i, loss of flow-induced [Ca2+]i signaling compared with normal kidney cells; GSK1016790A restored mechanosensitive Ca2+ signaling and TRPV4 function | ↓Protein, TRPV4 glycosylation, subcellular TRPV4 localization is shifted toward the apical membrane; GSK1016790A restored subcellular TRPV4 distribution | [64] |
Abbreviations: ENaC, epithelial Na+ channel; FSS, fluid shear stress; HSP, heat shock protein; KCNJ11, ATP-sensitive K+ channel, Kir 6.2; NKCC1, Na+-K+-2Cl− cotransporter; PANX1, Pannexin-1; TMEM16A, transmembrane member 16A; TRPV4, transient receptor potential vanilloid type 4.
CFTR in ADPKD
In ADPKD, cAMP level is increased, which activates apical CFTR and subsequently drives Cl−-dependent fluid secretion (Figure 2 and Table 1), creating a major pathway responsible for cystogenesis [21,24,35–37]. The existence of CFTR channels was reported in the cyst-lining ADPKD epithelial cells [35]; functionally active forskolin- or cAMP-stimulated Cl− currents were recorded there using patch-clamp technique. The apical expression of CFTR was further confirmed in the ADPKD kidney cysts from mice, humans, and dogs [35,38–40]. Small molecule CFTR inhibitors of the thiazolidinone, glycine hydrazide and quinoxalinedione chemical classes, have been shown to exhibit anti-secretory effects in animal models of ADPKD [41–44]. CFTR inhibitors were able to prevent ADPKD cyst growth and reduced the size of preformed cysts [41,43,44]. These compounds have lower IC50 (from nM to μM) compared with known non-selective CFTR blockers like glibenclamide, diphenylamine-2- carboxylate (DPC), and 5-nitro-2- (3-phenylpropylamino) benzoate (NPPB) with IC50 up to 100 μM [41–44]. To date, only short-term effects of CFTR inhibitors and modulators have been studied in vitro and, in more rare cases, in vivo in PKD models. To achieve therapeutic benefits, targeted and sufficient accumulation of the inhibitor in the kidneys along with low toxicity during long-term use are needed. In addition, extended use of CFTR inhibitors may induce lung or pancreatic pathology [41]. In summary, there is a lot of potential for establishing new CFTR-based approaches to the treatment of ADPKD, however, more research efforts are needed to develop pharmacology that is more effective and less toxic in vivo.
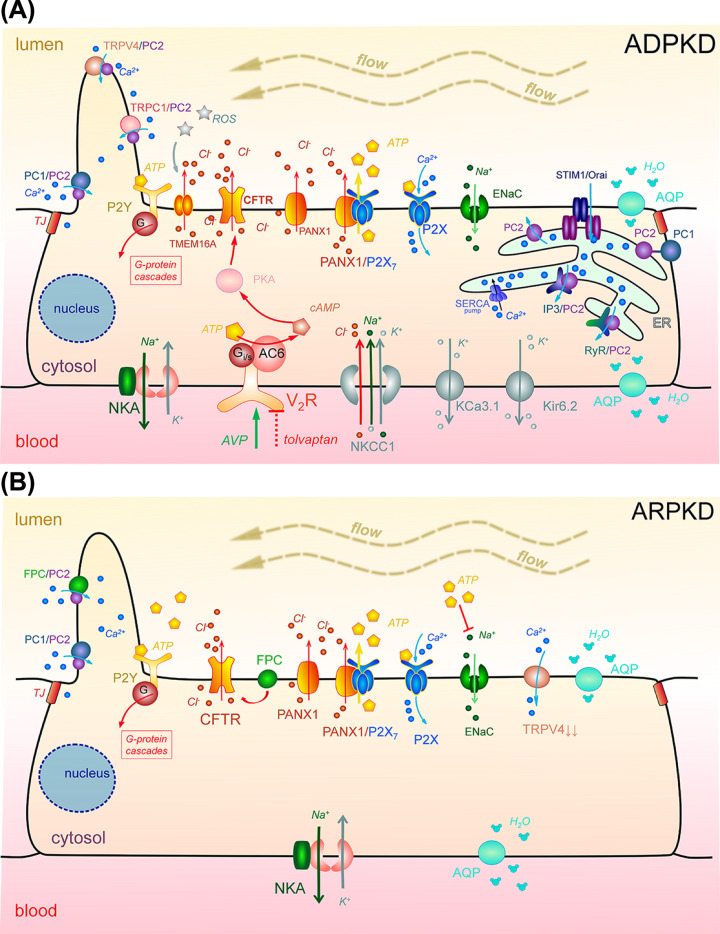
Figure 2. Major determinants of epithelial transport involved in the development of PKDs.
(A) In ADPKD: Loss of PC1 and/or PC2 function as well as abnormal activities of other Ca2+-permeable ion channels (e.g. PC1/PC2, TRPC1/PC2, TRPV4/PC2, IP3/PC2, RyR/PC2 complexes in the primary cilium or the ER) alter intracellular Ca2+ influx, which in turn can increase intracellular cAMP level. cAMP production can also be stimulated by binding of AVP (vasopressin) to its V2R receptors. Increased cytosolic concentration of cAMP activates apical CFTR (via PKA) and subsequently drives transepithelial fluid secretion, which is supported by the basolateral cotransporter NKCC1. The recycling of K+ for Na+-K+-ATPase and NKCC1 activity is provided by basolateral K+ channels (Kir6.2, KCa3.1). Remodeling of purinergic signaling (a shift from P2Y to P2X) may be another pathological mechanism driving cystogenesis and fluid accumulation. There is an abnormally high ATP level in the cyst; source is still debated, for instance, PANX1 may form a high conductance ATP-releasing channel with P2X7. High ATP level can inhibit ENaC-dependent reabsorption, and can also cause further imbalance in Ca2+ signaling by affecting metabotropic P2Y or ionotropic P2X receptors. Increased activity of the Ca2+-sensitive TMEM16A Cl− channel can be induced by high ATP level. Tolvaptan, the first drug which was shown to slow ADPKD progression (FDA-approved in 2018), is a selective antagonist of vasopressin receptor 2. (B) In ARPKD: abnormally high level of ATP may inhibit ENaC-dependent reabsorption (ENaC activity is still being debated in ARPKD models), and can also cause further Ca2+ signaling imbalance by affecting metabotropic P2Y or ionotropic P2X receptors. FPC and PC2 may form a complex in the plasma membrane and/or primary cilium and regulate Ca2+ response; dysfunction of FPC can lead to altered CFTR activity. TRPV4 activity was shown to be reduced in ARPKD animal model. The transcellular water transport in cystic epithelium can be mediated by water channels, aquaporins. Abbreviations: AC6, adenylyl cyclase 6; AQP, aquaporin; ATP, adenosine triphosphate; ENaC, epithelial Na+ channel; ER, endoplasmic reticulum; IP3, inositol 1,4,5-trisphosphate receptor; NKA, Na+/K+ ATPase; NKCC1, Na+-K+-2Cl− cotransporter; PANX1, Pannexin-1; PC1, polycystin 1; PC2, polycystin 2; PKA, protein kinase A; RyR, ryanodine receptor; TJ, tight junction; TRPC1, transient receptor potential canonical 1; TRPV4, transient receptor potential vanilloid type 4 channel; V2R, vasopressin receptor 2.
CFTR and ARPKD
Studies devoted to the role of CFTR in ARPKD are less extensive than in ADPKD. On the one hand, data on the bpk mouse model of ARPKD indicate that the loss of CFTR function (bpk−/−; cftr+/−) might not be required for the development and progression of this disease [45]. On the other hand, mice with a double knockout (bpk−/−; cftr−/−) exhibited greatly enlarged kidneys and died earlier than cystic control mice. In the recent work on Pkhd1del4/del4 cholangiocytes from ARPKD mice, which display progressive liver and pancreatic cyst formation, CFTR was found to be down-regulated [46]. Forskolin-induced cyst growth was reduced in these cells by CFTR Corrector VX-809, as well as by heat shock proteins (HSPs; by increasing HSP27 or reducing HSP90 or HSP70).
Overall, the data suggest fundamental differences in the mechanisms of anion secretion in ARPKD and ADPKD. The information about the expression of CFTR and action of different agonists or antagonists (inhibitors) on its function and cyst development in various PKD models is presented in Table 1.
Other regulators of Cl− transport in ADPKD
Along with the CFTR, Ca2+-activated TMEM16A (Transmembrane Member 16A, anoctamin 1) Cl− channels were shown to contribute to cyst enlargement in ADPKD (see Table 1; [40,47,48]). TMEM16A is activated by intracellular Ca2+ and mediates fundamental physiological processes such as regulation of Cl− secretion, neuronal and cardiac excitation, smooth muscle contraction, and sensory transduction [49]. Knockdown of either Pkd1 or Pkd2 in M1 CD cells caused an increase in the expression of TMEM16A, which enhanced intracellular Ca2+ release, cell proliferation, and Cl− secretion [40]. Pkd1/Tmem16a double knockout led to reduced cyst growth and cystic renal epithelial cell proliferation [40] and confirmed the involvement of TMEM16A channel in Cl− secretion in ADPKD cysts. The activation of TMEM16A was also shown following peroxidation of plasma membrane phospholipids in human and mouse kidney samples and in MDCK cells [50]. Several reactive oxygen species (ROS) scavengers and antioxidants tested (e.g., ferrostatin-1, glutathione, coenzyme Q10) were able to inhibit TMEM16A, thereby reducing fluid secretion and development of MDCK-derived cysts; moreover, the authors suggested that the epithelial CFTR-dependent Cl− transport may be regulated by TMEM16A [50].
Na+-K+-2Cl− cotransporter (NKCC1), which is widely distributed in different types of epithelial and non-epithelial cells, is an Na+-dependent Cl− transporter that was reported to be expressed in ADPKD cysts and cultured ADPKD cells, where it is responsible for basolateral Cl− entry (see Figure 2 and Table 1) [38]. NKCC1-positive ADPKD cysts also express CFTR, suggesting a regulatory pathway of Cl− secretion similar to secretory epithelia [38]. NKCC1 inhibitors (bumetanide or ethacrynic acid) and CFTR inhibitors (NPPB or CFTR inhibitor-172) were both shown to prevent cyst formation in a mouse CD cell line (mCCD-N21) [51]. In addition, Magenheimer et al. showed that CFTR inhibition or NKCC1 blockage/genetic deletion prevented cyst formation in Pkd1−/− metanephric kidneys [52].
