Abstract
Objectives. To test a tailored mobile health (i.e., mHealth) intervention for waterpipe tobacco cessation in young adults.
Methods. From 2018 to 2020 at 2 US sites, we conducted a randomized trial with 349 waterpipe tobacco smokers aged 18 to 30 years randomized to control (no intervention), untailored, or tailored intervention arms. Intervention arms received a 6-week mHealth intervention conveying risks of waterpipe tobacco through text and images and strategies to enhance motivation and support quitting. The tailored intervention was personalized to baseline measures and intervention text message responses. Risk appraisals, motivation to quit, waterpipe smoking frequency, and cessation were assessed at 6 weeks, 3 months, and 6 months.
Results. At 6 months, cessation was higher in the tailored (49%) than the control arm (29%; odds ratio = 2.4; 95% confidence interval = 1.3, 4.2) and smoking frequency was lower in the tailored (mean = 3.5 days) than the control arm (mean = 4.3 days; P = .006). At interim follow-ups, significant differences in other outcomes favored the tailored intervention.
Conclusions. Tailored mobile messaging can help young adult waterpipe tobacco smokers quit. This scalable intervention is poised for population implementation.
Waterpipe (i.e., hookah) smoking is a method of tobacco use in which tobacco (usually sweetened or flavored) is heated with charcoal, smoke passes through water, and the smoke is inhaled by the user. Waterpipe tobacco smoking poses risks of health harm (e.g., cancer, cardiovascular disease, respiratory disease) and addictiveness, and is understudied relative to other forms of tobacco use.1–8 Among US adults, the prevalence of waterpipe tobacco smoking is low overall, but it is more common in certain subgroups (e.g., some racial/ethnic and sexual minorities) and most common among young adults aged 18 to 30 years.9–12 In the Population Assessment of Tobacco and Health (PATH) Study (wave 1, total n = 45 971), 11% of young adults were past-30-day waterpipe tobacco smokers, and young adults comprised 78% and 88% of adults who smoked waterpipe tobacco daily or weekly and monthly, respectively.10 Prospective PATH data show that although most young adults who smoke waterpipe tobacco do so intermittently (i.e., nondaily), many sustain use over time.13 Young adults’ waterpipe tobacco smoking is influenced by multiple factors, including appealing flavors, marketing, and use in social settings.14,15 Importantly, young adults’ misperceptions that waterpipe tobacco is not harmful or addictive are major factors contributing to waterpipe tobacco smoking.14–19 Young adults also have low motivation to quit waterpipe tobacco smoking and believe quitting is easy, yet many develop dependence symptoms and have difficulty quitting.6,20
There is very limited research on waterpipe tobacco smoking cessation interventions in young adults. A 2015 Cochrane review found only 3 intervention studies, 1 of which focused on young adults.21 Subsequent reviews included additional intervention studies,22,23 but all found limited evidence for cessation interventions targeting young people. Furthermore, many interventions studied to date have low appeal and are less likely to benefit young adults because they focus on exclusive, daily waterpipe tobacco smokers.21–23 The growth in young adult waterpipe use, associations with cigarette smoking,24 and research gaps have produced calls to develop interventions addressing use patterns (i.e., nondaily smoking) and underlying misperceptions about risks in young adults.21,23
A recent study piloted a personally tailored, mobile health (i.e., mHealth) messaging cessation intervention for young adult waterpipe smokers.25 Results demonstrated acceptability and feasibility of the intervention and preliminary effects on behavioral outcomes.25 mHealth is a promising strategy for waterpipe tobacco cessation interventions among young adults for several reasons. First, most US young adults own a mobile phone and use their phone for text messaging,26,27 and they are receptive to mHealth interventions.26,28 This positions mHealth interventions for high reach in the target population. Second, mobile messaging systems can deliver messages with text and visual imagery (i.e., multimedia message service; MMS); this approach can enhance the effects of tobacco messaging.29 Third, mHealth interventions are scalable with the potential to be freely available to the US population. Finally, mobile messaging systems can also deliver interventions interactively and tailor content to individual characteristics. Tailored messaging increased the effects of online and mHealth interventions for cigarette smoking cessation in previous studies.30–32
Pilot research25 supports the use of personally tailored mHealth interventions for waterpipe tobacco cessation, but they have not been tested rigorously. The goal of this study was to test the efficacy of an interactive mHealth cessation intervention in young adult waterpipe tobacco smokers and examine if a personally tailored intervention had added effects compared with an untailored intervention. The primary outcomes were risk appraisals (i.e., perceived risk, worry), cessation, waterpipe tobacco smoking frequency, and motivation to quit at 6 months. We also report results of secondary outcomes at interim time points (6 weeks, 3 months) based on recommendations for tobacco cessation trials.33
METHODS
This study was a 2-site, 3-arm, parallel group randomized trial. All participants provided informed consent, and the participating institutions’ institutional review boards approved all procedures.
Participants
From 2018 to 2020, we recruited participants from the community at 2 academic medical centers in the US Mid-Atlantic region. Recruitment advertisements sought young adults for a study about waterpipe tobacco beliefs and behavior and directed interested individuals to a Web site with study details and a link to an eligibility screener. Eligible participants were young adults aged 18 to 30 years who reported smoking waterpipe tobacco in the past month and on at least a monthly basis. We chose these behavioral eligibility criteria based on young adults’ waterpipe tobacco smoking patterns and previous pilot work to ensure participants smoked waterpipe tobacco with sufficient frequency for a cessation intervention.10,25 Eligible participants also had to be able to complete study procedures in English and agree to use a personal mobile phone to send and receive study text messages. There were no other explicit exclusion criteria (e.g., for other medical conditions or alcohol or substance use).
Procedures
Eligible individuals provided informed consent online, completed an online baseline assessment, and received basic information on the risks of waterpipe tobacco smoking.34,35 Participants were randomized to 1 of the 3 trial arms: control, untailored intervention, or tailored intervention. Participants completed follow-ups online 6 weeks, 3 months, and 6 months after baseline. Participants received incentives for completing study milestones ($20 at baseline, $25 at 6 weeks, $25 at 3 months, and $30 at 6 months).
Randomization
We randomized participants in a 1:1:1 ratio to the 3 trial arms; the randomization sequence was prepared in blocks by a statistician not involved in the trial. We stratified randomization by whether participants reported infrequent (i.e., monthly) or frequent (i.e., daily or weekly) waterpipe tobacco smoking at baseline to ensure balance by the trial arms.
Control Arm
Participants in the control arm received no intervention; they completed assessments only.
Intervention Arms
The intervention was a 6-week mobile messaging intervention. Descriptions of the message content development, pretesting, and the intervention pilot were published previously.34,36 Messages were delivered on 2 days each week for 6 weeks, a frequency and duration based on patterns of young adult waterpipe tobacco smoking10 and recommendations for mHealth interventions.37–39 The content was scheduled for all participants so the first message day occurred early in the week (Tuesday) and the second occurred before the weekend (Friday).
We developed the intervention based on recommendations for mobile interventions,40 recommendations for waterpipe tobacco interventions,41 and research on young adults’ waterpipe tobacco beliefs and behavior.35,42–45 The message content communicated the short- and long-term health harms, toxicant exposure, and addictiveness of waterpipe tobacco use.40 The content was sequenced to avoid repetition and introduce new information over time.
We developed the 12 message themes to align with misperceptions about risks of waterpipe tobacco use in young adults from previous research.44 Messages conveyed risks of waterpipe tobacco through text and visual imagery (i.e., MMS) with images selected to convey the core risk communicated in text.34,36 The intervention was designed to enhance motivation to quit by building behavioral skills, increasing confidence, and providing strategies for behavior change.46–48 Over 6 weeks, this progressed from thinking about risks to planning to avoid waterpipe tobacco smoking, incorporating behavioral substitutes, and making a plan to quit.
The first day was an introductory message preparing participants to start. Each message day thereafter, participants first responded to a text message prompt that engaged participants by posing questions about waterpipe tobacco use or beliefs about risks. After responding to the prompts, participants received the MMS risk message content.
In the untailored intervention arm, all participants received the same prompts and message content. In the tailored intervention arm, we personalized the MMS message content to participants’ baseline waterpipe tobacco smoking frequency, baseline risk beliefs, and responses to the prompts during the intervention. For waterpipe tobacco smoking frequency, we categorized participants as infrequent (i.e., monthly) or frequent (i.e., daily or weekly) smokers at baseline. For risk beliefs, we used a 12-item measure of beliefs about the health harms and addictiveness of waterpipe tobacco at baseline to tailor the messages.44 Each risk belief aligned with 1 of the messages, and we categorized participants’ responses to each baseline risk belief item as “low” indicating they do not believe waterpipe tobacco smoking to be risky or “high” risk beliefs that waterpipe smoking has greater risks for tailoring.36 We also tailored the content to participants’ responses to the text message prompts, such as whether they reported smoking waterpipe tobacco. Example intervention messages are provided in Table A (available as a supplement to the online version of this article at http://www.ajph.org).
Measures
At baseline, we assessed age, gender, race, Hispanic ethnicity, educational attainment, employment status, and household income.49 We measured cigarette smoking at baseline, defining cigarette smokers as those who have smoked at least 100 cigarettes in their lifetime and now smoke cigarettes every day or some days.49 We assessed past-30-day use of other tobacco (large cigars, little cigars, cigarillos, smokeless tobacco, electronic cigarettes)49 and summarized responses as any other tobacco use in the past 30 days (yes or no).35 We also captured number of days in the past 30 days drinking alcohol.49
We assessed waterpipe tobacco risk appraisals at all time points using 4 items—2 for harms and 2 for addiction.34,35,43 Perceived risk of harms (i.e., chance of disease) from smoking waterpipe tobacco was based on a 1 (no chance) to 7 (certain to happen) scale. Worry about harms was also measured on a 1 (not at all) to 7 (very much) scale. We used 2 similar items to measure perceived risk of addiction (1–7 scale) and worry about addiction (1–7 scale). Based on previous studies,34,35,43 we created a summary risk appraisals outcome by averaging responses to the 4 items at each time point (Cronbach’s α = .72 at baseline, .76 at 6 weeks, .75 at 3 months, and .80 at 6 months). We also analyzed each item separately, the results of which are shown in Table B (available as a supplement to the online version of this article at http://www.ajph.org).
At baseline, we assessed waterpipe tobacco use frequency and dependence. We asked whether participants usually smoked waterpipe tobacco monthly, weekly, or daily and categorized participants as infrequent (i.e., monthly) or frequent (i.e., daily or weekly) smokers.25,34,35 We assessed use frequency as the number of days in the past 30 days that participants smoked waterpipe tobacco.9 We administered the 6-item Waterpipe Tobacco Dependence Scale8 and summed the items to create a score (range = 0–25) with higher values indicating greater dependence (Cronbach’s α = .77).8
At the follow-ups, we used a series of items to assess waterpipe tobacco smoking frequency and cessation.9 The first item assessed whether participants smoked waterpipe tobacco “even 1 or 2 puffs” since the last assessment. Among those responding no, the next item asked whether they completely stopped smoking waterpipe tobacco (yes or no). This captured cessation at each follow-up as point-prevalence abstinence.33 Among those who had not quit, we assessed waterpipe tobacco smoking frequency at the follow-ups as described previously. For those who quit, we coded waterpipe tobacco smoking as zero at follow-ups. We analyzed as outcomes whether participants reported that they quit smoking waterpipe tobacco completely (yes or no) and the number of days in 30 days participants smoked waterpipe tobacco at each time point.