The transcellular Cl− pathway is also mediated by Cl− exchangers such as Cl−/HCO3− and Cl−/OH−. Na-independent Cl−/HCO3− exchanger (pendrin, SLC26A4, PDS) is localized in type B cells of the cortical CDs, where it mediates Cl− absorption and secretion of HCO3− [53,54]. Despite the diversity of the transcellular pathway of Cl− secretion in PKD, fluid accumulation is mainly provided by cAMP-dependent Cl− secretion [36]. In some models, pendrin has been shown to act together with the Na-dependent Cl−/HCO3− exchanger (NDCBE) to regulate NaCl absorption; it is also known to regulate aldosterone-induced Na+ reabsorption by changing epithelial Na+ channel (ENaC) abundance and function [55]. Grantham et al. investigated the effect of chemical inhibitors on the primary and secondary transport of active solutes in epithelial cysts in vitro, particularly, it has been demonstrated that L-645,695 (NDCBE inhibitor) attenuates MDCK cyst growth [56]. There is currently insufficient evidence to support the claim that disturbance in transcellular Cl− transport can lead to or aggravate ADPKD, although it is an interesting and relatively unexplored target in PKD.
The paracellular Cl− transport provides reabsorption of approximately 70% of Cl− [57]. Paracellular movement of Cl− occurs through tight junctions, that contain the claudin proteins (CLDNs) [58]. Tight junction composition was shown to be altered in the epithelium of polycystic kidneys: CLDNs normally expressed in the CD (3, 4, 7, 8, and 10) were localized in small cysts, while only CLDN7 persisted at substantive levels in the dedifferentiated epithelium of large, likely late-stage cysts, where it was found both at the TJ and basolaterally [59]. Sassi et al. showed that CLDN8 interacts with the γ subunit of ENaC to regulate paracellular Na+ permeability in the renal CD, which allows to speculate regarding the involvement of this interaction in cystic disease [60]. Therefore, it is possible that CLDNs may contribute to the pathogenesis of ADPKD, however, very little is known about the mechanisms that might be involved in their regulation in the disease state, or whether pharmacological targeting of these proteins could be beneficial for attenuating cystogenesis.
Regulation of calcium handling in PKD: polycystins, cilia, calcium channels, transporters, and purinergic signaling-dependent calcium entry
Ca2+ levels in ADPKD and ARPKD
Ca2+ signaling and homeostasis are crucial determinants of cellular function and survival. Disruption of Ca2+ homeostasis has been directly linked to cystogenesis, however, there are controversial data about how dysregulation of the intracellular Ca2+ level ([Ca2+]i) is associated with cyst formation in ADPKD and ARPKD. It was reported that reduction in intracellular Ca2+ level affects the growth of ADPKD cystic cells (M-1 cells stably transfected with the C-tail of polycystin-1): calcium restriction in these cells can activate the B-Raf/ERK pathway, switching M1 cells to a phenotype that is growth-stimulated by cAMP [25]. A sustained increase in [Ca2+]i (achieved with either Bay K8644, a Ca2+ channel activator, or A23187, a Ca2+ ionophore) was shown to restore the normal anti-mitogenic response to cAMP in primary cultures of cyst-lining epithelial cells from both ADPKD and ARPKD kidneys [61]. Yamaguchi et al. [61] showed that steady-state [Ca2+]i levels are 20 nM lower in cyst-derived ADPKD cells (57 ± 2 nM) compared with normal human kidney cells (77 ± 2 nM). These findings are similar to the results of Tomilin et al., also obtained in ADPKD [62]. On the other hand, in ARPKD, using cystic monolayers freshly isolated from PCK rats, Palygin et al. demonstrated that basal concentration of Ca2+ is ∼120 nM, while Zaika et al. reported a lower (∼90 nM) level in the same model [63,64]. The question of calcium handling and levels in the cystic cell in ADPKD and ARPKD remains open, and further studies in different models are needed to draw overarching conclusions.
Polycystins and Ca2+ handling in ADPKD
Ca2+ dysregulation in ADPKD is largely mediated by polycystin 1 and polycystin 2 (PC1 and PC2), proteins expressed in the cilia and plasma membrane of the tubular cells [65]. PC1 is a transmembrane protein belonging to the transient receptor potential (TRP) family that consists of 11 transmembrane domains, a cytosolic C-terminus, and a long extracellular N-terminus. Its C-terminus contains a site for binding and activation of the heterotrimeric G proteins [66], which allows initiation and regulation of G protein-mediated signaling pathways [67]. PC2 is a Ca2+ permeable non-selective channel that also belongs to the TRP family. PC2 contains six transmembrane segments with cytosolic C- and N-termini [68]; it is involved in maintaining Ca2+ homeostasis and regulates a plethora of signaling pathways [61,69], and functions independently or as a part of the PC1/PC2 complex [70,71]. Importantly, PC2 is required for the processing and delivery of PC1 to the cell membrane [72]. Some research groups reported that PC1 can function as a channel by itself [73], however this statement is controversial. The dominant hypothesis postulates that PC1 and PC2 form functional heterotrimeric complexes (1:3, respectively) through the interaction of their coiled-coil domains [70,74–76]; in addition, PC2 can also form homotetramers [77]. It is known that a homomeric channel formed by PC2 alone is permeable to monovalent (Na+, Cs+, K+) and divalent cations (Ba2+, Mg2+, Ca2+). Importantly, PC2 is more permeable for divalent cations, and is characterized by high conductance for Ca2+ [78,79]. Furthermore, PC2 was shown to be capable of conducting larger organic cations (tetrabutylammonium, tetrapentylammonium, tetraethylammonium) [80].
A number of studies demonstrated that PC1 is necessary for the functioning of the PC1/PC2 complex [25,70,76]. However, Su et al. reported that PC1 may act as a dominant-negative subunit of PC2 due to the presence of three positively charged residues in the pore region of PC1/PC2, preventing any currents from passing through the complex [74]. Mutations in the pore regions of PC1 and PC2 significantly affect the ionic selectivity of the PC1/PC2 heteromeric and PC2 homomeric complexes, which is widely discussed in the literature [81–83]. Importantly, the formation of polycystin ion-conducting complexes does not exclude separate interactions of polycystins with other molecular partners [84,85]. Interestingly, it was suggested that the amount of PC1 and PC2 should be within a certain tight range to prevent cystogenesis. Yao et al. recently demonstrated that Pkd1-mutant mouse tissues exhibit increased expression of PC2, which is maintained by a new signaling axis involving histone deacetylase HDAC6 and GRP94 (a member of the HSP90 chaperone family) [86].
In addition to the PC2’s own function as a cation channel, it also can modulate Ca2+ signaling via interactions with other channels, including the TRP channel family (for instance, transient receptor potential canonical 1 (TRPC1), TRPV4), inositol 1,4,5-triphosphate receptor (IP3R) and ryanodine receptor (RyR), in the endoplasmic reticulum (ER) and on the plasma membrane [37,87]. For instance, the interaction of PC2 with IP3R happens in the ER. When IP3 receptor is activated, Ca2+ concentration in the ER is decreased, which is sensed by stromal interaction molecule 1 (STIM1). STIM1 then activates a Ca2+ release-activated Ca2+ channel modulator protein 1 (Orai1) in the plasma membrane, and likely other Ca2+ channels. Ca2+, which is entered in the cytosol, then returns to ER via the sarco/ER Ca2+-ATPase (SERCA) Ca2+ pump [88]. In their recent work, using PC1-null proximal tubule cells and an inducible PC1 knockout mouse model, Yanda et al. showed that high levels of STIM1 and IP3R play a role in stimulation of cyst growth, cAMP level increase and a release of Ca2+ from the ER [89]. It has also been hypothesized that PC1 can activate PC2, leading to entry of Ca2+ from extracellular space into the cytosol, and this Ca2+ then activates the RyR in a Ca2+-induced Ca2+ release mechanism [90]. In renal cilia, PC2 has been shown to form heterotetrameric complexes with TRPC1 channels [91,92] (Figure 2). Overall, the role of PC1/PC2-mediated Ca2+ signaling in PKD development, especially when considered in concert with ER calcium release and other Ca2+ channels, is a very complex, extensive and interesting topic with a multitude of interactions, and readers are encouraged to refer to seminal papers for further details [37,89,93–96].
FPC and calcium handling in ARPKD
ARPKD is caused by mutations in the PKHD1 gene encoding for FPC. FPC is a membrane protein with a small cytoplasmic C-tail and a long extracellular N-terminal region; its function is yet largely undefined. Wang et al. showed that the FPC and PC2 colocalize in the plasma membrane and primary cilium of the MDCK cells, and regulate Ca2+ response in renal epithelium [97]. Kim et al. demonstrated that the intracellular COOH– tail of the FPC physically interacts with the N-terminus of PC2 [98,99]; however, Wang et al. suggested that KIF3A (a subunit of the motor protein kinesin-2) is a mediator of the FPC–PC2 interaction [97]. Earlier work by Wu et al. reported that KIF3B provides physical and functional interaction between PC2 and FPC [100]. Additional evidence confirmed the interdependence of FPC and PC2; in primary epithelial cells of the Pkhd1−/− mouse kidneys, Kim et al. demonstrated that physical interaction between FPC and PC2 is able to prevent suppression of PC2 expression caused by loss of FPC [99]. These findings are consistent with the fact that FPC deficiency resulted in suppressed expression of PC2 in vivo in the renal cortex of Pkhd1−/− mice, but not vice versa [98]. Based on the established interaction between FPC and PC2, it was assumed that FPC is able to regulate the level of intracellular Ca2+, possibly by regulating the activity of PC2 [97,98,100,101]. On the other hand, FPC can regulate intracellular Ca2+ independently of PC2: Nagano et al., using protein library screening, found that FPC (specifically, its C-terminal region) interacts with the N-terminal region of the calcium modulating cyclophilin ligand (CAML), whereby participating in Ca2+ signaling in distal nephron [102]. Hiesberger et al. showed using mIMCD-3 (mouse inner medullary collecting duct) cells that intracellular Ca2+ release (in combination with activation of PKC) regulates proteolytic cleavage of FPC, although in HEK293 cells either Ca2+ release or PKC activation were also able to regulate FPC cleavage [103]. Taken together, these studies are very important and add to the pool of knowledge, but do not yet provide a clear understanding of the role and participation of FPC, PC2 or FPC-PC2 in the regulation of Ca2+ homeostasis in ARPKD.