We measured motivation to quit smoking waterpipe tobacco at baseline and at the follow-ups among those who did not report quitting using a single item with a 1 (not at all) to 7 (very) scale.35,44
Statistical Analysis
We used descriptive statistics to characterize the sample overall and by arm. For risk appraisals and motivation to quit, we tested mean differences by trial arm at each time point using general linear models. Levene’s test confirmed homogeneity of variance assumptions for each model (i.e., all P > .05). We interpreted the F statistic for trial arm and pairwise differences in least squares means using Tukey’s adjustment for multiple comparisons.
For frequency of use, we used the Wilcoxon rank sum test for differences by trial arm. We interpreted the Kruskal‒Wallis χ2 statistic for trial arm and the Wilcoxon z test for pairwise comparisons between arms.
We used logistic regression to test if cessation differed by arm at each time point. We interpreted the χ2 statistic for trial arm and the odds ratios (ORs) and 95% confidence intervals (CIs) for differences in cessation between arms. We ran 2 models for this outcome. The first model used data from those completing follow-ups only. The second model assumed that all those lost to follow-up had not quit smoking waterpipe tobacco.
For all outcomes, our primary comparison was the 6-month time point; earlier time points were prespecified as secondary. Sensitivity analyses controlling for baseline covariates that were not balanced by randomization (gender, race, cigarette smoking) did not differ for any outcomes, so we report unadjusted results.
Sample Size
We conducted a priori power calculations to determine the sample size needed to test for differences in the primary outcomes at 6 months between the trial arms assuming 2-tailed α of .05, 80% power, and 80% retention at 6 months. To detect mean differences as small as Cohen’s d of 0.37 between trial arms in risk appraisals, motivation to quit, and use frequency and differences in cessation as small as 19% between trial arms, we needed to enroll 330 participants at baseline.
RESULTS
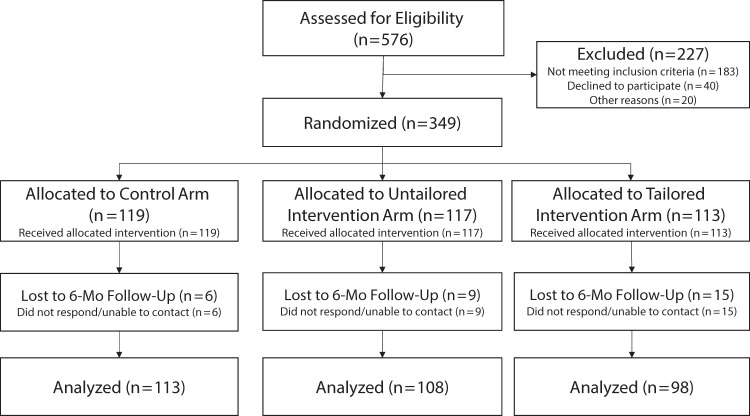
We screened 576 individuals for eligibility (Figure 1); 167 were ineligible (29%), 6 declined to participate (1%), 17 (3%) were withdrawn because they were later determined to be ineligible (e.g., provided inconsistent age), and we were unable to contact 37 (6%) after screening. In total, 349 participants enrolled and were randomized (Figure 1).
FIGURE 1—
Flow Diagram for Randomized Trial of a Mobile Messaging Intervention for Waterpipe Tobacco Cessation in Young Adults: United States, 2018‒2020
Table 1 displays baseline characteristics overall and by arm. Participants averaged 24.0 (SD = 3.4) years of age, 54% were female, 58% were non-White race, and 11.5% were Hispanic ethnicity. Nearly two thirds (65%) were frequent waterpipe smokers, and participants smoked waterpipe on average 11.5 (SD = 9.1) of the past 30 days. Overall, 29% were current cigarette smokers, and 68% reported other tobacco use. There were more cigarette smokers in the control arm, participants in the control arm were more likely to be female, and participants in the untailored arm were more likely to be White race.
TABLE 1—
Baseline Characteristics for Randomized Trial of a Mobile Messaging Intervention for Waterpipe Tobacco Cessation in Young Adults: United States, 2018‒2020
| Overall (n = 349), Mean ±SD or % (No.) |
Control (n = 119), Mean ±SD or % (No.) |
Untailored Intervention (n = 117), Mean ±SD or % (No.) |
Tailored Intervention (n = 113), Mean ±SD or % (No.) |
|
| Age | 24.0 ±3.4 | 23.9 ±3.4 | 23.7 ±3.5 | 24.6 ±3.5 |
| Gender | ||||
| Female | 53.6 (187) | 59.7 (71) | 48.7 (57) | 52.2 (59) |
| Male | 46.4 (162) | 40.3 (48) | 51.3 (60) | 47.8 (54) |
| Race | ||||
| White | 42.1 (147) | 42.9 (51) | 36.8 (43) | 46.9 (53) |
| Non-White | 57.9 (202) | 57.1 (68) | 63.2 (74) | 53.1 (60) |
| Hispanic ethnicity | ||||
| Yes | 11.5 (40) | 12.6 (15) | 12.0 (14) | 9.8 (11) |
| No | 88.3 (308) | 87.4 (104) | 88.0 (103) | 90.2 (101) |
| Education | ||||
| < college | 16.0 (56) | 13.4 (16) | 20.5 (24) | 14.2 (16) |
| Some college or higher | 84.0 (293) | 86.6 (103) | 79.5 (93) | 85.8 (97) |
| Employment | ||||
| Not full-time employed | 58.1 (203) | 56.3 (67) | 63.2 (74) | 46.0 (52) |
| Full-time employed | 44.7 (156) | 43.7 (52) | 36.8 (43) | 53.9 (61) |
| Annual household income, $ | ||||
| ≤ 50 000 | 65.3 (228) | 62.2 (74) | 63.2 (74) | 70.8 (80) |
| > 50 000 | 34.4 (120) | 37.8 (45) | 35.9 (42) | 29.2 (33) |
| Waterpipe smoking frequency | ||||
| Infrequent (i.e., monthly) | 34.7 (121) | 33.6 (40) | 35.0 (41) | 35.4 (40) |
| Frequent (i.e., weekly or daily) | 65.3 (228) | 66.4 (79) | 65.0 (76) | 64.6 (73) |
| Past-30-d waterpipe smoking, days | 11.3 ±9.1 | 11.1 ±9.2 | 10.6 ±8.7 | 12.2 ±9.3 |
| Waterpipe tobacco dependence | 6.7 ±5.3 | 7.0 ±5.6 | 6.4 ±4.9 | 6.7 ±5.4 |
| Motivation to quit waterpipe tobacco | 2.7 ±1.6 | 2.9 ±1.6 | 2.5 ±1.6 | 2.6 ±1.6 |
| Current cigarette smoker | 29.2 (102) | 37.8 (45) | 24.8 (29) | 24.8 (28) |
| Any other tobacco use, past 30 d | 67.9 (237) | 67.2 (80) | 69.2 (81) | 67.2 (80) |
| Days drinking alcohol, past 30 d | 7.5 ±7.0 | 8.4 ±7.6 | 6.7 ±6.1 | 7.4 ±7.2 |
Note. For some variables (e.g., Hispanic ethnicity), numbers for categories do not sum to the total sample size because of sporadic missing data (1 or 2 cases in each instance).