Fluid flow and mechanosensation in calcium homeostasis in PKD: TRPV4 and cilia
There is evidence that improper flow-mediated [Ca2+]i responses are involved in both ARPKD and ADPKD cystogenesis [37], potentially through the effects on the transduction of the flow sensation by the renal cilia, or mechanosensitive properties of the ion channels and transporters themselves. The transient receptor potential vanilloid type 4 (TRPV4) channels could play an important modulatory role in the process of mechanosensation [62,64]. TRPV4 is a Ca2+-permeable, nonselective cation channel that can be activated by various chemical, osmotic and mechanical signals. It is implicated in the regulation of multiple physiological processes in the kidneys, airways, hearts, brains, endothelial cells, and skin [104]. Functional activity of TRPV4 was reported to be inversely correlated with renal cystogenesis in a rat model of ARPKD [64], and decreased TRPV4 activity was also revealed in human ADPKD cells; Tomilin et al. suggested that TRPV4 activation may be useful to restore intracellular calcium homeostasis in cystic cells, thus inhibiting the progression of ADPKD [62] (see Figure 2, Table 1 ).
Several studies were devoted to a mechanosensitive regulation of intracellular Ca2+ in ARPKD as well. Rohatgi et al. investigated the effect of laminar flow on intracellular Ca2+ levels in conditionally immortalized human ARPKD renal cystic cells with reduced PKHD1 gene expression; they reported an excessive increase in the [Ca2+]i peak compared with control cells in response to shear stress [105].
The role of cilia in Ca2+ handling in PKD
The primary cilium is located on the apical membrane of epithelial cells, protruding into the luminal space of the renal tubules [106,107]. It is considered an immobile sensory organelle with a (9 + 0) axoneme that is involved in molecular signaling and tissue development, although some research demonstrated structural abnormalities in the primary cilium that are different from the generally accepted 9 + 0 paradigm [108]. Disturbances in the structure, development, and function of the primary cilium, including dysregulation of cilia-dependent signaling leading to disruption of Ca2+-regulated pathways, cause diseases collectively known as ciliopathies; PKD is considered a ciliopathy [85,109]. Abundant evidence has been collected that confirms the involvement of primary cilia in the regulation of Ca2+ signaling in PKD: the primary cilium, acting as a mechanosensor capable of detecting the presence of fluid flow, has been reported to regulate the influx of Ca2+ ions through the polycystins [90,110]. In contrast, Delling et al. observed no influx of Ca2+ ions when fluid flow (physiological and supraphysiological values) was applied to the primary cilium [111]. Siroky et al. examined the role of ciliary calcium handling in ARPKD: in orpk cilia-negative cells, which have disrupted expression of the Polaris, a protein required for ciliogenesis, Ca2+ permeation was markedly increased compared with the control group, and sensitivity to Gd3+ (a nonselective blocker of cationic channels) was observed [112]. Liu et al. reported an increase in intracellular Ca2+ in response to fluid flow; the magnitude of the response was dependent on the age of mutant orpk mice, 1-week-old orpk mutant mice were blunted in contrast with 2-week-old mice [113]. Therefore, the role of ciliary mechanosensation in the regulation of Ca2+ handling is still being debated. For more detailed information on the ciliary signaling disruptions that lead to PKD, please refer to a recent review by our group that highlighted this topic [114].
ATP-mediated regulation of Ca2+ entry and release in PKD
ATP levels in ADPKD and ARPKD
ATP is a crucial intracellular energy source; however, ATP molecules released extracellularly are also responsible for paracrine and autocrine signaling [115]. In healthy renal epithelia, extracellular ATP, acting through purinergic P2 receptors, plays a major role in regulation of intracellular Ca2+, hemodynamics, and epithelial ion transport [116]. In PKD, growing evidence suggests that ATP and related signaling may be key factors contributing to the formation of cysts [117]. Abnormally high ATP levels were shown in the renal cystic fluid from patients, as well as in model systems for ADPKD and ARPKD [117]. Higher amounts (up to 10 µM) of ATP were reported in fluid from microdissected human ADPKD cysts compared with the ∼400 nM ATP level typically found in tubular fluid of healthy controls [118]. Similar findings were reported in ARPKD; using highly ATP-sensitive enzymatic biosensors Palygin et al. showed that cystic fluid collected from PCK rat kidneys (snap-frozen sample) exhibited between five- and ten-times higher levels of ATP compared with cortex of healthy Sprague–Dawley rats (∼2 µM vs 200 nM, respectively) [63].
ATP acts through its purinergic P2 receptors, such as metabotropic P2Y and ionotropic P2X [117]. There are eight known G protein-coupled P2Y receptors (P2Y1, 2, 4, 6, and 11–14) that are stimulated by nucleotides such as ATP, ADP, UTP, UDP and UDP-glucose, and mediate ATP-induced release of intracellular Ca2+ [63,119]. There are seven P2X receptors (P2X1–7) that bind ATP as their principal ligand, and form Ca2+-permeable non-selective cation channels [120]. In the cystic tubular epithelium, intracellular ATP was shown to evoke an improper Ca2+ response ([63,121] see Table 2). For instance, high concentrations of ATP triggered Ca2+ response in freshly isolated ARPKD cystic epithelia (PCK rats) [63]. In ADPKD, reduced flow-sensitive ATP release in human epithelial cells from cysts was shown to contribute to the loss of flow-sensitive purinergic Ca2+ signaling that is typical for normal cells [121]. To date, the exact mechanisms of ATP-mediated Ca2+ regulation and release in PKD remain poorly understood, and there are many controversies, especially regarding the expression of the P2 receptors in normal and diseased states.
Table 2. Effects of P2 receptor antagonism/agonism on ARPKD and ADPKD development.
| Target | ADPKD or ARPKD | Species/model | Antagonist | Agonist | Action (effect) | Reference |
|---|---|---|---|---|---|---|
| Ionotropic P2X receptors | ||||||
| P2Xs | ADPKD | Human ADPKD epithelial primary cultures/in vitro | ATP αβmeATP bzATP |
Increased cytoplasmic [Ca2+]i, stimulated secretory Cl− transport | [140] | |
| MDCK-derived cysts (ADPKD)/in vitro | PPADS; Suramin (non-selective P2 receptor antagonist); Reactive blue2; Apyrase (ATP scavenger) |
bzATP | Reduced cyst growth and slowed disease progression | [141] | ||
| MDCK-derived cysts (ADPKD)/in vitro | Suramin Apyrase |
Reduced ATP-mediated fluid secretion and cyst development | [142] | |||
| P2X4 | ARPKD | PCK rat (ARPKD model)/in vivo | isoPPADS (non-selective P2X antagonist); 5-BDBD (P2X4) |
αβmeATP | Regulated [Ca2+]i | [63] |
| cpk/cpk ARPKD mice/in vitro | oxidized ATP (oxATP) | bzATP (P2X7); ATP; UTP |
bzATP reduced cyst development; oxATP abrogated this effect ATP, UTP had lesser effects than bzATP | [143] | ||
| PCK rat (ARPKD model) ex vivo | isoPPADS; AZ10606120 (P2X7) |
αβmeATP | Regulated [Ca2+]i | [63] | ||
| PCK rat (ARPKD model)/in vivo | P2rx7 knockout | P2rx7 knockout reduced cyst development through pannexin-1 channel and ATP accumulation in the cyst space, increased ENaC activity | [123] | |||
| P2X7 | ADPKD | Pkd2 morphant zebrafish/in vivo | A-438079; oxATP | Reduced cyst formation via ERK-dependent pathways | [144] | |
| Cultured human epithelial cells from ADPKD cysts/in vitro | oxATP | UTP; ATP; ADP |
Loss of flow-induced [Ca2+]i, reduced flow-sensitive ATP release; delayed and attenuated [Ca2+]i recovery was sensitive to oxATP | [121] | ||
|
Pkd1RC/RC mice (ADPKD)/in vivo M1 renal monolayers/in vitro |
αβmeATP | Pannexin-1 and P2X7 contributed to ATP release and decreased ENaC activity, promote cyst development | [122] | |||
| Metabotropic P2Y receptors | ||||||
| P2Ys | ADPKD | Human ADPKD epithelial primary cultures/in vitro | UTP UDP ADP |
Increased cytoplasmic [Ca2+]i, stimulated secretory Cl− transport | [140] | |
| MDCK-derived cysts (ADPKD)/in vitro | PPADS Suramin Reactive blue2 Apyrase |
Reduced cyst growth and slowed disease progression | [141] | |||
| P2Y1 | ADPKD | MDCK-derived cysts (ADPKD)/in vitro | PPADS Suramin Reactive blue2 MRS2179 (specific) |
Reduced cyst growth and slowed disease progression | [142] | |
Abbreviation: ENaC, epithelial Na+ channel.
Remodeling of purinergic signaling (for instance, a shift from metabotropic P2Y to ionotropic P2X receptors) may be one of the pathological mechanisms required for the progressive expansion of cysts. Table 2 reports possible involvement of P2 receptor signaling in PKD development, based on studies that tested pharmacological inhibition of certain receptors or genetic models. Data obtained for both ADPKD and ARPKD suggest a complex interplay of P2 receptor expression and function. For instance, a recent study done in Pkd1RC/RC mice, a model of ADPKD, demonstrated an increased expression of pannexin-1 (PANX1) and P2X7 in the cystic epithelium and enhanced ATP release into the luminal space, potentially through PANX1/P2X7 cooperation [122]. This group also showed the contribution of P2X7 in cyst development via PANX1-dependent ATP release in ARPKD (PCK rats) [123]. Later, Verschuren et al. also demonstrated the PANX1-mediated ATP release into the tubular lumen; this phenomenon was reported to be due to fluid shear stress, and established in two models of PKD: the Pkd1 knockout mice (iKsp-Pkd1−/−) and Pkd1−/− mouse distal convoluted tubule cells [124]. Moreover, they also found that PANX1 inhibition attenuated cyst growth in zebrafish PKD2 morphants. Recently, remodeling of the P2 receptor profile in PKD towards the loss of P2Y and increased expression of P2X4 and P2X7 receptors has also been described [63]. Thus, the understanding of ATP and purinergic signaling in PKD pathogenesis has expanded in recent years and may promote the development of novel therapeutic strategies.
Furthermore, extracellular ATP or UTP are able to activate a Ca2+-sensitive TMEM16A Cl− channel through stimulation of P2Y2 purinergic receptors, which promoted cell proliferation and growth of cysts in ARPKD [50,125]. Interestingly, gender-dependent differences were recently observed in intracellular Ca2+ concentration ([Ca2+]i) in ADPKD epithelium. Specifically, higher basal and ATP-induced [Ca2+]i levels were detected in primary renal epithelial cells isolated from male mice (vs female cells), which were hypothesized to result in the larger TMEM16A currents in males [126].
Sodium transport in PKD
Epithelial Sodium Channel (ENaC)
One of the ion channels that are established to play a role in PKD is the amiloride-sensitive ENaC (Table 1), expressed in the apical membrane of the renal CD and well-known to be involved in maintenance of water–salt homeostasis and the control of blood pressure. ENaC is heterotrimer consisting of three structurally related α, β, and γ subunits [127,128]. Dysfunction of ENaC in the CD has been demonstrated to contribute to the process of cyst development [122,129–131]. Despite numerous studies, there are conflicting data on how ENaC-mediated Na+ transport is specifically involved in the formation of cysts.
ADPKD
As established in the previous sections of this manuscript, cAMP-dependent Cl− secretion is the primary driving force of fluid secretion in renal cyst enlargement in ADPKD. Impaired Na+ transport may contribute to net solute accumulation within the cyst by removing the absorptive mechanism that opposes secretion. Yanda et al. showed a profound drop in ENaC expression during cystogenesis in ADPKD mice [39]. Their data also support the possibility of interaction between ENaC and CFTR. VX-809, a corrector of CFTR, was shown to reduce cyst growth, increase ENaC at the apical membrane, and thereby promote fluid absorption in Pkd1-knockout mice [132]. Another pathway that could regulate the activity of ENaC in cystic epithelia is purinergic signaling. Arkhipov et al. recently showed in Pkd1RC/RC mice that ENaC activity is reduced in the cystic cells as a result of ATP release via a complex between PANX1 and an ionotropic purinergic receptor P2X7 (Figure 2) [122].
ARPKD
Na+ absorption was found to be 50% higher in immortalized cells of ARPKD renal cysts compared with age-matched normal human fetal collecting tubule cells (HFCTs) [133]. In a cellular model of ARPKD and in a PCK rat, increased apical ENaC expression and enhanced Na+ reabsorption were attributed to mislocalization of the E3 ubiquitin-protein ligase Nedd 4-2 (Table 1) [131]. This was further corroborated in a study performed on cilium-deficient ARPKD principal cell monolayers, which also exhibited augmented ENaC expression [134]. These reports showing higher ENaC activity and/or expression are of particular interest, since they could explain early and severe hypertension in ARPKD patients. In contrast, Veizis et al. observed a 50% reduction in amiloride-sensitive ENaC-mediated Na+ absorption in the cells of ARPKD cysts from BPK mice vs. normal CD cells. They postulated that the decreased Na+ absorption may contribute to the accumulation of luminal fluid [135]. Moreover, the addition of the epidermal growth factor (EGF) to the apical bathing solution of cystic monolayers led to inhibition of Na+ transport, and a decrease in ENaC expression [136]. Pavlov et al. reported decreased levels of ENaC and aquaporin-2 (AQP-2), as well as lower ENaC activity in ARPKD cysts from PCK rats compared with normal nondilated tubules, as well as in vivo cyst expansion in response to ENaC blocker, diuretic benzamil [129]. In a recent study, Dr. A. Staruschenko’s lab revealed that ENaC expression is reduced in the salt-restricted PCK rats, which exhibit increased renal cortical cystogenesis (vs rats fed normal and high-salt diets) [130]. Using the same PCK rat model in another study, Arkhipov et al. demonstrated that the absence of an ionotropic purinergic receptor P2X7 increased ENaC activity in the cysts, and attenuated their growth [123]. The discrepancies in the literature regarding ENaC up- or down-regulation could possibly be explained by the variety of rodent models used, differences in diets, the stage of disease development when the tests were conducted, as well as by the stage of cyst growth; all of these factors could affect the observed ENaC-related phenomena (see Table 1 and Figure 2B). Further research is required to reach a consensus on this topic, with valuable insights gained from molecular biology approaches combined with patch-clamp experiments, since sole measurements of the changes in protein expression do not allow for a comprehensive assessment of the ion channel function.
Potassium transport in PKD
The data about the role of potassium transport in PKD are limited, and remain of particular interest. Using CT scans, Torres et al. showed in two patients with chronic hypokalemia that potassium deficiency is accompanied by enhanced renal cystogenesis [137]. Using primary cultures of cells derived from the cysts of ADPKD patients, Sullivan et al. investigated whether cystic cells contain a transmembrane K+ pathway that supports the electrochemical driving force for Cl− secretion [138]. ATP-sensitive inward rectifier K+ channels, Kir 6.2 (KCNJ11), were reported to be expressed in these cells. Glibenclamide, an inhibitor of Kir 6.2, blocked Cl− secretion, inhibited growth and formation of cultured cysts, and reduced proliferation (see Table 1). The important role of a Ca2+-dependent potassium KCa3.1 channels (also known as SK4, KCNN4) in CFTR-stimulated Cl− secretory flux was demonstrated in a study on monolayers of kidney cells derived from patients with ADPKD. TRAM-34, a specific KCa3.1 blocker, inhibited forskolin-induced Cl− current and ADPKD cyst growth [139]. To summarize, although there are limited data (Figure 2), we believe that potassium transport is a promising yet understudied new direction for PKD research, and we are looking forward to novel publications in this area.
Conclusions
The recent approval of tolvaptan for ADPKD treatment advanced the disease therapy: despite the high cost, this drug can effectively prolong the life of some patients. Nevertheless, it is not the cure, and in case of ARPKD, no treatments are available whatsoever. The more we learn about these diseases, the more obvious it is that PKDs, although arising from specific largely known mutations, are multifactorial and need to be treated as such. Years of research established that ion transport is clearly perturbed in PKD, and targeting channels and transporters in ADPKD and ARPKD remains a promising avenue of research. Although recent findings have somewhat advanced our understanding of how specific ion channels and transporters are involved in the disease’s progression, there is still a plethora of unanswered questions. It is important to emphasize that the contribution of electrolyte transport to ADPKD and ARPKD is likely different due to the nature of the forming cysts: generally, in ADPKD, isolated cysts fill with fluid due to Cl−-dependent fluid secretion, while in ARPKD, fluid secretion may be less important since the cystic tubules are still connected to the urinary space. Unfortunately, due to the complex nature of PKDs, and the variety of cell, organoid, and animal models that are being used in PKD research, the findings related to electrolyte handling in cystic epithelium remain controversial, and in many cases, obscure. For instance, despite decades of research, the role of Ca2+ in PKD is still debated; we have yet to come to a definite conclusion as to which downstream pathways are feasible targets that would not cause a backlash of side effects. Purinergic receptors and pannexins recently emerged as a potential novel therapeutic target for both ADPKD and ARPKD; availability of new pharmacology designed to inhibit or activate specific P2 receptors might allow for more successful therapies in future. The recent novel findings regarding PANX/P2X7 association and their role in cystogenesis are particularly exciting, however, there is an urgent need for the development of specific pharmaceuticals to target PANX and/or and PANX/P2X7 complex. The role of the ENaC-dependent sodium transport is yet to be fully determined in PKD, and we are looking forward to resolving the existing controversies regarding ENaC expression and activity in the AR and ADPKD cystic epithelium. One of the exciting but understudied pathways in PKD is potassium handling; currently, there is only a handful of studies that looked at potassium channels and transporters in this disease. It is particularly interesting if dietary potassium supplementation, or pharmacological manipulation of potassium channels, can affect cyst enlargement. Further, it should be noted that although clinical presentation and development of AR and ADPKD varies dependent on sex, very few studies have investigated the changes in cystic ion transport associated with sexual dimorphisms. Given the recently reported sex differences in renal channels and transporters along the nephron in healthy subjects, examination of these differences in PKD will expand our horizons toward treatments that might be more effective in individuals with predominantly male or female hormonal patterns. Along these lines, age-dependent changes in PKD are of particular interest as well. In summary, refining our knowledge of ion transport-related changes occurring in PKD will undoubtedly expand the therapeutic modalities, and more research is needed to decipher the complex and interdependent chain of events leading to cystogenesis. We expect that studies of electrolyte transport-related mechanisms, performed in a methodical and stratified manner, with the inclusion of biological variables such as sex and age, will move forward the discovery of new biomarkers of prognostic and therapeutic importance.
Acknowledgements
We thank Adam Jones (Augusta University) for thorough proofreading of the final version of the manuscript. Writing feedback was provided by Dr. R.J. Lambert in MUSC’s Center for Academic Excellence and Writing Center.
Abbreviations
- AC
adenylyl cyclase
- ADPKD
autosomal dominant polycystic kidney disease
- ARPKD
autosomal recessive polycystic kidney disease
- CD
collecting duct
- CFTR
cystic fibrosis transmembrane conductance regulator
- CLDN
Claudin
- ENaC
epithelial Na+ channel
- ER
endoplasmic reticulum
- FPC
fibrocystin; polyductin
- HSP
heat shock protein
- IP3R
inositol 1,4,5-triphosphate receptor
- MDCK
Madin–Darby Canine Kidney
- NDCBE
Na+-dependent Cl−/HCO3− exchanger
- NKCC1
Na+-K+-2Cl− cotransporter
- PANX1
Pannexin-1
- PC1; PC2
polycystin 1; polycystin 2
- PDE
phosphodiesterase
- PKD
polycystic kidney disease
- PKHD1
polycystic kidney and hepatic disease 1
- ROS
reactive oxygen species
- RyR
ryanodine receptor
- STIM1
stromal interaction molecule 1
- TMEM16A
transmembrane member 16A
- TRP
transient receptor potential
- TRPС1
transient receptor potential canonical 1
- TRPV4
transient receptor potential vanilloid type 4
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
This work was supported by the APS Lazaro J. Mandel Young Investigator Award, NIH grants NIDDK R00 DK105160, NHLBI R01HL148114; and the PKD Foundation Award [grant number 221G18a (all to D.V.I.)].