Retention was 93% at 6 weeks (n = 324), 93% at 3 months (n = 325), and 91% at 6 months (n = 319). Attrition at the 3-month and 6-month follow-ups was higher in the tailored intervention arm (11% and 13%) than the control (3% and 5%) and untailored intervention (7% and 8%) arms.
There were no significant differences in risk appraisals between trial arms at 3 months or 6 months (Table 2). At 6 weeks, the effect of trial arm was significant (F2324 = 3.1; P = .045). Risk appraisals were significantly greater in the tailored arm (mean = 4.2; 95% CI = 3.9, 4.4) than the control arm (mean = 3.8; 95% CI = 3.5, 4.0; P = .039). Results from analyses of individual items are shown in Table B.
TABLE 2—
Risk Appraisals, Past-30-Day Waterpipe Tobacco Smoking Frequency, and Motivation to Quit by Trial Arm: United States, 2018‒2020
| Baseline (n = 349), Mean (95% CI) | 6 Weeks (n = 324), Mean (95% CI) | 3 Months (n = 325), Mean (95% CI) | 6 Months (n = 319), Mean (95% CI) | |
| Risk appraisals | ||||
| Control (A) | 3.7 (3.5, 3.8) | 3.8C (3.5, 4.0) | 4.0 (3.7, 4.2) | 4.0 (3.8, 4.3) |
| Untailored (B) | 3.4 (3.2, 3.6) | 3.9 (3.6, 4.1) | 3.9 (3.7, 4.2) | 4.0 (3.8, 4.3) |
| Tailored (C) | 3.5 (3.3, 3.7) | 4.2A (3.9, 4.4) | 4.0 (3.8, 4.3) | 4.3 (4.0, 4.5) |
| Waterpipe tobacco smoking frequency | ||||
| Control (A) | 11.1 (9.4, 12.8) | 7.8C (6.2, 9.5) | 6.1B,C (4.6, 7.5) | 4.3C (3.0, 5.6) |
| Untailored (B) | 10.6 (9.0, 12.2) | 5.4 (4.0, 6.8) | 4.6A (3.2, 5.9) | 4.0 (2.6, 5.2) |
| Tailored (C) | 12.2 (10.4, 13.9) | 5.4A (4.0, 6.8) | 4.3A (3.0, 5.7) | 3.5A (2.0, 5.0) |
| Motivation to quit | ||||
| Control (A) | 2.9 (2.6, 3.2) | 3.3C (3.0, 3.6) | 3.8 (3.4, 4.2) | 4.0 (3.5, 4.4) |
| Untailored (B) | 2.5 (2.2, 2.8) | 3.8 (3.4, 4.2) | 3.9 (3.4, 4.3) | 4.0 (3.5, 4.4) |
| Tailored (C) | 2.6 (2.3, 2.9) | 4.1A (3.7, 4.5) | 3.9 (3.4, 4.4) | 3.5 (3.0, 4.1) |
Note. CI = confidence interval. For each time point, means for each outcome with different superscript letters differed significantly from the trial arm indicated (A = control; B = untailored; C = tailored) at P < .05. For risk appraisals and motivation to quit, comparisons of means are from general linear models with Tukey’s adjustment for pairwise comparisons. For waterpipe tobacco smoking frequency, comparison of means is from Wilcoxon rank sum test and z test P values for pairwise comparisons. Waterpipe tobacco smoking frequency included all participants with those who quit at a given time point coded as 0. Motivation to quit only included those who had not quit smoking waterpipe tobacco at a given time point.
At 6 months, waterpipe tobacco smoking frequency (Table 2) was significantly lower in the tailored arm (mean = 3.5 days; 95% CI = 2.0, 5.0) than the control arm (mean = 4.3 days; 95% CI = 3.0, 5.6; Kruskal‒Wallis χ2 for trial arm [2 df] = 9.2; P = .010; Wilcoxon z = −3.1; P = .006). At 6 weeks, smoking frequency was also significantly lower in the tailored arm than the control arm, and at 3 months it was significantly lower in the untailored and tailored arms than the control arm (Table 2).
Among those who did not quit smoking waterpipe tobacco, there were no significant differences in motivation to quit at 3 (F2240 = 0.08; P = .923) or 6 months by trial arm (F2195 = 0.93; P = .398; Table 2). Motivation to quit was significantly greater at 6 weeks in the tailored arm than the control arm (Table 2).
Table 3 shows outcomes for cessation. Using available data, at 6 months, cessation was significantly higher in the tailored arm (49%; OR = 2.4; 95% CI = 1.3, 4.2) than the control arm (29%; χ2 for trial arm [2 df] = 8.8; P = .012). At 6 weeks and 3 months, cessation was significantly higher in the untailored and tailored arms than the control arm (Table 3). Assuming those lost to follow-up continued smoking waterpipe tobacco (Table 3), at 6 months, cessation was significantly higher in the tailored arm (43%; OR = 1.9; 95% CI = 1.1, 3.3) than the control arm (28%) but the overall effect of arm was no longer significant (χ2 for trial arm [2 df] = 5.5; P = .064). At 6 weeks and 3 months, cessation was significantly higher in the untailored and tailored arms than the control arm (Table 3).
TABLE 3—
Waterpipe Tobacco Cessation by Trial Arm at Follow-Up Time Points: United States, 2018‒2020
| 6 Weeks (n = 324) | 3 Months (n = 325) | 6 Months (n = 319) | ||||
| % | OR (95% CI) | % | OR (95% CI) | % | OR (95% CI) | |
| Available data | ||||||
| Control | 10 | 1 (Ref) | 12 | 1 (Ref) | 29 | 1 (Ref) |
| Untailored | 24 | 2.8 (1.3, 5.8) | 28 | 2.9 (1.4, 5.7) | 38 | 1.5 (0.9, 2.6) |
| Tailored | 22 | 2.5 (1.2, 5.2) | 36 | 4.1 (2.1, 8.3) | 49 | 2.4 (1.3, 4.2) |
| Assume lost to follow up continued smoking | ||||||
| Control | 10 | 1 (Ref) | 12 | 1 (Ref) | 28 | 1 (Ref) |
| Untailored | 22 | 2.5 (1.2, 5.3) | 27 | 2.7 (1.4, 5.4) | 35 | 1.4 (0.8, 2.4) |
| Tailored | 20 | 2.3 (1.1, 4.8) | 33 | 3.7 (1.8, 7.2) | 43 | 1.9 (1.1, 3.3) |
Note. CI = confidence interval; OR = odds ratio. Table displays percentage reporting cessation and ORs (95% CIs) for cessation in the untailored and tailored intervention arms relative to the control arm at each time point. The first model with available data at each time point excludes those lost to follow-up. The second model at each time point assumes those lost to follow-up did not quit (i.e., continued smoking waterpipe tobacco).
DISCUSSION
Our results demonstrate that a tailored mHealth messaging intervention increased cessation and decreased waterpipe tobacco smoking frequency among young adults. Although both the tailored and the untailored interventions affected outcomes at interim time points, for behavioral outcomes only, the tailored intervention effects were sustained to 6 months. These results build on previous research on waterpipe tobacco risk messages34,35,45 by testing mHealth message delivery, demonstrating tailored messaging effects, and capturing behavioral outcomes.
There is limited research on waterpipe tobacco smoking cessation interventions for young adults21–23 even though this is the age group in the United States when waterpipe tobacco smoking is most common.9–12 This study is the first, to our knowledge, to demonstrate the efficacy of a tailored mHealth cessation intervention in young adult waterpipe tobacco smokers over a 6-month follow-up, filling a critical research gap. The mHealth intervention is highly scalable, aligning with major public health agencies’ efforts to make mobile cessation interventions freely available. For example, the National Cancer Institute offers mHealth cessation programs for cigarette smoking and smokeless tobacco cessation, but not waterpipe tobacco cessation.50 Our study provides the first evidence for a mHealth waterpipe tobacco smoking cessation intervention that can be implemented in this manner. Notably, waterpipe tobacco smoking is less prevalent than other forms of tobacco use (e.g., cigarette smoking) in the US population, but it is most common among young adults and it is associated with subsequent cigarette smoking initiation.24 From a public health perspective, this intervention could be impactful if it is made available with other interventions designed to prevent and reduce tobacco use in young people overall.
A recent prospective analysis of US young adults’ waterpipe tobacco smoking provides context for our findings.13 Sharma et al. examined past-12-month waterpipe tobacco smoking over 3 years of PATH Study data, finding that 42% of young adults who smoked waterpipe tobacco at wave 1 continued smoking over the 3-year period, 47% discontinued by wave 3, and 11% discontinued at wave 2 and resumed smoking at wave 3.13 This analysis examined past-12-month use, and it is unclear if “discontinuing” reflects cessation or intermittent use. However, the findings highlight the need to examine intervention outcomes over an extended follow-up. Some intervention effects we observed diminished over time, and assessing longer-term outcomes in the future will be important to determine if the effects are sustained and to assess maintenance of cessation and relapse.51,52 This can guide future steps to optimize our intervention, such as testing adaptive models that provide additional support for those who do not quit or who relapse.53
Notably, many young adult waterpipe tobacco smokers are dual or poly tobacco users of other tobacco products.13,54 In our sample, nearly one third were cigarette smokers, and roughly two thirds used other tobacco. Although we observed intervention effects on waterpipe tobacco smoking, it is unclear if the intervention reduced tobacco use overall. Smoking cessation research has focused predominantly on exclusive tobacco product users (e.g., cigarette smokers) and existing interventions do not address dual or poly use.55 Given the high prevalence of dual and poly use in young adults in general55 and in young adult waterpipe smokers,13,54 in future research it will be important to examine how interventions targeting waterpipe tobacco smoking affect other tobacco use outcomes in dual and poly users.