References
- 1.Verani R.R. and Silva F.G. (1988) Histogenesis of the renal cysts in adult (autosomal dominant) polycystic kidney disease: a histochemical study. Mod. Pathol. 1, 457–463 [PubMed] [Google Scholar]
- 2.Gunay-Aygun M., Font-Montgomery E., Lukose L., Tuchman Gerstein M., Piwnica-Worms K., Choyke P.et al. (2013) Characteristics of congenital hepatic fibrosis in a large cohort of patients with autosomal recessive polycystic kidney disease. Gastroenterology 144, 112.e2–121.e2 10.1053/j.gastro.2012.09.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Besse W., Roosendaal C., Tuccillo L., Roy S.G., Gallagher A.R. and Somlo S. (2020) Adult inactivation of the recessive polycystic kidney disease gene causes polycystic liver disease. Kidney360 1, 1068–1076 10.34067/KID.0002522020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burgmaier K., Kilian S., Bammens B., Benzing T., Billing H., Buscher A.et al. (2019) Clinical courses and complications of young adults with autosomal recessive polycystic kidney disease (ARPKD). Sci. Rep. 9, 7919 10.1038/s41598-019-43488-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Solazzo A., Testa F., Giovanella S., Busutti M., Furci L., Carrera P.et al. (2018) The prevalence of autosomal dominant polycystic kidney disease (ADPKD): a meta-analysis of European literature and prevalence evaluation in the Italian province of Modena suggest that ADPKD is a rare and underdiagnosed condition. PLoS ONE 13, e0190430 10.1371/journal.pone.0190430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Willey C.J., Blais J.D., Hall A.K., Krasa H.B., Makin A.J. and Czerwiec F.S. (2017) Prevalence of autosomal dominant polycystic kidney disease in the European Union. Nephrol. Dial. Transplant. 32, 1356–1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Torres V.E. and Ong A.C.M. (2020) Cellular signaling in PKD: foreword. Cell. Signal. 71, 109625 10.1016/j.cellsig.2020.109625 [DOI] [PubMed] [Google Scholar]
- 8.Cornec-Le Gall E., Torres V.E. and Harris P.C. (2018) Genetic complexity of autosomal dominant polycystic kidney and liver diseases. J. Am. Soc. Nephrol. 29, 13–23 10.1681/ASN.2017050483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Audrezet M.P., Cornec-Le Gall E., Chen J.M., Redon S., Quere I., Creff J.et al. (2012) Autosomal dominant polycystic kidney disease: comprehensive mutation analysis of PKD1 and PKD2 in 700 unrelated patients. Hum. Mutat. 33, 1239–1250 10.1002/humu.22103 [DOI] [PubMed] [Google Scholar]
- 10.Waldrop E., Al-Obaide M.A.I. and Vasylyeva T.L. (2019) GANAB and PKD1 variations in a 12 years old female patient with early onset of autosomal dominant polycystic kidney disease. Front. Genet. 10, 44 10.3389/fgene.2019.00044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Porath B., Gainullin V.G., Cornec-Le Gall E., Dillinger E.K., Heyer C.M., Hopp K.et al. (2016) Mutations in GANAB, encoding the glucosidase IIalpha subunit, cause autosomal-dominant polycystic kidney and liver disease. Am. J. Hum. Genet. 98, 1193–1207 10.1016/j.ajhg.2016.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris P.C. and Torres V.E. (1993) Polycystic Kidney Disease, Autosomal Dominant(Adam M.P., Ardinger H.H., Pagon R.A., Wallace S.E., Bean L.J.H. and Mirzaa G., eds), GeneReviews(R), Seattle (WA) [Google Scholar]
- 13.Pei Y. (2001) A “two-hit” model of cystogenesis in autosomal dominant polycystic kidney disease? Trends Mol. Med. 7, 151–156 10.1016/S1471-4914(01)01953-0 [DOI] [PubMed] [Google Scholar]
- 14.Puder S., Fischer T. and Mierke C.T. (2019) The transmembrane protein fibrocystin/polyductin regulates cell mechanics and cell motility. Phys. Biol. 16, 066006 10.1088/1478-3975/ab39fa [DOI] [PubMed] [Google Scholar]
- 15.Ziegler W.H., Soetje B., Marten L.P., Wiese J., Burute M. and Haffner D. (2020) Fibrocystin is essential to cellular control of adhesion and epithelial morphogenesis. Int. J. Mol. Sci. 21, 5140 10.3390/ijms21145140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mangoo-Karim R., Ye M., Wallace D.P., Grantham J.J. and Sullivan L.P. (1995) Anion secretion drives fluid secretion by monolayers of cultured human polycystic cells. Am. J. Physiol. 269, F381–F388 10.1152/ajprenal.1995.269.3.F381 [DOI] [PubMed] [Google Scholar]
- 17.Wallace D.P., Grantham J.J. and Sullivan L.P. (1996) Chloride and fluid secretion by cultured human polycystic kidney cells. Kidney Int. 50, 1327–1336 10.1038/ki.1996.445 [DOI] [PubMed] [Google Scholar]
- 18.Mangoo-Karim R., Uchic M., Lechene C. and Grantham J.J. (1989) Renal epithelial cyst formation and enlargement in vitro: dependence on cAMP. Proc. Natl. Acad. Sci. U.S.A. 86, 6007–6011 10.1073/pnas.86.15.6007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mangoo-Karim R., Uchic M.E., Grant M., Shumate W.A., Calvet J.P., Park C.H.et al. (1989) Renal epithelial fluid secretion and cyst growth: the role of cyclic AMP. FASEB J. 3, 2629–2632 10.1096/fasebj.3.14.2480260 [DOI] [PubMed] [Google Scholar]
- 20.Ye M. and Grantham J.J. (1993) The secretion of fluid by renal cysts from patients with autosomal dominant polycystic kidney disease. N. Engl. J. Med. 329, 310–313 10.1056/NEJM199307293290503 [DOI] [PubMed] [Google Scholar]
- 21.Davidow C.J., Maser R.L., Rome L.A., Calvet J.P. and Grantham J.J. (1996) The cystic fibrosis transmembrane conductance regulator mediates transepithelial fluid secretion by human autosomal dominant polycystic kidney disease epithelium in vitro. Kidney Int. 50, 208–218 10.1038/ki.1996.304 [DOI] [PubMed] [Google Scholar]
- 22.Belibi F.A., Wallace D.P., Yamaguchi T., Christensen M., Reif G. and Grantham J.J. (2002) The effect of caffeine on renal epithelial cells from patients with autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol. 13, 2723–2729 10.1097/01.ASN.0000025282.48298.7B [DOI] [PubMed] [Google Scholar]
- 23.Sullivan L.P. and Grantham J.J. (1996) Mechanisms of fluid secretion by polycystic epithelia. Kidney Int. 49, 1586–1591 10.1038/ki.1996.230 [DOI] [PubMed] [Google Scholar]
- 24.Jouret F. and Devuyst O. (2020) Targeting chloride transport in autosomal dominant polycystic kidney disease. Cell. Signal. 73, 109703 10.1016/j.cellsig.2020.109703 [DOI] [PubMed] [Google Scholar]
- 25.Yamaguchi T., Wallace D.P., Magenheimer B.S., Hempson S.J., Grantham J.J. and Calvet J.P. (2004) Calcium restriction allows cAMP activation of the B-Raf/ERK pathway, switching cells to a cAMP-dependent growth-stimulated phenotype. J. Biol. Chem. 279, 40419–40430 10.1074/jbc.M405079200 [DOI] [PubMed] [Google Scholar]
- 26.Wang Q., Cobo-Stark P., Patel V., Somlo S., Han P.L. and Igarashi P. (2018) Adenylyl cyclase 5 deficiency reduces renal cyclic AMP and cyst growth in an orthologous mouse model of polycystic kidney disease. Kidney Int. 93, 403–415 10.1016/j.kint.2017.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanaoka K. and Guggino W.B. (2000) cAMP regulates cell proliferation and cyst formation in autosomal polycystic kidney disease cells. J. Am. Soc. Nephrol. 11, 1179–1187 10.1681/ASN.V1171179 [DOI] [PubMed] [Google Scholar]
- 28.Rees S., Kittikulsuth W., Roos K., Strait K.A., Van Hoek A. and Kohan D.E. (2014) Adenylyl cyclase 6 deficiency ameliorates polycystic kidney disease. J. Am. Soc. Nephrol. 25, 232–237 10.1681/ASN.2013010077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pinto C.S., Raman A., Reif G.A., Magenheimer B.S., White C., Calvet J.P.et al. (2016) Phosphodiesterase isoform regulation of cell proliferation and fluid secretion in autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol. 27, 1124–1134 10.1681/ASN.2015010047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sussman C.R., Ward C.J., Leightner A.C., Smith J.L., Agarwal R., Harris P.C.et al. (2014) Phosphodiesterase 1A modulates cystogenesis in zebrafish. J. Am. Soc. Nephrol. 25, 2222–2230 10.1681/ASN.2013040421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ye H., Wang X., Sussman C.R., Hopp K., Irazabal M.V., Bakeberg J.L.et al. (2016) Modulation of polycystic kidney disease severity by phosphodiesterase 1 and 3 subfamilies. J. Am. Soc. Nephrol. 27, 1312–1320 10.1681/ASN.2015010057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang X., Yamada S., LaRiviere W.B., Ye H., Bakeberg J.L., Irazabal M.V.et al. (2017) Generation and phenotypic characterization of Pde1a mutant mice. PLoS ONE 12, e0181087 10.1371/journal.pone.0181087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Csanady L., Vergani P. and Gadsby D.C. (2019) Structure, gating, and regulation of the Cftr anion channel. Physiol. Rev. 99, 707–738 10.1152/physrev.00007.2018 [DOI] [PubMed] [Google Scholar]
- 34.Cohen-Cymberknoh M., Shoseyov D. and Kerem E. (2011) Managing cystic fibrosis: strategies that increase life expectancy and improve quality of life. Am. J. Respir. Crit. Care Med. 183, 1463–1471 10.1164/rccm.201009-1478CI [DOI] [PubMed] [Google Scholar]
- 35.Hanaoka K., Devuyst O., Schwiebert E.M., Wilson P.D. and Guggino W.B. (1996) A role for CFTR in human autosomal dominant polycystic kidney disease. Am. J. Physiol. 270, C389–C399 10.1152/ajpcell.1996.270.1.C389 [DOI] [PubMed] [Google Scholar]
- 36.Rajagopal M. and Wallace D.P. (2015) Chloride secretion by renal collecting ducts. Curr. Opin. Nephrol. Hypertens. 24, 444–449 10.1097/MNH.0000000000000148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu M. and Yu S. (2016) New insights into the molecular mechanisms targeting tubular channels/transporters in PKD development. Kidney Dis. (Basel) 2, 128–135 10.1159/000444839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lebeau C., Hanaoka K., Moore-Hoon M.L., Guggino W.B., Beauwens R. and Devuyst O. (2002) Basolateral chloride transporters in autosomal dominant polycystic kidney disease. Pflugers Arch. 444, 722–731 10.1007/s00424-002-0880-3 [DOI] [PubMed] [Google Scholar]