Limitations
This study has several important strengths, including a carefully developed mHealth intervention, rigorous trial design, and high retention. However, the findings should be interpreted in light of study limitations. We used remote (e.g., online, mobile) procedures for recruitment, data collection, and intervention delivery. These methods are increasingly used to improve efficiency of smoking cessation trials56; however, they are subject to limitations (e.g., potential reporting biases) that should be considered when interpreting the findings. We measured cessation by self-report. Although biochemically verified cessation is a gold standard in clinical trials,57 established biomarkers (e.g., exhaled carbon monoxide, cotinine) cannot verify waterpipe tobacco smoking cessation in a population in which use of other combustible (e.g., cigarettes) and noncombustible (e.g., electronic cigarettes) products is common. Finally, assessing outcomes over a longer follow-up will provide more robust evidence on long-term intervention effects. We examined outcomes to 6 months as recommended for cessation trials,33 but this will be important to understand if the effects are sustained.
Public Health Implications
This trial is the first, to our knowledge, to demonstrate the efficacy of a tailored mHealth messaging intervention for waterpipe tobacco smoking cessation in young adults. This is a scalable intervention model that aligns with ongoing efforts to make mHealth cessation interventions freely available to populations that need them. This study advances the science on waterpipe tobacco smoking cessation interventions, and the results suggest several important areas for further study. This includes examining long-term outcomes to assess if the effects are sustained and identify intervention optimization strategies for those who do not quit or who relapse, and examining intervention effects on other tobacco use in young adult dual and poly users.
ACKNOWLEDGMENTS
This work was supported by the National Cancer Institute of the National Institutes of Health (NIH) under award R01CA217861.
The results of this study were presented at the 2021 Annual Meeting of the Society for Research on Nicotine and Tobacco.
The data for this study were collected while L. Phan was a postdoctoral fellow at Georgetown University Medical Center.
Note. The study sponsors had no role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to disclose.
HUMAN PARTICIPANT PROTECTION
The study protocol was approved by the institutional review boards at Georgetown University and Duke University. The protocol for data analysis was also approved by the institutional review board at The Ohio State University.
Footnotes
See also Busch et al., p. 1567.
REFERENCES
- 1.El-Zaatari ZM, Chami HA, Zaatari GS. Health effects associated with waterpipe smoking. Tob Control. 2015;24(suppl 1):i31–i43. doi: 10.1136/tobaccocontrol-2014-051908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haddad L, Kelly DL, Weglicki LS, Barnett TE, Ferrell AV, Ghadban R. A systematic review of effects of waterpipe smoking on cardiovascular and respiratory health outcomes. Tob Use Insights. 2016;9:13–28. doi: 10.4137/TUI.S39873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Montazeri Z, Nyiraneza C, El-Katerji H, Little J. Waterpipe smoking and cancer: systematic review and meta-analysis. Tob Control. 2017;26(1):92–97. doi: 10.1136/tobaccocontrol-2015-052758. [DOI] [PubMed] [Google Scholar]
- 4.Shihadeh A, Schubert J, Klaiany J, El Sabban M, Luch A, Saliba NA. Toxicant content, physical properties and biological activity of waterpipe tobacco smoke and its tobacco-free alternatives. Tob Control. 2015;24(suppl 1):i22–i30. doi: 10.1136/tobaccocontrol-2014-051907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Waziry R, Jawad M, Ballout RA, Al Akel M, Akl EA. The effects of waterpipe tobacco smoking on health outcomes: an updated systematic review and meta-analysis. Int J Epidemiol. 2017;46(1):32–43. doi: 10.1093/ije/dyw021. [DOI] [PubMed] [Google Scholar]
- 6.Aboaziza E, Eissenberg T. Waterpipe tobacco smoking: what is the evidence that it supports nicotine/tobacco dependence? Tob Control. 2015;24(suppl 1):i44–i53. doi: 10.1136/tobaccocontrol-2014-051910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bahelah R, DiFranza JR, Fouad FM, Ward KD, Eissenberg T, Maziak W. Early symptoms of nicotine dependence among adolescent waterpipe smokers. Tob Control. 2016;25(e2):e127–e134. doi: 10.1136/tobaccocontrol-2015-052809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sidani JE, Shensa A, Shiffman S, Switzer GE, Primack BA. Behavioral associations with waterpipe tobacco smoking dependence among US young adults. Addiction. 2016;111(2):351–359. doi: 10.1111/add.13163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kasza KA, Ambrose BK, Conway KP, et al. Tobacco-product use by adults and youths in the United States in 2013 and 2014. N Engl J Med. 2017;376(4):342–353. doi: 10.1056/NEJMsa1607538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robinson JN, Wang B, Jackson K, Donaldson E, Ryant C. Characteristics of hookah tobacco smoking sessions and correlates of use frequency among US adults: findings from wave 1 of the Population Assessment of Tobacco and Health (PATH) Study. Nicotine Tob Res. 2018;20(6):731–740. doi: 10.1093/ntr/ntx060. [DOI] [PubMed] [Google Scholar]
- 11.Salloum RG, Thrasher JF, Kates FR, Maziak W. Water pipe tobacco smoking in the United States: findings from the National Adult Tobacco Survey. Prev Med. 2015;71:88–93. doi: 10.1016/j.ypmed.2014.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang TW, Asman K, Gentzke AS, et al. Tobacco product use among adults—United States, 2017. MMWR Morb Mortal Wkly Rep. 2018;67(44):1225–1232. doi: 10.15585/mmwr.mm6744a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma E, Bansal-Travers M, Edwards KC, et al. Longitudinal pathways of exclusive and polytobacco hookah use among youth, young adults and adults in the USA: findings from the PATH Study Waves 1‒3 (2013‒2016) Tob Control. 2020;29(suppl 3):s155–s162. doi: 10.1136/tobaccocontrol-2020-055625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akl EA, Jawad M, Lam WY, Co CN, Obeid R, Irani J. Motives, beliefs and attitudes towards waterpipe tobacco smoking: a systematic review. Harm Reduct J. 2013;10(1):12. doi: 10.1186/1477-7517-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akl EA, Ward KD, Bteddini D, et al. The allure of the waterpipe: a narrative review of factors affecting the epidemic rise in waterpipe smoking among young persons globally. Tob Control. 2015;24(suppl 1):i13–i21. doi: 10.1136/tobaccocontrol-2014-051906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elton-Marshall T, Driezen P, Fong GT, et al. Adult perceptions of the relative harm of tobacco products and subsequent tobacco product use: longitudinal findings from waves 1 and 2 of the Population Assessment of Tobacco and Health (PATH) study. Addict Behav. 2020;106:106337. doi: 10.1016/j.addbeh.2020.106337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hair E, Rath JM, Pitzer L, et al. Trajectories of hookah use: harm perceptions from youth to young adulthood. Am J Health Behav. 2017;41(3):240–247. doi: 10.5993/AJHB.41.3.3. [DOI] [PubMed] [Google Scholar]
- 18.Sidani JE, Shensa A, Naidu M, Yabes J, Primack B. Initiation, progression, and sustained waterpipe use: a nationally representative longitudinal study of US young adults. Cancer Epidemiol Biomarkers Prev. 2017;26(5):748–755. doi: 10.1158/1055-9965.EPI-16-0687-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Villanti AC, Cobb CO, Cohn AM, Williams VF, Rath JM. Correlates of hookah use and predictors of hookah trial in US young adults. Am J Prev Med. 2015;48(6):742–746. doi: 10.1016/j.amepre.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 20.Mays D, Tercyak KP, Rehberg K, Crane MK, Lipkus IM. Young adult waterpipe tobacco users’ perceived addictiveness of waterpipe tobacco. Tob Prev Cessat. 2017;3:133. doi: 10.18332/tpc/80133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maziak W, Jawad M, Jawad S, Ward KD, Eissenberg T, Asfar T. Interventions for waterpipe smoking cessation. Cochrane Database Syst Rev. 2015;(7):CD005549. doi: 10.1002/14651858.CD005549.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gardner K, Kearns R, Woodland L, et al. A scoping review of the evidence on health promotion interventions for reducing waterpipe smoking: implications for practice. Front Public Health. 2018;6:308. doi: 10.3389/fpubh.2018.00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jawad M, Jawad S, Waziry RK, Ballout RA, Akl EA. Interventions for waterpipe tobacco smoking prevention and cessation: a systematic review. Sci Rep. 2016;6(1):25872. doi: 10.1038/srep25872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Al Oweini D, Jawad M, Akl EA. The association of waterpipe tobacco smoking with later initiation of cigarette smoking: a systematic review and meta-analysis exploring the gateway theory. Tob Control. 2019 doi: 10.1136/tobaccocontrol-2018-054870. [DOI] [PubMed] [Google Scholar]
- 25.Mays D, Phan L, Johnson AC, et al. Results of a single arm pilot study of a mobile messaging intervention for hookah tobacco cessation in young adults. Tob Use Insights. 2020;13:1179173x20915200. doi: 10.1177/1179173X20915200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Villanti AC, Johnson AL, Ilakkuvan V, Jacobs MA, Graham AL, Rath JM. Social media use and access to digital technology in US young adults in 2016. J Med Internet Res. 2017;19(6):e196. doi: 10.2196/jmir.7303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mobile fact sheet. Internet & Technology. Washington, DC: Pew Research Center; 2019. [Google Scholar]
- 28.Abroms LC, Ahuja M, Kodl Y, et al. Text2Quit: results from a pilot test of a personalized, interactive mobile health smoking cessation program. J Health Commun. 2012;17(suppl 1):44–53. doi: 10.1080/10810730.2011.649159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noar SM, Hall MG, Francis DB, Ribisl KM, Pepper JK, Brewer NT. Pictorial cigarette pack warnings: a meta-analysis of experimental studies. Tob Control. 2016;25(3):341–354. doi: 10.1136/tobaccocontrol-2014-051978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Head KJ, Noar SM, Iannarino NT, Grant Harrington N. Efficacy of text messaging-based interventions for health promotion: a meta-analysis. Soc Sci Med. 2013;97:41–48. doi: 10.1016/j.socscimed.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 31.Spohr SA, Nandy R, Gandhiraj D, Vemulapalli A, Anne S, Walters ST. Efficacy of SMS text message interventions for smoking cessation: a meta-analysis. J Subst Abuse Treat. 2015;56:1–10. doi: 10.1016/j.jsat.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 32.Ybarra ML, Jiang Y, Free C, Abroms LC, Whittaker R. Participant-level meta-analysis of mobile phone-based interventions for smoking cessation across different countries. Prev Med. 2016;89:90–97. doi: 10.1016/j.ypmed.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Piper ME, Bullen C, Krishnan-Sarin S, et al. Defining and measuring abstinence in clinical trials of smoking cessation interventions: an updated review. Nicotine Tob Res. 2020;22(7):1098–1106. doi: 10.1093/ntr/ntz110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mays D, Johnson AC, Phan L, Tercyak KP, Rehberg K, Lipkus I. Effect of risk messages on risk appraisals, attitudes, ambivalence, and willingness to smoke hookah in young adults. Health Psychol Behav Med. 2020;8(1):96–109. doi: 10.1080/21642850.2020.1730844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mays D, Tercyak KP, Lipkus IM. The effects of brief waterpipe tobacco use harm and addiction education messages among young adult waterpipe tobacco users. Nicotine Tob Res. 2016;18(5):777–784. doi: 10.1093/ntr/ntv223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson AC, Lipkus I, Tercyak KP, et al. Development and pretesting of risk-based mobile multimedia message content for young adult hookah use. Health Educ Behav. 