- 39.Yanda M.K., Cha B., Cebotaru C.V. and Cebotaru L. (2019) Pharmacological reversal of renal cysts from secretion to absorption suggests a potential therapeutic strategy for managing autosomal dominant polycystic kidney disease. J. Biol. Chem. 294, 17090–17104 10.1074/jbc.RA119.010320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cabrita I., Kraus A., Scholz J.K., Skoczynski K., Schreiber R., Kunzelmann K.et al. (2020) Cyst growth in ADPKD is prevented by pharmacological and genetic inhibition of TMEM16A in vivo. Nat. Commun. 11, 4320 10.1038/s41467-020-18104-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Verkman A.S., Synder D., Tradtrantip L., Thiagarajah J.R. and Anderson M.O. (2013) CFTR inhibitors. Curr. Pharm. Des. 19, 3529–3541 10.2174/13816128113199990321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Snyder D.S., Tradtrantip L., Yao C., Kurth M.J. and Verkman A.S. (2011) Potent, metabolically stable benzopyrimido-pyrrolo-oxazine-dione (BPO) CFTR inhibitors for polycystic kidney disease. J. Med. Chem. 54, 5468–5477 10.1021/jm200505e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tradtrantip L., Sonawane N.D., Namkung W. and Verkman A.S. (2009) Nanomolar potency pyrimido-pyrrolo-quinoxalinedione CFTR inhibitor reduces cyst size in a polycystic kidney disease model. J. Med. Chem. 52, 6447–6455 10.1021/jm9009873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang B., Sonawane N.D., Zhao D., Somlo S. and Verkman A.S. (2008) Small-molecule CFTR inhibitors slow cyst growth in polycystic kidney disease. J. Am. Soc. Nephrol. 19, 1300–1310 10.1681/ASN.2007070828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nakanishi K., Sweeney W.E. Jr, Macrae Dell K., Cotton C.U. and Avner E.D. (2001) Role of CFTR in autosomal recessive polycystic kidney disease. J. Am. Soc. Nephrol. 12, 719–725 10.1681/ASN.V124719 [DOI] [PubMed] [Google Scholar]
- 46.Yanda M.K., Tomar V. and Cebotaru L. (2021) Therapeutic potential for CFTR correctors in autosomal recessive polycystic kidney disease. Cell Mol. Gastroenterol. Hepatol. 10.1016/j.jcmgh.2021.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Buchholz B., Faria D., Schley G., Schreiber R., Eckardt K.U. and Kunzelmann K. (2014) Anoctamin 1 induces calcium-activated chloride secretion and proliferation of renal cyst-forming epithelial cells. Kidney Int. 85, 1058–1067 10.1038/ki.2013.418 [DOI] [PubMed] [Google Scholar]
- 48.Cabrita I., Buchholz B., Schreiber R. and Kunzelmann K. (2020) TMEM16A drives renal cyst growth by augmenting Ca(2+) signaling in M1 cells. J. Mol. Med. (Berl.) 98, 659–671 10.1007/s00109-020-01894-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang Y.D., Cho H., Koo J.Y., Tak M.H., Cho Y., Shim W.S.et al. (2008) TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature 455, 1210–1215 10.1038/nature07313 [DOI] [PubMed] [Google Scholar]
- 50.Schreiber R., Buchholz B., Kraus A., Schley G., Scholz J., Ousingsawat J.et al. (2019) Lipid peroxidation drives renal cyst growth in vitro through activation of TMEM16A. J. Am. Soc. Nephrol. 30, 228–242 10.1681/ASN.2018010039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Montesano R., Ghzili H., Carrozzino F., Rossier B.C. and Feraille E. (2009) cAMP-dependent chloride secretion mediates tubule enlargement and cyst formation by cultured mammalian collecting duct cells. Am. J. Physiol. Renal Physiol. 296, F446–F457 10.1152/ajprenal.90415.2008 [DOI] [PubMed] [Google Scholar]
- 52.Magenheimer B.S., St John P.L., Isom K.S., Abrahamson D.R., De Lisle R.C., Wallace D.P.et al. (2006) Early embryonic renal tubules of wild-type and polycystic kidney disease kidneys respond to cAMP stimulation with cystic fibrosis transmembrane conductance regulator/Na(+),K(+),2Cl Co-transporter-dependent cystic dilation. J. Am. Soc. Nephrol. 17, 3424–3437 10.1681/ASN.2006030295 [DOI] [PubMed] [Google Scholar]
- 53.Soleimani M. (2015) The multiple roles of pendrin in the kidney. Nephrol. Dial. Transplant. 30, 1257–1266 10.1093/ndt/gfu307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Soleimani M., Greeley T., Petrovic S., Wang Z., Amlal H., Kopp P.et al. (2001) Pendrin: an apical Cl-/OH-/HCO3- exchanger in the kidney cortex. Am. J. Physiol. Renal Physiol. 280, F356–F364 10.1152/ajprenal.2001.280.2.F356 [DOI] [PubMed] [Google Scholar]
- 55.Leviel F., Hubner C.A., Houillier P., Morla L., El Moghrabi S., Brideau G.et al. (2010) The Na+-dependent chloride-bicarbonate exchanger SLC4A8 mediates an electroneutral Na+ reabsorption process in the renal cortical collecting ducts of mice. J. Clin. Invest. 120, 1627–1635 10.1172/JCI40145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grantham J.J., Uchic M., Cragoe E.J. Jr, Kornhaus J., Grantham J.A., Donoso V.et al. (1989) Chemical modification of cell proliferation and fluid secretion in renal cysts. Kidney Int. 35, 1379–1389 10.1038/ki.1989.137 [DOI] [PubMed] [Google Scholar]
- 57.Sansom S.C., Weinman E.J. and O’Neil R.G. (1984) Microelectrode assessment of chloride-conductive properties of cortical collecting duct. Am. J. Physiol. 247, F291–F302 10.1152/ajprenal.1984.247.2.F291 [DOI] [PubMed] [Google Scholar]
- 58.Hou J., Renigunta A., Yang J. and Waldegger S. (2010) Claudin-4 forms paracellular chloride channel in the kidney and requires claudin-8 for tight junction localization. Proc. Natl. Acad. Sci. U.S.A. 107, 18010–18015 10.1073/pnas.1009399107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yu A.S., Kanzawa S.A., Usorov A., Lantinga-van Leeuwen I.S. and Peters D.J. (2008) Tight junction composition is altered in the epithelium of polycystic kidneys. J. Pathol. 216, 120–128 10.1002/path.2392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sassi A., Wang Y., Chassot A., Komarynets O., Roth I., Olivier V.et al. (2020) Interaction between epithelial sodium channel gamma-subunit and Claudin-8 modulates paracellular sodium permeability in renal collecting duct. J. Am. Soc. Nephrol. 31, 1009–1023 10.1681/ASN.2019080790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yamaguchi T., Hempson S.J., Reif G.A., Hedge A.M. and Wallace D.P. (2006) Calcium restores a normal proliferation phenotype in human polycystic kidney disease epithelial cells. J. Am. Soc. Nephrol. 17, 178–187 10.1681/ASN.2005060645 [DOI] [PubMed] [Google Scholar]
- 62.Tomilin V., Reif G.A., Zaika O., Wallace D.P. and Pochynyuk O. (2018) Deficient transient receptor potential vanilloid type 4 function contributes to compromised [Ca(2+)]i homeostasis in human autosomal-dominant polycystic kidney disease cells. FASEB J. 32, 4612–4623 10.1096/fj.201701535RR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Palygin O., Ilatovskaya D.V., Levchenko V., Klemens C.A., Dissanayake L., Williams A.M.et al. (2018) Characterization of purinergic receptor expression in ARPKD cystic epithelia. Purinergic Signal. 14, 485–497 10.1007/s11302-018-9632-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zaika O., Mamenko M., Berrout J., Boukelmoune N., O'Neil R.G. and Pochynyuk O. (2013) TRPV4 dysfunction promotes renal cystogenesis in autosomal recessive polycystic kidney disease. J. Am. Soc. Nephrol. 24, 604–616 10.1681/ASN.2012050442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mangolini A., de Stephanis L. and Aguiari G. (2016) Role of calcium in polycystic kidney disease: From signaling to pathology. World J. Nephrol. 5, 76–83 10.5527/wjn.v5.i1.76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Parnell S.C., Magenheimer B.S., Maser R.L., Rankin C.A., Smine A., Okamoto T.et al. (1998) The polycystic kidney disease-1 protein, polycystin-1, binds and activates heterotrimeric G-proteins in vitro. Biochem. Biophys. Res. Commun. 251, 625–631 10.1006/bbrc.1998.9514 [DOI] [PubMed] [Google Scholar]
- 67.Parnell S.C., Magenheimer B.S., Maser R.L., Pavlov T.S., Havens M.A., Hastings M.L.et al. (2018) A mutation affecting polycystin-1 mediated heterotrimeric G-protein signaling causes PKD. Hum. Mol. Genet. 27, 3313–3324 10.1093/hmg/ddy223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Grieben M., Pike A.C., Shintre C.A., Venturi E., El-Ajouz S., Tessitore A.et al. (2017) Structure of the polycystic kidney disease TRP channel Polycystin-2 (PC2). Nat. Struct. Mol. Biol. 24, 114–122 10.1038/nsmb.3343 [DOI] [PubMed] [Google Scholar]
- 69.Li Y., Wright J.M., Qian F., Germino G.G. and Guggino W.B. (2005) Polycystin 2 interacts with type I inositol 1,4,5-trisphosphate receptor to modulate intracellular Ca2+ signaling. J. Biol. Chem. 280, 41298–41306 10.1074/jbc.M510082200 [DOI] [PubMed] [Google Scholar]
- 70.Ha K., Nobuhara M., Wang Q., Walker R.V., Qian F., Schartner C.et al. (2020) The heteromeric PC-1/PC-2 polycystin complex is activated by the PC-1 N-terminus. eLife 9, e60684 10.7554/eLife.60684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Luo Y., Vassilev P.M., Li X., Kawanabe Y. and Zhou J. (2003) Native polycystin 2 functions as a plasma membrane Ca2+-permeable cation channel in renal epithelia. Mol. Cell. Biol. 23, 2600–2607 10.1128/MCB.23.7.2600-2607.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gainullin V.G., Hopp K., Ward C.J., Hommerding C.J. and Harris P.C. (2015) Polycystin-1 maturation requires polycystin-2 in a dose-dependent manner. J. Clin. Invest. 125, 607–620 10.1172/JCI76972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Babich V., Zeng W.Z., Yeh B.I., Ibraghimov-Beskrovnaya O., Cai Y., Somlo S.et al. (2004) The N-terminal extracellular domain is required for polycystin-1-dependent channel activity. J. Biol. Chem. 279, 25582–25589 10.1074/jbc.M402829200 [DOI] [PubMed] [Google Scholar]