2019;46(2 suppl):97–105. doi: 10.1177/1090198119874841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Evans W, Nielsen P, Szekely D, et al. Dose‒response effects of the text4baby mobile health program: randomized controlled trial. JMIR Mhealth Uhealth. 2015;3(1):e12. doi: 10.2196/mhealth.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Free C, Phillips G, Galli L, et al. The effectiveness of mobile-health technology-based health behaviour change or disease management interventions for health care consumers: a systematic review. PLoS Med. 2013;10(1):e1001362. doi: 10.1371/journal.pmed.1001362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Naughton F, Jamison J, Boase S, et al. Randomized controlled trial to assess the short-term effectiveness of tailored web- and text-based facilitation of smoking cessation in primary care (iQuit in practice) Addiction. 2014;109(7):1184–1193. doi: 10.1111/add.12556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abroms LC, Whittaker R, Free R, Mendel Van Alystne J, Schindler-Ruwisch J. Developing and pretesting a text messaging program for health behavior change: recommended steps. JMIR Mhealth Uhealth. 2015;3(4):e107. doi: 10.2196/mhealth.4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O’Neill N, Dogar O, Jawad M, Kellar I, Kanaan M, Siddiqi K. Which behavior change techniques may help waterpipe smokers to quit? An expert consensus using a modified Delphi technique. Nicotine Tob Res. 2018;20(2):154–160. doi: 10.1093/ntr/ntw297. [DOI] [PubMed] [Google Scholar]
- 42.Hornik RC, Volinsky A, Mannisb S, et al. Validating the Hornik & Wolfe approach to choosing media campaign themes: do promising beliefs predict behavior change in a longitudinal study? Commun Methods Meas. 2019;13(1):60–68. doi: 10.1080/19312458.2018.1515902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lipkus IM, Eissenberg T, Schwartz-Bloom RD, Prokhorov AV, Levy J. Affecting perceptions of harm and addiction among college waterpipe tobacco smokers. Nicotine Tob Res. 2011;13(7):599–610. doi: 10.1093/ntr/ntr049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lipkus IM, Mays D. Comparing harm beliefs and risk perceptions among young adult waterpipe tobacco smokers and nonsmokers: implications for cessation and prevention [erratum in Addict Behav Rep. 2021;13:100333]. Addict Behav Rep. 20187103–110. 10.1016/j.abrep.2018.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lipkus IM, Mays D, Tercyak KP. Characterizing young adults’ susceptibility to waterpipe tobacco use and their reactions to messages about product harms and addictiveness. Nicotine Tob Res. 2017;19(10):1216–1223. doi: 10.1093/ntr/ntw251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baer JS, Kivlahan DR, Donovan DM. Integrating skills training and motivational therapies. Implications for the treatment of substance dependence. J Subst Abuse Treat. 1999;17(1-2):15–23. doi: 10.1016/S0740-5472(98)00072-5. [DOI] [PubMed] [Google Scholar]
- 47.Tobacco Use and Dependence Guideline Panel. Treating tobacco use and dependence: 2008 Update. Rockville, MD: US Department of Health and Human Services; 2008. [Google Scholar]
- 48.Vidrine JI, Reitzel LR, Figueroa PY, et al. Motivation and problem solving (MAPS): motivationally based skills training for treating substance use. Cogn Behav Pract. 2013;20(4):501–516. doi: 10.1016/j.cbpra.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hu SS, Neff L, Agaku IT, et al. Tobacco product use among adults—United States, 2013‒2014. MMWR Morb Mortal Wkly Rep. 2016;65(27):685–691. doi: 10.15585/mmwr.mm6527a1. [DOI] [PubMed] [Google Scholar]
- 50.National Cancer Institute. 2021. https://smokefree.gov/tools-tips/text-programs
- 51.Ockene JK, Emmons KM, Mermelstein RJ, et al. Relapse and maintenance issues for smoking cessation. Health Psychol. 2000;19(1 suppl):17–31. doi: 10.1037/0278-6133.19.Suppl1.17. [DOI] [PubMed] [Google Scholar]
- 52.Piasecki TM. Relapse to smoking. Clin Psychol Rev. 2006;26(2):196–215. doi: 10.1016/j.cpr.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 53.Collins LM, Nahum-Shani I, Almirall D. Optimization of behavioral dynamic treatment regimens based on the Sequential, Multiple Assignment, Randomized Trial (SMART) Clin Trials. 2014;11(4):426–434. doi: 10.1177/1740774514536795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Osibogun O, Taleb ZB, Bahelah R, Salloum RG, Maziak W. Correlates of poly-tobacco use among youth and young adults: findings from the Population Assessment of Tobacco and Health study, 2013‒2014. Drug Alcohol Depend. 2018;187:160–164. doi: 10.1016/j.drugalcdep.2018.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pacek LR, Villanti AC, Mcclernon FJ. Not quite the rule, but no longer the exception: multiple tobacco product use and implications for treatment, research, and regulation. Nicotine Tob Res. 2020;22(11):2114–2117. doi: 10.1093/ntr/ntz221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dahne J, Tomko RL, McClure EA, Obeid JS, Carpenter MJ. Remote methods for conducting tobacco-focused clinical trials. Nicotine Tob Res. 2020;22(12):2134–2140. doi: 10.1093/ntr/ntaa105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Benowitz NL, Bernert JT, Foulds J, et al. Biochemical verification of tobacco use and abstinence: 2019 update. Nicotine Tob Res. 2020;22(7):1086–1097. doi: 10.1093/ntr/ntz132. [DOI] [PMC free article] [PubMed] [Google Scholar]