- 74.Su Q., Hu F., Ge X., Lei J., Yu S., Wang T.et al. (2018) Structure of the human PKD1-PKD2 complex. Science 361, eaat9819 10.1126/science.aat9819 [DOI] [PubMed] [Google Scholar]
- 75.Yu Y., Ulbrich M.H., Li M.H., Buraei Z., Chen X.Z., Ong A.C.et al. (2009) Structural and molecular basis of the assembly of the TRPP2/PKD1 complex. Proc. Natl. Acad. Sci. U.S.A. 106, 11558–11563 10.1073/pnas.0903684106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hanaoka K., Qian F., Boletta A., Bhunia A.K., Piontek K., Tsiokas L.et al. (2000) Co-assembly of polycystin-1 and -2 produces unique cation-permeable currents. Nature 408, 990–994 10.1038/35050128 [DOI] [PubMed] [Google Scholar]
- 77.Ferreira F.M., Oliveira L.C., Germino G.G., Onuchic J.N. and Onuchic L.F. (2011) Macromolecular assembly of polycystin-2 intracytosolic C-terminal domain. Proc. Natl. Acad. Sci. U.S.A. 108, 9833–9838 10.1073/pnas.1106766108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gonzalez-Perrett S., Kim K., Ibarra C., Damiano A.E., Zotta E., Batelli M.et al. (2001) Polycystin-2, the protein mutated in autosomal dominant polycystic kidney disease (ADPKD), is a Ca2+-permeable nonselective cation channel. Proc. Natl. Acad. Sci. U.S.A. 98, 1182–1187 10.1073/pnas.98.3.1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kleene S.J. and Kleene N.K. (2021) Inward Ca(2+) current through the polycystin-2-dependent channels of renal primary cilia. Am. J. Physiol. Renal Physiol. 320, F1165–F1173 10.1152/ajprenal.00062.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Anyatonwu G.I. and Ehrlich B.E. (2005) Organic cation permeation through the channel formed by polycystin-2. J. Biol. Chem. 280, 29488–29493 10.1074/jbc.M504359200 [DOI] [PubMed] [Google Scholar]
- 81.Wang Z., Ng C., Liu X., Wang Y., Li B., Kashyap P.et al. (2019) The ion channel function of polycystin-1 in the polycystin-1/polycystin-2 complex. EMBO Rep. 20, e48336 10.15252/embr.201948336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shen P.S., Yang X., DeCaen P.G., Liu X., Bulkley D., Clapham D.E.et al. (2016) The structure of the polycystic kidney disease channel PKD2 in lipid nanodiscs. Cell 167, 763.e11–773.e11 10.1016/j.cell.2016.09.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zheng W., Yang X., Hu R., Cai R., Hofmann L., Wang Z.et al. (2018) Hydrophobic pore gates regulate ion permeation in polycystic kidney disease 2 and 2L1 channels. Nat. Commun. 9, 2302 10.1038/s41467-018-04586-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Peyronnet R., Martins J.R., Duprat F., Demolombe S., Arhatte M., Jodar M.et al. (2013) Piezo1-dependent stretch-activated channels are inhibited by Polycystin-2 in renal tubular epithelial cells. EMBO Rep. 14, 1143–1148 10.1038/embor.2013.170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Saigusa T., Yue Q., Bunni M.A., Bell P.D. and Eaton D.C. (2019) Loss of primary cilia increases polycystin-2 and TRPV4 and the appearance of a nonselective cation channel in the mouse cortical collecting duct. Am. J. Physiol. Renal Physiol. 317, F632–F637 10.1152/ajprenal.00210.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yao Q., Outeda P., Xu H., Walker R., Basquin D., Qian F.et al. (2021) Polycystin-1 dependent regulation of polycystin-2 via GRP94, a member of HSP90 family that resides in the endoplasmic reticulum. FASEB J. 35, e21865 10.1096/fj.202100325RR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Brill A.L. and Ehrlich B.E. (2020) Polycystin 2: a calcium channel, channel partner, and regulator of calcium homeostasis in ADPKD. Cell. Signal. 66, 109490 10.1016/j.cellsig.2019.109490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Deng X., Wang Y., Zhou Y., Soboloff J. and Gill D.L. (2009) STIM and Orai: dynamic intermembrane coupling to control cellular calcium signals. J. Biol. Chem. 284, 22501–22505 10.1074/jbc.R109.018655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yanda M.K., Liu Q., Cebotaru V., Guggino W.B. and Cebotaru L. (2019) Role of calcium in adult onset polycystic kidney disease. Cell. Signal. 53, 140–150 10.1016/j.cellsig.2018.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nauli S.M., Alenghat F.J., Luo Y., Williams E., Vassilev P., Li X.et al. (2003) Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat. Genet. 33, 129–137 10.1038/ng1076 [DOI] [PubMed] [Google Scholar]
- 91.Zhang P., Luo Y., Chasan B., Gonzalez-Perrett S., Montalbetti N., Timpanaro G.A.et al. (2009) The multimeric structure of polycystin-2 (TRPP2): structural-functional correlates of homo- and hetero-multimers with TRPC1. Hum. Mol. Genet. 18, 1238–1251 10.1093/hmg/ddp024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tsiokas L., Arnould T., Zhu C., Kim E., Walz G. and Sukhatme V.P. (1999) Specific association of the gene product of PKD2 with the TRPC1 channel. Proc. Natl. Acad. Sci. U.S.A. 96, 3934–3939 10.1073/pnas.96.7.3934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wilkes M., Madej M.G., Kreuter L., Rhinow D., Heinz V., De Sanctis S.et al. (2017) Molecular insights into lipid-assisted Ca(2+) regulation of the TRP channel Polycystin-2. Nat. Struct. Mol. Biol. 24, 123–130 10.1038/nsmb.3357 [DOI] [PubMed] [Google Scholar]
- 94.Liu X., Vien T., Duan J., Sheu S.H., DeCaen P.G. and Clapham D.E. (2018) Polycystin-2 is an essential ion channel subunit in the primary cilium of the renal collecting duct epithelium. eLife 7, e33183 10.7554/eLife.33183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Di Mise A., Ranieri M., Centrone M., Venneri M., Tamma G., Valenti D.et al. (2018) Activation of the calcium-sensing receptor corrects the impaired mitochondrial energy status observed in renal polycystin-1 knockdown cells modeling autosomal dominant polycystic kidney disease. Front. Mol. Biosci. 5, 77 10.3389/fmolb.2018.00077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kuo I.Y., Brill A.L., Lemos F.O., Jiang J.Y., Falcone J.L., Kimmerling E.P.et al. (2019) Polycystin 2 regulates mitochondrial Ca(2+) signaling, bioenergetics, and dynamics through mitofusin 2. Sci. Signal. 12, eaat7397 10.1126/scisignal.aat7397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang S., Zhang J., Nauli S.M., Li X., Starremans P.G., Luo Y.et al. (2007) Fibrocystin/polyductin, found in the same protein complex with polycystin-2, regulates calcium responses in kidney epithelia. Mol. Cell. Biol. 27, 3241–3252 10.1128/MCB.00072-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kim I., Fu Y., Hui K., Moeckel G., Mai W., Li C.et al. (2008) Fibrocystin/polyductin modulates renal tubular formation by regulating polycystin-2 expression and function. J. Am. Soc. Nephrol. 19, 455–468 10.1681/ASN.2007070770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kim I., Li C., Liang D., Chen X.Z., Coffy R.J., Ma J.et al. (2008) Polycystin-2 expression is regulated by a PC2-binding domain in the intracellular portion of fibrocystin. J. Biol. Chem. 283, 31559–31566 10.1074/jbc.M805452200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wu Y., Dai X.Q., Li Q., Chen C.X., Mai W., Hussain Z.et al. (2006) Kinesin-2 mediates physical and functional interactions between polycystin-2 and fibrocystin. Hum. Mol. Genet. 15, 3280–3292 10.1093/hmg/ddl404 [DOI] [PubMed] [Google Scholar]
- 101.Yang J., Zhang S., Zhou Q., Guo H., Zhang K., Zheng R.et al. (2007) PKHD1 gene silencing may cause cell abnormal proliferation through modulation of intracellular calcium in autosomal recessive polycystic kidney disease. J. Biochem. Mol. Biol. 40, 467–474 10.5483/BMBRep.2007.40.4.467 [DOI] [PubMed] [Google Scholar]
- 102.Nagano J., Kitamura K., Hujer K.M., Ward C.J., Bram R.J., Hopfer U.et al. (2005) Fibrocystin interacts with CAML, a protein involved in Ca2+ signaling. Biochem. Biophys. Res. Commun. 338, 880–889 10.1016/j.bbrc.2005.10.022 [DOI] [PubMed] [Google Scholar]
- 103.Hiesberger T., Gourley E., Erickson A., Koulen P., Ward C.J., Masyuk T.V.et al. (2006) Proteolytic cleavage and nuclear translocation of fibrocystin is regulated by intracellular Ca2+ and activation of protein kinase C. J. Biol. Chem. 281, 34357–34364 10.1074/jbc.M606740200 [DOI] [PubMed] [Google Scholar]
- 104.Toft-Bertelsen T.L. and MacAulay N. (2021) TRPing to the point of clarity: understanding the function of the complex TRPV4 ion channel. Cells 10, 165 10.3390/cells10010165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rohatgi R., Battini L., Kim P., Israeli S., Wilson P.D., Gusella G.L.et al. (2008) Mechanoregulation of intracellular Ca2+ in human autosomal recessive polycystic kidney disease cyst-lining renal epithelial cells. Am. J. Physiol. Renal Physiol. 294, F890–F899 10.1152/ajprenal.00341.2007 [DOI] [PubMed] [Google Scholar]
- 106.Crayen M.L. and Thoenes W. (1978) Architecture and cell structures in the distal nephron of the rat kidney. Cytobiologie 17, 197–211 [PubMed] [Google Scholar]
- 107.Liu W., Xu S., Woda C., Kim P., Weinbaum S. and Satlin L.M. (2003) Effect of flow and stretch on the [Ca2+]i response of principal and intercalated cells in cortical collecting duct. Am. J. Physiol. Renal Physiol. 285, F998–F1012 10.1152/ajprenal.00067.2003 [DOI] [PubMed] [Google Scholar]
- 108.Sun S., Fisher R.L., Bowser S.S., Pentecost B.T. and Sui H. (2019) Three-dimensional architecture of epithelial primary cilia. Proc. Natl. Acad. Sci. U.S.A. 116, 9370–9379 10.1073/pnas.1821064116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pazour G.J., Dickert B.L., Vucica Y., Seeley E.S., Rosenbaum J.L., Witman G.B.et al. (2000) Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J. Cell Biol. 151, 709–718 10.1083/jcb.151.3.709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lee K.L., Guevarra M.D., Nguyen A.M., Chua M.C., Wang Y. and Jacobs C.R. (2015) The primary cilium functions as a mechanical and calcium signaling nexus. Cilia 4, 7 10.1186/s13630-015-0016-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Delling M., Indzhykulian A.A., Liu X., Li Y., Xie T., Corey D.P.et al. (2016) Primary cilia are not calcium-responsive mechanosensors. Nature 531, 656–660 10.1038/nature17426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Siroky B.J., Ferguson W.B., Fuson A.L., Xie Y., Fintha A., Komlosi P.et al. (2006) Loss of primary cilia results in deregulated and unabated apical calcium entry in ARPKD collecting duct cells. Am. J. Physiol. Renal Physiol. 290, F1320–F1328 10.1152/ajprenal.00463.2005 [DOI] [PubMed] [Google Scholar]
- 113.Liu W., Murcia N.S., Duan Y., Weinbaum S., Yoder B.K., Schwiebert E.et al. (2005) Mechanoregulation of intracellular Ca2+ concentration is attenuated in collecting duct of monocilium-impaired orpk mice. Am. J. Physiol. Renal Physiol. 289, F978–F988 10.1152/ajprenal.00260.2004 [DOI] [PubMed] [Google Scholar]
- 114.Vasileva V.Y., Sultanova R.F., Sudarikova A.V. and Ilatovskaya D.V. (2021) Insights into the molecular mechanisms of polycystic kidney diseases. Front. Physiol. 12, 693130 10.3389/fphys.2021.693130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Corriden R. and Insel P.A. (2010) Basal release of ATP: an autocrine-paracrine mechanism for cell regulation. Sci. Signal. 3, re1 10.1126/scisignal.3104re1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vallon V., Unwin R., Inscho E.W., Leipziger J. and Kishore B.K. (2020) Extracellular nucleotides and P2 receptors in renal function. Physiol. Rev. 100, 211–269 10.1152/physrev.00038.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ilatovskaya D.V., Palygin O. and Staruschenko A. (2016) Functional and therapeutic importance of purinergic signaling in polycystic kidney disease. Am. J. Physiol. Renal Physiol. 311, F1135–F1139 10.1152/ajprenal.00406.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Palygin O., Levchenko V., Ilatovskaya D.V., Pavlov T.S., Ryan R.P., Cowley A.W. Jret al. (2013) Real-time electrochemical detection of ATP and HO release in freshly isolated kidneys. Am. J. Physiol. Renal Physiol. 305, F134–F141 10.1152/ajprenal.00129.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Geyti C.S., Odgaard E., Overgaard M.T., Jensen M.E., Leipziger J. and Praetorius H.A. (2008) Slow spontaneous [Ca2+] i oscillations reflect nucleotide release from renal epithelia. Pflugers Arch. 455, 1105–1117 10.1007/s00424-007-0366-4 [DOI] [PubMed] [Google Scholar]
- 120.Jacobson K.A. and Muller C.E. (2016) Medicinal chemistry of adenosine, P2Y and P2X receptors. Neuropharmacology 104, 31–49 10.1016/j.neuropharm.2015.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Xu C., Shmukler B.E., Nishimura K., Kaczmarek E., Rossetti S., Harris P.C.et al. (2009) Attenuated, flow-induced ATP release contributes to absence of flow-sensitive, purinergic Cai2+ signaling in human ADPKD cyst epithelial cells. Am. J. Physiol. Renal Physiol. 296, F1464–F1476 10.1152/ajprenal.90542.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Arkhipov S.N. and Pavlov T.S. (2019) ATP release into ADPKD cysts via pannexin-1/P2X7 channels decreases ENaC activity. Biochem. Biophys. Res. Commun. 513, 166–171 10.1016/j.bbrc.2019.03.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Arkhipov S.N., Potter D.L., Geurts A.M. and Pavlov T.S. (2019) Knockout of P2rx7 purinergic receptor attenuates cyst growth in a rat model of ARPKD. Am. J. Physiol. Renal Physiol. 317, F1649–F1655 10.1152/ajprenal.00395.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Verschuren E.H.J., Rigalli J.P., Castenmiller C., Rohrbach M.U., Bindels R.J.M., Peters D.J.M.et al. (2020) Pannexin-1 mediates fluid shear stress-sensitive purinergic signaling and cyst growth in polycystic kidney disease. FASEB J. 34, 6382–6398 10.1096/fj.201902901R [DOI] [PubMed] [Google Scholar]
- 125.Schreiber R., Ousingsawat J. and Kunzelmann K. (2020) Targeting of intracellular TMEM16 proteins to the plasma membrane and activation by purinergic signaling. Int. J. Mol. Sci. 21, 4065 10.3390/ijms21114065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Talbi K., Cabrita I., Schreiber R. and Kunzelmann K. (2021) Gender-dependent phenotype in polycystic kidney disease is determined by differential intracellular Ca(2+) signals. Int. J. Mol. Sci. 22, 10.3390/ijms22116019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kleyman T.R. and Eaton D.C. (2020) Regulating ENaC’s gate. Am. J. Physiol. Cell Physiol. 318, C150–C162 10.1152/ajpcell.00418.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Noreng S., Posert R., Bharadwaj A., Houser A. and Baconguis I. (2020) Molecular principles of assembly, activation, and inhibition in epithelial sodium channel. eLife 9, e59038 10.7554/eLife.59038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pavlov T.S., Levchenko V., Ilatovskaya D.V., Palygin O. and Staruschenko A. (2015) Impaired epithelial Na+ channel activity contributes to cystogenesis and development of autosomal recessive polycystic kidney disease in PCK rats. Pediatr. Res. 77, 64–69 10.1038/pr.2014.145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ilatovskaya D.V., Levchenko V., Pavlov T.S., Isaeva E., Klemens C.A., Johnson J.et al. (2019) Salt-deficient diet exacerbates cystogenesis in ARPKD via epithelial sodium channel (ENaC). EBioMedicine 40, 663–674 10.1016/j.ebiom.2019.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kaimori J.Y., Lin C.C., Outeda P., Garcia-Gonzalez M.A., Menezes L.F., Hartung E.A.et al. (2017) NEDD4-family E3 ligase dysfunction due to PKHD1/Pkhd1 defects suggests a mechanistic model for ARPKD pathobiology. Sci. Rep. 7, 7733 10.1038/s41598-017-08284-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yanda M.K., Liu Q. and Cebotaru L. (2018) A potential strategy for reducing cysts in autosomal dominant polycystic kidney disease with a CFTR corrector. J. Biol. Chem. 293, 11513–11526 10.1074/jbc.RA118.001846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rohatgi R., Greenberg A., Burrow C.R., Wilson P.D. and Satlin L.M. (2003) Na transport in autosomal recessive polycystic kidney disease (ARPKD) cyst lining epithelial cells. J. Am. Soc. Nephrol. 14, 827–836 10.1097/01.ASN.0000056481.66379.B2 [DOI] [PubMed] [Google Scholar]
- 134.Olteanu D., Yoder B.K., Liu W., Croyle M.J., Welty E.A., Rosborough K.et al. (2006) Heightened epithelial Na+ channel-mediated Na+ absorption in a murine polycystic kidney disease model epithelium lacking apical monocilia. Am. J. Physiol. Cell Physiol. 290, C952–C963 10.1152/ajpcell.00339.2005 [DOI] [PubMed] [Google Scholar]
- 135.Veizis E.I., Carlin C.R. and Cotton C.U. (2004) Decreased amiloride-sensitive Na+ absorption in collecting duct principal cells isolated from BPK ARPKD mice. Am. J. Physiol. Renal Physiol. 286, F244–F254 10.1152/ajprenal.00169.2003 [DOI] [PubMed] [Google Scholar]
- 136.Veizis I.E. and Cotton C.U. (2005) Abnormal EGF-dependent regulation of sodium absorption in ARPKD collecting duct cells. Am. J. Physiol. Renal Physiol. 288, F474–F482 10.1152/ajprenal.00227.2004 [DOI] [PubMed] [Google Scholar]
- 137.Torres V.E., Young W.F. Jr, Offord K.P. and Hattery R.R. (1990) Association of hypokalemia, aldosteronism, and renal cysts. N. Engl. J. Med. 322, 345–351 10.1056/NEJM199002083220601 [DOI] [PubMed] [Google Scholar]
- 138.Sullivan L.P., Wallace D.P., Gover T., Welling P.A., Yamaguchi T., Maser R.et al. (2002) Sulfonylurea-sensitive K(+) transport is involved in Cl secretion and cyst trowth by cultured ADPKD cells. J. Am. Soc. Nephrol. 13, 2619–2627 10.1097/01.ASN.0000034220.09301.9C [DOI] [PubMed] [Google Scholar]
- 139.Albaqumi M., Srivastava S., Li Z., Zhdnova O., Wulff H., Itani O.et al. (2008) KCa3.1 potassium channels are critical for cAMP-dependent chloride secretion and cyst growth in autosomal-dominant polycystic kidney disease. Kidney Int. 74, 740–749 10.1038/ki.2008.246 [DOI] [PubMed] [Google Scholar]
- 140.Schwiebert E.M., Wallace D.P., Braunstein G.M., King S.R., Peti-Peterdi J., Hanaoka K.et al. (2002) Autocrine extracellular purinergic signaling in epithelial cells derived from polycystic kidneys. Am. J. Physiol. Renal Physiol. 282, F763–F775 10.1152/ajprenal.0337.2000 [DOI] [PubMed] [Google Scholar]
- 141.Turner C.M., King B.F., Srai K.S. and Unwin R.J. (2007) Antagonism of endogenous putative P2Y receptors reduces the growth of MDCK-derived cysts cultured in vitro. Am. J. Physiol. Renal Physiol. 292, F15–F25 10.1152/ajprenal.00103.2006 [DOI] [PubMed] [Google Scholar]
- 142.Buchholz B., Teschemacher B., Schley G., Schillers H. and Eckardt K.U. (2011) Formation of cysts by principal-like MDCK cells depends on the synergy of cAMP- and ATP-mediated fluid secretion. J. Mol. Med. (Berl.) 89, 251–261 10.1007/s00109-010-0715-1 [DOI] [PubMed] [Google Scholar]
- 143.Hillman K.A., Woolf A.S., Johnson T.M., Wade A., Unwin R.J. and Winyard P.J. (2004) The P2X7 ATP receptor modulates renal cyst development in vitro. Biochem. Biophys. Res. Commun. 322, 434–439 10.1016/j.bbrc.2004.07.148 [DOI] [PubMed] [Google Scholar]
- 144.Chang M.Y., Lu J.K., Tian Y.C., Chen Y.C., Hung C.C., Huang Y.H.et al. (2011) Inhibition of the P2X7 receptor reduces cystogenesis in PKD. J. Am. Soc. Nephrol. 22, 1696–1706 10.1681/ASN.2010070728 [DOI] [PMC free article] [PubMed] [Google Scholar]